Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Antiplatelet agents are the gold standard for secondary prevention of non-cardioembolic stroke.
Aspirin, aspirin plus dipyridamole, or clopidogrel, ticagrelor, and possibly cilostazol are acceptable therapeutic alternatives.
The combination of aspirin plus clopidogrel should not be used for long-term secondary stroke prevention.
For prevention of stroke recurrence early after minor stroke or high-risk transient ischemic attack, the combination of aspirin and clopidogrel or aspirin and ticagrelor are superior to monotherapy. Such dual antiplatelet therapy produces excessive bleeding if given for more than 21 days.
Stroke is the second-leading cause of death worldwide, after ischemic heart disease, and is the sixth leading cause of adult disability-adjusted life-years worldwide. The predominant mechanisms for ischemic stroke are cardioembolic, thromboembolic, and small-vessel disease. The rest are due to unusual mechanisms or are classified as cryptogenic.
Cerebral ischemia tends to recur after a primary episode of either transient ischemic attack (TIA) or stroke. Most commonly, cerebral ischemia is caused by thromboemboli that form on damaged vascular surfaces of extracranial or intracerebral arteries. Local activation of platelets on the walls of diseased arteries initiates thrombus formation under conditions of high flow typical of arteries because activated platelets not only clump together but also directly catalyze thrombin generation. These thrombi are classically considered “white clots”; that is, they are composed mainly of platelets plus some fibrin. These platelet-rich thrombi that form on atherosclerotic plaques may either occlude small arterioles directly or embolize into intracerebral end arteries, producing vascular occlusion that results in neurologic dysfunction.
Because platelet activation is causally linked to episodes of cerebral arterial ischemia, therapies that diminish or block the early steps in hemostasis that are platelet-dependent are used in patients with TIAs or stroke to prevent further episodes of cerebral ischemia.
Stroke prevention can be considered under the rubrics of primary prevention and secondary prevention, which can be divided into (1) early and (2) late. Risk-factor management is of the utmost importance in primary prevention of stroke—control of hypertension, for example, is associated with a 30%–40% reduction in stroke—although antiplatelet drug use may have a role in primary prevention in certain populations.
However, despite the use of a variety of antithrombotic therapies for secondary prevention of stroke, risk reduction has been disappointing; it has been only about 15%–20% in most large clinical trials. , Increasing the intensity of treatment, either antiplatelet therapy or anticoagulation (or both together), has the important adverse effect of increasing both intracerebral and systemic hemorrhage and negates the net therapeutic benefit. The success of antiplatelet drugs in reducing the recurrence of ischemic stroke further incorporates the fact that bleeding is less common during antiplatelet therapy than during anticoagulant therapy.
In contrast to arterial thrombi, venous thrombi form under conditions involving vascular stasis, consist mainly of fibrin and erythrocytes, and are much less dependent on platelet activation. Venous thrombosis, including cerebral venous thrombosis, is thus better prevented by anticoagulation than by antiplatelet therapy. This is also true for cardioembolic strokes associated with atrial fibrillation (AF). However, the distinctions between factors contributing to arterial versus venous thrombi are far from absolute. Both platelet-dependent phases and coagulation-factor-dependent phases of hemostasis intermingle to a considerable extent, for example, because of the prominent role played by platelets in catalyzing thrombin generation.
Moreover, combining antiplatelet and anticoagulant agents in the search for more powerful effects during long-term, secondary prevention of cerebral ischemia has been notably unsuccessful because an unacceptably high incidence of intracerebral and extracranial bleeding ensues when the drug combinations are used at doses that effectively block both platelet function and blood coagulation.
The evidence is clear that persons at increased risk of stroke have excessively active platelets and that even normal platelet activation in the setting of arterial disease imparts thromboembolic risk. This provides the pathophysiologic basis for antiplatelet therapy in the secondary reduction of stroke risk. To understand the rationale for using particular antiplatelet drugs, it is important first to consider how platelets regulate normal hemostasis. Hemostasis is defined as the appropriate physiologic response to vascular injury that provides prompt control of blood loss. In contrast, thrombosis is excessive or inappropriate blood clotting. Platelet hemostatic function needs then to be contrasted with platelet prothrombotic function, that is, how platelets promote inappropriate formation of blood clots in the setting of arterial vascular disease. Because platelets interact with the blood vessel wall in both hemostasis and thrombosis, the status of the arterial endothelial lining is an important determinant of platelet behavior. Normal vascular endothelium is non-thrombogenic and prevents platelet interactions with it and with other platelets or leukocytes by multiple mechanisms including secretion of prostaglandins (PGs) and nitric oxide (NO), surface expression of anticoagulant heparan sulfate and adenosine-diphosphate (ADP)-metabolizing enzymes, and facilitation of smooth, nonturbulent blood flow.
Normal platelets circulate for about 7–10 days after being released from megakaryocytes in the bone marrow, even though they lack nuclei and are almost incapable of protein synthesis. The youngest platelets are the most hemostatically active. The relatively long life of platelets in the blood has permitted effective, once-daily dosing with several antiplatelet drugs. Circulating platelets do not normally interact with one another, with other blood cells, or with the surface of the normal vascular endothelium. If a blood vessel is injured and endothelial continuity is broken, however, platelets undergo, within seconds, a rapid series of coordinated activation changes that quickly leads to a hemostatic platelet plug. This primary hemostatic plug prevents bleeding at the injury site without blocking blood flow through the vessel ( Fig. 63.1 ).
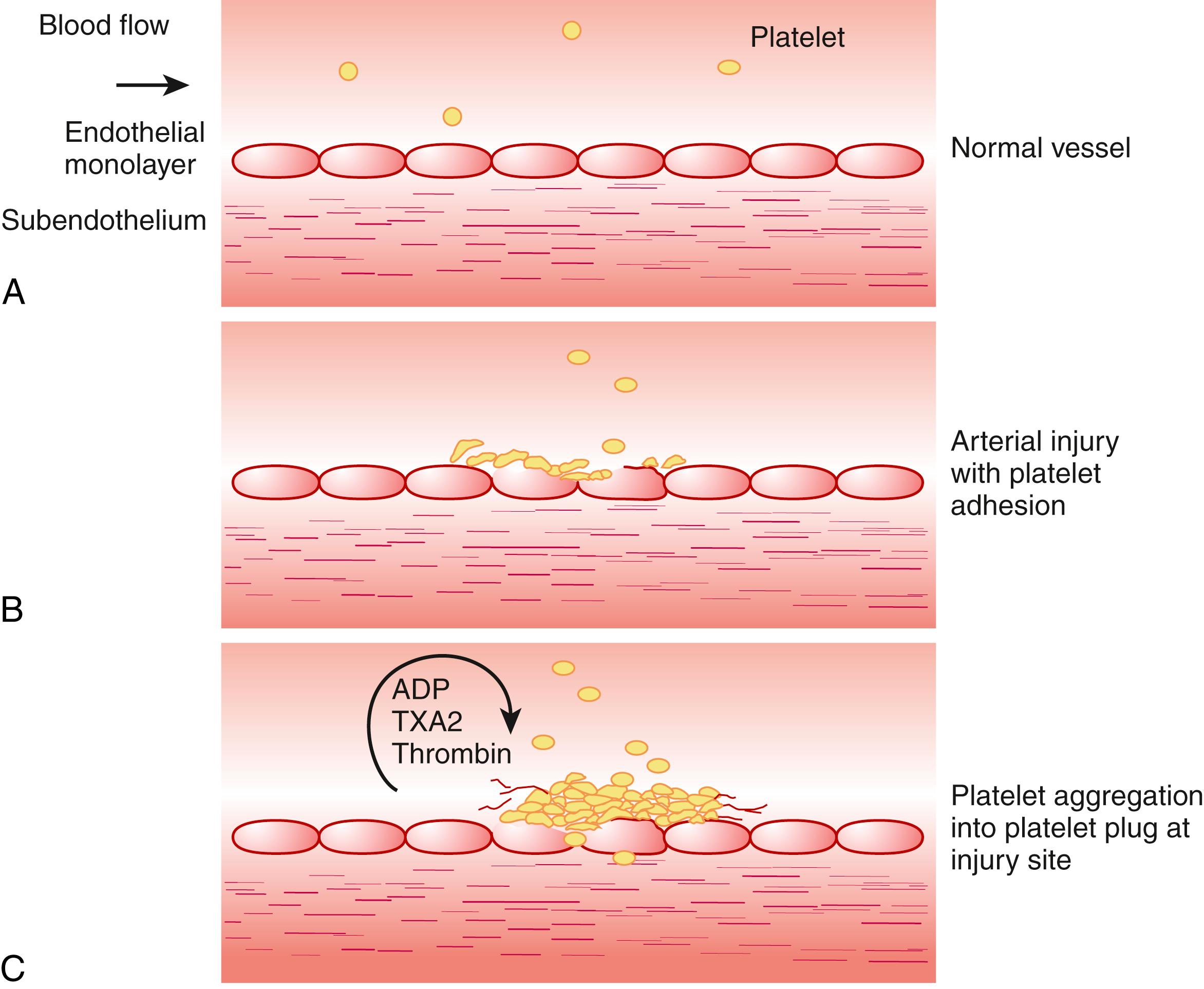
These activation steps start with adhesion of single platelets to the damaged vessel wall, in which the platelets change in shape from flat, unreactive disks to “spiny spheres” that spread out over the surface. Next is aggregation, in which additional platelets join into masses or clumps on top of the original spread layer of platelets, blocking blood loss. During this process, the now activated platelets release vasoactive substances from storage granules, including adhesive glycoproteins (GPs), procoagulants, agonists for platelet activation, enzymes, and inflammatory mediators ( Table 63.1 ). Moreover, once platelets are activated, vasoactive lipids, such as thromboxane A 2 (TXA 2 ) and leukotrienes, are rapidly synthesized and released. Many of these substances either recruit other platelets or produce vascular contraction, which are functions that help stop bleeding promptly at the injury site. Most importantly, the surface membranes of activated platelets catalyze thrombin generation with great efficiency, such that activated platelets serve to initiate, augment, and localize fibrin formation, further amplifying thrombotic potential as well as participating in clot stabilization.
| Dense granule contents | Adenosine diphosphate, adenosine triphosphate, Ca 2+ , serotonin |
| α Granule contents | Adhesive proteins: fibronectin, fibrinogen, thrombospondin, vitronectin |
| Coagulation factors: von Willebrand factor, factor V, factor X | |
| Growth factors: fibroblast growth factor, platelet-derived growth factor, transforming growth factor-β | |
| Membrane proteins: P-selectin, amyloid precursor protein | |
| Others: albumin, immunoglobulin G, antibacterial proteins, platelet inhibitor activator-1 | |
| Lysosomes | Acid hydrolases, neutral proteases, elastase, complement-activating enzymes, heparitinase |
| Peroxisomes | Catalase |
| Lipid mediators (not preformed) | Prostaglandin endoperoxides, thromboxane A 2 , prostaglandin D 2 , 12-hydroxyeicosatetraenoic acid, isoprostanes |
In both hemostasis and thrombosis, the major signal for platelet activation is a local injury to endothelium, which leads to exposure of prothrombotic components to the blood, usually localized in the subendothelial matrix. Whereas normal, intact endothelium displays numerous antithrombotic functions and repels platelets, subendothelium is rich in prothrombotic substances such as collagen and adhesive molecules, including von Willebrand factor (vWF), thrombospondin, and fibronectin. In addition, subtle endothelial dysfunction produced by turbulent blood flow, hyperlipidemia, inflammation, or atherosclerosis—without any physical discontinuity in the endothelial monolayer lining the blood vessels—can also activate platelets. Moreover, once activated, platelets themselves recruit additional platelets by releasing vasoactive mediators and catalyzing thrombin formation; in turn, thrombin is a potent platelet activator. Platelet activation is, therefore, an exponential and interactive, rather than linear, process.
Platelet activation involves changes in both the morphologic and biochemical state of the platelet membrane, including conformational changes in adhesion receptors, binding of adhesive proteins, mobilization of intracellular granule contents, interactions with the cytoskeleton, initiation of signaling, and platelet–platelet interaction. GP platelet adhesion receptors mediate attachment of platelets to substrate proteins. Adhesion receptors that are important for normal hemostasis, thus representing potential therapeutic targets for thrombosis prevention, include the following: GPIa, a collagen receptor; GPIb, a receptor for vWF; the GPIIb/IIIa complex, a major receptor for fibrinogen, fibronectin, and vWF; GPIV, a thrombospondin receptor, required for irreversible platelet activation; GPs V and IX; and α v β 3 , a receptor for vitronectin, fibrinogen, and fibronectin. These receptors are all functional on resting platelets except for GPIIb/IIIa, which requires platelet activation to undergo the conformational change that permits this complex to bind fibrinogen.
Many of the adhesive GPs that are ligands for these receptors share the peptide sequence RGD ( R, arginine; G, glycine; D, aspartic acid), which is directly involved in cell–cell adhesion.
Adhesion of platelets to other platelets, to the subendothelium exposed by vascular injury, or to activated endothelium is the first major step in the platelet activation sequence. Platelet adhesion is mediated by a complex of GPIb–V–IX, which binds to matrix vWF at high shear rates and is also a binding site for thrombin, thus acting to amplify platelet responses to thrombin. , GPIb–V–IX is mainly involved in platelet activation resulting from abnormal shear stress, such as is found in arteries narrowed by atherosclerosis. Changes in conformation of the GPIbα component of the complex or of vWF can induce interaction between these two molecules. Binding of vWF to collagen induces small conformational changes in vWF that permit its binding to GPIb. Furthermore, binding of vWF to GPIb causes redistribution of the GPIb–V–IX GP complex within platelets, linking the complex to the cytoskeleton and activating phosphorylating enzymes that regulate actin polymerization and the activation of the GPIIb/IIIa complex. Therefore, inhibition of platelet adhesion should be antithrombotic. Peptides that block GPIb–V–IX function are under development as novel antiplatelet drugs. Activation of GPIb–V–IX in a thromboxane-independent manner accounts for part of the “resistance” to aspirin that is observed in connection with the presence of atherosclerotic risk factors. Absence or dysfunction of GPIb is characteristic of a rare platelet disorder involving soft-tissue bleeding, the Bernard-Soulier syndrome. At low shear, the GPIIb/IIIa complex also participates in platelet adhesion to surfaces through binding of fibrinogen.
Aggregation is the step in the platelet activation process most relevant to the pathogenesis of occlusive vascular events in an oxygen-sensitive arterial bed. During aggregation, activated platelets clump together atop an initial, adherent layer of platelets deposited at a site of injury or diseased vascular wall. These white thrombi, composed mainly of platelets and fibrin, may be transient or may become stabilized by further fibrin deposition, forming the nidus of bulkier clots in which erythrocytes and leukocytes also become trapped. During normal hemostasis, such platelet plugs halt bleeding from injured microvessels without obstructing blood flow, but in diseased vessels (e.g., in atherosclerosis or vasculitis or after irradiation), excessive platelet plug formation can occlude cerebral vasculature, producing TIA or stroke.
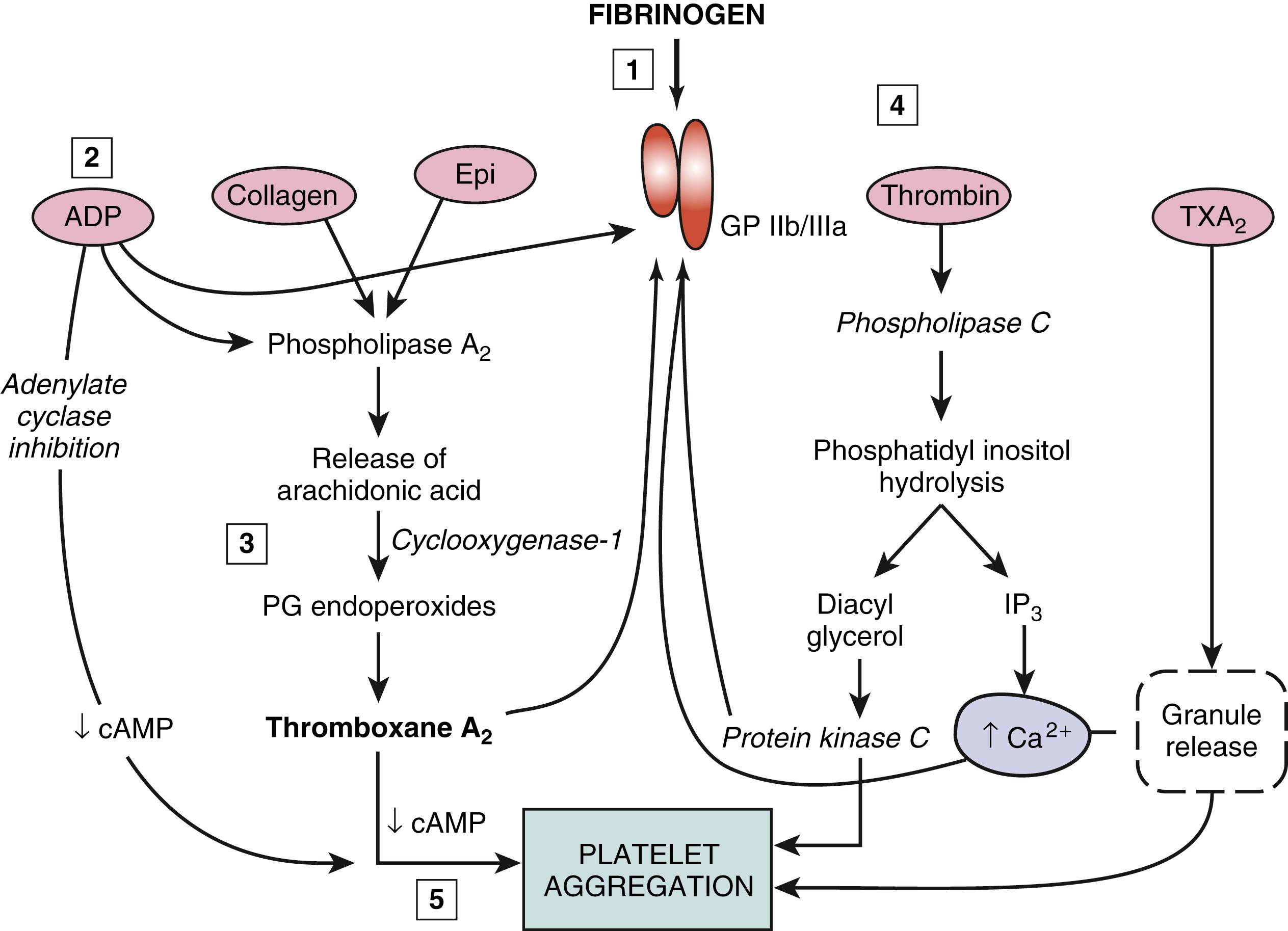
The most important membrane receptor for aggregation is the integrin GPIIb/IIIa, a bimolecular membrane complex unique to platelets and megakaryocytes. Platelet aggregation by all known pathways depends on conformational changes in GPIIb/IIIa induced by platelet agonists. , Three main physiologic pathways independently triggered by different ligands can activate the GPIIb/IIIa complex; one pathway is activated by arachidonic acid leading to TXA 2 formation, the second by ADP, and the third by thrombin ( Fig. 63.2 ). All of these signaling pathways converge in a final common mechanism to produce a conformational change in GPIIb/IIIa that exposes a high-affinity fibrinogen binding site. The activated GPIIb/IIIa complex thus markedly increases fibrinogen binding, becomes associated with cytoskeletal proteins and signaling kinases (e.g., pp60[c-src]), forms receptor clusters, and becomes phosphorylated. All of these functions favor platelet–platelet interactions. Both outside–inside signaling (e.g., agonist-driven) and inside–outside signaling (i.e., kinase/phosphatase-driven) are involved in this activation process.
Because the final common pathway for platelet activation requires the GPIIb/IIIa complex, therapeutic blockade of the complex inhibits all further platelet activation. In contrast, drugs that inhibit only one of the three pathways, such as aspirin inhibition of the thromboxane pathway or clopidogrel inhibition of the ADP pathway, do not prevent GPIIb/IIIa activation. Drugs that directly block the functions of the GPIIb/IIIa complex thus have profound inhibitory effects on platelet function and are currently used to treat acute cardiac ischemia. Development of the monoclonal antibody abciximab (ReoPro), of peptides such as eptifibatide (Integrilin), and of peptidomimetics such as tirofiban (Aggrastat), which inhibit GPIIb/IIIa function when administered intravenously, has permitted highly successful antiplatelet therapy in acute cardiac interventions. Used over a short interval in combination with heparin and aspirin, these GPIIb/IIIa inhibitors prevent early thrombosis and deter vascular reocclusion after coronary angioplasty and stent placement. Unfortunately, the use of oral GPIIb/IIIa inhibitors for long-term prevention of cardiac thrombosis has been unsuccessful to date in numerous clinical trials, in which administration of such agents has been accompanied by higher rates of thrombosis or bleeding. A single clinical trial testing the safety of abciximab in treatment of acute stroke has been published (see later). Indeed, the Blockage of the Glycoprotein IIb/IIIa Receptor to Avoid Vascular Occlusion (BRAVO) study of lotrafiban (an oral GPIIb/IIIa inhibitor) and heparin for prevention of cardiovascular and cerebrovascular events, initiated in 2000, had to be discontinued because of excess occurrences of thrombosis.
Each of the three independent biochemical pathways of platelet aggregation provides a separate potential for therapeutic intervention. One major pathway involves metabolism of arachidonic acid, which is released from membrane phospholipids during platelet activation (see Fig. 63.2 , center left ). This pathway is sensitive to aspirin and other nonsteroidal antiinflammatory drugs (NSAIDs). Receptor engagement by various platelet agonists activates phospholipase C to cleave membrane-bound phosphatidyl inositol to inositol 1,4,5-triphosphate (IP 3 ) and diacylglycerol. IP 3 in turn releases stored calcium ions, permitting activation of platelet phospholipase A 2 , which releases esterified arachidonic acid from membrane phospholipids. The enzyme cyclooxygenase-1 in platelets rapidly converts the released arachidonic acid to PG endoperoxides that are isomerized to TXA 2 , a potent platelet agonist and vasoconstrictor. The TXA 2 diffuses from the platelets and binds to its specific seven-transmembrane receptor on the platelet surface, signaling further activation of phospholipase C. Concomitantly, the liberated diacylglycerol activates protein kinase C, which translocates to the platelet plasma membrane and triggers activation of GPIIb/IIIa, exposing its fibrinogen binding site, thereby permitting platelet aggregation and secretion of platelet granule contents (see Fig. 63.2 , right side ). Aspirin, which irreversibly binds to cyclooxygenase-1, blocks the formation of TXA 2 for the entire life span of circulating platelets because platelets are incapable of synthesizing new cyclooxygenase protein.
Platelets that cannot produce TXA 2 can still be activated via the ADP-dependent and thrombin-dependent activation pathways. Platelet aggregation can be initiated via two interacting ADP receptors by ADP that is released by platelets or derived from extraplatelet sources such as red blood cells, even in the presence of aspirin. , Thrombin acts on several specific protease-activated membrane receptors (PARs) that signal the activation of phospholipase C and irreversible platelet aggregation and the release reaction that is independent of arachidonic acid metabolism. Similarly, platelet activation by the phosphorylcholine-derivative platelet-activating factor (PAF), which is produced by leukocytes or by disturbed endothelial cells, is also aspirin-insensitive. The existence of these separate pathways for platelet aggregation may be regarded as representing fail-safe or redundant mechanisms to avoid hemorrhages.
Release of vasoactive contents of platelet storage granules normally accompanies platelet activation, augments platelet aggregation, and accelerates localized clot formation, vasoconstriction, and the initiation of wound healing. Not only are preformed GP mediators, ADP, vasoactive amines, growth factors, calcium ions, and serotonin released into the blood or displayed on the surface membrane, but also short-lived lipid mediators are synthesized and released during platelet activation. The extent of the release reaction can be modulated by antiplatelet drugs.
Several types of granules rich in substances that participate in blood coagulation, cell–cell interactions, and wound repair are present in platelets (see Table 63.1 ). The different granule types—dense granules, α granules, lysosomes, and peroxisomes—can be morphologically distinguished and are also functionally characterized by their contents and the ease of release of their contents. For example, weak platelet stimuli, such as ADP, adenosine triphosphate (ATP), serotonin, and calcium—all of which participate in potentiating irreversible platelet aggregation—release the contents of the dense granules. By contrast, strong stimuli are required for release of α granule contents: fibrinogen, fibronectin, vWF, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), transforming growth factor-β (TGF-β), platelet factor-4 (PF-4), and β-thromboglobulin. α Granules also contain albumin, immunoglobulins, antibacterial proteins, and a complement inhibitor. The membrane of the α granules contains P-selectin and the amyloid precursor protein; on platelet activation, P-selectin is transferred to the surface of the platelets, where it mediates cell–cell interactions with leukocytes and plays an important role in inflammatory reactions. Platelet lysosomes, the last granule type to be released, contain acid hydrolases, neutral proteases, elastase, enzymes that activate complement, and a heparitinase. Peroxisomes contain catalase.
In general, release of components of both dense bodies and α granules usually accompanies platelet aggregation but may also occur from platelets that are adherent to damaged endothelium or subendothelium, even without formation of platelet aggregates.
In contrast to the release of preformed proteins or amines from platelet granules, vasoactive lipid mediators are not stored by platelets, but instead are rapidly synthesized and released on platelet activation. Most of these lipids are oxygenated metabolites of arachidonic acid, which is mobilized from membrane phospholipids when agonists interact with platelet membrane receptors. The released arachidonate is the substrate for several different metabolic pathways producing eicosanoids and hydroxylated fatty acids. TXA 2 is the major platelet product of arachidonic acid metabolism by the eicosanoid pathway, and its synthesis occurs very rapidly—within seconds—on platelet activation. G-protein linked, seven membrane-spanning receptors for TXA 2 are present on platelets, leukocytes, and vascular cells. These receptors bind TXA 2 and also its endoperoxide precursors, which have similar vasoconstrictor properties. Signal transduction via TXA 2 receptors initiates platelet activation and causes vasoconstriction.
Platelets also synthesize hydroxylated fatty acids from arachidonate via a separate enzymatic pathway involving lipoxygenase. The major product of this pathway in platelets is 12-hydroxyeicosatetraenoic acid (12-HETE), an inflammatory mediator that is chemotactic for leukocytes, stimulates vascular smooth muscle proliferation, and can be converted to additional inflammatory mediators, di-HETEs, by leukocytes. In contrast to the rapid burst of TXA 2 synthesis by activated platelets, the production of 12-HETE occurs continuously over a long period. Nonenzymatic, vasoactive metabolites of arachidonate, isoprostanes are also formed by oxidation; they can inactivate vascular or platelet NO.
Interactions among different cell types can produce additional arachidonic acid products via transcellular metabolism, yielding products that are not made by a single cell type alone. Thus, platelet-released arachidonic acid can be converted into other vasoactive products by leukocytes or endothelial cells in close proximity. As mentioned previously, leukocytes can convert 12-HETE into various di-HETEs that have chemotactic and inflammatory properties. Endothelial cells can convert platelet-derived arachidonic acid or endoperoxide into prostacyclin, an antiplatelet substance that is a vasodilator. Similarly, endoperoxides released by endothelial cells can be converted by normal or aspirin-treated platelets into TXA 2 .
Platelets responding to strong agonists such as collagen, TXA 2 , and thrombin accelerate both primary hemostatic plug formation and catalysis of blood coagulation, efficiently localized on the surface of the activated platelets. By providing specific sites, receptors, procoagulant factors, and lipid cofactors for the assembly of the key complexes of procoagulant enzymes, the activated platelet surface markedly accelerates the rate of localized thrombin generation, increasing by more than 200,000-fold the reaction rate in the fluid phase. Furthermore, activated platelets themselves contribute coagulation factors such as factor V and factor X released from their granules into a local clot as well as antifibrinolytic factors such as plasminogen activator inhibitor-1 (PAI-1). Because thrombin is a strong stimulus for platelet activation, the release reaction, and TXA 2 formation, the initial production of trace amounts of thrombin at the site of vascular injury directly stimulates further platelet activation and promotes coagulation. Microparticles shed from activated platelets also catalyze thrombin generation. Platelets additionally participate in clot stabilization by releasing the fibrin cross-linking protein factor XIII and by supporting clot retraction.
During fibrinolysis, blood clots are dissolved by the protease plasmin, which cleaves insoluble fibrin. Platelets both promote and inhibit fibrinolysis, and the products of fibrinolysis can affect platelet function. Activated platelet surfaces favor fibrinolysis by localizing plasminogen and promoting its activation. Thus, platelets bind plasminogen and plasminogen activators (both urokinase and tissue-type activators) via the GPIIb/IIIa complex. Thrombospondin released from platelet granules and then displayed on the platelet surface also binds plasminogen and enhances the activation of bound plasminogen. Because plasmin is more efficiently formed on a surface than in the fluid phase, activated platelets provide an alternative surface for promoting fibrinolysis. Platelet-bound plasminogen, therefore, is more readily activated by either tissue plasminogen activator or streptokinase than is free plasminogen, which suggests a mechanism by which platelets can enhance local fibrinolysis. In turn, plasmin at low concentrations enhances and at high concentrations depresses platelet activation.
Platelets also contain and secrete two fibrinolytic antagonists, PAI-1 and α 2 -antiplasmin. The net effects of these different platelet activities are that platelet-rich thrombi resist fibrinolysis and thrombolysis and that platelets become activated during therapeutic thrombolysis.
For clinical practice, the coexistence of multiple pathways mediating platelet activation means that drugs inhibiting only one pathway—for example, aspirin inhibiting cyclooxygenase, thus blocking TXA 2 formation—only partially block platelet activation. The existence of multiple pathways of platelet activation thus protects against hemorrhage but permits thrombosis. Several natural mechanisms exist in normal blood vessels to limit the extent of platelet activation. These mechanisms are also vasodilatory. They are (1) plasma ADPase enzymes and endothelial ectoADPases that metabolize ADP to adenosine, a vasodilator and inhibitor of platelet activation; (2) prostacyclin, the vasodilator metabolite of arachidonic acid released by endothelial cells, which stimulates platelet adenylate cyclase, raising intraplatelet cyclic adenosine monophosphate (cAMP) levels, blocking calcium release, and inhibiting platelet aggregation and secretion; and (3) NO produced by endothelial cells, platelets, and monocytes via NO synthases. ,
NO stimulates platelet guanylate cyclase and raises levels of cyclic guanosine monophosphate (cGMP), thus inhibiting platelet activation. It also relaxes vascular smooth muscle via stimulation of cGMP in the vessel wall. NO is formed from l -arginine, an amino acid that has been shown to inhibit platelet aggregation. In arterial vascular disease, it is clear that NO-mediated mechanisms are diminished. At present, development of soluble ADPases and stimulators of NO synthase or NO donors as clinical antithrombotic agents is being actively pursued, but such drugs have not yet entered the clinical trial phase of development.
Activated platelets recruit leukocytes to sites of vascular injury, setting up an inflammatory response that assists in repair of damaged tissue after hemostasis but promotes atherosclerotic progression in vascular disease. Numerous substances released from platelets are directly chemotactic for neutrophils (12-HETE), monocytes (PF-4, PDGF), or both. Platelet proteases release chemotactic complement fragment C5a from plasma C5 and potentiate C3 activation, enhancing leukocyte function. Platelet P-selectin translocated to the surface membrane of activated platelets mediates interactions between platelets and leukocytes, including initial leukocyte rolling on vascular endothelium, and P-selectin also augments leukocyte activation. Activated platelets remain coated with P-selectin, which permits long-lasting inflammatory effects in the vicinity of platelet plugs, whereas display of P-selectin on activated endothelium is a transient phenomenon. Activation of endothelium, however, induces platelet thrombus formation.
Adhesive proteins released by platelets promote inflammation as well as hemostasis; therefore, they contribute to thrombosis. Circulating levels of vWF, fibrinogen, thrombospondin, and PDGF rise during inflammation and are chronically elevated in patients with occlusive vascular disease. The growth factors released by aggregating platelets are chemotactic for leukocytes, smooth muscle cells, and endothelial cells and, during wound healing, stimulate cell division of smooth muscle cells and fibroblasts. Among these factors is TGF-β. Platelets are a major source of circulating TGF-β, which is released on their activation. TGF-β stimulates the synthesis of matrix proteins, a component of atherosclerotic lesions. Because these growth factors can also be released by single platelets adhering to an abnormal vascular wall, they can stimulate excessive proliferative responses in the vessel wall that favor both restenosis after revascularization and progression of atherosclerosis. Platelets are rich in cholesterol, which is also released during platelet activation and can be incorporated into the arterial wall. Therefore, antiplatelet therapy that depresses release of platelet granule contents has potential anti-atherosclerotic as well as direct antithrombotic value.
Patients at risk of occlusive stroke often have activated platelets. , Their platelet hyperreactivity most likely reflects the interactions between platelets and an abnormal vascular surface. Most frequently atherosclerosis, but also vasculitis, infection, trauma, or congenital anomaly, may lead to vascular endothelial dysfunction. Stroke risk factors such as hypertension, hypercholesterolemia, smoking, diabetes mellitus, and inflammation contribute both to increased platelet reactivity and to abnormal endothelial behavior with consequently higher risk of arterial thrombosis. In addition, turbulent blood flow that accompanies atherosclerotic or hypertensive arterial changes (e.g., endothelial dysfunction, stiff vascular wall, atherosclerotic plaque, or altered pressure gradients) further contributes to increased platelet reactivity in the absence of actual breaches in the endothelium. High levels of platelet activation markers have been associated with carotid artery wall thickening.
Plasma factors may also contribute to increased platelet reactivity in stroke-prone patients. The higher levels of circulating catecholamines associated with advancing age and with stress contribute to platelet reactivity. Catecholamines are weak direct platelet agonists but augment platelet aggregation by other agonists, oppose the effects of natural antiplatelet factors such as prostacyclin and NO, and offset some of the antiplatelet efficacy of aspirin. A circadian rhythm in acute myocardial infarction (MI), stroke, and sudden death has been observed in which the peak incidence is seen in the early morning, soon after patients awaken. , Platelet aggregability is highest soon after waking and correlates with elevations in plasma catecholamines and free fatty acids. A diminution in the morning excess of cardiovascular events in patients who take aspirin points to platelet involvement in the process.
In clinical settings marked by stress, in which catecholamine levels are increased, plasma fibrinogen and vWF–factor VIII are also elevated, which contributes to platelet activation. Elevated plasma fibrinogen by itself is an independent risk factor for stroke and is known to increase platelet aggregability. Recent infection may also contribute to the risk of recurrent cerebral ischemia. In the elderly, a seasonal rise in stroke incidence during the winter months may reflect a higher incidence of infection. ,
High platelet counts may, but do not necessarily, represent a stroke risk factor. In situations in which platelet turnover is continually enhanced, a compensatory increase in megakaryocyte size may result in release of platelets that are “younger,” larger, and more hemostatically active than usual. Young platelets have been found to be larger than average, to produce more prothrombotic factors, and to aggregate in response to lower concentrations of agonists. Large platelets are a risk factor for death and recurrent vascular events after MI. Platelet volume is greater in patients with acute stroke than in age- and sex-matched control subjects, and the high platelet volume may persist for months.
In myeloproliferative diseases, platelets are often very large, and platelet mass is increased even more than platelet count; platelet numbers, therefore, underestimate total platelet mass. The greater platelet mass probably contributes to the higher thrombotic risk observed in these diseases. High platelet counts in a setting of myeloproliferative disease are associated with increased risk of thrombosis, including cerebral thrombosis, and lowering the platelet count with chemotherapy appears to reduce the risk of both stroke and thrombosis in these diseases. Low-dose aspirin therapy also decreases stroke risk in patients with myeloproliferative diseases, especially if the platelet count has been normalized. In contrast, secondary thrombocytosis that accompanies inflammation or follows splenectomy, for example, is not usually associated with a higher risk of thrombosis or stroke.
The question of whether greater platelet reactivity in patients with stroke merely reflects the body’s inflammatory response to tissue damage is, however, clearly answered with a no. Increases in platelet activation not only may follow an acute stroke for many months but also may be chronically present before an episode of cerebral ischemia. In adults, increased platelet aggregability after atherosclerotic stroke has been demonstrated to continue for at least 3–9 months after the acute event in 60% of patients; this time extends well beyond that required for resolution of the acute inflammatory changes typical of cerebral tissue damage. Similar changes in platelet aggregability are not observed after stroke caused by cardiac emboli, even though brain tissue damage may be extensive. A long-term increase in platelet aggregation after acute cerebral ischemia correlates with a poorer outcome. In some unusual circumstances, oxidative stress has been correlated with chronic platelet activation. In familial stroke, infants seen with stroke can be shown to have a chronic platelet hyperreactivity that is not blocked by NO; such infants lack the plasma enzyme glutathione peroxidase and, therefore, have low blood antioxidant potential that counters the protective effect of NO on platelets. In most cases of adult ischemic stroke, however, no such simple correlation between oxidative stress and platelet hyperreactivity can be made.
The pathologic consequences of greater platelet reactivity in stroke-prone patients clearly show that more is not better. In normal arteries, hemostasis rapidly controls bleeding, and the damaged area is soon precisely repaired, whereas in a diseased arterial bed, even this normal hemostatic response may have the unwanted consequences of arterial thrombosis. This dysequilibrium between hemostasis and thrombosis appears to be particularly true in vessels vulnerable to even temporary occlusion, such as the end arteries of the brain. Furthermore, normal platelet contributions to wound repair can similarly be detrimental in stroke-prone patients, in whom platelet-derived mediators promote excessive vascular cell proliferation that accelerates intimal hyperplasia and vascular narrowing. ,
The sequential steps in platelet activation are sensitive to different pharmacologic interventions. Antiplatelet drugs that mainly affect platelet aggregation and mediator release tend to block platelet-initiated thrombosis without much depression of hemostasis and, therefore, involve a smaller chance of hemorrhagic complications. On the other hand, antiplatelet drugs that block platelet adhesion, the initial step in platelet activation at a vascular surface, or the GPIIb/IIIa complex and, thus, all aggregation pathways, are highly effective in preventing thrombosis; thus far, however, they continue to impose an unacceptable risk of bleeding, particularly in the brain. Only direct inhibitors of thrombin, factor Xa, or both block the capacity of platelets to catalyze thrombin generation. Such agents might pose a major bleeding risk with long-term use in secondary prevention of cerebral ischemia and have not been tested for prevention of non-cardioembolic stroke.
Antiplatelet drugs in current use for secondary prevention of stroke include aspirin, clopidogrel, ticagrelor, dipyridamole, cilostazol, and various combinations of these agents ( Table 63.2 ). In clinical settings in which platelets are not major factors in the production of vasoocclusive arterial emboli, anticoagulation is an effective form of secondary prevention of stroke. The foremost of such settings is cardioembolic stroke associated with nonvalvular AF and valvular heart disease. Other important causes of stroke that are relatively independent of platelets are cholesterol emboli released from ulcerated atherosclerotic plaques, patent foramen ovale, fibrin-rich emboli from intracardiac mural thrombus, “bland” cardiac valve vegetations in inflammatory disease, thalassemia, and sickle cell disease , , ; these causes together represent about 15% of cerebral ischemic episodes. Even in some of these settings, antiplatelet agents may have a role in stroke prevention if anticoagulation alone does not suffice or if the patient also has atherosclerotic risk factors for stroke.
| Activity | Effect on Platelets | Drug |
|---|---|---|
| Inhibition of membrane GPIb receptor | Block GPI-V-IX function, prevent adhesion and aggregation | S-nitroso-AR545C a |
| Inhibition of membrane GPIIb/IIIa receptor | Prevent bending of fibrinogen Prevent platelet–platelet interaction |
Abciximab RGD peptide analogues Disintegrins |
| Inhibition of membrane ADP P2Y 12 receptor | Prevent binding of ADP Prevent ADP-mediated aggregation Decrease GPIIb/IIIa activation |
Ticlopidine, clopidogrel, ticagrelor AR-C69931 MX b |
| Inhibition of membrane ADP P2Y 1 receptor | Prevent binding of ADP | Adenosine 3′-phosphate, 5′-phosphosulfate b |
| Prevent calcium mobilization | ||
| Catabolism of ADP | Prevent ADP-mediated aggregation | Soluble recombinant CD39 a |
| Foster disaggregation | ||
| Inhibition of cyclooxygenase-1 | Prevent TXA 2 generation | Aspirin (irreversible effect) |
| Inhibit arachidonic-acid-mediated platelet aggregation and secretion | NSAIDs (competitive effect) | |
| Stimulate adenylate cyclase | Raise platelet cAMP, preventing aggregation and secretion | Epoprostenol, Iloprost Fish oils, ω3 fatty acids a |
| Inhibition of phosphodiesterase | Maintain elevated cAMP once raised | Dipyridamole, cilostazol |
| Methylxanthines a | ||
| Stimulate guanylate cyclase | Raise platelet CGMP, preventing aggregation and secretion | NO, NO donors (e.g., S -nitrosoglutathione, nitroglycerin) |
| Inhibit calcium flux | Prevent calcium mobilization | Ca 2+ channel blockers a |
| Decrease aggregation and secretion | Local anesthetics a | |
| β-blockers a | ||
| Inhibit thrombin generation or action | Inhibit aggregation and secretion | Heparins, hirudin |
| Inhibit procoagulant activity | Antithrombin peptides b |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here