Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Aspirin therapy (initial dose of 325 mg and subsequently 81 mg a day) should be initiated to patients within 24–48 hours after acute ischemic stroke. Patients receiving thrombolytic therapy should delay treatment until after 24 hours. Antiplatelet agents remain the preferred antithrombotic therapy for most patients with stroke not secondary to cardioembolism and should be initiated prior to discharge from the hospital.
A 21-day course of dual antiplatelet therapy with aspirin and clopidogrel, or for up to 3 months in patients with low risk of bleeding, started with clopidogrel loading following transient ischemic attack or minor stroke is safe and effective and should be initiated within 24 hours.
Parenteral administration of anticoagulants or antiplatelet agents as a primary therapy to halt neurologic worsening or improve outcomes in the setting of acute ischemic stroke is not effective. These agents also are associated with an increased risk of intracerebral hemorrhage. These agents should not be considered as an alternative to treatment of acute ischemic stroke with intravenous thrombolysis and/or endovascular interventions.
Parenteral administration of anticoagulants or antiplatelet agents as an adjunctive therapy to intravenous thrombolysis and/or endovascular interventions is under study.
Among immobilized patients following stroke, deep vein thrombosis prophylaxis should be started early using low-molecular-weight heparin, heparin, and/or intermittent compression stockings. The duration of therapy is unclear.
Oral anticoagulants for long-term prevention of recurrent ischemic stroke in patients with cardioembolism should be initiated, but the timing of starting treatment is unclear. The size of the ischemic stroke demonstrated by brain imaging should be considered in this decision. The new oral anticoagulants are associated with a lower risk of intracerebral hemorrhage but have a more rapid onset of action than vitamin K antagonists. These medications have not been tested in patients with acute stroke. The plan for treatment with oral anticoagulants should be finalized prior to discharge from the hospital following a stroke.
Because most ischemic strokes are secondary to arterial thromboembolism, antithrombotic agents (i.e., anticoagulants and antiplatelet agents) are a mainstay of medical treatment to prevent ischemic stroke. The utility of these medications in long-term management to prevent stroke or recurrent stroke is well established. Oral anticoagulants, including vitamin K antagonists (VKAs) and new oral anticoagulants (NOACs), are of confirmed usefulness in the prevention of cardioembolic stroke among patients with high-risk heart diseases, including most patients with atrial fibrillation with prior stroke or transient ischemic attack (TIA). Antiplatelet agents are the standard medical therapy for lowering the risk of stroke among patients with arterial diseases, including those patients with either intracranial or extracranial atherosclerosis. Because of their established efficacy in long-term care and because most strokes are secondary to formation of clots, there continues to be considerable interest in the emergency use of either anticoagulant or antiplatelet agents in the setting of acute stroke. A number of antithrombotic agents can be considered ( Table 54.1 ). These agents have been used as the primary intervention or as an adjunct to other measures aimed at restoring perfusion to the brain. The rationale for these medications includes halting the propagation of an intra-arterial thrombus, forestalling early recurrent embolization, and maintaining collateral flow to the area of ischemia. In addition, their adjunctive use after mechanical or pharmacologic thrombolysis centers on preventing re-thrombosis or reocclusion after recanalization. Antithrombotic agents also are a potential therapy for those patients with stroke who are bedridden and have an increased risk of deep vein thrombosis (DVT) and pulmonary embolism (PE), which add to the morbidity and potential mortality of the neurologic event. The antithrombotic medications are used both to prevent and to treat these thromboembolic events. The timing of initiation of long-term anticoagulant therapy following stroke remains unclear, while antiplatelet therapy should be started within 24 hours with the exception of delayed treatment after intravenous (IV) tissue plasminogen activator (t-PA).
| Anticoagulants | Benefit (Level of Evidence) | Increased Risk of ICH |
|---|---|---|
| Glycosaminoglycans | ||
| Heparin | Low-dose DVT prevention (1A) | Yes |
| Low-molecular-weight heparins | Low-dose DVT prevention (1A) | Yes |
| Danaparoid | No | Yes |
| Direct Thrombin Inhibitors | ||
| Argatroban | IIIB | Yes |
| Dabigatran | Prevention in AF (1A) | |
| Xa Inhibitors | ||
| Fondaparinux | NT | |
| Rivaroxaban, apixaban, edoxaban | Prevention in AF (1A) | |
| Antiplatelet Agents | ||
| Aspirin (ASA) | Yes (1A) | Yes (low) |
| Clopidogrel | Stroke prevention (1A) | Uncertain |
| Dipyridamole | No data | |
| Cilostazol | Stroke prevention (1A) | No |
| Ticagrelor | Not compared to ASA | No |
| Prasugrel | No | No |
| Vorapaxar | No | Yes |
| Terutroban | Not compared to ASA | No |
| Combined Antiplatelets | ||
| ASA+clopidogrel | Yes for minor stroke duration up to 90 days (1A) | No up to one month ; yes 31–90 days |
| ASA+cilostazol vs. ASA+clopidogrel |
Pending | Pending |
| ASA+clopidogrel+dipyridamole | No | Yes |
| Glycoprotein IIb/IIIa Receptor Blockers | ||
| Abciximab | No | Yes |
| Eptifibatide (Integrilin) | Uncertain | No |
| Tirofiban | Not compared to ASA | No |
Recent guidelines address the use of antithrombotic agents for treatment of acute ischemic stroke ( https://www.nice.org.uk/guidance/cg68/chapter/1-Guidance#pharmacological-treatments-for-people-with-acute-stroke ). , Several reviews have also discussed the role of antithrombotic therapy in preventing recurrent ischemic stroke. ,
Heparin , low-molecular-weight (LMW) heparins , and danaparoid have been used for more than 70 years. Heparin is a mixture of glycosaminoglycans that is usually obtained from porcine or bovine sources. Its molecular weight ranges from 5000 to 30,000 daltons (D). Because of the high risk of local bleeding at the injection site, heparin is given either subcutaneously or intravenously, not intramuscularly. The antithrombotic effects of heparin are immediate if it is given intravenously in a bolus dose followed by a continuous IV infusion.
Heparin binds to plasma proteins, platelet-derived proteins, and endothelial cells. Differences in levels of those proteins might explain variations in clinical responses to heparin among patients. Heparin alters the conformation of antithrombin, which in turn increases the ability of antithrombin to inactivate thrombin. Heparin binds to the amino terminus of the molecule site of antithrombin, which leads to a conformational change that increases the ability of antithrombin to inactivate thrombin by a ratio of 1000–4000 times. It also inhibits activated factor X (factor Xa) and activated factor IX (factor IXa). Heparin does not directly affect either thrombin or factor Xa already incorporated in a formed thrombus; thus, it does not have thrombolytic effects. The ratio of inhibition of activated thrombin to factor Xa is 1:1. Heparin also prevents fibrin formation through its inhibition of thrombin-induced activation of platelets and factors V and VIII. It does not affect factor Xa already bound to platelets. Heparin also inactivates thrombin through heparin cofactor II—an action that occurs at high concentrations and is independent from its effects on antithrombin. In addition, the high-molecular-weight components of heparin alter endothelial modulation of clotting factors and interact with platelet factor 4 (PF4). The binding of heparin to von Willebrand factor (vWF) also affects platelet function.
Heparin also attaches to macrophages and endothelial cells. This binding, which is saturable, relates to the rapid clearance of heparin from the circulation. Heparin has a narrow therapeutic window; differences between safe but effective doses and dangerous levels are small. There is a strong association between the risks of serious bleeding with increases in the dose of heparin. Some patients are relatively insensitive to the actions of heparin (heparin resistance). This response may be secondary to a deficiency of antithrombin, increased heparin clearance or to an elevation of heparin-binding proteins, including fibrinogen, factor VIII, or PF4. Heparin does not cross the placenta.
The activated partial thromboplastin time (aPTT) is the most widely used test to monitor the biologic (antithrombotic) effects of heparin. This test measures responses to heparin-induced inhibition of thrombin, factor Xa, and factor IXa. The optimal level of anticoagulation is uncertain but is assumed to be approximately 1.5 times control values. The aPTT test has a number of serious limitations. Variability in reagents between institutions may lead to spurious results. Patients with the lupus anticoagulant–antiphospholipid antibody syndrome often have falsely elevated aPTT values; in this situation, monitoring heparin therapy with the test may be problematic, and alternative ways to assess heparin’s activity include measuring inhibition of factor Xa or measuring heparin levels via neutralization with protamine sulfate. The usual therapeutic level of heparin is inhibition of factor Xa to 0.3–0.7 U/mL.
To prevent DVT, most high-risk patients receive 5000 units of heparin, administered subcutaneously, two to three times a day. To maintain full anticoagulation, the usual daily dose is approximately 24,000–30,000 units. Traditionally, a bolus of heparin (usually 5000 units) is given to start treatment; thereafter, heparin is given as a continuous IV infusion. Initially, the aPTT value is markedly prolonged, so follow-up assessments are usually delayed until 6 hours after the initiation of therapy. The patient’s weight is an important variable that affects biologic responses to heparin. As a result, weight-based nomograms are now used to administer heparin.
Heparin also has subtle anti-inflammatory actions that may differ from its effects on coagulation factors. While heparin may have effects on major neurotransmitters of the brain, the interactions of these effects and the potential utility of heparin for treatment of patients with acute brain ischemia are not obvious.
Because of the many limitations of unfractionated (traditional) heparin, other parenterally administered, rapidly acting anticoagulants have been developed. The leading alternatives are the LMW heparins and danaparoid. The LMW heparins weigh approximately 1000–10,000 D. The reduction into low-weight compounds leads to lessened binding to platelets, proteins, endothelial cells, and macrophages. This probably explains the longer duration of effect and the relatively predictable responses to the use of the LMW heparins.
The LMW heparins and danaparoid have a reduced effect on thrombin function compared with unfractionated heparin but more selective inhibition of factor Xa. The ratio for thrombin and factor Xa is approximately 1:2–1:4. Because these agents do not affect thrombin activity except in very high concentrations, assessment of the responses by the use of the aPTT is unreliable. Rather, measuring inhibition of factor Xa tests the antithrombotic effects of these medications. The desired levels are 0.3–0.7 units/mL. Because these agents are excreted renally, the level of anticoagulation may be high among patients with renal failure. Clinical trials have demonstrated that neonates and infants need a relatively higher dose of the LMW heparins compared with older children or adults to achieve targeted levels for inhibition of factor Xa.
Although the LMW heparins and danaparoid may be given intravenously, most clinical studies have focused on subcutaneously administered regimens using a weight-based nomogram, particularly in the scenario of preventing DVT. The responses to specific LMW heparins generally are similar, but the specific pharmacologic effects differ; thus, these agents should be evaluated individually. In particular, the ratio of antithrombin activity to antifactor Xa activity varies among the several compounds.
Another group of parenteral anticoagulants that inhibit factor Xa, mediated via antithrombin, but do not interact with PF4 are the pentasaccharides (fondaparinux, idraparinux, idrabiotaparinux) . They are renally excreted and may be safe for patients with heparin-induced thrombocytopenia (HIT). Fondaparinux is used for prophylaxis and treatment of venous thromboembolism (VTE) as an alternative to LMW heparins. Idraparinux has a longer half-life and was tested for stroke prevention in atrial fibrillation (AMADEUS trial), but the study was terminated because of excess bleeding, as was a trial of idrabiotaparinux (BOREALIS-AF), which was terminated by the manufacturer for commercial reasons.
Because of the lag between the initiation of therapy with VKA and an antithrombotic response, these agents are not used as an intervention for treatment of an acute ischemic stroke. In addition, these medications may have an initial and transient prothrombotic effect through their initial inhibition of the actions of proteins C and S, which limit their applicability. However, oral agents with direct or indirect inhibition of activated factors Xa have a more rapid onset of action. These agents include rivaroxaban , apixaban , and edoxaban . , Double-blind trials have tested Xa inhibitors for stroke prevention in atrial fibrillation; these are ARISTOTLE and AVERROES (apixaban), ROCKET-AF (rivaroxaban), and ENGAGE-AF TIMI 48 (edoxaban). They showed similar efficacy in preventing stroke compared to VKA and are approved by the US Food and Drug Administration (FDA) for this indication. Otamixaban is a parenteral Xa inhibitor with a short half-life being studied in acute coronary syndrome (ACS), but it was associated with a high risk of bleeding and hence further development is unlikely.
Direct thrombin inhibitors may affect unbound thrombin and do not require antithrombin, resulting in a more reliable antithrombotic effect than unfractionated heparin. These agents also do not affect platelet function and do not interact with PF4, so they are suitable for patients with or at risk of HIT. They may be bivalent like the agent Hirudin —originally derived from the salivary gland of the medicinal leech—which is now available with the use of recombinant technology ( lepirudin, desirudin ). It is a potent and irreversible inhibitor of thrombin function. The anticoagulant activity of hirudin is monitored by the aPTT. Antihirudin antibodies may develop within 5 days of treatment in 40%–70% of patients treated with lepirudin. These antibodies may increase drug potency or cause anaphylaxis with re-exposure. Desirudin and lepirudin have been used in the treatment of patients with ACS, but they have not been used to treat patients with stroke. Lepirudin is no longer available. Bivalirudin ( Hirulog ), another direct thrombin inhibitor that is reversible, has been used to treat patients undergoing percutaneous coronary interventions (PCI), particularly in those patients at risk of HIT. There is a lower risk of bleeding compared with heparin, but there is little evidence to show an overall net benefit.
Argatroban is a univalent selective thrombin inhibitor that competitively acts at the active site of thrombin; it has an immediate antithrombotic effect. Argatroban is metabolized by the liver and has a short half-life (approximately 50 minutes); thus, anticoagulation may be initiated or terminated more rapidly than with either unfractionated heparin or the LMW heparins. It also is monitored by the aPTT. It is approved for treatment of HIT and is being tested as an adjunctive for IV thrombolytic therapy , and endovascular interventions in patients with acute ischemic stroke. It is being tested in a large clinical trial.
Dabigatran is an oral direct thrombin inhibitor that was as effective as warfarin in preventing thromboembolism with less risk of bleeding when administered at a lower dose. At a higher dose, it was more effective than warfarin in preventing stroke and systemic embolism but had a similar rate of bleeding and is FDA-approved for stroke prevention. A large clinical trial tested large doses of dabigatran among persons with prosthetic cardiac valves but was stopped because of an increased risk of bleeding and no evidence of superiority over warfarin in preventing embolization.
Aptamers are small RNA or DNA oligonucleotides that bind target molecules through high-affinity interactions. The advantage of these molecules is that they can be inactivated rapidly by a complementary antidote aptamer. A hybrid aptamer of RNA/DNA (ARC1779) binds to the A1 domain of activated vWF, blocking the interaction of vWF with the receptor GP1b ( Fig. 54.1 ). Pegnivacogin is an RNA aptamer that blocks factors IX and Xa and can be rapidly reversed by a complementary RNA aptamer, anivamersen. However, due to severe allergic reactions in a phase 3 trial of PCI, further drug development has been stopped.
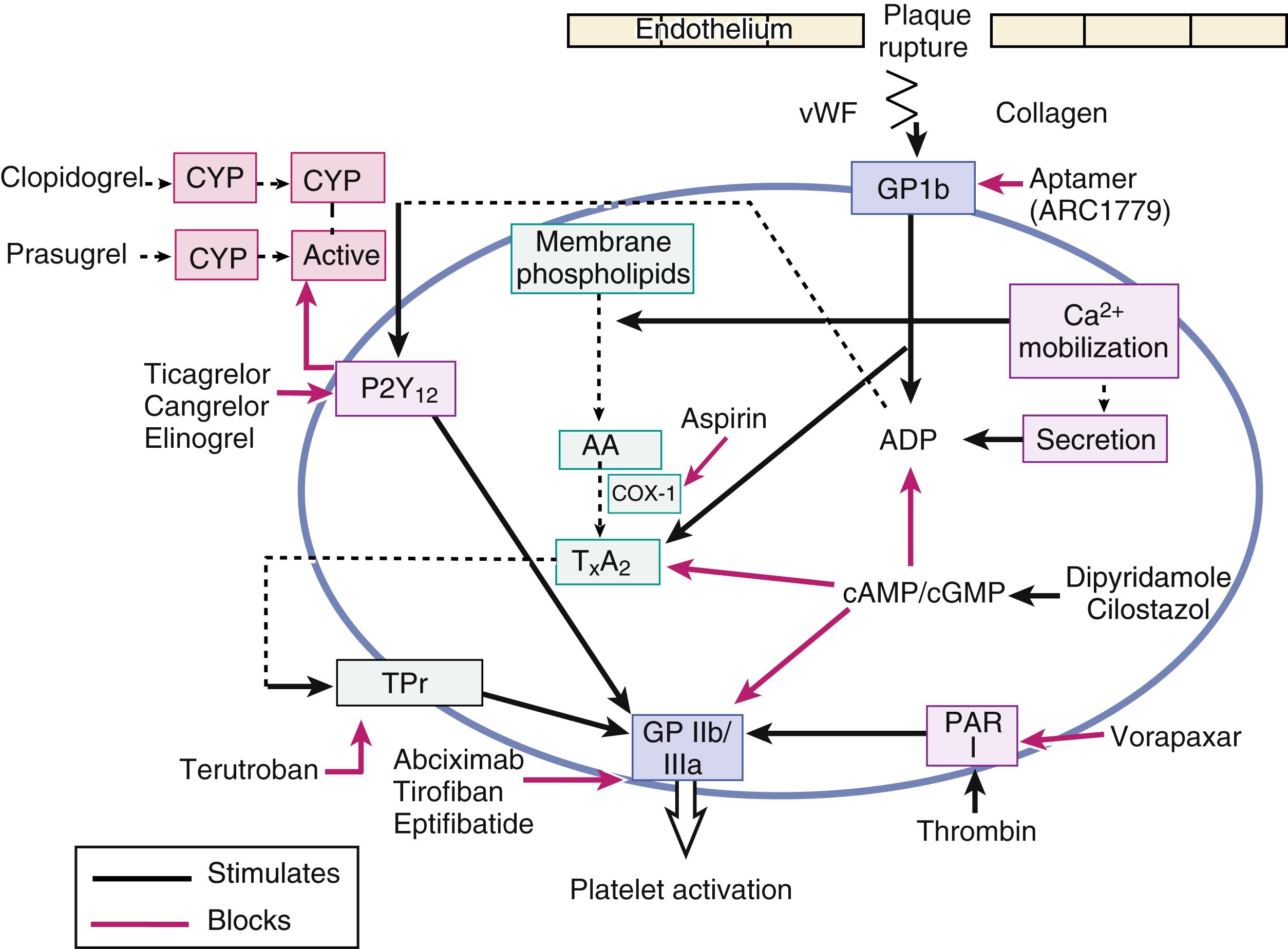
Antiplatelet agents target different receptors to prevent platelet aggregation (see Fig. 54.1 ). Aspirin irreversibly blocks the cyclo-oxygenase (COX) activity of prostaglandin (PG) H synthase 1 (COX-1) and 2 (COX-2) by acetylation of a serine residue of the COX channel. Aspirin’s actions on platelet COX-1 are approximately 170-fold more potent than on monocyte COX-2. It produces a permanent defect of thromboxane-A 2 -dependent platelet function that induces platelet aggregation and causes vasoconstriction. Aspirin also alters endothelial production of prostacyclin—an agent that inhibits platelet aggregation and prevents vasoconstriction. The potentially prothrombotic effects of prostacyclin inhibition appear to be less relevant clinically because the endothelial cells may regenerate new COX, unlike the anuclear platelet, in which COX inhibition is irreversible.
The antithrombotic actions of aspirin occur over a broad range of doses. Aspirin is readily absorbed, so peak plasma levels are reached within 30–40 minutes. The portal circulation is the point of contact with platelets with aspirin, which has a half-life of 15–20 minutes. Enteric coating of the tablet does delay absorption with peak levels at 3–4 hours. There is not a lower risk of gastrointestinal (GI) bleeding with this formulation, suggesting that it is the systemic effect of aspirin to inhibit the production of prostaglandins responsible for gastric protection that causes GI side-effects. , The risk of GI bleeding is directly related to the dose of aspirin. A single dose of 100 mg of aspirin has an almost immediate effect on platelet aggregation. Lower doses of aspirin (<100 mg) may take longer than 24 hours to achieve maximal suppression of COX. Subsequently, lower daily doses are able to maintain the antiplatelet effects. A meta-analysis of recent evidence does not support the use of aspirin as prophylaxis against stroke or myocardial infarction among asymptomatic, healthy adults. However, current guidelines recommend low-dose aspirin for secondary prevention of stroke or coronary events. Thus, the minimum initial dose of aspirin appears to be at least 160 mg; that is the dose used in the Chinese Acute Stroke Trial (CAST). Most nonsteroidal anti-inflammatory drugs (NSAIDs) reversibly bind COX receptors with a stronger affinity than aspirin so that these receptors are occupied when aspirin is exposed to platelets in the portal circulation if NSAIDs are taken prior to aspirin. NSAIDs should be taken 30 minutes after aspirin or at least 8 hours before aspirin. Other potential antiplatelet actions may be the result of platelet activation by neutrophils, which is mediated by nitric oxide and enhancement of the production of nitric oxide by endothelial cells. The utility of aspirin as a neuroprotective agent for use in acute stroke is not established, but there is evidence that strokes may be less severe among patients who have previously used aspirin, and infarct volume may be reduced. There have been concerns that patients may develop resistance to aspirin and that higher doses of the medication may be needed to achieve antiplatelet effects. , Reasons for aspirin treatment failure include nonadherence, high platelet turnover due to an inflammatory process such as atherosclerosis, interaction with medications such as NSAIDs, alternative pathway of platelet activation, tachyphylaxis with reduced effect of aspirin with prolonged use, and genetic polymorphisms with the first three reasons probably the most important contributory causes. There is no evidence that switching to an alternative antiplatelet agent decreases the risk of stroke.
Terutroban is a selective thromboxane-prostaglandin receptor antagonist. A clinical trial comparing it to aspirin in stroke prevention (PERFORM) was stopped prematurely because of futility and an increased risk of minor bleeding.
Dipyridamole and cilostazol block the actions of phosphodiesterase, resulting in decreased reuptake of adenosine and elevated levels of cAMP and gAMP. These agents prolong platelet survival, reduce inflammation, scavenge free radicals, and produce vasodilation. Bioavailability of dipyridamole is low unless it is combined with tartaric acid to provide an acid environment for absorption in the extended-release formulation. The interval from initiation of treatment with extended-release dipyridamole to the achievement of antiplatelet effects may be too slow to make this medication useful to treat acute ischemic stroke, although in combination with aspirin, it is effective in preventing recurrent stroke. Cilostazol has been investigated for the secondary prevention of stroke. An observational study in Japan showed that the addition of cilostazol to other antiplatelet agents following carotid endarterectomy was associated with fewer ischemic brain lesions on imaging. In a meta-analysis, cilostazol was more effective than aspirin in preventing recurrent stroke (odds ratio [OR] 0.64; 95% confidence interval [CI], 0.47–0.88) and had a lower risk of hemorrhage. A trial of the combination of cilostazol with either aspirin or clopidogrel for nonlacunar stroke is in progress. (NCT01995370)
The thienopyridines, ticlopidine , clopidogrel , prasugrel , ticagrelor , elinogrel , and cangrelor , inhibit platelet aggregation induced by adenosine diphosphate by blocking the platelet receptor P2Y12, which interacts with fibrinogen. The ability of clopidogrel to limit platelet aggregation occurs in a dose-dependent manner. The inhibition of platelet function starts within 2 hours of a 600 mg loading dose of clopidogrel and is irreversible. Inhibition of platelet aggregation is greater with a 600 mg loading dose than a 300 mg dose, but a trial of the 600 mg loading dose followed by double-dose clopidogrel (150 mg) did not show any additional benefit in preventing recurrent vascular events compared with a 300 mg loading dose and 75 mg dose in patients with ACS. The risk of stent thrombosis in those patients undergoing PCI was lower with the high-dose regime. Clopidogrel is a prodrug that requires a two-step activation process by the cytochrome P-450 (CYP) isoenzymes. Inhibition of these enzymes, particularly CYP2C19, by other drugs such as fluoxetine or by competition for the catalytic site on CYP2C19 by proton pump inhibitors (PPI) can lead to interindividual variability in platelet aggregation. However, in one randomized study of co-administration of clopidogrel and omeprazole in patients with ACS, there was more than 50% reduction of GI bleeding with PPI use and no increased risk of vascular events. While PPI decreases the efficacy of platelet inhibition by clopidogrel in vitro, there are conflicting data about whether co-administration of PPI with clopidogrel increases the risk of thrombotic events, and guidelines suggest selective use of PPI in patients at high risk of GI bleeding. , Smoking increases the clinical benefit of clopidogrel by inducing the activity of CYP1A2, another isoenzyme that activates clopidogrel. There are also genetic variations that affect the antiplatelet response to clopidogrel. There are significant ethnic differences in the frequency of these alleles, with 65% of East Asians and 35% of Western populations having a loss-of-function (LOF) allele. Pan et al. showed that in Asian patients with one LOF allele, the risk of recurrent stroke was 1.8, while with 2 LOF alleles, the risk increased to 2.5. In the CHANCE study, polymorphisms of the ABCB1 gene that decreases the intestinal absorption of clopidogrel were associated with an increased risk of recurrent stroke. The clinical usefulness of genotyping patients or performing tests of platelet inhibition routinely to select the appropriate antiplatelet agent or to adjust the dose is controversial and perhaps should be restricted to patients who have recurrent events while taking clopidogrel. Prasugrel has a 10-fold to 100-fold greater and irreversible inhibition of platelet aggregation than clopidogrel. Prasugrel also has more rapid effects on platelet function. It is also a prodrug but requires only one-step activation by CYP enzymes and is not affected by genetic variability of these isoenzymes. Prasugrel was approved for the treatment of patients with ACS because there was a significant reduction in ischemic events when it was compared with clopidogrel. However, in patients with prior stroke or transient ischemic attack (TIA), there was an increased risk of ICH (2.3% vs. 0%). The PRASTRO-I trial compared prasugrel to clopidogrel in patients with non-cardioembolic ischemic stroke and failed to confirm the non-inferiority of prasugrel, including those with genetic variants of CYP2C19. Ticagrelor, unlike clopidogrel and prasugrel, binds reversibly to the P2Y12 receptor and has a half-life of 7–8 hours. It is not a prodrug, has a rapid onset of action, and is more potent than clopidogrel. It is more effective than clopidogrel in preventing vascular death, MI, or stroke among patients with ACS (PLATO trial). It was used with low-dose aspirin (<100 mg) as efficacy improved. A subgroup analysis of patients with prior stroke or TIA that comprised 6.2% of randomized patients showed similar benefit in preventing thrombotic events and no increased risk of ICH (0.9% vs. 0.7%). A secondary prevention trial in ACS patients (PEGASUS) compared ticagrelor to aspirin; while patients with stroke or TIA were excluded, the trial demonstrated a reduction in subsequent stroke and no increase in intracerebral hemorrhage (ICH). A large clinical trial did not demonstrate the superiority of ticagrelor when compared with aspirin in stroke prevention. However, a subgroup analysis of outcomes among patients with stroke secondary to atherosclerotic carotid disease in the SOCRATES trial did demonstrate the superiority of ticagrelor. A trial comparing aspirin to the combination of aspirin and ticagrelor (THALES) is currently in progress. While ticagrelor is being used as an adjunctive agent to endovascular therapy in patients with stroke, no clinical trials have tested the agent. Cangrelor is an IV adenosine triphosphate analogue that reversibly binds to the P2Y12 platelet receptor. It has a half-life of 3–6 minutes, and the antiplatelet effect is gone within 60 minutes after the infusion is stopped. Studies have demonstrated that cangrelor is equal to prasugrel in efficacy.
The glycoprotein (integrin) IIb/IIIa receptor antagonists are potent blockers of platelet aggregation by affecting the binding of fibrinogen to the platelets. These agents do not affect platelet adhesion, but they do limit the formation of a clot. Abciximab is a monoclonal chimeric murine-human antibody that blocks the receptor. A bolus dose of abciximab may block more than 80% of the platelet receptors and maintain antiplatelet effects for several hours. Platelet function is inhibited within 2 hours of administration of a bolus dose. The usual loading dose of abciximab is 0.25 mg/kg, and the maintenance infusion is 0.125 mcg/kg/min (maximum, 10 mcg/min). Platelet function recovers within 48 hours following an infusion lasting 12 hours. The antiplatelet effects of abciximab are potentiated by the concomitant administration of aspirin. Abciximab also may have some effect on the formation of thrombin. Abciximab, in conjunction with other antithrombotic medications, has been used to treat patients with ACS, including those undergoing PCI. A clinical trial of abciximab in treatment of acute ischemic stroke was halted for lack of efficacy and an increased risk of intracranial hemorrhage (AbESTT).
Tirofiban is a nonpeptide derivative of tyrosine. It selectively and reversibly blocks the glycoprotein IIb/IIIa receptor. Marked inhibition of platelet aggregation and prolongation of the bleeding time occur within 5 minutes of the start of an IV infusion of the medication. Platelet function is normal within 2–6 hours after the infusion is halted. Tirofiban is used to treat ACS. Administration of tirofiban is being used as an adjunct to endovascular procedures in treatment of acute ischemic stroke. , , The risk of bleeding is relatively low, and outcomes are improved. Further research is underway.
Eptifibatide is a heptapeptide that has a high affinity and specificity for the glycoprotein IIb/IIIa receptor. It also affects integrin-mediated binding of the smooth muscle cells to thrombospondin and prothrombin. After administration of a bolus dose followed by an IV infusion, platelet aggregation is diminished markedly within 15 minutes. Eptifibatide also affects thrombin generation and markedly prolongs the bleeding time. Platelet function returns to normal within 4 hours after withdrawal of the drug. The agent is used to treat patients with ACS and is the most widely used of the IIb/IIIa receptor antagonists. The usual dose is 180 mcg/kg (maximum, 22.6 mg), given over 1–2 minutes, followed by a continuous infusion of 2 mcg/kg/min (maximum dose, 15 mg/h). All the IIb/IIIa receptor antagonists can cause an immune-mediated thrombocytopenia. Preliminary clinical studies of eptifibatide as an adjunctive therapy in patients with stroke treated with recombinant tissue plasminogen activator or endovascular interventions have provided evidence that the agent is safe and may be associated with improved outcomes. , Further research is underway.
Thrombin mediates platelet activation via an interaction with the protease-activated receptor 1 (PAR-1) on the platelet. Vorapaxar is a competitive and selective inhibitor of the PAR-1 receptor without affecting the production of fibrin by thrombin. The (TRA 2°P)-TIMI 50 trial was a double-blind, placebo-controlled trial that tested the addition of vorapaxar to standard therapy in patients with MI, peripheral vascular disease, or stroke. The trial was halted prematurely for the subgroup of patients with prior TIA or stroke because of an associated increased risk of ICH.
Novel antiplatelet agents are being developed that target the interaction between vWF and the platelet receptor glycoprotein 1b. An aptamer (ARC1779) was tested in a small pilot study of patients undergoing carotid endarterectomy and showed reduced embolic signals. ,
Physicians are concerned about the safety of emergency administration of antithrombotic medications in the treatment of patients with acute ischemic stroke. Intracranial hemorrhage, including hemorrhagic transformation of the infarction, is a potential complication of any therapy aimed at restoring or improving perfusion to the brain. Bleeding complications also may occur in other locations in the body. In addition, some of the antithrombotic agents also may be associated with nonhemorrhagic complications.
A 2015 meta-analysis found that heparin is associated with an increased risk of bleeding. Two doses of subcutaneously administered heparin were tested in the International Stroke Trial. Intracranial bleeding was diagnosed in 16 of 2429 patients (0.7%) given the lower dose and in 43 of 2426 patients (1.8%) receiving the higher dose. In a subgroup of patients with atrial fibrillation, the rates of hemorrhage were 1.3% and 2.8% for the lower and higher doses of heparin. The risk of bleeding with heparin is generally associated with the level of anticoagulation and the dose of medication. A significantly increased risk of bleeding has also been observed with low-dose anticoagulation used for prophylaxis of VTE, and Lederle et al. found that heparin prophylaxis among patients with acute stroke was associated with an increased major bleeding risk (OR, 1.66; 95% CI, 1.20–2.28), which was an absolute increased risk of 6 events per 1000 people treated.
Nonneurologic hemorrhages also may complicate heparin. In the International Stroke Trial, extracranial hemorrhage was diagnosed in 33 of 2426 patients (1.4%), given the higher dose of heparin, and in 10 of 2429 patients (0.4%) receiving low-dose heparin. The most common locations for serious bleeding are the GI tract, urinary system, retroperitoneal space, and joints.
Heparin should be discontinued if a patient experiences severe bleeding. Protamine sulfate also may be administered. The calculated dosage of protamine is based on the assumption that heparin has a half-life of approximately 60 minutes and that the dosage of the antidote corresponds to the amount of heparin given in the previous 90 minutes. Approximately 1 mg of protamine sulfate negates the effects of 100 units of heparin. IV protamine sulfate should be infused slowly (over at least 10 minutes) because it can induce hypotension. Anaphylaxis may also complicate administration of protamine.
A severe, autoimmune-mediated thrombocytopenia (HIT) associated with antibodies to anti-PF4/heparin complex may appear 5–15 days after heparin is started. Prior use of heparin may sensitize a patient, and a second exposure may induce a severe thrombocytopenia within hours. The autoimmune reaction is not related to the dose of heparin. The diagnosis of HIT is based on an unexplained drop in the platelet count of at least 50% or skin lesions at sites of heparin injection and the presence of anti-PF4/heparin complex antibodies. The secondary white clot syndrome may lead to myocardial or cerebral ischemia. A study of patients with acute ischemic stroke treated with heparin found a 1.7% incidence of definite HIT. Following ischemic stroke, patients receiving heparin should have platelet counts checked every 2–3 days from days 4 to 14 (or until heparin is stopped). Patients with HIT with thrombosis should be treated with lepirudin, fondaparinux, danaparoid, or argatroban. An NOAC such as dabigatran or factor Xa inhibitors are some potential options.
Bleeding is the most likely potential complication of treatment with an LMW heparin or danaparoid. Because of a more predictable dose–response relationship, the risk of bleeding appears to be less with these agents than with unfractionated heparin. The long duration of their pharmacologic actions is a potential disadvantage if serious bleeding does happen. There is no effective antidote.
The safety of these agents in patients with acute ischemic stroke has been evaluated in several clinical trials. Most of the trials tested subcutaneous administration. A trial performed in Hong Kong found no increase in the risk of intracranial or extracranial hemorrhage with the administration of nadroparin. However, a second trial of nadroparin found a significantly increased risk of serious bleeding. In a study comparing aspirin or dalteparin in patients with atrial fibrillation and recent stroke, symptomatic intracranial hemorrhage was diagnosed in 2.7% of the patients, given the LMW heparin, and in 1.8% of patients receiving aspirin. Extracranial hemorrhage also was more common among the patients treated with dalteparin. A German trial tested four doses of certoparin; the risks of symptomatic intracranial or extracranial bleeding were the greatest among the group that received the highest dose of the medication. In a trial that tested two doses of tinzaparin in comparison with aspirin, the risks for symptomatic intracranial hemorrhage were 0.2% for aspirin, 0.6% for low-dose tinzaparin, and 1.4% for the highest dose of tinzaparin. Wong et al. compared the utility of aspirin or an LMW heparin, nadroparin, in patients with recent stroke associated with large-artery occlusive disease. The rates of hemorrhagic transformation of the infarction and severe adverse events were similar in both treatment groups.
One trial tested the IV administration of danaparoid in the treatment of patients with acute ischemic stroke. The medication was given with a bolus, followed by a continuous IV infusion, and subsequent treatment was adjusted according to levels of inhibition of factor Xa. Enrollment of patients with more than a moderately severe stroke (as indicated by a National Institutes of Health Stroke Scale [NIHSS] score of 15 or greater) was halted because of an unacceptably high rate of symptomatic intracranial hemorrhage. Overall, symptomatic hemorrhage occurred in six of 628 patients given placebo (0.9%) and in 19 of 638 patients treated with danaparoid (2.9%). A meta-analysis of the randomized trials of anticoagulants among patients with cardioembolism concluded that early administration of anticoagulants is associated with a significant increase in the risk of symptomatic ICH (2.5% vs 0.7%; OR, 2.89; 95% CI, 1.19–7.01).
A clinical trial compared the utility of enoxaparin or unfractionated heparin in preventing DVT after stroke. The trial, which involved entry within 48 hours after stroke, demonstrated no difference in neurologic outcomes in the two groups. Symptomatic ICH occurred in 0.69% of the heparin group and 0.46% of the enoxaparin group. Another clinical trial compared the utility of certoparin or unfractionated heparin in preventing VTE among patients treated within 24 hours of stroke. The rates of major bleeding complications were 1.1% with certoparin and 1.8% with heparin. A systematic review that compared aspirin or LMW heparin after stroke found that the anticoagulant was associated with a significant increase in major extracranial hemorrhage. Among patients treated within 24 hours, symptomatic ICH was significantly more common among patients treated with LMW heparin. In a prospective cohort of 215 children with acute ischemic stroke, full-dose anticoagulation was associated with a 4% risk of symptomatic ICH. A recent meta-analysis concluded that the LMW heparins and danaparoid were not superior to conventional heparin when administered among patients with recent stroke. Thrombocytopenia is a potential complication of treatment with the LMW heparins and danaparoid, but the risk of this complication is lower than with unfractionated heparin. Because of the potential for cross-reactivity, patients who have HIT should not receive the LMW heparins.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here