Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Optical coherence tomography (OCT) can provide micron-level high-resolution images of the cornea and anterior segment without touching the eye.
Anterior segment OCT can map the corneal and epithelial thicknesses; both are useful for keratoconus screening and classification of corneal irregularities.
Anterior segment OCT can visualize laser in situ keratomileusis (LASIK) flaps for LASIK evaluation, refractive surgery enhancement, or LASIK complication management.
Anterior segment OCT can measure actual posterior corneal power, which is important for improving intraocular lens power calculation in post–refractive surgery patients.
Anterior segment OCT can precisely measure the depth and location of corneal opacities.
Anterior segment OCT is useful for planning and evaluation in various surgical procedures such as phototherapeutic keratectomy, corneal transplantation, or refractive implants.
Anterior segment OCT is useful in ocular surface tumor diagnosis and management.
Anterior segment OCT angiography (OCTA) is useful to detect increased tumor vascularity in iris melanomas.
Optical coherence tomography (OCT) was initially developed by Fujimoto, Huang, et al. as a way to obtain near-histologic–resolution images of tissue without biopsy. Izatt et al. reported the first application of OCT in imaging of the cornea and anterior segment in 1994. The earliest generation of OCT systems used time-domain technology that scans one depth at a time. Current OCT systems use Fourier-domain technology, which detects signal from all depths in parallel, resulting in greatly improved speed and signal-to-noise ratio. Table 17.1 lists the commercially available OCT platforms for anterior segment imaging. An important consideration is the wavelength; longer wavelength light penetrates deeper into the sclera and iris but has coarser resolution, and the best operating wavelength will depend on the intended application. Several commercial OCT systems operating in the 840-nm wavelength range can provide an axial resolution of 5 μm (in tissue) or even better and are capable of visualizing fine corneal structures such as the corneal epithelium, Bowman layer, and corneal endothelium ( Fig. 17.1 ).
| Name | Manufacturer | Type | Wavelength | Resolution | Scan Speed |
|---|---|---|---|---|---|
| Visante | Carl Zeiss Meditec | TD-OCT | 1310 nm | 18 μm | 2 kHz |
| iVue | Optovue | FD-OCT | 840 nm | 5 μm | 26 kHz |
| Avanti XR | Optovue | FD-OCT | 840 nm | 5 μm | 70 kHz |
| Cirrus | Carl Zeiss Meditec | FD-OCT | 840 nm | 5 μm | 27 kHz |
| Spectralis | Heidelberg Engineering | FD-OCT | 820 nm | 3.9 μm | 40 kHz |
| Envisu | Bioptigen | FD-OCT | 840 nm | 2.4–7.5 μm | 10–32 kHz |
| Copernicus REVO | Optopol | FD-OCT | 830 nm | 5 μm | 27–80 kHz |
| Triton | Topcon | FD-OCT | 1050 nm | 8 μm | 100 kHz |
| Casia2 | Tomey | FD-OCT | 1310 nm | 10 μm | 50 kHz |
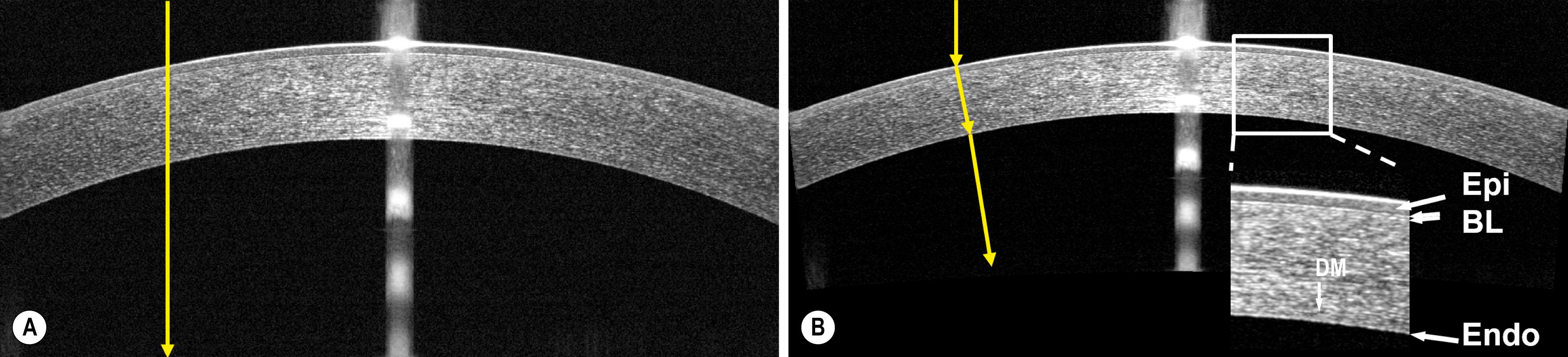
OCT uses light to probe the eye. Anterior segment OCT images are distorted by refraction because light changes its propagation direction at surfaces of tissue index transition, such as the air-cornea and cornea-aqueous interfaces. Moreover, OCT records the optical path length that the light travels rather than the physical dimensions. “Dewarping” algorithms were developed to correct the beam refraction and to transform optical delay into actual physical dimensions (see Fig. 17.1 ). Accurate dewarping requires that the OCT images contain the anterior corneal boundary. Commercial corneal OCT systems use two-dimensional dewarping, which further requires that the scans be radials—the scans line should pass through the corneal vertex.
Keratoconus is an ectatic disorder of the cornea characterized by progressive stromal thinning and steepening of the cornea leading to a loss of visual acuity. Moderate to advanced keratoconus is easily recognizable by several distinctive clinical features, but the diagnosis of forme fruste keratoconus can be challenging. Keratoconus evaluations, done through corneal topography-based algorithms, can sometimes be ambiguous. When a patient presents with normal vision and shows only a slight inferior steepening on topography, the clinician is left to wonder whether subclinical keratoconus is present.
OCT-generated pachymetry (corneal thickness) ( Fig. 17.2 ) could help to confirm the diagnosis by detecting the eccentric focal corneal thinning characteristic of keratoconus. Studies used five pachymetric variables to evaluate the presence of asymmetry in corneal thickness and thinning: minimum, minimum-median, superior-inferior (S-I), superonasal-inferotemporal (SN-IT), and the vertical location of the thinnest cornea (Ymin). A keratoconus risk score table (downloadable version available at www.coollab.net/resources ) was designed based on these pachymetric variables.
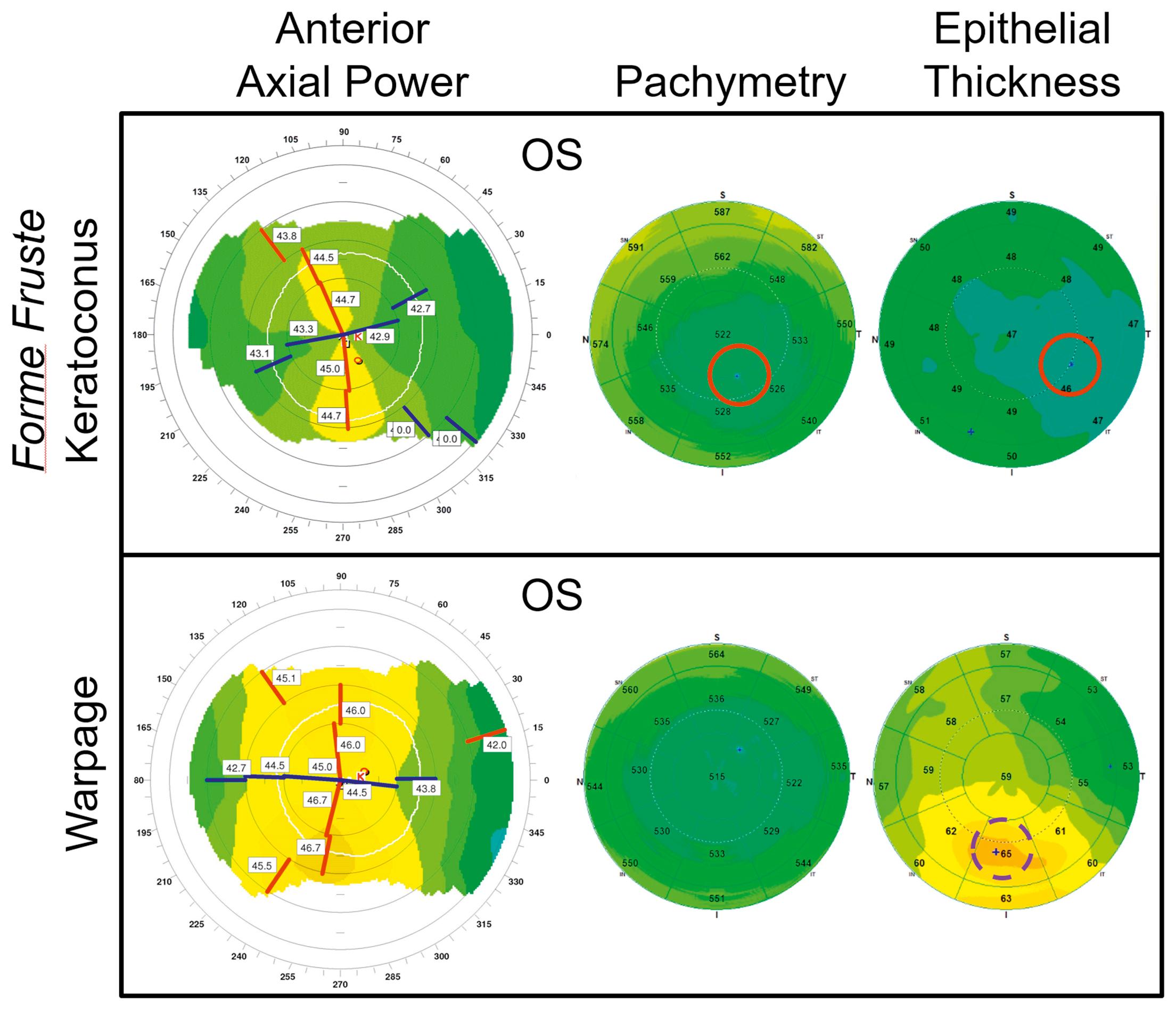
OCT also has sufficient resolution to map the corneal epithelial thickness (see Fig. 17.2 ), which is not possible with slit scanning and Scheimpflug camera technologies. In keratoconus, the epithelium thins at the apex of the cone to reduce focal steepening. , This compensatory focal thinning reduces the surface distortion detectable by corneal topography. An epithelial thickness–based pattern standard deviation (epi-PSD) variable is shown to be very sensitive for detecting uneven epithelium in any type of corneal shape irregularities. , Analyzing the corneal and epithelial thickness patterns together can further distinguish keratoconus from other corneal irregularities (see Fig. 17.2 ). In corneal irregularities due to secondary epithelial compensation, which include keratoconus, stromal scars, and stromal dystrophies, areas of topographic steepening are associated with focal epithelial thinning . In contrast, in primary epithelial deformations due to contact lens-related warpage, dry eye, or epithelial basement membrane dystrophy, areas of topographic steepening are associated with focal epithelial thickening (see Fig. 17.2 ).
OCT allows for the visualization of laser in situ keratomileusis (LASIK) flaps in the immediate postoperative period and is useful for the evaluation of microkeratome performance ( Fig. 17.3 ). Flap morphology analyzed with OCT demonstrated that flaps created with a femtosecond laser are more uniform in thickness whereas those created with a mechanical microkeratome are thinner in the center.
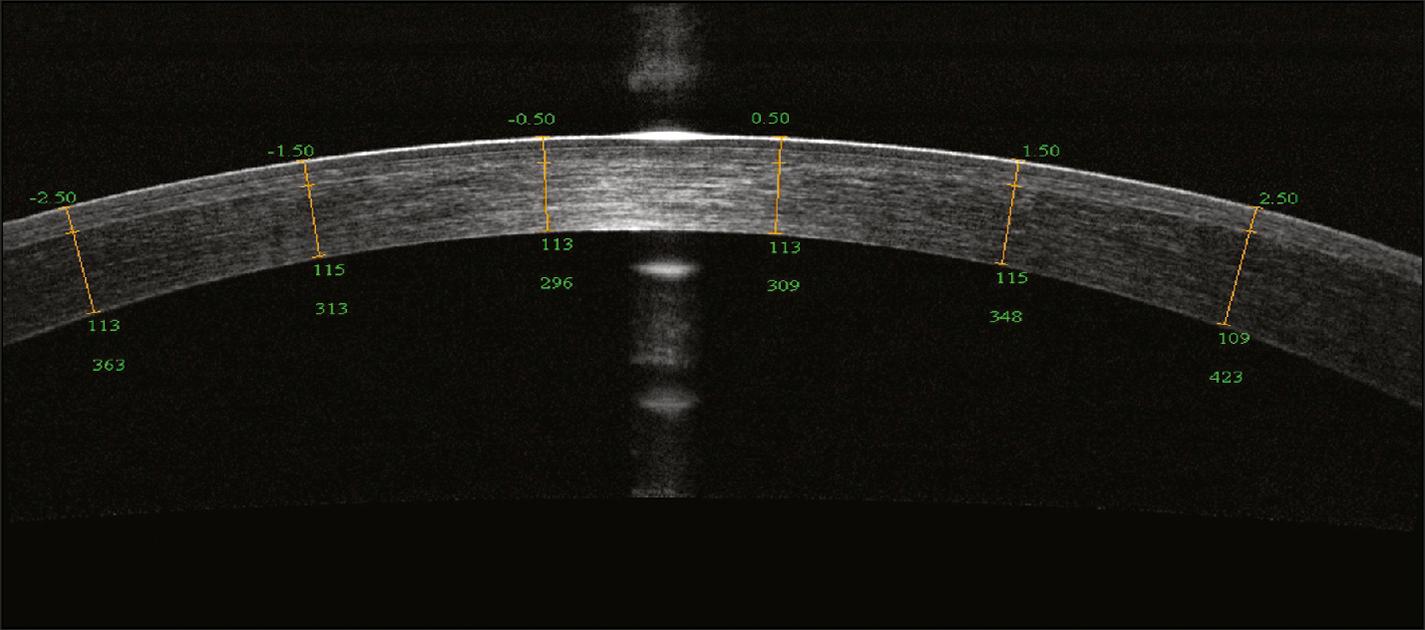
Corneal ectasia occurring after laser vision correction is a well-documented complication, and an important risk factor is residual stromal bed thickness less than 250 μm. The capability to analyze flaps several years after LASIK using OCT becomes useful in refractive enhancement evaluations. Accurate measurement of the residual stromal bed thickness can help to determine whether a LASIK enhancement can be safely performed without the risk of causing corneal ectasia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here