Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Open surgery for the treatment of aortic pathologies is technically demanding for surgeons and invasive for the patient. It is a major physiological insult often resulting in significant morbidity and mortality. Mortality rates from elective surgery of open thoracic and abdominal aneurysms are in the region of 8% and 5%, respectively. Additionally, the cohort of patients requiring aortic intervention commonly have a significant number of comorbidities, including ischaemic heart disease, cerebrovascular, renovascular and peripheral vascular disease, as well as respiratory impairment.
These considerations have driven the development of minimally invasive endovascular therapies for aortic disease. Endovascular repair of the aorta using covered stents (stent-grafts) was first described in 1991 and is generally associated with a smaller physiological insult and lower early mortality than open surgical repair. Although the current generation of stent-grafts are easier to insert and perform better in the long term compared with their predecessors, they are by no means a panacea for all aortic pathology.
A multidisciplinary approach to aortic disease is vital and should include interventional radiologists, cardiothoracic and vascular surgeons, vascular anaesthetists and device specialists (e.g. company representatives). This multidisciplinary approach ensures that the patient receives the most appropriate treatment, be it open surgery, endovascular repair or medical management.
This chapter will discuss the endovascular management of commonly encountered aortic pathologies, with particular focus on the indications, the anatomical and technical considerations as well as the complications.
The management of patients with aortic disease is complex and should not be undertaken without adequate supportive infrastructure, staffing, processes and governance. The decision on how (and whether) to treat relies on detailed anatomical, physiological and clinical information. Patients should undergo a formal assessment of their general cardiovascular fitness (such as a cardiopulmonary exercise test [CPET]) with treatment and optimisation of any underlying comorbidities. Once clinical information and data on aortic morphology and cardiovascular fitness are available, the patient should be discussed at a multidisciplinary team meeting and a plan for treatment agreed. In an emergency, such detailed work-up is clearly impossible, although there is occasionally time to optimise the patient's physiology to some extent.
Endovascular aortic repair should be carried out by experienced staff within a sterile environment of theatre standard, with optimal imaging facilities and equipment to convert rapidly to open repair in an emergency. The ideal is an angiography room/hybrid theatre with fixed C-arm image intensification and access to fusion imaging to guide treatment. Ideally, there should be availability of CO 2 and gadolinium angiography for patients with contrast allergy or risk of contrast-induced renal failure. Three-dimensional imaging or on-table Cone beam computed tomography (CBCT) ( Fig. 79.1 ) can offer additional on-table valuable information and immediate post-procedure assessment, especially during complex aortic procedures. Postoperative care in an intensive care or high-dependency unit should be available. Vascular surgical, anaesthetic and radiological support should be available on a 24/7 basis.
The principal aim of stent-grafting procedures is to line the inside of a vessel. In the case of an aneurysm, the stent-graft is designed to line the aneurysm sac so that it is isolated from the circulation to prevent sac expansion and subsequent aneurysm rupture. Over the years, innovation in stent-graft technology has improved the ease of deployment, extending the range of patients suitable for endovascular treatment.
The basic stent-graft ( Fig. 79.2 ) is composed of a fabric tube, usually woven polyester or expanded polytetrafluoroethylene (PTFE). The scaffold of stent-grafts is composed of circular, Z -shaped or crown-shaped metal struts [‘ring stents’], usually made of nitinol or Elgiloy and is attached to the fabric tube using sutures or glue.

Some devices have uncovered stents at one end to provide additional support and fixation above or below the covered portion of the device. The stent-graft is held in place by the radial force of the ring stents, which produce friction against the aortic wall and prevent distal device migration. Some devices have small hooks (usually at the top end), which engage with the aortic wall to prevent migration. Stents can vary in shape depending on indication and location of use. Most abdominal aortic aneurysm (AAA) stent-graft systems comprise a main body with a long limb on one side and a short limb (or ‘contralateral gate’) on the other ( Fig. 79.3 ). Once the main body is deployed, the short limb is cannulated from the opposite side. A second limb is then inserted over a wire into the contralateral gate, sealing inside the main body at a flow divider mimicking the aortoiliac arterial anatomy.
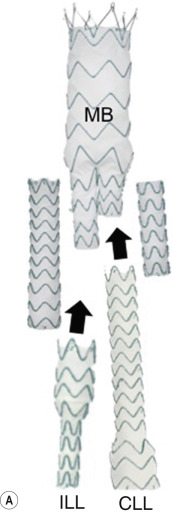
In addition to straight tube and trouser grafts, there are now complex fenestrated and branched stent-grafts. These devices are useful in peri- and transvisceral aneurysms (Type IV Crawford classification discussed later in the chapter) and also thoracic aortic repairs where the proximal graft end has to extend proximal to main branches including the carotid and subclavian arteries. Other devices include parallel stent-grafts, a group of devices (chimney, snorkels and sandwich stent-grafts) that share a common feature whereby a stent-graft is deployed within the target branch vessel and follows a path within the aorta parallel to the main stent-graft device ( Fig. 79.4 ). Other devices that have been utilised include endovascular-sealing system (EVAS) ( Fig. 79.5 ). These systems, which are currently for limited use only, are tube stents with a circumferential plastic bag delivered via a femoral arterial access into position and then filled with a polymer. Device adjuncts are also available on the market, such as Endoanchors, which are designed to anchor the fabric to the aortic wall using a corkscrew-like device and are used to prevent and treat proximal type 1 endoleaks.
Most manufacturers make a range of stent-graft diameters and lengths to allow ‘off-the-shelf’ device selection. Complex devices (such as fenestrated stent-grafts or in sizes outside the normally manufactured ranges) can be ordered but these are expensive with an associated manufacturing lead-time. Newer, flexible fenestrated devices may soon also become available. Currently, the approximate cost of a typical single thoracic aortic stent-graft is approximately £10k, a bifurcated abdominal aortic device costs approximately £6k and a custom-made device can cost £15k or more plus VAT.
Preoperative planning using good-quality high-resolution imaging is required to choose the correct stent-graft for a particular aneurysm. This is usually achieved by thin-section contrast-enhanced arterial phase computed tomography (CT) to image the whole of the aorta and the access vessels in a volumetric acquisition. Magnetic resonance (MR) angiography is an alternative that is currently increasingly being utilised. Multiplanar reformatting software allows measurement of true axial diameters and vessel length. Expert systems are available to automate this process, though many clinicians prefer to do it by hand.
To achieve a good ‘seal’ between the device and the aortic wall, the stent-graft is usually oversized relative to the aorta by 15% to 20%.
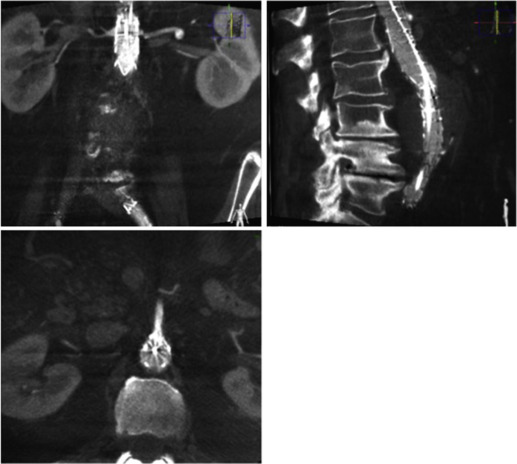
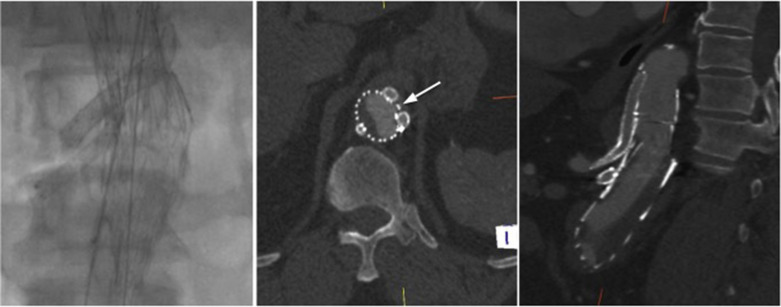
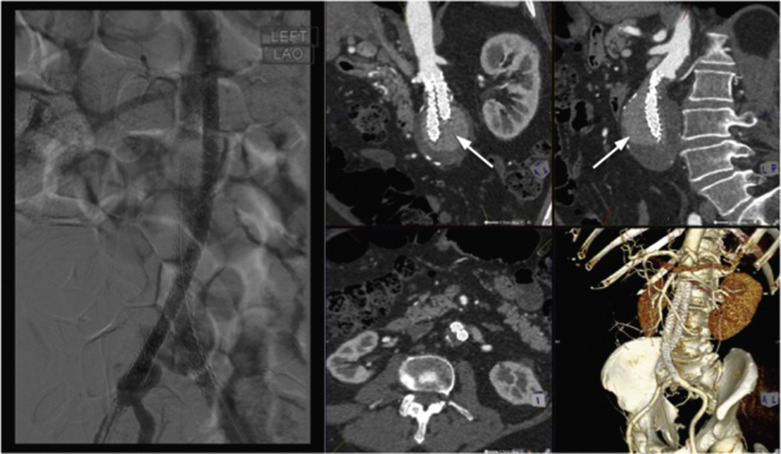
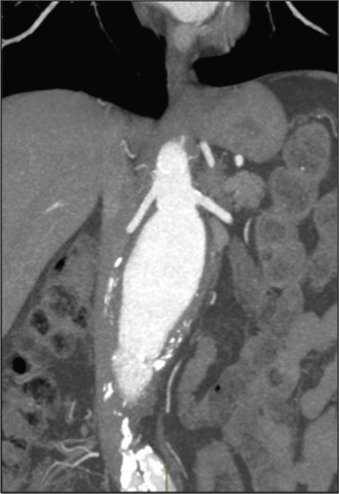
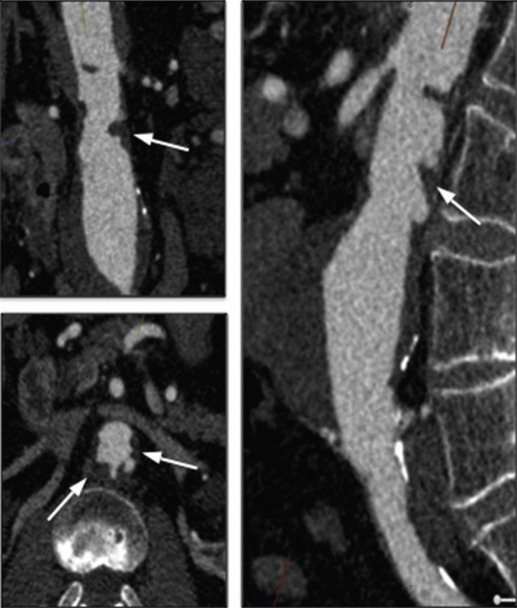
The proximal aorta seal-point (sometimes called the ‘neck’) should ideally be relatively disease free and straight. The neck length is the distance between the point of aortic pathology (e.g aneurysm) and the most adjacent branch artery intended to be preserved and kept uncovered by the graft fabric material (e.g most inferior renal artery in the case of an infrarenal endovascular repair for AAA). Most stent-grafts require a neck length of 8 to 15 mm to achieve a seal in the abdominal aorta and 20 mm in the thoracic aorta. Shorter necks ( Fig. 79.6 ) and diseased necks (e.g. those containing marked atheroma or thrombus, Fig. 79.7 ) risk a suboptimal seal and leakage between the device and the aortic wall. A neck that is sharply angulated relative to the more distal aorta ( Fig. 79.8 ) risks the stent-graft not deploying truly perpendicular to the aortic wall. This can increase the risk of suboptimal apposition and leak, although modern devices are designed to accept a greater degree of neck angulation than earlier designs, and some devices are specifically marketed for greater neck angulation ( Fig. 79.9 ). Finally, markedly barrel or conical-shaped necks ( Fig. 79.10 ) increase the risk of a poor seal as the degree of oversize needed to achieve a seal at the narrower portion of the neck may not be sufficient to achieve seal at the wider portion. The considerations outlined above for the ‘neck’ apply equally to the distal sealing point or distal ‘landing zone’.
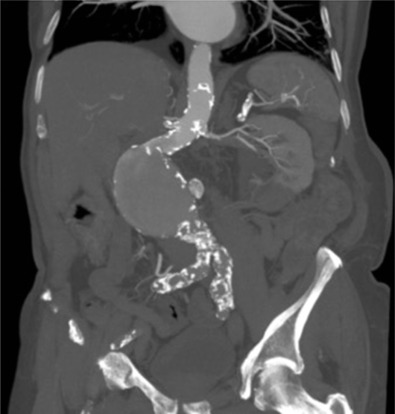
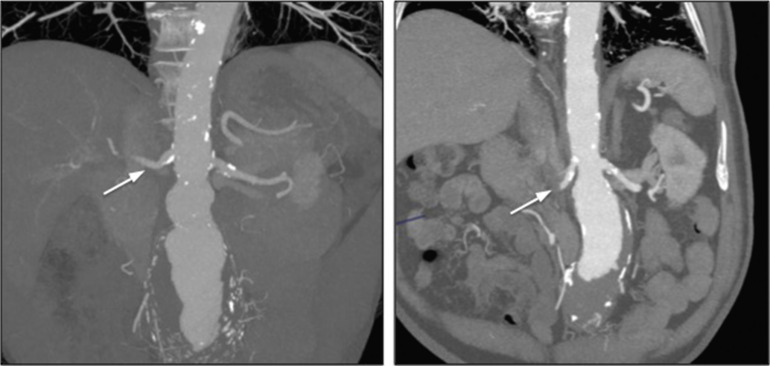
Approximately 40% of AAAs are unsuitable for conventional endovascular repair (as opposed to fenestrated or branched) because of adverse morphology. A description of the degree to which an operator can compromise on an ‘ideal’ neck (minimal angulation, straight-sided, long and disease free) is beyond the scope of this chapter.
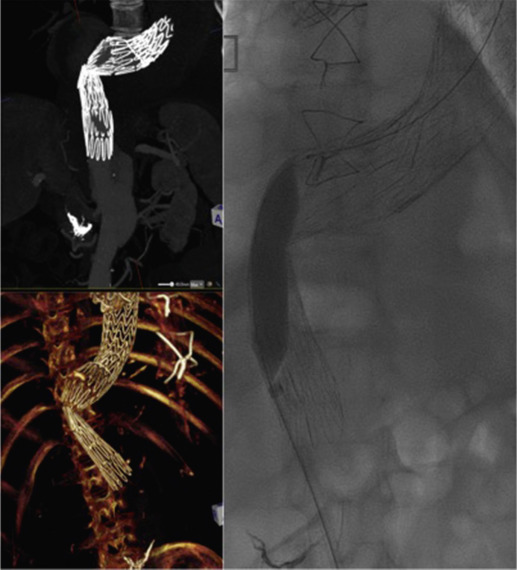
Stent-grafts are supplied preloaded on a deployment system. They are usually constrained within a sheath, which, at deployment, is gradually withdrawn, allowing the stent to expand under its own radial force ( Fig. 79.11 ). In the absence of dissection, a moulding balloon is usually inflated at the sealing zones and grafts’ junctional zones (points of overlapping stent grafts) to improve sealing. Some delivery systems have mechanisms to allow partial deployment and repositioning before final release.

The accurate positioning of the graft is paramount to achieve satisfactory results. The ‘windsock’ effect of systolic pressure during the cardiac cycle or during the balloon moulding can force a partially deployed device distally from its intended deployment position. This is occasionally problematic, although it is usually only of major significance in the proximal thoracic aorta. Some thoracic delivery systems have mechanisms to constrain the proximal portion of the stent-graft until the remainder of the device is fully deployed to minimise this windsock effect. Where the proximal positioning of a thoracic stent-graft is critical (e.g. in short or angulated necks), overdrive cardiac pacing or pharmacological manipulation to lower blood pressure may be used as the device is deployed. Once completely released, a device cannot be retrieved or repositioned without open surgery.
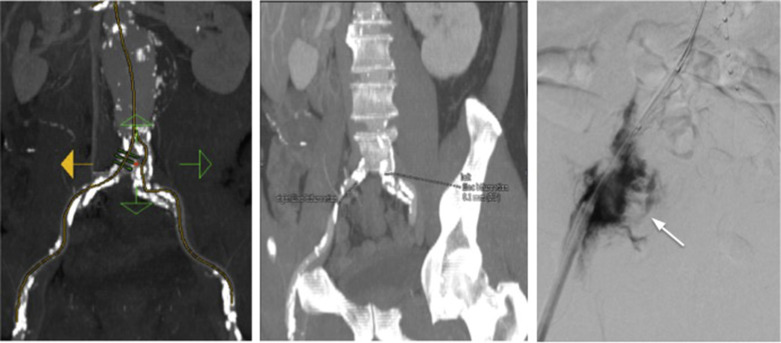
Deployment systems vary in diameter between 14 and 25 F (approximately 4 to 9 mm). Patients with narrower access vessels (usually common femoral and iliac arteries) than the diameter of the delivery system cannot undergo endovascular repair without adjunctive procedures such as angioplasty or stenting. A technique called ‘Pave and Crack’ is sometimes employed where the artery is deliberately over-dilated to rupture the artery having placed a large diameter stent-graft across the area first. Other options include the placement of a temporary surgical conduit to allow device insertion from above the level of stenosis. Heavily calcified or tortuous access vessels may also preclude endovascular repair. Attempts to force a large delivery system through suboptimal access can result in access vessel rupture or avulsion with potentially disastrous consequences ( Fig. 79.12 , Table 79.1 ).
| Comment | |
|---|---|
| Contraindication | |
| Absolute | |
| Lack of patient consent | |
| Limited life expectancy | Where the risks of intervention outweigh the benefits |
| Poor cardiopulmonary reserve or other comorbid condition | Where the risks of intervention outweigh the benefits |
| Unstable patients | Where they are deemed unable to withstand the small delay associated with imaging of the pathology |
| Ascending aortic pathology | Unless there is absolutely no surgical alternative |
| Relative | |
| Contrast allergy | Procedures can be performed with carbon dioxide angiography or intravascular ultrasound as imaging guidance |
| Renal insufficiency | Procedures can be performed with carbon dioxide angiography or intravascular ultrasound as imaging guidance |
| Suboptimal proximal seal zone (‘neck’) | Proceed accepting an increased risk of endoleak; a longer than usual neck will help mitigate to an extent or use fenestrated or branched device |
|
|
| Poor access vessels | Can be mitigated with angioplasty, ‘Pave and Crack’ or proximal surgical conduit |
|
|
| Children | Where physiological growth may result in loss of seal and device dislocation |
| Young patients | As long-term durability of endovascular repair has not yet been established |
| Not Contraindications | |
| Rupture | |
| Malignancy | |
| Mycotic aneurysms | |
Evolution of endovascular technology has produced smaller-profile devices and a suture-based percutaneous closure device, allowing total percutaneous endovascular aneurysm repair (PEVAR) to now be widely practiced, reducing the need for a traditional surgical cutdown. For many patients, this provides a better vascular access option with shorter operative time, less postoperativr pain, and shorter hospital stay by minimising the potential complications of a conventional femoral cut down.
For the purposes of thoracic endovascular aortic repair (TEVAR), thoracic aortic pathology is best classified according to its location relative to the left subclavian artery (the Stanford classification, described originally for thoracic aortic dissection— Fig. 79.13 ). Pathology affecting the aortic root and ascending aorta (Stanford A disease) is generally unsuitable for primary endovascular repair.
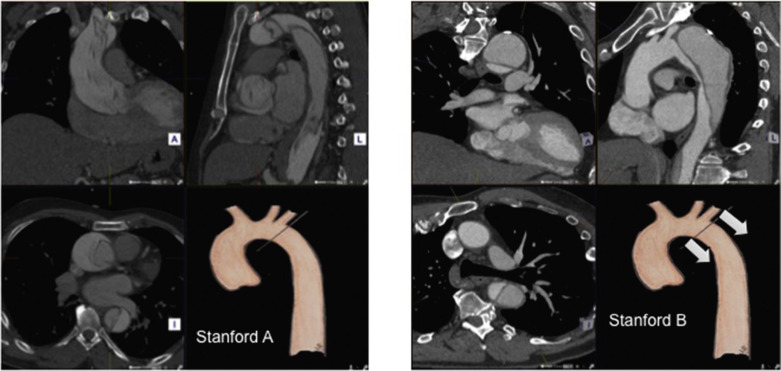
Disease affecting the aorta distal to the left subclavian artery (Stanford B disease) is amenable to endovascular repair, assuming there is enough disease-free aorta to achieve a seal at the proximal end (usually 20 mm). The left subclavian artery can be covered to increase the available usable neck proximal to the diseased aorta but may need a prior bypass to reduce the risk of stroke. An even more proximal seal can be achieved by extending the proximal landing zone by prior surgical bypass of 1, 2 or all 3 of the supra-aortic arteries ( Fig. 79.14 ). Carotid-subclavian and carotid–carotid bypass can be performed via incisions in the neck, but complete arch debranching requires a median sternotomy (though not cardiopulmonary bypass).
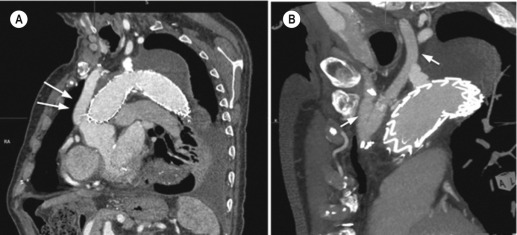
Angulation is a particular problem in the aortic arch and proximal descending thoracic aorta where the arch is not a smooth curve (like a Norman arch) but, rather, is peaked with an apex (like a Gothic arch; Fig. 79.15 ). This can lead to type 1 endoleak, particularly around the inner curve of the aorta if there is a marked ‘stand-off’ between the device and the inner curve of the aorta (resulting in ‘beaking’). The stent-graft can be folded in on itself by the action of blood flowing around the stent-graft rather than through it. Device placement such that the seal zone is in a straight segment of aorta a few centimetres either proximal or distal to the point of angulation will avoid issues of poor seal and stand-off but, frequently, such placement is impossible and a compromise must be struck between neck length and angulation. Modern devices conform to angled aortic morphology more readily than their predecessors but marked angulation remains a problem.
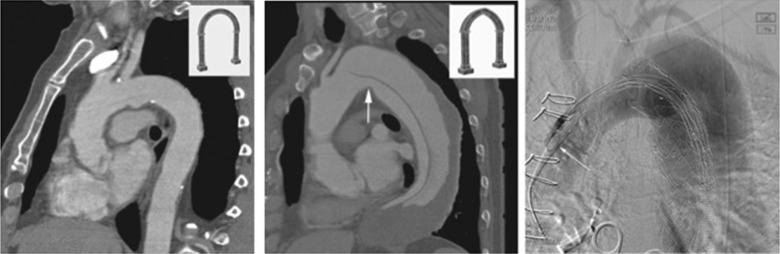
The blood supply to the spinal cord arises from the anterior spinal artery, which is a branch of the vertebral artery usually arising from the first part of the subclavian artery. Additionally, there is supply directly from the intercostal arteries as they arise from the descending thoracic aorta and enter the spinal canal as radicular (or medullary) arteries with the dorsal nerve roots. There is frequently a dominant radicular artery—the artery of Adamkiewicz—arising between T8 and L1, usually on the left. Repair of thoracic aortic pathology (whether open or endovascular) carries a risk of coverage (and therefore occlusion) of some or all of this spinal supply with resultant spinal cord ischaemia and paraplegia. Open repair offers the possibility of intercostal vessel reimplantation, but endovascular repair does not. The overall risk of paraplegia with endovascular repair of thoracic aortic aneurysm (TAA) is in the region of 3%. The risks are higher if there is an aortic dissection, if the left subclavian is covered, if longer lengths of the thoracic aorta are covered and if there has been prior abdominal aortic repair. A prophylactic lumbar cerebrospinal fluid drain is protective during open and endovascular repair of TAA where the risks of cord ischaemia are thought to be high, although most operators prefer to place a drain only if symptoms develop post procedure due to the risks associated with routine spinal drainage.
The incidence of TAA is approximately 10 per 100,000 people per year, with the incidence increasing as the population ages. Most (70%) involve the ascending aorta. They present frequently as incidental findings on chest x-ray (CXR) or other imaging. They are usually asymptomatic or may infrequently cause hoarseness, stridor, dyspnoea, dysphagia or pain due to local mass effects. The natural history of TAA is one of progressive expansion, the rate of which depends upon the location of the aneurysm and its underlying cause. Rupture presents with chest pain and shock and patients with rupture rarely survive to hospital. The risk of rupture of a TAA is determined by the size, site, rate of growth and association with genetic syndromes such as Marfan. Untreated, most TAAs eventually rupture, but the annual risk of rupture is difficult to accurately quantify. Retrospective review of outcomes for patients with TAA demonstrated annual rupture risk to be 0.3% for TAAs 4 to 4.9 cm in diameter, 1.7% for TAAs 5 to 5.9 cm in diameter and 3.6% for TAAs 6 cm or above.
Repair should be considered when the risks of rupture exceed the risks of intervention. Current guidelines suggest that TEVAR should be considered when an asymptomatic descending aortic TAA reaches 5.5 cm, or 4.3 cm for patients with Marfan syndrome. A higher threshold (6 cm) is suggested for open repair, given its greater risks. Rapidly expanding aneurysms (a growth rate of 1 cm/year or faster) or symptomatic aneurysms usually require treatment whatever their size. Pseudo-aneurysms, saccular aneurysms and mycotic aneurysms are perceived to be at a greater risk of rupture than fusiform degenerative aneurysms and generally mandate early intervention.
The aim of TEVAR is to achieve a proximal and distal seal in disease-free segments of the aorta using a stent-graft resulting in complete exclusion of the aneurysm sac from the circulation. Frequently, the length of the aneurysm may require several devices to be ‘telescoped’ one into the other to provide coverage. This may require tapered devices as well as considerable overlap of components to prevent subsequent dislocation and type 3 endoleak (see below).
Registry data indicate that the overall 30-day mortality of TEVAR is 2% to 5% dependent on the type of the repair necessary, with emergency repair associated with higher mortality compared to elective repair (17.1% vs. 1.8%, respectively). Complications include stroke (2.4%), spinal cord ischaemia and paraplegia (2% to 4%) which is permanent in 1.4% of patients, access vessel damage (2% to 5%), myocardial infarction (2% to 4%), and respiratory failure (5%). Endoleaks (see below) usually occur early and are seen in up to 7%; device migration occurs in approximately 3%, although the requirement for secondary intervention to treat these problems is low (6% to 8%). At 3 years, freedom from reintervention is 90.3%. Aneurysm-related mortality at 1 year following endovascular repair of TAA is low (2% to 3%).
There are no randomised studies comparing open surgical and endovascular repair of TAAs. Data from non-randomised studies indicate that TEVAR can be performed with high technical success, low postoperative morbidity and overall 30-day, 1-year, 2-year and 3-year survival rates in 96.0%, 80.3%, 77.3% and 74.0%, respectively. The long-term durability of stent-grafts in the management of thoracic aortic disease is unknown, but 15 years of worldwide experience gives no reason to doubt their structural integrity in the lifespan of the patient. Newer generations of stent-grafts are predicted to perform better long term than their predecessors, but this is unproven.
Acute aortic syndrome is a general term used to encompass three aortic pathologies with similar presentation: aortic dissection, intramural haematoma (IMH) and penetrating atherosclerotic ulcer (PAU). The exact pathological relationship between these entities is unclear, but they share some features. In aortic dissection, there is intimal disruption that allows blood to track through a dissection plane in the media. The initiating event for this may be a direct tear in the intima (owing to shear stresses on the aortic wall) or intramural haemorrhage (from vasa vasorum) weakening the intima at the site of the tear. Thrombosis of the false lumen of a dissection may give rise to appearances identical to IMH, IMH itself may be caused by microscopic dissection-like intimal tears. Bleeding at the base of a PAU could give rise to IMH or act as a focus for dissection. The clinical presentation of acute aortic syndromes is usually abrupt onset severe chest pain, the classical description is of ‘tearing interscapular’ pain, but pain may be anterior, abdominal or migratory. The symptom complex overlaps with many other potential diagnoses (e.g. myocardial infarction) and diagnosis relies significantly on a high index of suspicion. A small proportion of patients have a clinically silent dissection. Associated presenting features may be cerebrovascular accident, renal failure, acute ischaemia of the bowel or limbs (from branch vessel occlusion), myocardial infarction, acute aortic regurgitation (due to involvement of the aortic root or coronary ostia), pericardial tamponade, massive haemothorax with profound shock (due to rupture) or progression to aneurysm formation. These complicating features are more common with dissection than with IMH.
Aortic dissection: intimal disruption that allows blood to track through a dissection plane in the media.
Intramural haematoma: intramural haemorrhage from vasa vasorum or bleeding at the base of a penetrating ulcer (can appear similar to thrombosis of a dissection false lumen).
Penetrating aortic ulcer: ulcerating atherosclerotic lesion that penetrates into the media.
The diagnosis is usually made by cross-sectional imaging. A normal CXR does not exclude aortic dissection, IMH or PAU, although it may confirm an alternative diagnosis in patients with low clinical risk. Of patients presenting with acute aortic syndrome, three-quarters have dissection, with 10% to 20% having IMH and a small proportion having PAU. Dissection involves the ascending aorta and arch in three-quarters of cases. IMH involves the descending thoracic aorta more frequently and tends to occur in older patients. PAU usually occurs in markedly atheromatous aortic segments, normally the descending thoracic aorta. Whatever the exact pathological relationship between these entities, the principles of management are broadly similar ( Fig. 79.16 ).
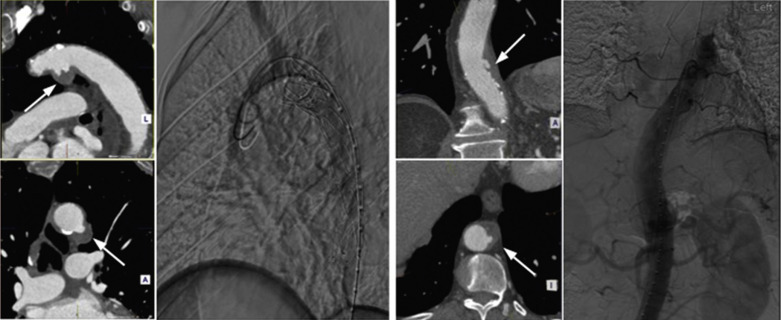
The incidence of thoracic aortic dissection is approximately 5 to 10 per 100,000 people per year and is increasing with an ageing population. Dissection is associated with hypertension (especially if uncontrolled), inherited disorders of elastin such as Marfan syndrome and certain vasculitides. TAA is a risk factor for dissection and vice versa. Classification of dissection is important for management. It is classified according to its age (acute, less than 14 days old; subacute, 14 days to 2 months; chronic, older than 2 months), the presence of associated complications, and location (Stanford A or B).
Untreated, the prognosis of aortic dissection is poor, with 20% of patients dying before they reach hospital. The mortality for type A dissection is higher than for type B, with an approximately 1% to 3% mortality per hour for the first 48 hours after presentation. The 30-day mortality of medically treated type A dissection is approximately 50% and approximately 17% for surgically treated patients. This increases with age and in patients with organ malperfusion. The 30-day mortality rate of uncomplicated type B dissection is approximately 10%; however, complications occur in 30% and these are associated with significantly higher mortality (20% by 48 hours, 30% by 30 days).
All patients with aortic dissection should be managed in a high-dependency environment with invasive monitoring. They should have aggressive management of heart rate (HR) and blood pressure to reduce aortic wall stress (HR <60 bpm, sBP <120 mm Hg)—although this can sometimes be difficult, requiring a combination of agents. Intravenous β-blockade and nitrates are commonly used and titrated to an adequate response. Pain control is also essential.
As discussed earlier, disease affecting the ascending aorta is generally unsuitable for endovascular repair and definitive management should be surgical. For Stanford type B dissections (involving the descending aorta beyond the left subclavian artery), close clinical observation and serial imaging is mandatory to guide further definitive management. Until recently, the management of uncomplicated type B thoracic aortic dissection was considered best with medical therapy alone. However, results from the INSTEAD trial showed that TEVAR repair in addition to optimal medical treatment is associated with improved 5-year aorta-specific survival and delayed disease progression and should be considered over best medical therapy alone. Prognostic features to aid decision-making include false lumen expansion rate (1 mm per month), partial thrombosis of false lumen, size of entry tear (>1 cm), false lumen diameter (>2.2 cm), early combined diameter >4 cm, and the influence of tear configuration on the false and true lumen flow haemodynamics. Unfortunately, not all patients benefit and there remains debate on who or when to intervene.
Uncontrolled pain, persistent hypertension, rapid expansion, rupture and major branch vessel occlusion with ischaemia (malperfusion syndrome) are indications for urgent treatment. The main aim of TEVAR is to treat the complications of acute dissection: namely, malperfusion syndrome, aneurysm formation and rupture. Restoration of branch vessel flow may be achieved by closure (i.e. coverage with a stent-graft) of the primary proximal entry tear, with the aim of depressurising the false lumen (FL) and allowing true lumen (TL) re-expansion. Where branch vessels have been occluded dynamically (i.e. where the dissection flap has prolapsed across their aortic true lumen ostium— Fig. 79.17 ), depressurisation of the FL allows the flap to move away from the ostium, with restoration of flow. Where the dissection has extended into a branch vessel (static occlusion—see Fig. 79.17 ) or where dynamic obstruction is not adequately relieved, additional branch vessel stenting or the deliberate formation of holes in the flap (‘fenestration’) may be required.
| Indications for urgent treatment of type B aortic dissection (complicated type B aortic dissection) | |
| Rupture with blood outside the vessel wall | Absolute indication |
| Major vessel occlusion with malperfusion syndrome | Absolute indication |
| Rapid expansion to a total aortic diameter of 4.5 cm or greater. | Absolute indication |
| Uncontrolled pain | Relative indication |
| Worsening radiological findings | Relative indication |
| Prognostic factors to aid decision to treat uncomplicated type B thoracic aortic dissection | |
| False lumen expansion rate | >1 cm/year |
| False lumen diameter | >2.2 cm |
| Early combined diameter | >4 cm |
| Tear configuration | Entry tear diamete >1 cm |
| Partial false lumen thrombosis | |
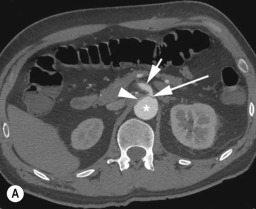
There is usually a main entry tear in the proximal descending aorta, although the main entry tear may be in the lower thoracic aorta or even in the abdominal aorta for dissections limited to the abdominal aorta. If the dissection extends below the diaphragm, there are often fenestrations at the level of the renal arteries and/or in the infrarenal aorta.
The standard TEVAR technique is the coverage of the main proximal entry tear in the thoracic aorta with a stent-graft. If other significant entry tears in the thoracic aorta are present, endograft coverage may be extended distally to cover these as well. Most operators do not extend endograft coverage below the diaphragm at the initial treatment, although this may be required in conjunction with adjunctive therapy to preserve flow to the visceral arteries if late complications affect the abdominal aorta.
Ideally, the thoracic FL should collapse entirely or should at least thrombose ( Fig. 79.18 ). False lumen thrombosis protects against rupture and aneurysm formation and is associated with good long-term outcomes. Ongoing FL perfusion after stent-graft coverage of the primary tear ( Fig. 79.19 ) may occur via uncovered fenestrations, retrogradely from abdominal aortic fenestrations or via back bleeding from branch vessels.
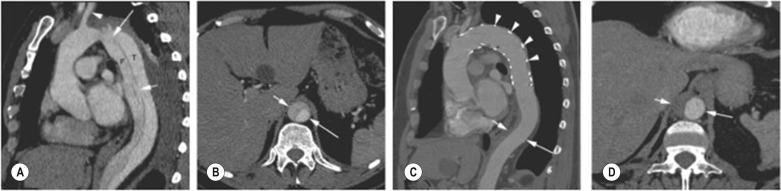
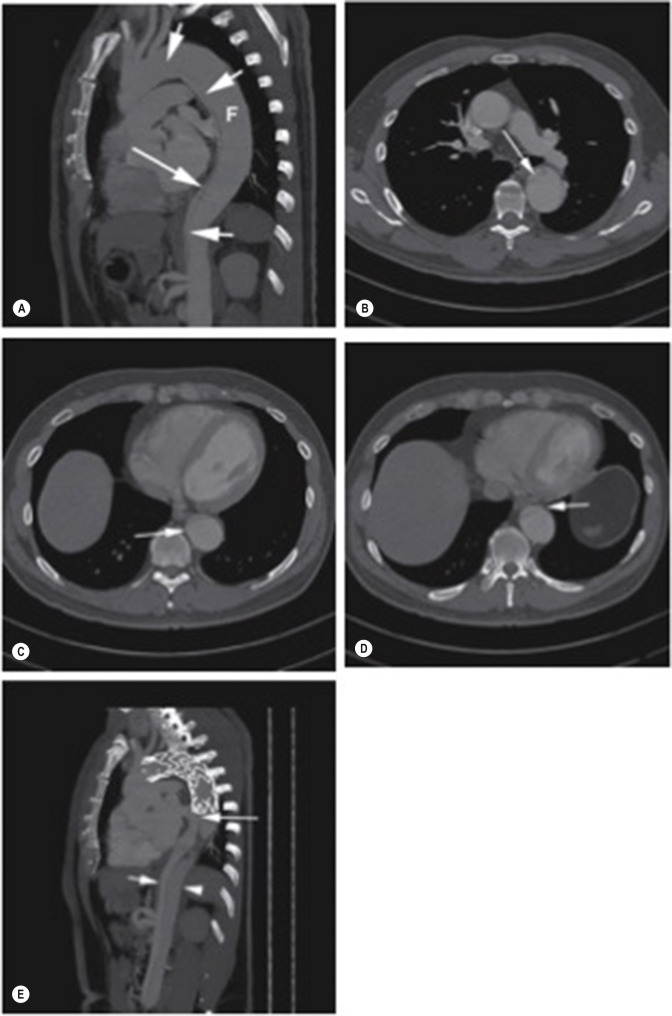
Uncommonly, patients with abdominal malperfusion syndrome, who are not suitable for endograft closure of the primary tear, can be treated by percutaneous fenestration of the dissection flap in the abdominal aorta to equalise pressure between the abdominal TL and FL. A number of interventional radiology methods have been used, including needle puncture of the flap and ‘cheese-wire’ cutting of the dissection. However, percutaneous fenestration techniques are rarely required with modern endograft technology.
The main complication of chronic dissection is aneurysm formation involving the thoracic and/or the abdominal aorta. Late aneurysm formation occurs in up to 50% of patients. Management of aneurysmal chronic dissection requires exclusion of flow from the FL by the closure of as many fenestrations between the TL and FL as possible with endografts. In some patients, closure of all fenestrations is necessary, which may require complex (fenestrated or branched) endografts or surgical bypass of the major aortic branches to preserve blood flow to the visceral arteries.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here