Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Renal transplantation is the preferred treatment for most end-stage renal diseases. Transplantation’s success, however, has been counterbalanced, by its dependence on immunosuppressive drugs with their related infectious, metabolic, and malignant complications. Consequently, a common goal throughout the history of clinical transplantation has been the minimization and individualization of immunosuppressive therapy. Typically, drugs with highly specific mechanisms of action have been preferred over drugs with broad effects, and the search for increasingly specific drugs has provided a major impetus for the development of immunosuppressive therapies in general, and of antibodies and fusion proteins in particular.
Antibodies and other glycoprotein cell surface receptors are defined by their ability to bind to a particular ligand with unambiguous specificity. Although they may mediate diverse effects through associated downstream signaling pathways, their function is characterized by fidelity to distinct binding motifs. This trait has been long recognized as having great potential for targeted therapeutic use with minimal unintended effects, and organ transplantation historically has been a preferred testing ground for receptor-based therapeutics, such as monoclonal antibodies (MAbs), polyclonal antibody preparations, and engineered glycoprotein receptor–antibody hybrids known as fusion proteins; these are collectively known as biologics . The initial success of biologics in transplantation has more recently led to an explosion in the number developed for clinical use. In addition to transplant-related indications, biologics have been developed for the treatment of many oncologic and autoimmune conditions, and now at least 300 preparations are in some level of clinical or preclinical development. Importantly, although renal allograft rejection was the original indication for MAb therapy, most modern development has been spurred by indications serving larger population bases. In addition to using agents developed for transplantation, clinicians are increasingly adopting therapies from other immunologically relevant indications. This so-called off-label use is now increasingly common and is becoming a primary means of biologics development for transplantation.
This chapter provides an overview of antibody- and receptor-based therapies for kidney transplantation. Drugs developed and approved for use in transplantation are described, as well as drugs with relevant actions developed for other indications but evaluated in transplantation. In addition, clinically tested investigational agents are reviewed.
Early experiences in renal transplantation were marked by high rates of rejection and complications related to the effects of the two available immunosuppressants of the day, glucocorticosteroids and azathioprine; this, combined with the recognition that lymphocytes were the predominant effectors in rejection, stimulated interest in alternative lymphocyte-directed strategies. By the mid-1960s, several investigators had shown that animals injected with lymphocytes would produce sera containing lymphocyte-specific antibodies, which could be used to reduce the lymphocyte counts when injected into other experimental animals. This technology gave rise to the initial lymphocyte depletion trials using antilymphocyte antibody preparations: antilymphocyte serum, antilymphocyte globulin, and antithymocyte globulin. These agents were collectively called polyclonal preparations because they were composed of antibodies with many, largely undefined specificities. Their ability to prevent and reverse rejection, particularly in patients refractory to the drugs of the day, led to their increasing use over the ensuing decade.
The increased use of polyclonals made many of their limitations apparent. The imprecise in vivo methods for producing polyclonal antibodies resulted in preparations with promiscuous binding to many nonlymphocyte cell types. Although each antibody in the preparation bound to a single target, collectively the preparation bound to a broad array of cell surface molecules. Cross-reactivity with many hematopoietic cells made anemia, neutropenia, and thrombocytopenia dose-limiting. The method of production also led to wide batch-to-batch variability. The clinical effect of the agent varied considerably, making it difficult to establish prospectively proper dosages and to estimate the magnitude of anticipatable side effects. In addition, because the preparations were made in animals, usually rabbits or horses, they contained proteins that were antigenic to humans. They had the potential to induce a neutralizing antibody response and evoke adverse effects, such as serum sickness or anaphylaxis. Finally, some lymphocyte cell surface receptors, when bound by antibody, would induce cell activation, leading to a release of anaphylatoxins and cytokines, producing a syndrome of flu-like and, in extreme cases, septic-like symptoms, subsequently termed cytokine release syndrome .
In the 1970s, Kohler and Milstein presented a landmark development in the field of protein therapeutics—a means of producing antibody preparations with a single, genetically defined monoclonal specificity. The development of MAbs addressed many of the shortcomings associated with polyclonal preparations, particularly specificity and variability. The first such preparation approved for clinical use in 1985 was muromonab (OKT3), a MAb of mouse origin specific for human cluster of differentiation (CD) 3 (described later). OKT3 rapidly and specifically cleared T cells from the peripheral circulation and was shown to be an effective treatment for allograft rejection. Although many of the problems associated with the diffuse nature of polyclonal antibodies were addressed, some were not. The immune response against heterologous animal proteins and the cytokine release syndrome remained. OKT3’s heightened specificity for the T cell receptor (TCR) not only produced more reliable T cell clearance but also more reliable T cell activation and cytokine release. The antimouse antibody response also limited prolonged dosing in a subset of patients.
With the genetic engineering advances of the 1980s, the production of MAbs became much more efficient, theoretically allowing any surface molecules to be targeted. Effort was redirected from pan-T cell depletion toward fine targeting of relevant T cell subsets and blockade of functions unique to effector T cell activation. An example was the high-affinity interleukin (IL)-2 receptor CD25 (described later), expressed predominantly on activated T cells. Additionally, methods of genetic engineering were developed to allow DNA encoding for binding sites from heterologous proteins to be grafted onto genetic sequences encoding the monomorphic scaffold of human antibodies to create chimeric or humanized MAbs. These techniques also allowed for unique fusion proteins to be created, combining the Fc portions of antibodies with nonantibody receptors and ligands and allowing for cell surface molecules to be created in a soluble form with prolonged half-lives.
The humanization of antibodies and the use of human-derived receptors has practically eliminated the problem of antibody clearance and opened the possibility for prolonged treatment regimens. More recently, the production of fully human antihuman antibodies has become a practical reality. Techniques including phage display mutagenesis and the transgenic production of mice containing human immunoglobulin genes that respond to immunization with human antibody now offer the promise of highly specific, nonimmunogenic, well-tolerated protein reagents. Human and humanized biologics are now making possible prolonged therapy with highly specific therapeutic agents.
Multiple surface molecules have been targeted by biologics investigationally, and several are now accepted as clinical therapies in transplantation and other indications. Biologic therapy is being increasingly adopted into standard practice, with 95% of kidney transplants performed in the US now using some form of prophylactic antibody therapy. Despite this trend, however, it has not been established whether this strategy is necessary in all cases. Although antibody induction reduces acute rejection rates in the first year after transplantation, the lasting effects of induction remain incompletely defined. The modern era is now characterized by the availability of many promising agents and the challenge of understanding their most appropriate clinical use.
The clinical effects of MAbs in transplantation relate closely to the physiologic effects and structural characteristics of antibodies in general. Antibodies are one of two common glycoprotein antigen receptors that result from somatic gene rearrangements in specialized lymphocytes, the other being TCRs. Five different heavy-chain loci (μ, γ, α, ε, and δ) and two light-chain loci (κ and λ), each with variable, diversity, or junctional (V, D, or J) and constant (C) regions, are brought together randomly by the recombination-associated gene (RAG)-1 and RAG-2 apparatus to form a functional antigen receptor with highly variable binding ability. Antibodies have a basic structure of two identical heavy chains and two identical light chains ( Fig. 19.1 ). The heavy-chain usage defines the immunoglobulin type as being IgM, IgG, IgA, IgE, or IgD. This structure forms two identical antigen-binding sites brought together on a common region known as the Fc portion of the antibody. Although all of these subtypes have therapeutic potential, IgG antibodies have been the most commonly used clinically. IgG molecules are the most common result of peripheral immunization and are structurally easier to produce and manipulate.
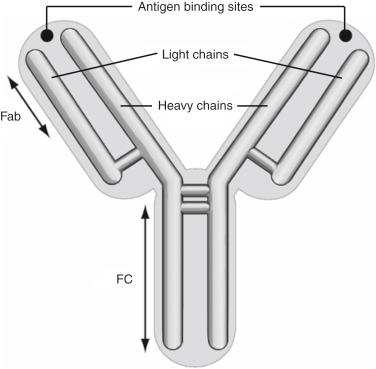
Physiologically, antibodies exist as surface molecules on B cells, facilitating their antigen-specific activation and, importantly, are secreted into the serum to bind to and neutralize circulating antigens. Heterologous nonhuman antibodies are sufficiently similar to their human counterparts to facilitate most physiologic effector functions when used in humans. Antibodies produced by mice, rabbits, and horses can be used in humans and still evoke biologically important effects. There is no animal that is a priori superior, however, and all heterologous antibodies have the potential to induce a neutralizing antibody response.
Antibodies can have a broad range of effects when they bind ( Fig. 19.2 ). They can mimic the native ligand of a molecule and lead to signal transduction, or they can bind to the molecule in such a way as to prevent it from binding to its intended ligand. Antibodies can be either activating or inhibiting, and the predominant effect can be determined only through empirical in vivo analysis. Antibodies can bind to cells in such a way as to have no appreciable effect. Thus antibody binding cannot be equated with functional significance. In some cases, a combined effect occurs whereby the antibody activates the targeted molecule but induces surface molecule internalization, effectively clearing the molecule from the cell surface and inhibiting its subsequent function. This transient activation effect can lead to a burst of target cell activity (e.g., cytokine release), resulting in undesirable side effects, or it can simply lead to surface modulation of the targeted molecule. Antibodies cannot target molecules that are not present on the cell surface. Although they can influence intracellular pathways, they cannot directly bind intracellular molecules in vivo.
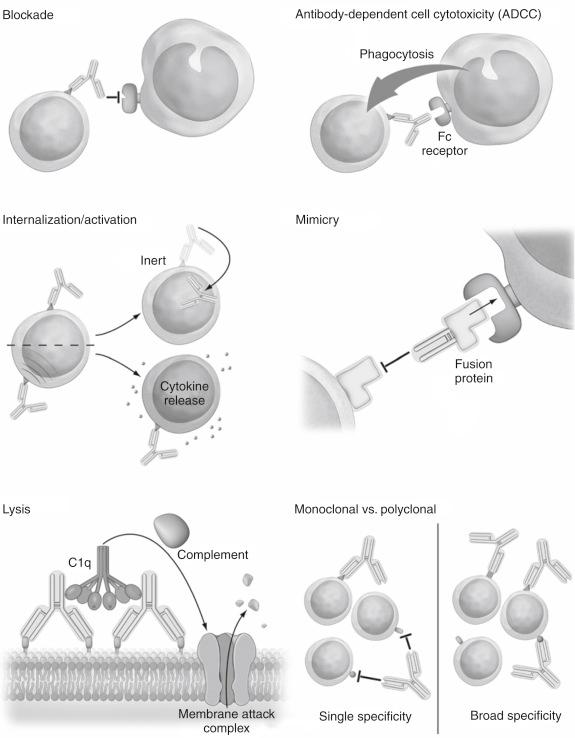
Antibodies also activate the classical complement cascade and in doing so can induce complement-mediated lysis of a targeted cell. In addition, many phagocytic cells have receptors for the constant Fc region of antibodies and preferentially engulf cells coated with antibody through a process known as antibody-dependent cellular cytotoxicity (ADCC). Both of these activities facilitate the most noticeable effect of antibody therapies: target cell depletion. Depletion is only the most obvious effect of antibody therapy, however, and should not be assumed to be the most relevant or desired. Additionally, these effects depend on their antigen-binding region and their nonvariable Fc region for effectiveness. The importance of Fc segment effects is shown by nonspecific antibody infusion, which can mediate important effects, presumably by neutralizing complement or saturating Fc receptors.
It has become apparent that the maturation state of the targeted cells also can influence the response to antibody treatments. Specifically, cells that have matured into a memory phenotype have some degree of resistance to antibody-mediated depletion. The mechanisms involved in depletion resistance remain to be defined, but memory cells differ from naïve cells in many potentially relevant ways, including enhanced antiapoptotic and complement regulatory gene expression. The ultimate effect of antibody therapy may vary not only with the antibody preparation but also with the phenotype of the targeted cell and even the recipient’s immune history.
All of these effects can alter the function of molecules and cells, giving antibodies broad therapeutic potential. This array of effects makes antibody development difficult, however. Minor changes in antibody structure can radically alter the effect, and at present it is impossible to predict an antibody’s properties based solely on structure. Certain IgG isotypes support complement and ADCC functions better than others, but generally an antibody must be tested in vivo to determine which of its many potential effects would be dominant. Specifically, new antibodies undergo detailed and rigorous analysis of structure, posttranslational modifications, and cellular functions during development. This can, at times, have serious consequences that make the introduction of new antibodies into phase I clinical trials challenging.
Fusion proteins are molecules engineered from a single receptor targeting a ligand of interest fused to another protein that provides another salutary property. In transplantation, this secondary molecule is typically the Fc portion of an IgG molecule that gives the receptor an antibody-like half-life and/or opsonization properties. Fusion proteins also can involve the fusion of a specific toxin to a MAb to facilitate epitope-directed drug delivery. Fusion proteins are similar to MAbs in that they have a single homogeneous specificity and can be composed of human or humanized components, limiting their immune clearance and opening their use for prolonged administration. Currently, only a single fusion protein is approved for transplantation, belatacept, which is a mutated form of the receptor CTLA4 (CD152) fused to IgG Fc domain. Belatacept, along with notable examples of transplant-relevant fusion proteins in development, will be discussed subsequently.
Immunosuppressive regimens used for organ transplantation can be generally characterized as induction, maintenance, or rescue therapies. Induction immunosuppression is intense treatment designed to inhibit immune responsiveness prophylactically at the time of transplantation. It is usually potent to the point that prolonged use is prohibitively toxic. Maintenance immunosuppression is of lesser potency, but is tolerable for long-term use and forms the basis of most immunosuppressive regimens. Rescue therapy is similar to induction in that it is intense, effective, and chronically intolerable, but differs in that it is used to reverse established rejection. Immunosuppressive medications can conceivably fall into any or all of these categorizations based on the dose and route used. Currently, biologics are primarily indicated as rescue agents and are used in approximately 30% of all acute rejection episodes. Their use as induction agents predominates: 95% of patients undergoing kidney transplantation now receive biologic induction.
Antibody preparations have been generally classified as depleting or nondepleting based on whether or not they deplete cells expressing the targeted antigen. Generally, T cell depleting antibody preparations are indicated for the treatment of refractory (e.g., steroid-resistant) acute cellular rejections, acute rejections occurring in high-risk settings (e.g., marginal kidneys), and particularly aggressive vascular (e.g., Banff grade 2 or 3) rejections. Depleting antibodies are also used as induction agents, although this is often an off-label use. Nondepleting antibody preparations and fusion proteins have been most commonly studied as induction agents and typically have less efficacy in rescue indications. Applications of biologics have been made possible by the ability to produce human biologics and so are gradually being incorporated into maintenance immunosuppression. The first biologic maintenance agent, belatacept, was approved for use as a calcineurin inhibitor (CNI) replacement for kidney transplantation in June 2011. It is a fusion protein with specificity for the B7 costimulatory molecules and will be considered fully later in this chapter.
Randomized trials have studied many depleting and nondepleting antibody preparations. These agents are efficacious in reducing the rate of acute rejection when used as induction agents and combined with standard maintenance regimens, compared with bolus methylprednisolone induction. Few prospective studies compare the prominent agents, however, and no agent has distinguished itself as clearly superior in all clinical circumstances. Most trials have used the surrogate end-point of acute rejection, rather than more definitive outcome measures, such as patient or graft survival.
When considered as a whole, biologics have been convincingly shown to be more effective than steroids in reversing first acute cellular rejection, but offer minimal benefit for humoral rejection episodes. When used as induction agents, they reduce the incidence of acute rejection in the first 6 months of transplantation in kidney recipients, particularly recipients who are sensitized or experiencing delayed graft function, compared with the historical standard of bolus methylprednisolone induction and maintenance with cyclosporine, azathioprine, and prednisone. Despite these benefits, there is no evidence that biologics alter long-term patient or graft survival in the era of modern immunosuppression. Additionally, the use of biologics increases the risk of patient toxicity, including cytomegalovirus (CMV) disease, thrombocytopenia, and leukopenia. Long-term analysis suggests that a measurable effect in kidney transplantation disappears after 5 years. This analysis may indicate that the side effects of maintenance therapy or patient comorbidities supersede early graft outcome and are the dominant determinants of outcome over time.
Antibody preparation use does not generally influence the rate of technical complications but seems to reduce the risk of graft thrombosis in children. Several induction strategies, in particular polyclonal antibodies and OKT3, have been shown to measurably increase the risk of posttransplantation lymphoproliferative disease (PTLD) and death from malignancy when combined with conventional maintenance immunosuppression. PTLD is a product of the intensity of the overall immunosuppressive therapy in combination with the recipient’s preexisting immunity to the causative agent, Epstein-Barr virus (EBV). Specifically, the expected PTLD rate is 0.5% in patients who do not receive antibody induction or who receive CD25-specific therapy. OKT3 induction carries a significantly higher rate of 0.85%, as does polyclonal depletion at 0.81%, particularly in recipients newly exposed to EBV at transplantation. Interestingly, the use of alemtuzumab, a CD52-specific MAb with potent depletional properties similar in magnitude but differing in spectrum to polyclonal preparations or OKT3, has been shown to have a low PTLD incidence that approximates that of the nondepletional CD25-specific MAbs. This likely relates to alemtuzumab’s B cell depleting properties, with B cells being the dominant reservoir of EBV, although practice patterns associated with alemtuzumab also may account for the difference.
Other early complications, including cardiovascular and infectious deaths, correlate with antibody use, but the interpretation of this relationship is confounded by the preferential use of antibodies in high-risk patients. Viral infection is a substantial concern, however, when using potent antibody therapy, particularly agents associated with T cell depletion. When used for induction or rescue, antibody preparations should be accompanied by broad prophylaxis against opportunistic infection. Antiviral therapy, such as ganciclovir or acyclovir, should be initiated and continued for at least 3 months. The choice of agent is based on the pretransplant status of the donor and recipient. Oral candidiasis prophylaxis with nystatin or clotrimazole and Pneumocystis therapy with trimethoprim/sulfamethoxazole also should be considered for several months. Individual clinical risks often dictate substantially longer periods of prophylaxis. Each antibody preparation has a unique side effect profile and indication, which are discussed subsequently.
The use of antibody preparations for maintenance therapy had been limited until more recently by the immune response formed against the antibody itself. Recombinant humanized or chimeric antibodies and fusion proteins have essentially eliminated this as a concern. Long-term benefits with belatacept as a maintenance agent are now gaining recognition and other antibodies are currently in clinical trials for sustained preventive therapy (discussed later in this chapter).
Heterologous antibody preparations can be derived from many animals immunized with human tissues, cells (e.g., human lymphocytes), or cell lines (e.g., Jurkatt cells). When reinfused into humans, these antibodies bind to antigens expressed on the original immunogen, where they mediate the effects discussed earlier. Given that these preparations are produced through whole-cell immunization, the resulting preparations contain a vast array of antibodies binding many epitopes expressed on the immunogen cells—some intended, and some not. Because each animal produces a unique immune response to an antigen, clinical-grade preparations are generally the result of pooled responses from many animals. For practical reasons, most polyclonal preparations are derived from rabbit or horse immunizations.
Ideally, a single renewable cell type equivalent to the effector cell in rejection could be used as a reproducible immunogen free from elements such as stromal tissue and neutrophils. No such cell has yet to be identified or developed. Commercially available polyclonal preparations continue to be made using heterogeneous cell populations or tissues such as thymus obtained from deceased donors or surgical specimens or from the Jurkatt T cell line, which is thought to approximate the antigenic spectrum of allospecific T cells. After immunization, immunized animals are bled to obtain hyperimmune serum. The serum is typically absorbed against platelets, erythrocytes, and selected proteins to remove antibodies that could result in undesirable effects such as thrombocytopenia. Historically, hyperimmune serum was administered without additional purification, but now all commercially available products are purified to obtain only IgG isotypes. Even so, polyclonal antibody preparations are not fractionated to separate relevant from irrelevant antibodies preexistent from the environmental immune responses of the immunized animals. More than 90% of antibodies found in polyclonal preparations are likely not involved in therapeutically relevant antigen binding.
Many groups have prepared polyclonal antibody preparations for their own institutional use, and this practice gave rise to a highly variable literature with little standardization or objective comparison between products. More recently, three dominant commercial polyclonal preparations have emerged: two rabbit-derived antibody preparations, antithymocyte globulin–rabbit (ATG-R, Thymoglobulin, Genzyme-Sanofi) and antithymocyte globulin–Fresenius (ATG-F, Fresenius), and one horse-derived product (ATGAM, Pfizer). Of these, Thymoglobulin is used most commonly in North America, with both rabbit preparations used in Europe. ATGAM is primarily used for the treatment of aplastic anemia, but can be used for patients who have a hypersensitivity to rabbits.
As discussed earlier, antibodies can mediate many effects when they bind to their target antigen, and a significant factor determining their effect is the antigenic specificity of the preparation. By their very nature, polyclonal preparations are composed of a wide variety of antibodies, and complete characterization has remained elusive. Detected specificities include many T cell molecules involved in antigen recognition (CD3, CD4, CD8, and TCR), adhesion (CD2, lymphocyte function antigen (LFA)-1, and intracellular adhesion molecule (ICAM)-1), costimulation (CD28, CD40, CD80, CD86, and CD154), non-T cell molecules (CD16, CD38, CD138, and CD20) and class I and II major histocompatibility complex (MHC) molecules ( Fig. 19.3 ). Although all of these targets hypothetically can influence an immune response, and when studied individually they do, it is unclear which of these specificities is crucial to the ultimate therapeutic effect. This broad reactivity with adhesion molecules and other receptors upregulated on activated endothelium has led many authors to advocate the preferential use of polyclonal antibody preparations in situations, such as prolonged ischemic times, where endothelial activation and ischemia–reperfusion injury is anticipated.
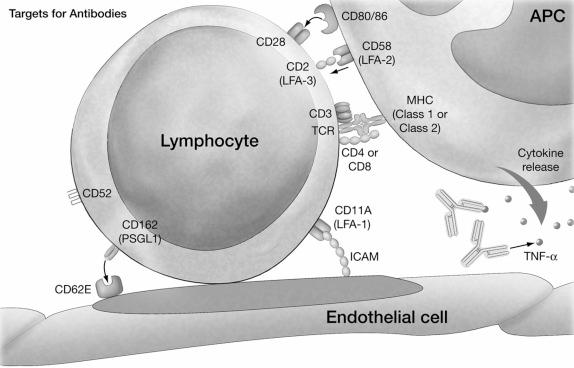
Most polyclonal antibodies have prolonged serum half-lives of several weeks. Nondepleted cells have been shown to be coated with heterologous antibody for months, suggesting that these preparations could influence the function of lymphocytes long after treatment has stopped. Lymphocyte subsets are abnormal for years after therapy, with particularly low CD4 + T cell counts. It also is reasonable to assume that antibodies targeting differing specificities would have variable effective half-lives based on the rates of surface molecule recycling, the affinity of the binding interaction, and the mechanism of action. Stimulating antibodies may have effects whenever they are bound, whereas inhibitory compounds could mediate an effect only when the natural ligand being antagonized is present. Polyclonal preparations likely have mechanisms of action that vary by batch, circumstance of use, and degradation state. It is unlikely that any single generalized mechanism exists. For the purposes of following the clinical effect, bulk T cell depletion is used as a general estimate of antibody potency, and polyclonal antibody preparations are considered depletional agents.
Polyclonal antibody preparations have been used in transplantation to achieve immunosuppression since the 1960s. They are used as induction and rescue therapies, but the immune response to the proteins has precluded attempts to use them as maintenance drugs. As discussed previously, no single mechanism of action has been established, and they likely mediate their antirejection properties through depletion and other effects, including costimulation blockade, adhesion molecule modulation, and, to a lesser extent, B cell depletion.
Historically, polyclonal antibody preparations were used to bolster the effect of steroids and azathioprine in an attempt to reduce the unacceptably high rejection rates typical of the 1960s and 1970s. Generally, a 2- to 3-week course of a polyclonal antibody delayed the onset of acute rejection and reduced the requirement for high-dose steroids in the early postoperative period without significantly altering long-term survival. After the introduction of cyclosporine, the use of polyclonal antibody induction fell from favor with the realization that this potent combination was associated with increased infectious and malignant morbidity. With improved viral prophylaxis, a better understanding of the infectious etiology of PTLD, and more standardized commercial polyclonal products, there has been a marked resurgence of interest in polyclonal antibody induction.
Most modern trials have evaluated polyclonal antibodies added to an otherwise rigorous maintenance regimen (typically triple immunosuppressive therapy). This intense regimen has statistically reduced acute rejection rates, but has reciprocated with increased infectious morbidity without changing the long-term outcome. The increased infectious risk may be acceptable in selected higher-risk patient populations, such as recipients of donation after cardiac death donors, recipients of organs with kidney donor profile index >85%, formerly extended criteria donors, and patients with a high risk of rejection, such as African American recipients, retransplant recipients, and recipients with delayed graft function, particularly when avoidance of prolonged CNIs is desired. A seminal randomized trial between ATG-R and CD25-specific (basiliximab) induction showed that ATG-R reduced the incidence and severity of acute rejection but not the incidence of delayed graft function. Recently, another randomized study with ATG-R and daclizumab induction revealed sustained superiority of ATG-R to prevent acute rejection in highly sensitized patients. In contrast, a randomized trial for rapid steroid withdrawal in nonsensitized patients showed no superiority of ATG-R over basiliximab in preventing acute rejection at 1 year after renal transplantation. Although early patient and graft survival were not influenced by the choice of induction regimen, long-term studies have suggested both a patient and graft survival benefit with ATG-R.
Other trials have attempted to address the increased infectious risk by pairing aggressive polyclonal induction with substantially reduced maintenance therapy. Two pilot studies have shown that ATG-R induction facilitates reduced maintenance immunosuppression in highly selected, closely followed patients, leading to graft and patient survivals comparable to the current standard. These studies have emphasized administration before reperfusion, theoretically to take maximal advantage of antiadhesion molecule effects, and relatively high-dose therapy, to limit the proinflammatory effects of reperfusion and to achieve rapid and lasting T cell depletion. Although these studies indicate that such an approach is possible, it remains to be seen if it can be generalized to noninvestigational settings.
Although induction therapy remains an off-label indication for polyclonal antibodies, their use for the treatment of steroid-refractory rejection is an established indication. Many polyclonal preparations have shown their utility in this setting, spanning several decades of associated maintenance regimens. The first randomized trial showing that antilymphocyte serum was superior to high-dose steroids for the treatment of established rejection was reported in 1979. In the context of azathioprine and prednisone maintenance immunosuppression, antilymphocyte serum reversed rejection faster than bolus glucocorticosteroids, reduced the rate of recurrent rejection, and led to improved survival at 1 year. Most rejection episodes in the cyclosporine era and beyond respond to bolus steroids. Polyclonal agents have been indicated as a second-line therapy for steroid-resistant acute cellular rejection. Recurrent rejection can be treated with repeated courses of polyclonal antibodies in situations where antirabbit (or antihorse) antibodies have not formed.
Of the currently available polyclonal preparations, ATG-R is used most commonly for rescue. It has been shown to be superior to ATGAM in terms of reversal of steroid-resistant rejection and persistence of a rejection-free state. This difference has not been shown, however, to influence patient or graft survival.
Non-T cell-specific polyclonal antibody preparations also reverse established cellular acute rejection. Although not typically considered alongside T cell depleting polyclonal antibody preparations, high-dose human IgG fractions (intravenous immunoglobulin) are polyclonal antibodies of random specificity pooled from human donors. Because they are not derived from animals, are not the products of heterologous immunization, and do not target a specific cell type, most of the adverse effects associated with polyclonal antibodies are not applicable. Nevertheless, high-dose human IgG fractions have been shown to reverse rejection despite the absence of any T cell depleting abilities. Although a course of polyclonal anti-T cell antibody typically consists of 5 to 20 mg/kg given over several days, intravenous immunoglobulin is infused at much higher doses, 500 to 1000 mg/kg over 1 to 3 days, and at this dose has been shown to reverse established rejection with the same overall reversal rate as OKT3. At least at high dose, nonspecific antibody infusion can modulate immune responses, perhaps through complement sequestration and Fc receptor binding with resultant downregulatory effects of Fc receptor-expressing antigen-presenting cells (APCs).
The polyclonal preparations used in modern clinical practice are generally given through a large-caliber central vein to avoid thrombophlebitis. In experienced hands, a dialysis fistula can be accessed for this purpose. Some reports have suggested that polyclonal antibodies can be administered peripherally when diluted and formulated with heparin, hydrocortisone, or bicarbonate solutions. An in-line filter is recommended to prevent infusion of precipitates that may develop during storage. The protein content should not exceed 4 mg/mL, and dextrose-containing solutions should be avoided because they induce protein precipitation.
Given the weeks-long half-lives of polyclonal antibodies, divided doses are not required for steady-state levels. The tolerability of these compounds is markedly improved, however, by spaced dosing. The rate of infusion is associated with the severity of side effects, and the course of therapy is generally over several days, with individual doses given over 4 to 6 hours. This time course depends on the dose used and is most applicable to the standard doses of ATG-R and ATG-F (1.5 mg/kg/dose for a total of 7.5–10 mg/kg) or ATGAM (15 mg/kg/dose for a total of 75–100 mg/kg). More recent investigational induction studies have employed substantially higher doses given over 12 to 24 hours or, alternatively, while the patient is anesthetized with comparable safety profiles. With a growing emphasis being placed on reduced length of stay after transplantation, larger infusions over fewer days are being employed.
Generally, rabbit-derived polyclonal preparations seem to be significantly better tolerated and more efficacious than ATGAM when used in a quadruple regimen for renal transplantation. The most common acute symptoms associated with polyclonal antibody use are the result of transient cytokine release. Chills and fever occur in at least 20% of patients and are generally treatable by premedication with methylprednisolone, antipyretics, and antihistamines. The use of polyclonal antibodies, particularly in the treatment of rejection, has been associated with an increase in the reactivation and development of primary viral disease caused by CMV, herpes simplex virus, EBV, and varicella. It is likely, however, that this is not a class-specific association, but rather an indication of more intensive immunosuppression in general.
Dosage adjustment is warranted to counter leukopenia and thrombocytopenia. Peripheral cell counts drawn immediately after infusion tend to exaggerate cytopenic effects, and most side effects are promptly remedied by time. T cell counts or, more easily, absolute lymphocyte counts can be monitored to ensure that the preparation is achieving its desired effect. Absolute lymphocyte counts less than 100 cells/μL are typical. Attempts to tailor therapy to a specific peripheral cell count have been made to limit the use of these costly preparations. Rejection can occur and persist with very low T cell counts, however, and there is little evidence that dose variation by cell count alters efficacy.
As discussed earlier, polyclonal antibody preparations evoke a humoral immune response to themselves. This response can be detected by enzyme-linked immunosorbent assay for antirabbit or antihorse antibody, but these tests are typically unavailable in clinical settings. Failure to achieve significant T cell depletion suggests the presence of these antibodies. Serum sickness and anaphylaxis also can occur. Preemptive skin testing is not often practiced because these tests have not correlated well with clinical outcome. Rather, slow infusion rates should be employed during initial exposure. Xenospecific antibodies are most likely to occur in individuals with prior exposure to the preparation involved, but can also exist in individuals with significant prior exposure to the animals themselves.
The most common adverse symptoms related to polyclonal antibodies are fever, urticaria, rash, and headache. These are most likely related to the release of pyrogenic cytokines, such as tumor necrosis factor (TNF)-α, IL-1, and IL-6, which result from activating antibody binding to targeted cell surface receptors and subsequent cell lysis. Infrequently, pulmonary edema and severe hypertension or hypotension can result in death. As the number of target cells decreases with repeated dosing, this response typically abates. The most concerning response is within the first 24 hours of the first dose, and patients should be monitored closely during this period. The response is limited considerably by methylprednisolone premedication. The rash associated with polyclonal antibody administration, conversely, tends to occur late in treatment or, at times, after the last dose. It is generally self-limiting and requires only symptomatic treatment for urticaria. Antiendothelial antibodies in polyclonal antibodies have been suggested to bind to donor endothelia and activate complement, inducing humoral rejection in some patients.
MAb preparations differ from polyclonal preparations in that all antibody molecules are derived from a single genetic template and are identical. Batch-to-batch variation is eliminated, allowing the mechanism of action and half-life to be extrapolated based on a single ligand receptor interaction (although this still can be influenced by many individualized circumstances). This preparation narrows the scope of effect, however, making the use of these drugs more dependent on precise knowledge of the pathology involved.
Historically, MAbs are the product of clonally immortalized B cell hybridomas. More recently, genetically engineered mammalian cells have been the source. Alternative production methods, including other eukaryotic cells such as yeast, prokaryotic bacteria, viruses, or even plant cells, are being utilized. As the production cell becomes increasingly distant from human, the resultant antibodies have increasingly aberrant glycosylation, which can radically alter their efficacy. Regardless of the production cell, the resultant antibody can be purified of any extraneous proteins or other antibodies and used as an infused drug.
The most common method for deriving a MAb is typically to immunize a mouse with a cell or cell fraction containing the antigen desired. Splenocytes are isolated from the immunized animal and fused with an immortalized cell, producing many diverse antibody-producing cells. These cells are cloned (grown from single-cell suspensions), and the supernatant from each clone is tested for reactivity against the desired antigen. A single robust clone with the desired antibody production characteristics is chosen and grown either in vitro or in a carrier animal. The supernatant from the clone is purified for therapeutic use. Because many MAbs are made by mouse B cells, they are mouse antibodies. Similar to animal-derived polyclonal antibodies, they can be cleared from the circulation by an antibody-directed immune response. This immune response can cause anaphylaxis and neutralize the effect of the MAb in subsequent administrations.
To improve the efficiency of antibody production and eliminate animal-derived protein epitopes, the gene fragment encoding the binding site of murine antibodies can be isolated and engineered onto the gene that encodes for nonpolymorphic regions of a human antibody, such as IgG1. The resultant hybrid antibody gene can be transfected into a high-expressing eukaryotic cell line and grown in vitro to produce antibodies that are predominantly human antibody, yet still bind to a specific human epitope ( Fig. 19.4 ). These hybrid antibodies can be considered chimeric, if the entirety of the murine antibody-binding site is used in the construct, or humanized, if the only murine portion is the specific complementary determining regions of the parent antibody. Generally, chimeric antibodies preserve the specificity of the original antibody better, whereas humanized antibodies have less chance of evoking a neutralizing response. Practically speaking, both are effective strategies that help avoid the problem of antibody clearance.
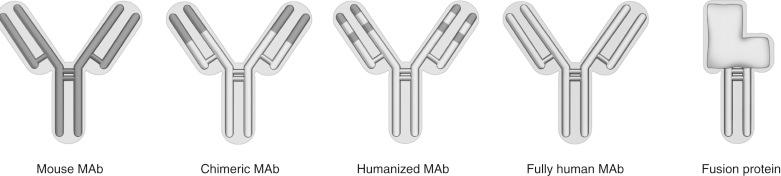
The entire IgG gene has been transgenically expressed in a mouse. This animal, when immunized, makes a human, not mouse, antibody, which can be prepared for monoclonal production. This method is likely to be more efficient for producing truly human antihuman antibodies without the need to engineer each antibody individually.
When approved for clinical use, MAbs must be named based on their structural characteristics ( Table 19.1 ). The generic name of a MAb gives the practitioner a reasonable understanding of the origins and specificity of the MAb.
| Target | Source | Suffix | |||
|---|---|---|---|---|---|
| Varies based on preference of developer | -v(i)- | Viral | -u- | Human | -mab |
| -b(a)- | Bacterial | -o- | Mouse | ||
| -l(i)- | Immune | -a- | Rat | ||
| -les- | Inflammatory lesions | -e- | Hamster | ||
| -c(i)- | Cardiovascular | -i- | Primate | ||
| -t(u)- | Melanoma, colonic, testicular, ovarian, mammary, prostate, miscellaneous tumors | -xi- | Chimeric | ||
| -f(u)- | Fungus | -zu- | Humanized | ||
| -gr(o)- | Growth factor | -axo- | Rat/mouse | ||
| -tox(a)- | Toxin | ||||
| -k(i)- | Interleukin | ||||
| -s(o)- | Bone | ||||
| -ne(u)(r)- | Nervous system | ||||
a The naming of antibodies follows the general guideline with the target and source preceding the suffix -mab.
Because each MAb has a singular specificity, each agent available for general clinical use is considered individually (see Fig. 19.3 ). Most MAbs are defined based on their targeted cell surface protein, and these are generally classified based on the CD nomenclature. A numerical CD designation does not define an antigen, but rather defines a molecule or group of molecules. MAbs that bind to the same CD molecule can bind to the same or different epitopes and have similar or different effects.
OKT3 (muromonab) is no longer available for clinical use. However, it is considered here given its unique place in the historical continuum of MAb therapy. OKT3 is CD3-specific; CD3 is a transmembrane complex of proteins that links to the TCR and conveys its activating signal to the nucleus via a calcineurin-dependent pathway, thus serving as the fundamental signal in antigen-specific T cell activation. CD3 is present on essentially all T cells, defining the cell type. The TCR signal is generally known as signal 1 because it is primarily required for T cell activation and defines the antigen specificity of the T cell. Given that T cells are a crucial mediator of acute cellular rejection, CD3 was one of the first molecules to be targeted with MAbs, and OKT3 (muromonab) was the first MAb to gain clinical approval for therapeutic use in humans.
Although the molecular target of OKT3 is singular and precise, its effects are many. The mechanism by which OKT3 mediates its immunosuppressive effect remains ill defined. OKT3 is an IgG2a mouse antibody that binds to the ε component of human CD3. On binding, the antibody mediates complement-dependent cell lysis and ADCC and, in doing so, rapidly clears T cells from the peripheral circulation. This binding event also leads to pan-T cell activation before their elimination, resulting in systemic cytokine release. The result is a marked cytokine release syndrome responsible for most of the adverse effects associated with the drug (see later).
When antigen binds to the TCR, TCR-CD3 internalization occurs; physiologically, this ensures that antigen binding is reflective of antigen burden and avoids activation mediated by continuous binding of a low-prevalence antigen. Similarly, OKT3 binding to CD3 leads to TCR-CD3 internalization. Uncleared T cells are often rendered void of surface TCR, are incapable of receiving a primary antigen signal, and are immunologically inert.
Bulk T cell clearance likely is not the primary mechanism of action of OKT3. Clinical rejection can occur with exceptionally low T cell counts achieved by other means, and stable graft function can occur with large T cell infiltrates within the graft itself. Although the peripheral circulation is rapidly cleared by OKT3, many T cells can be found in the periphery and in the allograft itself. A substantial amount of the rapid T cell clearance from the circulation is likely related to lymphocyte marginalization, perhaps induced by the cytokines released and by the methylprednisolone that is given with OKT3. The overall effect of OKT3 is likely an aggregate effect of interrupted TCR binding, TCR internalization, cytokine-mediated regulatory changes, disrupted trafficking, and cell depletion. OKT3 has proven efficacy as an induction and rescue agent. Its immunogenicity has prevented its use as a maintenance agent, and the drug is effective only in combination with other immunosuppressive compounds.
Initial trials with OKT3 have shown that this MAb is an efficacious induction agent in kidney transplantation, but only when combined with otherwise effective maintenance immunosuppression. OKT3 cannot prevent rejection beyond the period of its actual infusion without additional maintenance therapy. Its usefulness as an induction agent is most pronounced in sensitized patients and patients with delayed graft function, in whom it facilitates the delay of CNI administration and the resultant nephrotoxicity. It reduces the number of acute rejection episodes and the time to first rejection episode. In more recent literature, OKT3 has been shown to reduce acute rejection episodes compared with cyclosporine, azathioprine, or mycophenolate mofetil (MMF) and steroids without changing patient or graft survival, but to be equivalent to intravenous cyclosporine induction in children. Despite its early prominence, the use of OKT3 as an induction agent dramatically declined subsequently, primarily as a result of its side effect profile, and this led to its voluntary withdrawal from the market in 2009.
Because OKT3 is an entirely mouse-derived antibody, its use leads to the development of an antibody response directed against OKT3 in a significant percentage of patients. The development of antimouse antibodies varies based on the concomitant immunosuppression given, but is seen in at least 30% of patients.
The primary indication for OKT3 was for the treatment of biopsy-proven, steroid-refractory, acute cellular rejection. In this indication, the side effect profile was deemed justifiable, and the efficacy of OKT3 was undeniable. OKT3 was successful in providing sustained reversal of approximately 80% of these vigorous rejections. It was effective even in the presence of prior aggressive lymphocyte depletion, suggesting that its mechanism of action was not primarily a result of bulk T cell depletion. The incidence of steroid-refractory rejection, defined as failure to respond to 3 consecutive days of bolus methylprednisolone (e.g., 500 mg daily), declined considerably with improved maintenance immunosuppressive agents, as did the incidence of rejection in general. Thus the need for OKT3 was reduced considerably. That, combined with its unfavorable side effect profile relative to newer agents, led to its withdrawal from the US market in 2009. Sporadic use based on existing stocks of the drug continued into 2010.
The receptor for IL-2 is composed of three chains (α, β, and γ), of which the α and γ chains are constitutively expressed, and the β chain is induced with activation. The presence of the β chain, now designated as CD25, indicates prior T cell activation and identifies cells that have undergone some degree of effector maturation. CD25 has been targeted to suppress activated cells, while sparing resting cells.
Two commercially available anti-CD25 antibodies have been developed, both of which have been engineered to avoid antimurine antibody responses. Daclizumab is a humanized anti-CD25 IgG1, and basiliximab is a chimeric mouse–human anti-CD25 IgG1. Both agents avoid immune clearance and can be used for prolonged periods without inducing a neutralizing antibody. CD25 was the first molecule to be targeted successfully with a humanized MAb in transplantation. These agents also avoid the serum sickness associated with mouse-, rabbit-, or horse-derived proteins. Daclizumab was voluntarily withdrawn in 2009, largely based on market rather than biologic considerations. A subcutaneous form of daclizumab that may have future implications in transplantation was reintroduced and US Food and Drug Administration (FDA)-approved in 2016 for relapsing multiple sclerosis. Currently, basiliximab remains the only CD25-specific MAb available for use in transplantation. Studies for both of these agents are considered as the efficacy and mechanisms of action of these agents appear to be practically interchangeable.
Anti-CD25 antibodies are thought to work primarily through steric hindrance of IL-2 binding to CD25 and deprive T cells of this cytokine during early activation. There is little evidence for a depletional effect, or if there is one, it is limited to a few cells. More recently, it has become clear that CD25 induction is involved not only in the activation of cytotoxic T cells but also in the activation of cells with potentially salutary effects on the allograft, such as T regulatory cells. Previously activated T cells that are responding in an anamnestic response are less dependent on IL-2 for proliferation. Heterologous responses (cross-reactive responses between a previously encountered pathogen and an alloantigen) or memory alloimmune responses seem not to be affected significantly by CD25 interruption. Given this biology, primarily focused on naïve T cell early activation, CD25-directed antibodies have found a role in induction, but have no role in the treatment of established rejection. Although there has been anecdotal experience using these antibodies for maintenance immunosuppression in the setting of CNI toxicity with recurrent rejection, no study has formally evaluated this approach.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here