Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Antimicrobial susceptibility testing is one of the most important tasks of the clinical microbiology laboratory. Antimicrobial resistance is common, and early recognition of patients with resistant pathogens and appropriate optimization of their antimicrobial therapy significantly improves outcomes.
This chapter reviews the key aspects of antimicrobial susceptibility testing (AST) of bacteria, mycobacteria, and yeast. In most circumstances, AST is performed by evaluating the effect of antimicrobial agents on the growth of an organism, in culture. The relative effect (measured as either a zone of inhibition surrounding a disk containing the antimicrobial, or a minimum inhibitory concentration [MIC] for dilution methods) is interpreted using clinical breakpoints set by international standards organizations. Standardization of AST is achieved through use of consistent inoculum concentrations, test media, and incubation conditions. The concepts of surrogate agent testing, detection of resistance mechanisms, use of commercial systems, and molecular methods for susceptibility testing are discussed. In some instances, additional testing is required to detect important resistance, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Key concepts for testing bacteria, fungi, and mycobacteria are presented. Common antimicrobials used in clinical practice are also discussed.
Accurate and timely performance of AST is one of the most important functions of the clinical microbiology laboratory. This testing serves to both confirm the susceptibility of an organism to a given antimicrobial and to detect antimicrobial resistance. While there are examples of organisms for which knowledge of the identification is sufficient to inform therapy (e.g., Streptococcus pyogenes is universally susceptible to penicillin), the number of organisms with predictable antimicrobial susceptibility has dwindled over the past several decades. Of concern, the prevalence of “extensively” and “pan-drug” resistant organisms is increasing at an alarming rate across the world. Patients infected with these organisms have very high mortality rates, and some fear return to the pre-antimicrobial era of medicine, if practices to halt the spread of antimicrobial resistance are not implemented.
For patients with severe infection, timeliness of appropriate (i.e., active) antimicrobial therapy is directly related to survival. Studies have demonstrated that for patients with bacterial sepsis, there was a 7.6% increase in mortality for every hour that passed before an active antimicrobial was administered. Similarly, use of an inactive antimicrobial in the first 6 hours of onset of hypotension was associated with a fivefold increase in mortality. However, the etiologic agent of infection, let alone its antimicrobial susceptibility profile, are almost never available when patients with suspected infections are first evaluated. Clinicians must therefore infer the most likely pathogens based on the patient’s medical history, severity of illness, and location of infection and treat with antimicrobials that are likely to cover those pathogens. Antimicrobial prescribing based on these estimates is called empiric therapy . Once culture and susceptibility testing results are available, antimicrobial prescribing can be adjusted to target the isolated pathogen and its susceptibility profile, that is, directed therapy . Unfortunately, use of broad empiric therapy to mitigate the risk of choosing an inactive antimicrobial can be problematic because unnecessarily broad therapy is associated with adverse drug events (i.e., toxicity), risk for developing Clostridioides difficile infection, emergence of antimicrobial resistance, and increased cost. Furthermore, broad-spectrum agents may be less efficacious at treating the infection than is the targeted therapy. For example, patients with oxacillin-susceptible S. aureus blood stream infections treated with vancomycin have increased mortality rates compared with those patients treated with oxacillin. However, oxacillin resistance rates are 50% or greater in many US health care settings, precluding use of oxacillin for empiric therapy. Oxacillin resistance rates among S. aureus differ by geography and may be low in some regions, allowing empiric oxacillin use (e.g., <1.0% in many Scandinavian countries).
Together, these factors highlight the critical role of the laboratory to rapidly and accurately perform AST. These data are used by not only the clinician when making treatment decisions, but also to guide the activities of antimicrobial stewardship programs (ASPs) and hospital epidemiology (infection prevention) programs. This chapter will review key antimicrobials used to treat bacterial infections and the general principles of AST.
The beginning of the modern antibiotic era is typically associated with Paul Ehrlich and his idea of a “magic bullet” that could selectively target disease-causing microbes but spare the host of toxic side effects. This concept led to a systematic evaluation of compounds with activity against the spirochete Treponema pallidum, and his ultimate discovery of Salvasran in 1909, a compound that cured syphilis-infected rabbits. Substances with anti-infective properties have been used medicinally for thousands of years. For instance, more than 2500 years ago, the moldy soybean curd was used by the ancient Chinese for the treatment of carbuncles, boils, and other infections. Similarly, tetracycline has been found in the skeletal remains of humans from archaeologic sites in Egypt, Sudan, and Jordan, indicating they were exposed to this antimicrobial via fermented food products. Currently, numerous antimicrobials are available for the treatment of infections. This is the result of successive rounds of antimicrobial development that have broadened the range of infections that could be cured with antimicrobials. For example, when a novel antimicrobial agent is discovered, structural modifications may be undertaken in an attempt to improve spectrum of activity and/or safety profile of the agent; these are then referred to as “structurally related” antimicrobials. Antimicrobials can be further classified by their mode of action, including those that inhibit synthesis of the cell wall, proteins, nucleic acids, or those that inhibit bacterial metabolism ( Fig. 85.1 ). An additional way to distinguish between antimicrobial agents is whether they exhibit a cidal (killing) or static (inhibitory) effect. This determination is often largely based on in vitro studies; however, the true efficacy of the agent in vivo depends upon a number of other variables including the organism itself, bacterial density, and host defense mechanisms. The pharmacologic properties of an antimicrobial are also critical to in vivo activity; these are evaluated by studying two properties: pharmacodynamics (PD) and pharmacokinetics (PK). In simple terms, PD is the effect of the drug on the target organism at the site of infection over time, which is counterbalanced by the drug’s effect on the host over time (i.e., toxicity). PK is the study of how a drug’s concentration changes over time in the patient, through absorption, distribution, metabolism, and elimination in the body. For additional discussion on PD and PK, see Chapter 42 .
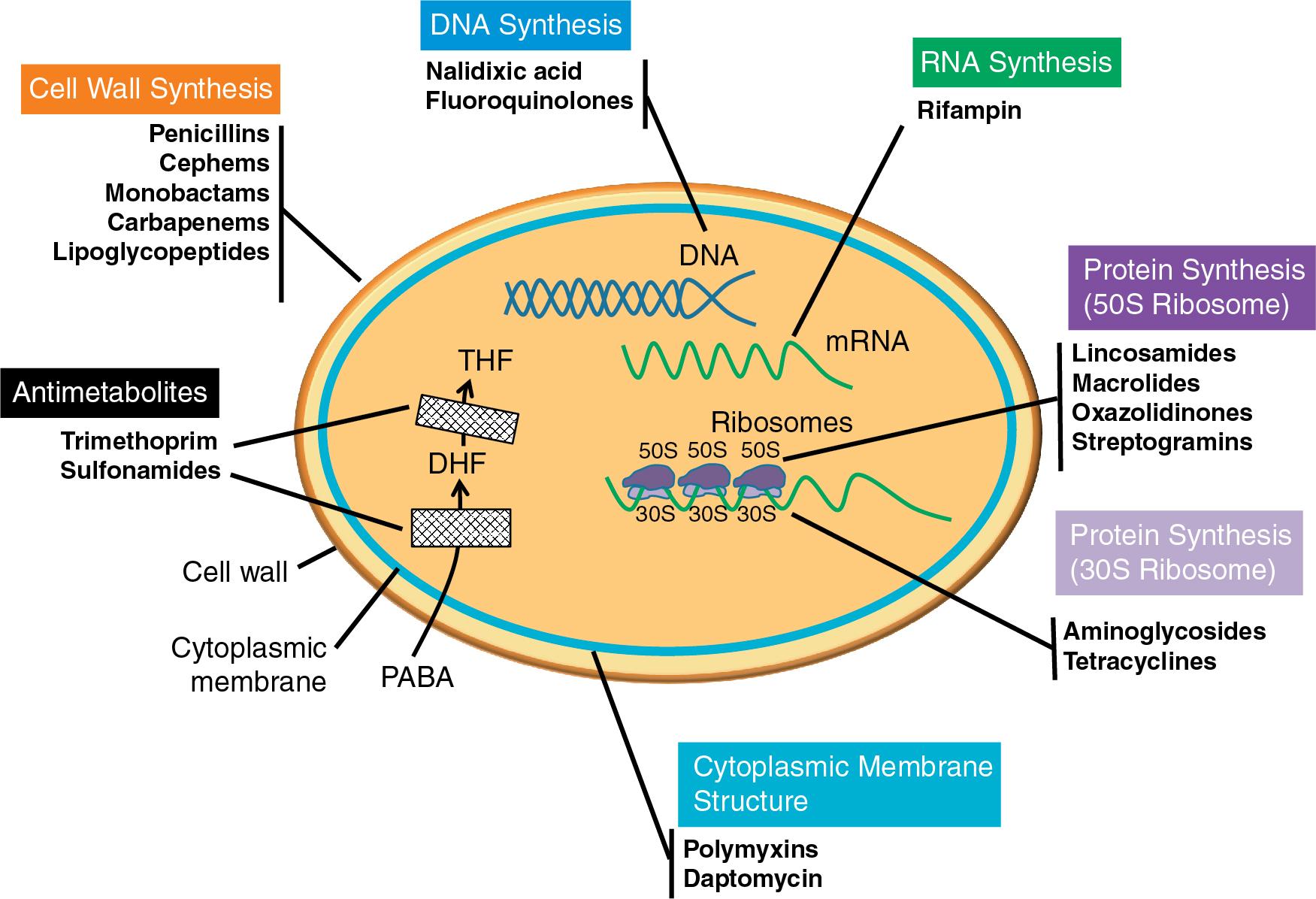
Herein we will discuss the most common antibacterial agents used in clinical practice. For a discussion of antifungal agents, see Chapter 87 .
Peptidoglycan is a major structural component of the bacterial cell wall, providing a rigid mesh-like protective layer. Peptidoglycan is composed of a chain of 10 to 64 disaccharide residues, which consist of alternating molecules of N -acetylglucosamine and N -acetylmuramic acid. These chains are cross-linked by a pentapeptide bridge. The building of these chains and cross-links is catalyzed by the penicillin-binding proteins (PBPs).
β-Lactams are among the most widely prescribed antimicrobials. These agents share a common structural feature, the β-lactam ring. Due to their structural similarity to pentapeptides, PBPs mistakenly incorporate the β-lactam agent into the cell wall during synthesis. The β-lactam then prevents further transpeptidation or cross-linking of the glycan strands. This decreases rigidity in the cell wall, rendering it unable to maintain osmotic stability; this ultimately ends in autolysis of the cell and accounts for the bactericidal activity of this antimicrobial class. , Resistance to β-lactams can occur in three distinct ways: expression of a β-lactamase enzyme that degrades the β-lactam, alteration of the β-lactam binding site, and reduced outer membrane permeability of the agent. The first two mechanisms are used by both gram-positive and gram-negative bacteria, whereas the third is exclusive to gram-negative bacteria. When these mechanisms occur in concert, for example, overexpression of efflux and/or porin loss along with β-lactamase production, the result is high-level β-lactam resistance. Given the frequency with which β-lactams are used clinically, β-lactamase production is an issue of significant public concern.
The β-lactam class of antimicrobials is subdivided based on structure and spectrum of activity ( Table 85.1 ). Penicillins are used against non–β-lactamase–producing bacteria. β-Lactam/β-lactamase inhibitor combinations are used against β-lactamase–producing isolates, using an inhibitor compound (e.g., clavulanate, sulbactam, tazobactam, avibactam, vaborbactam, relebactam) in combination with a penicillin, cephalosporin, or carbapenem. Often the inhibitor has minimal or no antibacterial activity on its own; rather, it binds and inactivates the β-lactamase, restoring activity of the β-lactam. Cephems, which encompass both cephalosporins and cephamycins, are typically active against penicillinase-producing isolates. The cephalosporins are further divided based on their generation, a classification that loosely separates the agents by spectrum of activity. Cefiderocol is a unique iron-chelating cephalosporin that enters the bacterial cell through iron transporters. Carbapenems are structurally similar to the penicillins and have broad-spectrum activity against both aerobic gram-positive and gram-negative organisms, and certain members (e.g., ertapenem) are active against anaerobic bacteria. Monobactams, such as aztreonam, are active only against aerobic gram-negative bacteria.
| Antimicrobial Class | Subclass | Example Agents |
|---|---|---|
| Penicillins | Penicillinase-labile penicillins |
|
| Penicillinase-stable penicillins |
|
|
| β-Lactam/β-lactamase inhibitor combinations |
|
|
| Cephems (parenteral) | Cephalosporin I |
|
| Cephalosporin II |
|
|
| Cephalosporin III |
|
|
| Cephalosporin IV |
|
|
| Cephalosporins with anti-MRSA activity |
|
|
| Cephamycins |
|
|
| Cephems (oral) | Cephalosporins |
|
| Monobactams |
|
|
| Penems | Carbapenem |
|
Glycopeptide and lipoglycopeptide agents have bactericidal activity against gram-positive bacteria, including anaerobes. Glycopeptides are used as first line therapy for the treatment of multidrug-resistant gram-positive bacterial infections, whereas widespread use of the newer lipoglycopeptides is limited by comparison. Glycopeptides are large molecules that inhibit cell wall synthesis by binding to the d -alanine, d -alanine terminal end of the peptidoglycan pentapeptide cross-bridge. β-Lactams inhibit the PBP transpeptidases directly, whereas glycopeptides such as vancomycin bind to the transpeptidase substrate, thereby hindering cross-linking. This compromises the structural integrity of the cell and results in cell lysis. Lipoglycopeptides are mechanistically similar to glycopeptides; however, due to the addition of a lipophilic side chain, these agents have the added feature of depolarizing the cell membrane. Two distinct mechanisms are responsible for resistance to glycopeptides and lipoglycopeptides: alteration of the pentapeptide terminus, thereby preventing transpeptidase binding, or enhancement of the cell wall thickness, which is thought to limit glycopeptide access to target pentapeptides. The first of these is responsible for vancomycin resistance in enterococci (i.e., van genes), whereas the second is seen in vancomycin-intermediate staphylococci. These two resistance mechanisms are discussed in greater depth in their respective sections later in this chapter.
This class of antibiotics consists of agents that are either anionic or cationic in nature. Their mechanism of action is unique in that these agents rely on electrostatic interactions to bind directly to the cell membrane, ultimately causing depolarization and cell death. The main mechanism for reduced susceptibility is believed to occur when the bacterial surface or surrounding environmental charge is altered. This modification ultimately serves to shield the bacterium from interaction with the antibiotic, via electrostatic repulsion.
The polymyxins are positively charged molecules that have a strong affinity for the negatively charged lipopolysaccharide (LPS) found in the outer membrane of aerobic gram-negative bacteria. Due to dosing constraints and concerns over renal toxicity and neurotoxicity, polymyxins are often used as an agent of last resort against multidrug-resistant organisms. In contrast, daptomycin is an anionic molecule that interacts with calcium in the host to become positively charged. Daptomycin possesses activity against gram-positive bacteria and is most commonly used to treat MRSA and VRE. The exact genetic mechanisms of acquired lipopeptide resistance are complex and not well understood. However, modifications to the cell wall that inhibit a strong binding interaction of the drug have been shown to play a role in the development of resistance to these agents. For the polymyxins, prevalence of acquired resistance remains low and LPS modification appears to be the main contributor. A novel plasmid-mediated polymyxin resistance mechanism carried by the mcr-1 gene has emerged in China and is of significant concern. As with the polymyxins, reduced susceptibility to daptomycin is often attributed to a change in the cell envelope charge and correlates strongly with preexposure to vancomycin and reduced vancomycin susceptibility.
Nitroimidazole agents are active against anaerobic bacteria; the most commonly prescribed agent in this class is metronidazole. Once inside the target bacterium, these agents are converted to a compound that inhibits DNA synthesis and elicits DNA damage through strand breakage, ultimately resulting in cell death. Several mechanisms of resistance have been proposed, but the primary mechanisms appear to be decreased uptake and altered reduction efficiency. Transferable resistance genes (termed nim genes) capable of converting the nitroimidazoles to a nontoxic by-product have been described in both gram-positive and gram-negative obligate anaerobes.
As a class, quinolones and fluoroquinolones are bactericidal against virtually all bacteria; however, activity differs greatly based upon the agent. Members of the quinolone class are divided by generation, which, like the β-lactams, loosely allows for separation into groups with distinct spectra of activity. The core, first-generation quinolones (e.g., nalidixic acid) display activity against aerobic gram-negative bacteria. Modification of the core quinolone nucleus improves the potency and spectrum of activity; second-, third-, and fourth-generation quinolones contain a fluorine atom added to the C-6 residue and are called the fluoroquinolones. This modification expanded coverage to include improved aerobic gram-negative activity and activity against gram-positive bacteria. Commonly used fluoroquinolones in clinical practice are ciprofloxacin and levofloxacin. Third- and fourth-generation fluoroquinolones were developed to specifically target gram-positive bacteria including the pneumococci (e.g., gatifloxacin) and anaerobic bacteria (e.g., moxifloxacin), respectively.
Quinolones interfere with the maintenance of chromosomal supercoiling, DNA strand breakage, and rejoining interactions that are part of the DNA synthesis process. Specifically, the quinolone class targets DNA gyrase and topoisomerase IV, arresting synthesis and ultimately leading to bacteriostasis and cell death. Resistance to quinolones occurs by either mutation of the target site, changes in regulatory expression of efflux pumps, or by acquisition of resistance-conferring genes (e.g., qnr ) that bind to and protect the DNA topoisomerase from inhibition by fluoroquinolones.
Rifamycins have a broad range of indications that vary based on the particular agent. Rifampin, also known as rifampicin, is an agent primarily used to treat Mycobacterium infections but has also found a role in combination therapy for aerobic gram-positives, intracellular bacteria, and some multidrug-resistant gram-negative bacteria. Although use of rifampin against nonmycobacterial infections remains controversial, the agent has been shown to aid in clearance of infections where the presence of a biofilm is suspected. Rifampin acts on the bacterial target by inhibiting DNA-dependent RNA polymerase. Resistance to rifampin emerges as a result of mutations in the rpoB gene that encodes the RNA polymerase β-chain. Resistance to the agent occurs quickly during monotherapy; therefore the agent must always be used in combination with another active agent.
Aminoglycosides are primarily used for treatment of aerobic gram-negative bacteria but may be used in combination with a cell wall–targeting agent to enhance killing of certain gram-positive organisms (e.g., enterococci, as discussed later) and for certain mycobacterial infections. Most members of the class are bactericidal but may only exhibit a bacteriostatic effect at low concentrations. The most commonly used agents in the aminoglycoside class include gentamicin, streptomycin, tobramycin, amikacin, and neomycin. Aminoglycosides irreversibly bind to the 30S ribosome and interfere with translation and translocation, thereby halting protein synthesis. Resistance to aminoglycosides can develop in a number of ways, including alteration of the target site, decreased permeability, and acquisition of aminoglycoside-modifying enzymes. Ribosome alteration occurs via the introduction of mutations that result in either low- or high-level resistance and mainly impact streptomycin activity. The remaining two mechanisms potentially affect all aminoglycosides. Reduced drug uptake is mainly seen in nonfermentative gram-negative bacteria (e.g., Pseudomonas aeruginosa ), whereas modifying enzymes capable of dissemination on transposons and plasmids have been found in a variety of bacterial species. Enzymes capable of inactivating aminoglycosides are categorized into three main types: acetyltransferases (AAC), adenyltransferases (ANT), and phosphotransferases (APH), and they often result in high-level resistance.
The lincosamide antibiotic class is used to treat a broad spectrum of infections. They possess bacteriostatic activity against most aerobic and anaerobic gram-positive bacteria. These agents block protein synthesis by binding to the 23S ribosomal RNA of the 50S ribosomal subunit, interfering with transpeptidation, and the initial elongation phase. When resistance mechanisms are present, they confer complete resistance to the class (e.g., clindamycin and lincomycin). Aerobic gram-negative organisms are intrinsically resistant to lincosamides as a result of poor permeability of the outer membrane. The main mechanism of acquired resistance is modification of the target via methylation of the 23S rRNA gene or mutations at the binding site. Modification by methylation can either be constitutive or inducible expression, hampering detection of resistance. Methylation of the target site by genes such as erm in staphylococci can result in cross-resistance of functionally related antibiotics, namely macrolides and streptogramin B. Methods used in the laboratory to detect this mechanism are discussed later in this chapter. Other mechanisms of resistance include modification of the antibiotic itself or efflux of the agent.
Macrolides are mainly active against aerobic gram-positive organisms. Their utility for treatment of gram-negative infections is limited with the exception of some fastidious and/or intracellular bacteria (e.g., Chlamydia , Legionella, and Campylobacter ). Agents within the class display slightly different spectra of activity. Generally speaking, the first agent of the class, erythromycin, has a relatively limited activity profile. However, binding stability and spectrum of activity was enhanced as other first-generation macrolides were developed. The next generations of macrolides, the ketolides and fluoroketolides, have several structural modifications, including a keto group where earlier agents contain a cladinose residue. These alterations further improved the activity profile of the class. Macrolides typically exert bacteriostatic effects; however, bactericidal activity by some members of this class can occur when pharmacokinetic parameters are optimized.
Macrolides inhibit protein synthesis by binding to the 50S ribosome and sterically hindering growth of the polypeptide chain. Resistance emerges in three distinct ways. First, target site modification, either by methylation or mutation, confers cross-resistance among the macrolides and functionally related antibiotics (e.g., lincosamides). The second mechanism, efflux, is a common mechanism that contributes to intrinsic resistance observed in gram-negative bacteria. This mechanism of macrolide resistance is also well documented in gram-positive organisms following acquisition via mobile genetic elements. The genes responsible for efflux differ between staphylococci and streptococci, as does the resulting susceptibility phenotype. The third mechanism of macrolide resistance is drug modification. This mechanism is the least clinically relevant but when present, it confers resistance only to structurally related macrolides and leaves functionally related agents unaffected.
Oxazolidinones primarily target aerobic gram-positive and anaerobic organisms by impairing early protein synthesis. Specifically, oxazolidinones bind to the peptidyl transferase center of the 50S ribosome and block positioning of tRNA, thereby halting peptide elongation. Resistance to oxazolidinones is best characterized for linezolid, where mutations of the 23S rRNA gene are the main contributor to resistance. In addition, methylation of rRNA has been shown to affect linezolid susceptibility in S. pneumoniae and staphylococci. Methylation conferred by the cfr gene is one of the described transferable resistance mechanisms for linezolid. The newer oxazolidinones, including tedizolid, have additional binding sites in the peptidyl transferase center that render their activity largely unaffected in the presence of the cfr -resistance mechanism.
Streptogramins are agents with activity against gram-positive bacteria. They are composed of A and B components that act synergistically, where alone they are bacteriostatic, but when combined may exhibit bactericidal activity. An example streptogramin is quinuspristin-dalfopristin. The two subgroups bind to distinct areas of the 50S ribosome and inhibit separate steps of protein synthesis. Type A streptogramins bind to the peptidyl transferase center preventing early-stage elongation, whereas type B streptogramins prevent extension of and early incomplete release of peptide chains. With regard to resistance, because streptogramin A and streptogramin B are chemically unrelated and have different target sites, the mechanisms of resistance are different. The main mechanism against group A is inactivation by AAC, although efflux has also been demonstrated. Although inactivating enzymes also exist against group B, the main mechanism of concern is modification of the target by 23S rRNA methylase by genes such as erm. This so-called MLS B mechanism is widely distributed among gram positives, affecting macrolides, lincosamides, and group B streptogramins. Expression of some erm genes requires induction, meaning that resistance can be masked if steps are not taken to unmask the resistance phenotype during susceptibility testing.
Tetracyclines are broad-spectrum agents that exhibit activity against gram-positive and gram-negative organisms. The class can be separated into two structural groups: tetracyclines (first- and second-generation) and the most recently discovered group termed glycylcyclines (i.e., tigecycline). Tetracyclines are strong chelating agents, a feature that affects their PK properties and mechanism of action. Tetracyclines bind to the 30S ribosomal subunit, blocking binding of aminoacyl-tRNA to the ribosome thereby preventing addition of new amino acids into the growing polypeptide chain.
The ability to traverse membrane systems is critical for all antibiotics that inhibit protein synthesis. As such, efflux is a common mechanism of resistance used by bacteria and is the most widespread mechanism of tetracycline resistance. Some efflux mechanisms confer cross-resistance to all members of the tetracycline class; however, many efflux mechanisms only target tetracycline itself and spare later-developed agents such as doxycycline, minocycline, tigecycline, and eravacycline. For this reason, if an isolate is susceptible to tetracycline, this often signifies it is susceptible to later derivatives. Conversely, if an isolate is resistant to tetracycline, resistance cannot be inferred for later derivatives. The second mechanism of tetracycline resistance is ribosomal protection. Ribosomal protection proteins bind to the tetracycline-ribosome complex and effectively dislodge the tetracycline, leaving the binding site available for continuation of protein synthesis. This mechanism confers a wider spectrum of resistance, often leading to complete resistance across the tetracycline class. Tetracycline resistance (tet) genes and oxytetracycline resistance (otr) genes are responsible for efflux or ribosomal protection mechanisms.
Inhibitors of the folate biosynthetic pathway typically have broad-spectrum activity against aerobic gram-positive and gram-negative bacteria. Trimethoprim is a competitive inhibitor, meaning it binds to a bacterial enzyme and prevents it from acting upon its intended target. Here, trimethoprim binds to dihydrofolate reductase, inhibiting activity of the enzyme. Resistance to trimethoprim occurs either when the enzyme is modified to prevent or limit binding by the antibiotic, or expression of the bacterial dihydrofolate reductase is increased to overwhelm the antibiotic concentration. To limit emergence of resistance, trimethoprim is often combined with a second agent that targets an alternative step in the folate synthesis pathway. Sulfonamides are competitive inhibitors of dihydropteroate synthase. Unfortunately, resistance to both trimethoprim and sulfonamides is prevalent and can be found together on transferable genetic elements. Mutations or acquisition of genes that result in overproduction or modification of the target contributes to the resistance seen in a variety of bacteria.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here