Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Complete ACL rupture: >5 mm of increased anterior tibial translation, positive pivot shift test.
Profile patient for desired future activity level.
High-risk activities (pivoting, cutting, twisting, and turning): reconstruct.
Acute ACL rupture and concomitant displaced bucket-handle meniscus tear: surgery within 2 to 3 weeks to repair meniscus, usually concurrent ACL reconstruction.
Low-risk activities, willing to avoid strenuous activities that place the knee at increased risk for giving-way episodes: conservative treatment.
Successful ACL reconstruction reduces risk of subsequent injuries.
Partial ACL tears: <50% fibers torn: conservative treatment; >50% torn in athlete: consider graft augmentation.
Patients presenting with acute complete ruptures of the anterior cruciate ligament (ACL) (>5 mm of increased anterior tibial translation and positive pivot shift test) are treated with rehabilitation until pain and swelling subside and joint motion and muscle function are restored. This delay markedly reduces the incidence of postoperative complications of knee motion limitations and muscle weakness. All patients with acute ruptures are profiled with regard to their desired future activity level to determine whether ACL reconstruction is warranted. Those who are highly motivated to return to high-risk activities, including strenuous athletics or occupations involving pivoting, cutting, twisting, and turning, are considered for reconstruction. In patients with acute ACL ruptures and a concomitant displaced bucket-handle meniscus tear, surgery within 7 to 10 days is required to reduce the meniscus to a normal location and repair the tear. The ACL reconstruction may be performed at the same setting; however, knees with excessive swelling and pain undergo a staged meniscus repair first. After an appropriate period of rehabilitation, ACL reconstruction is then performed.
Patients involved in low-risk activities or who are willing to avoid strenuous athletic and occupational activities that place the knee at increased risk for giving-way episodes may not require ACL reconstruction. These patients are placed into a conservative treatment program that includes rehabilitation to regain muscle strength and neuromuscular function and counseling on the risk of future giving-way reinjuries and potential damage to the joint. Even with surgical reconstruction, patients are informed that an ACL rupture is a serious injury and it is unlikely that they will ever have a truly normal knee joint. The injury may also involve a bone bruise and chondral damage, with sequelae for future joint symptoms. The goal of conservative management is to prevent recurrent giving-way reinjuries that are deleterious to the joint because they frequently result in meniscus tears and subsequent meniscectomy. It is important to categorize the knee joint regarding the extent of the injury that occurred. Repairable meniscus tears almost always indicate a concurrent ACL reconstruction. Otherwise, the success of the meniscus repair may be compromised. DeHaven and coworkers documented higher failure rates of meniscus repairs in knees with ACL deficiency than in knees with normal ACL function (46% and 5%, respectively) in a 10-year follow-up study. A grade III pivot shift and grossly positive Lachman test results (increased ≥10 mm anterior tibial translation) indicate involvement of the secondary ligamentous restraints and, in our experience, an increased risk of giving-way reinjuries with recreational activities. Patients with physiologic laxity of other ligaments or partial tears (second-degree) of medial or lateral ligaments frequently have a grossly positive pivot shift, as discussed in Chapter 3 .
The rationale for the indications for ACL reconstruction is based on a study of 103 patients with chronic ACL ruptures. These knees had no other ligament injuries and had not undergone reconstruction. The patients were evaluated for a mean of 5.5 years after the initial knee injury; a subgroup of 39 patients presented an average of 11 years postinjury. This study was not a natural history study, because all patients sought treatment because of symptoms. After the initial injury, 82% of the patients returned to sports activities, which gave an initial false impression of the seriousness of the ACL disruption. After 5 years only 35% were continuing athletics, often with recurrent symptoms of pain or swelling. A significant reinjury occurred in 36 patients within 6 months and in 53 patients within the first year following the initial injury. Moderate to severe symptoms of pain, swelling, and/or giving-way were reported in 32 patients during walking, in 45 patients during activities of daily living, and in 73 patients during turning or twisting athletic activities. Giving-way occurred in 22 patients during walking, in 34 patients during recreational sports, and in 66 patients during strenuous sports. Patients who had undergone meniscectomy had a twofold to fourfold increase in pain and swelling symptoms. Radiographic findings lagged behind clinical symptoms. Forty-four percent of patients in the subgroup 11 years from injury had moderate to severe arthritic changes. The “ring” sign was noted, which represents an osteophyte ( Fig. 7-1 ) that forms around the circumference of the lateral tibial plateau. This is associated with an absence of a pivot shift phenomena because the lateral femoral condyle translation is restrained by the osteophytes and increased concavity of the lateral tibial plateau.
In a follow-up study, 84 of the initial 103 patients underwent a repeat evaluation 3 years after initiating a conservative and activity modification treatment program. The frequently quoted “rule of thirds” represents the outcome of this study regarding the effects of an ACL injury on subsequent functional activities. Approximately one third of the patients showed improvement in symptoms and functional limitations with daily or recreational activities, one third did not improve, and one third became worse with continuing symptoms and limitations. Thirty percent of the patients (labeled “knee abusers”) were noncompliant with the activity modification recommendations and stated they would continue athletics despite symptoms and the increased risk for future arthritis. These patients had the poorest overall prognosis with conservative management. This general rule of thirds may not always be specific to individual patients with chronic ACL insufficiency because it does not take into account the presence of meniscus and arthritis grading, compliance with activity modification, and maintenance of a muscle strengthening program. The frequency of giving-way episodes correlated with chronic pain, swelling, and functional limitations. The conclusion was reached that any giving-way episode with athletics, even if followed by periods of no giving-way or other symptoms, was to be absolutely avoided because meniscus tears and joint deterioration may occur. The results of this program show the importance of counseling the patient on the possible expected levels of athletic activity and following closely those who do not initially choose to have surgery to lessen the chance of significant arthritis in future years.
Recent systematic reviews have demonstrated that ACL reconstruction reduces the incidence of subsequent meniscus injuries, reduces the need for further operations, and results in greater improvements in activity levels. In patients who undergo reconstruction and in whom the menisci are retained, there is a lower incidence of knee osteoarthritis (OA). Mather and associates reported that, in the short term, ACL reconstruction was less costly (cost reduction of $4503) and more effective compared with rehabilitation. In the long term, the mean lifetime cost to society for a patient undergoing ACL reconstruction was calculated to be $38,121 compared with $88,538 for nonoperative treatment with rehabilitation.
Partial ACL tears occur more frequently than suspected and present in a manner similar to complete ACL ruptures. These injuries frequently occur from a noncontact giving-way episode during jumping or pivoting and are often accompanied by a popping sensation and acute hemarthrosis. There is a limitation of full extension with pain on even gentle attempts to passively achieve 0 degrees, mimicking a torn displaced meniscus, which requires magnetic resonance imaging (MRI) to exclude a mechanical block to knee extension. In a study reported by the senior author (F.R.N.) and associates, of 32 knees with a partial ACL tear verified arthroscopically, a tear to one or both menisci occurred in 53% of the knees. In an average of 67 months postinjury (range, 24-110 months), 38% had progressed to complete ACL deficiency. The arthroscopic evaluation of the extent of ACL disruption was related to the prognosis and progression to complete ACL deficiency. Even though the arthroscopic evaluation does not define the true extent of ligament injury on a microscopic or functional basis, 86% of knees in which three fourths of the ACL was torn progressed to ACL deficiency. One half of the knees with an estimated injury involving half of the ligament progressed to ACL deficiency, even though major portions of the ACL were still intact, preventing a pivot shift phenomena. Only 12% of knees that had an estimated one fourth or less of the ligament torn progressed to complete ACL deficiency; therefore these knees had a reasonably good prognosis for return to athletics.
Messner and Maletius followed 22 consecutive patients with partial ACL ruptures (no more than 50% of the ACL fibers torn) a mean of 12 years postinjury; all but one were also reexamined for a mean of 20 years postinjury. No patient required ACL reconstruction. Patients decreased their level of activity from contact sports to recreational activities. Few had an increase in anterior translation or improvement in the pivot shift test, and few differences were noted in symptoms between follow-up evaluations.
Based on these data, the following rules apply for the management of partial ACL tears. Knees with tears of 50% or less of the ACL fibers are not candidates for reconstructive surgery. The patients are advised that they need to be followed yearly. The chance for progression to complete ACL deficiency in the more than 50% tear group is at least one in two knees. In the athletic individual, a hamstring graft augmentation is a consideration (made on a case-by-case basis) to provide stability and retain the remaining function of the ACL. In the more sedentary patient who may wish to choose a nonoperative treatment of a complete ACL rupture, a reconstruction is not warranted.
Sedentary patients, no symptoms, little exposure to high-risk activities
Patients unable to participate in postoperative rehabilitation program
Patients with preexisting severe loss of patellofemoral or tibiofemoral compartment joint space
Marked muscle atrophy
Complex regional pain syndrome
Obesity
Prior joint infection
Patients who are sedentary and do not experience giving-way or swelling episodes with daily activities and have little exposure to strenuous or high-risk activities are treated conservatively. These patients are advised to maintain an ideal body weight and have periodic knee examinations. In addition, patients who are willing to modify their activity level to avoid high-risk knee motions such as pivoting and cutting are placed in a conservative treatment program. Patients who are unable to participate or be compliant with postoperative rehabilitation are not surgical candidates.
The presence of symptomatic patellofemoral or tibiofemoral arthritis is a general contraindication to ACL surgery because pain symptoms remain postoperatively. Although pain symptoms may be lessened by restoring knee stability, the joint damage will still limit daily activities. Weight-bearing 45-degree posteroanterior (PA) views are important to determine the millimeters of remaining medial or lateral tibiofemoral joint space. In chronic ACL injuries, patients are frequently surprised to learn that the joint space is absent or nearly absent, because their symptoms have not yet limited daily activities. In these knees, conservative measures are instituted until such time that partial or total joint replacement is warranted.
Patients with ACL deficiency, symptomatic mild or moderate medial tibiofemoral arthritis, and varus malalignment require corrective valgus-producing high tibial osteotomy (HTO). These patients often do not require subsequent ACL surgery because there are limitations in activities owing to the joint damage. This principle applies to both ACL-deficient knees and those who had a prior failed ACL reconstruction. These patients often report improved joint instability because the varus thrust gait pattern is eliminated with osteotomy.
Patients with marked lower extremity muscle atrophy require a delay in surgery for many months until adequate muscle function has been restored. These patients have an increased risk for postoperative complications, including quadriceps muscle shutdown, patella infera, and arthrofibrosis. In some cases, the restoration of muscle function may lead to increased knee stability, avoiding ACL revision.
The presence of residual signs and symptoms of a complex regional pain syndrome (CRPS) requires careful examination and questioning regarding the presence of burning pain, abnormal skin hypersensitivity, extremity discoloration, and intolerance to cold and ice. Even after resolution of a prior CRPS, there is an increased frequency for return of the syndrome in these knees that must be promptly recognized in the postoperative period (see Chapter 40 ).
Patients with a body mass index of 30 or greater are usually not surgical candidates. A history of prior infection with subsequent joint arthritis often contraindicates ACL surgery. There may be associated medical conditions contraindicating surgery. The use of nicotine products is strongly discouraged and absolutely contraindicated if osteotomy alignment procedures are required.
ACL primary restraint anterior tibial translation.
ITB, mid medial capsule, mid lateral capsule, MCL, FCL combined secondary restraint.
ACL resists coupled motions anterior tibial translation and internal tibial rotation along with the lateral structures.
Secondary restraints may become lax after reinjuries, may be physiologically loose or tight.
Loss of ACL and lateral structures increases anterior tibial translation and internal tibial rotation.
Increase in coupled motions shifts center of rotation to medial compartment.
Concurrent injury to medial structures causes center of rotation to shift outside medial compartment, gross anterior subluxation of both compartments.
Some authors provide evidence of two-bundle division of ACL, others argue that ACL fiber function is too complex to be artificially divided into two bundles.
Characterization of ACL into two bundles represents gross oversimplification not supported by laboratory studies.
With substantial anterior tibial loading or coupled motions, majority of ACL fibers are in a load-sharing configuration with different percentages as to fiber tensile loads.
ACL is not isometric, all ACL fibers anterior to transitional zone lengthen with knee flexion, and posterior fibers lengthen with knee extension.
Function ACL fibers determined primarily by anterior-to-posterior direction (knee at extension), and secondarily by proximal-to-distal femoral attachment and anterior-to-posterior tibial attachment.
Placement of graft too far in an anterior or posterior femoral position has a large effect on deleterious lengthening and graft failure.
Several studies report transtibial drilling techniques select a posterior tibial position, resulting in a vertical ACL graft orientation.
Variation in ACL anatomy requires surgeon to outline the size and shape of the ACL attachment in each patient, if possible.
Landmarks for ACL tibial attachment: medial tibial spine, posterior interspinous ridge (RER), attachment of lateral meniscus. PCL is poor landmark.
Place guide pin eccentric and 2 to 3 mm anterior and medial to ACL center, avoiding posterior tibial attachment location.
Anterior notchplasty often required with central ACL graft placement.
Landmarks for ACL femoral attachment: posterior articular cartilage, Blumenstaat line, ACL attachment on lateral femoral wall.
Central anatomic ACL placement: femoral guide pin 2 to 3 mm above the midpoint of the proximal-to-distal length of the ACL attachment, 8 mm from the posterior articular cartilage edge. Define anatomic attachment with knee 20 to 30 degrees of flexion.
Majority in vitro robotic studies compare double-bundle graft to a less than ideal vertically placed single graft in a high femoral and posterior tibial location. This graft placement is not recommended.
Studies of single ACL grafts in the more ideal central tibial and femoral anatomic attachment show restoration of rotational knee stability.
There appears to be no distinct advantage of the more complex double-bundle technique in comparison to a well-placed central anatomic single graft technique.
Both ACL bundles function synergistically to resist medial and lateral compartment subluxations during Lachman and pivot shift tests.
Assessment of ACL graft function takes into account restoration of normal coupled motion limits of anterior tibial translation and internal tibial rotation. No current reliable measuring system is available to measure pivot shift test.
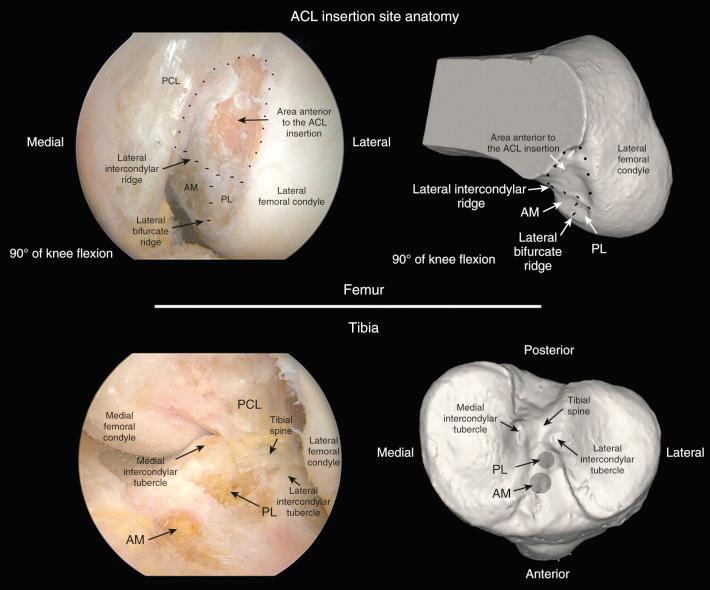
Important landmarks for the ACL tibial attachments are the medial tibial spine, posterior interspinous ridge of the proximal PCL fossa, and the attachment of the lateral meniscus. ACL tibial attachment location that we recommended for a single graft is directly adjacent to the lateral meniscus anterior horn attachment.
Important landmarks for the femoral attachment are the posterior articular cartilage, Blumenstaat line, and identification of the ACL attachment on the lateral femoral wall of the notch.
For single grafts, we recommend a central anatomic ACL placement with the femoral guide pin 2 to 3 mm above the midpoint of the proximal-to-distal length of the ACL attachment (30 degrees of knee flexion) and 8 mm from the posterior articular cartilage edge.
The function of the ACL is discussed in Chapter 3 . Additional information regarding the restoration of ACL function related to surgical techniques and graft placement issues are discussed in this section. The ACL is the primary restraint to anterior tibial translation, providing 87% of the total restraining force at 30 degrees of knee flexion and 85% at 90 degrees of flexion. The iliotibial band (ITB), mid medial capsule, mid lateral capsule, medial collateral ligament (MCL), and fibular collateral ligament (FCL) provide a combined secondary restraint to anterior tibial translation. The posteromedial (PM) and posterolateral (PL) capsule structures provide added resistance with knee extension. The secondary restraints may become deficient after an ACL injury or repeat giving-way episodes resulting in increased symptoms.
The ACL resists the coupled motions of anterior tibial translation and internal tibial rotation along with the lateral structures. In our laboratory, Wroble and colleagues measured in ACL-deficient knees the simulated effect of lateral soft tissue injuries on internal tibial rotation and anterior tibial translation. The experimental design consisted of a six degrees of freedom (DOF)-instrumented spatial linkage and loading of the knee joint under defined loads of 100-N anteroposterior (AP), 15 N-m varus-valgus, and 5 N-m internal-external tibial rotation torque. The limits of knee motions were determined in the intact knee and then after sectioning the ACL, ITB, lateral capsule (anterolateral ligament), popliteus tendon, popliteofibular ligament, and PL capsule.
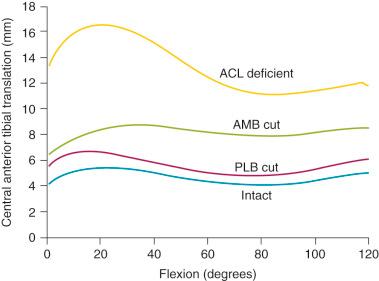
The effect of sectioning the ACL first, followed by the ITB and lateral capsule, is shown in Figure 7-2 . Two distinct responses were measured. In the first set of knees (see Fig. 7-2, A ), there were only moderate increases in anterior tibial translation after ACL sectioning and negligible further increases after sectioning the ITB and lateral capsule. However, in the second set of knees (see Fig. 7-2, B ), much larger increases were noted in anterior tibial translation after sectioning the ACL, and even greater increases were found after sectioning the ITB and lateral capsule.
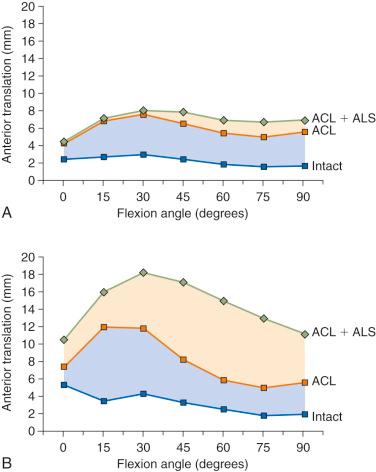
These findings are compatible with clinical tests after ACL injury in which some knees have only moderate increases in anterior tibial translation, whereas other knees have much larger increases. This concept is discussed in Chapter 3 , using the analogy of the bumper model to simulate the resistance of the secondary restraints on motion limits once the ACL is removed. Clinically, an ACL graft would be expected to be subjected to greater in vivo forces in the second set of knees without the unloading provided by relatively “tight” secondary restraints. The increase in the magnitude of anterior translation and internal rotation in these physiologically loose knees after loss of the anterolateral structures results in a grade III pivot shift phenomena. These knees require a high-strength, securely fixated ACL graft to resist these major displacements, and autografts are highly recommended in these grossly unstable knees, with preference for a bone-patellar tendon-bone (B-PT-B) graft.
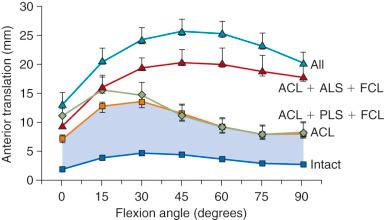
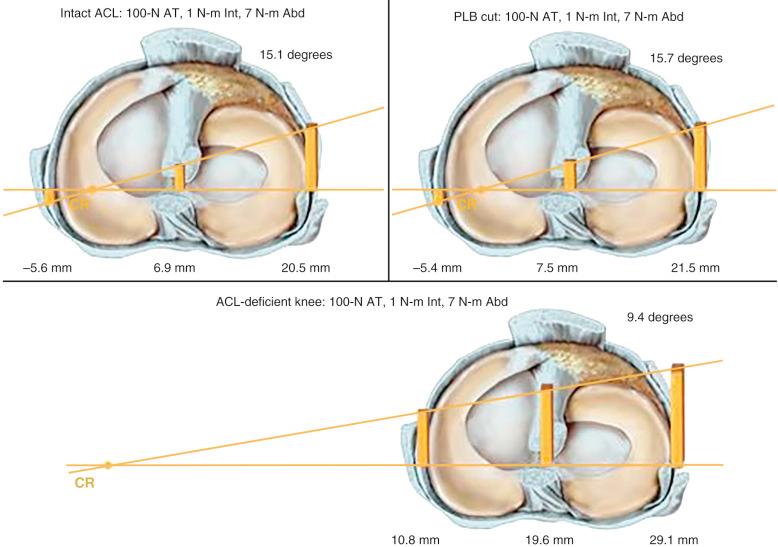
The effect of sectioning the lateral and posterolateral structures (PLS) is shown in Figure 7-3 . Sectioning of the FCL or PLS (popliteus tendon, popliteofibular ligament, PL capsule) produced small but statistically significant increases in anterior tibial translation at low flexion angles. The combined ACL-ALS-FCL (where ALS are the anterolateral structures comprising the ITB and anterior and middle portions of the lateral capsule) sectioned knee had large increases in anterior tibial translation, with the greatest changes occurring at higher flexion angles.
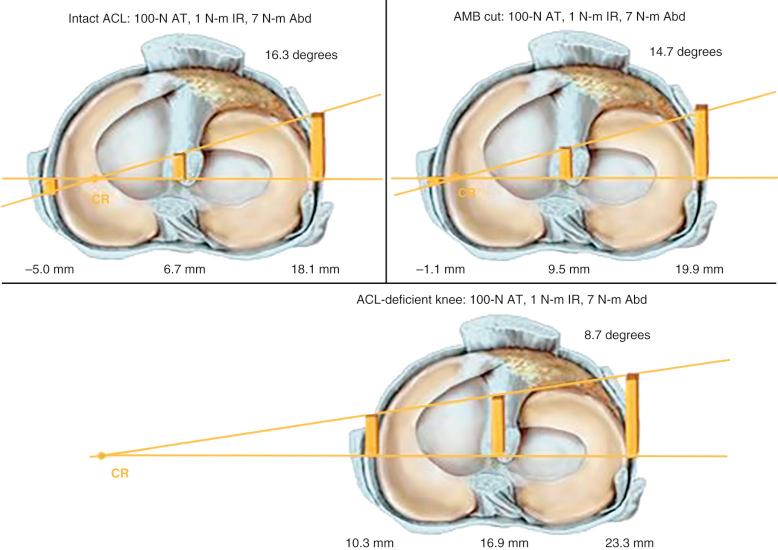
The effect of sectioning the ACL and lateral structures on the limits of internal tibial rotation are shown in Figure 7-4 . Sectioning of the ACL produced only a small increase in the final internal rotation limit. Sectioning of the ALS and the FCL produced sequentially larger increases in internal tibial rotation. In select revision knees that have large increases in internal tibial rotation, the data support the role of a lateral extraarticular procedure discussed later to unload the ACL graft and restore stability.
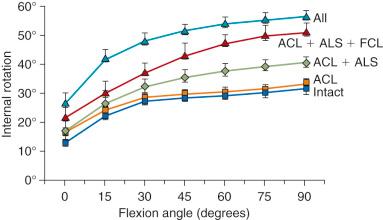
The conclusions from this study support the ACL as the primary restraint to anterior tibial translation. The data show that the ultimate limit for internal tibial rotation is resisted by the lateral extraarticular structures that are tightened by the internal tibial rotation. This is an important concept because clinical and biomechanical studies frequently attempt to measure increases in internal tibial rotation after ACL surgery in an effort to quantify ACL graft function. Accordingly, this approach would not be expected to be successful. When the ITB and lateral capsule are physiologically slack, the FCL and ACL assume increased importance in limiting internal rotation. In summary, ACL function is ideally described by the restraining effect of lateral and medial tibial plateau translation and not by internal rotation limits. A positive pivot shift test represents the effect of abnormal compartment translations and anterior subluxation of both tibiofemoral compartments.
The motions that occur during the pivot shift maneuver are shown in Figure 7-5 . At the beginning of the tests, the lower extremity is simply supported against gravity. After ACL disruption, both anterior tibial translation and internal tibial rotation occur as the femur drops back and externally rotates with subluxation of the lateral tibiofemoral compartment. This position is accentuated as the tibia is lifted anteriorly. At approximately 30 degrees of knee flexion, the tibia is pushed posteriorly, reducing the tibia into a normal position with the femur. From position C to position A (see Fig. 7-5, B ), the knee is extended to again produce the subluxated position.
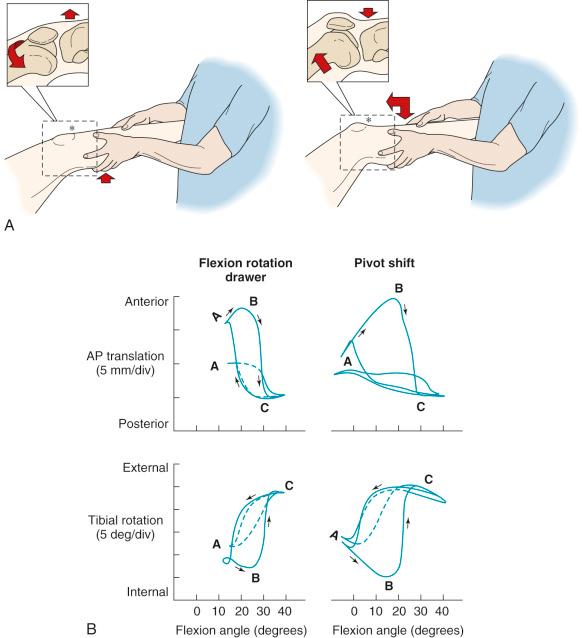
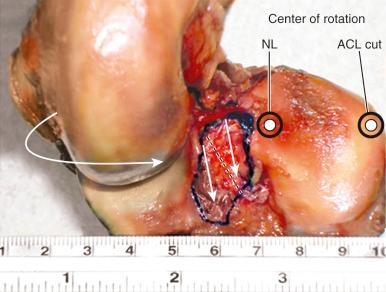
The effect of the ACL in providing rotational stability is shown in Figure 7-6 . After ACL sectioning, there is an increase in medial and lateral compartment translation as the center of rotation shifts from inside the knee joint to outside the medial compartment. The medial ligamentous structures influence the new center of rotation. With injury to the medial ligament structures, the center of rotation shifts so far medially that a tibial rotational displacement is essentially lost, resulting in a gross anterior subluxation of both compartments. The data show the important surgical concept of restoring injured medial and lateral ligament structures to restore anterior knee stability in conjunction with the ACL.
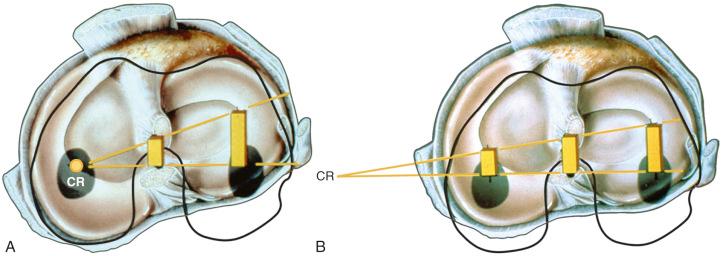
There have been numerous in vitro and in vivo studies published over the past decade on ACL function in regard to providing rotational knee stability. Based on the results of these studies, authors have provided recommendations for ACL single- and double-bundle graft reconstructions, graft placement, and tensioning. These investigations involved a simulated Lachman test and, more importantly, the pivot shift test, which correlates with patient subjective giving-way symptoms. In our opinion, there are problematic errors in many studies that the surgeon should be aware of in terms of application of the results to surgical treatment of ACL ruptures. For example, nearly all published studies describe rotational knee stability according to the changes in degrees of internal tibial rotation, without measuring the center of tibial rotation or the position or subluxation of the tibiofemoral compartments. We believe that providing only the changes in degrees of tibial rotation, without specification of the medial and lateral tibiofemoral compartment position or subluxation, is an inadequate descriptor of rotational knee stability. In fact, after ACL rupture, there is a change of only a few degrees of internal tibial rotation as long as the lateral extraarticular structures remain intact as already described.
A second problem we have identified in a majority of in vivo and in vitro pivot shift studies is the loading profile used to induce anterior tibial subluxation. Studies commonly report, in the pivot shift simulation, an abnormal anterior tibial translation after ACL sectioning that is only half that obtained in the Lachman test. However, under clinical conditions, a positive pivot shift test produces greater anterior tibial subluxation than the Lachman test. Accordingly, these prior studies were conducted under low knee displacement conditions in which the loading profile involved an internal rotation-valgus condition. There was an absence of a simultaneous combined anterior tibial loading, which is the major reason the clinical pivot shift test induces anterior tibial subluxation. A prior published study from our institution that studied orthopedic surgeons performing a pivot shift test on an instrumented lower extremity reported that two types of clinical pivot shift loadings were induced: (1) a predominant internal rotational-valgus torque; and (2) a combined anterior tibial loading with internal rotation-valgus torque. The combined pivot shift loading test produced the greatest amount of anterior tibial subluxation of both tibiofemoral compartments. The predominant internal rotational-valgus torque loading constrained anterior tibial subluxation by reducing the medial tibiofemoral compartment translation.
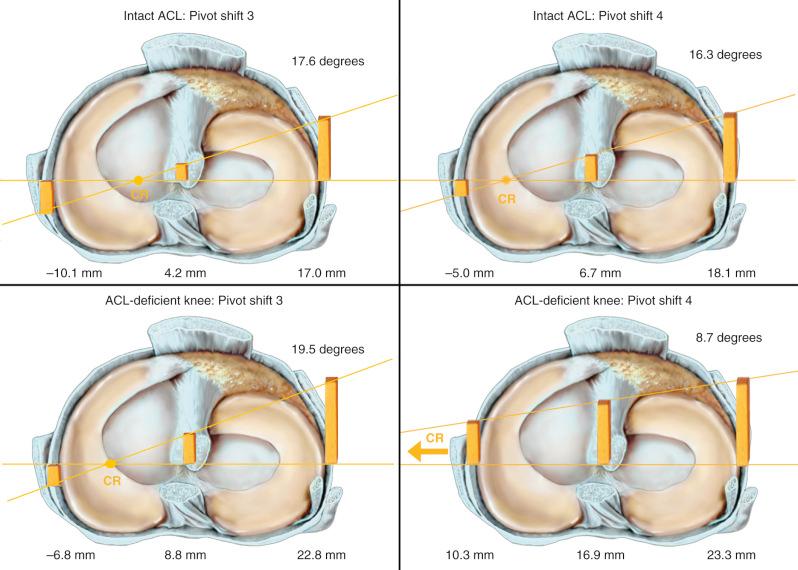
For reasons not apparent, pivot shift loading with a predominant internal rotation-valgus torque, but without a coupled anterior tibial loading, has been the typical loading profile in nearly all cadaveric studies on ACL function and surgical reconstructions even though this limits anterior tibial subluxation of the medial compartment. In Figure 7-7 , the results of a typical cadaveric knee specimen are shown under two pivot shift loading conditions. Three points are important. In the two simulated pivot shift tests, there is a greater anterior tibial subluxation of the lateral and medial compartment, with the pivot shift subluxation produced by a coupled anterior translation-internal rotation during the valgus loading as the knee approaches extension (pivot shift [PS] 4), compared with an absence of the anterior tibial loading component (PS3). Secondly, high tibial internal rotation torques in the pivot shift reduces the medial tibiofemoral compartment (see Fig. 7-7 ), constraining the amount of medial and central compartment translation. Third, an analysis of rotational knee stability requires knowledge of the resulting subluxations (translations) of the medial and lateral tibiofemoral compartments (see Chapter 3 ). We recommend that future in vitro and in vivo pivot shift studies use loading profiles of coupled anterior tibial translation and internal tibial rotation, along with valgus loading, to induce a maximum anterior tibial subluxation of both tibiofemoral compartments. Further, rotational torques should remain low to not constrain the maximum subluxation of the medial compartment.
Controversy exists in published studies on the division of the ACL into two distinct fiber bundles. Some authors provide evidence of both an anatomic and functional division, whereas others doubt this division exists and argue that ACL fiber function is too complex to be artificially divided into two bundles. In some studies, the anteromedial (AM) bundle is identified functionally at its femoral location as the proximal half of the attachment (knee in extension) that tightens with knee flexion. The PL bundle is identified as the distal half of the ACL femoral attachment that tightens with knee extension. The PL bundle is described to relax with knee flexion, as the ACL femoral attachment changes from a vertical to a horizontal structure. The problem is that this classical description of a reciprocal tightening and relaxation of the AM and PL bundles occurs only under low anterior loading conditions. With substantial anterior tibial loading, and particularly with a coupled motion of anterior translation and internal tibial rotation, the majority of the ACL fibers are brought into a load-sharing configuration to a differing percentage as is presented later.
We believe the characterization of the ACL into two fiber bundles represents a gross oversimplification not supported by biomechanical length-tension laboratory studies. Zavras and coworkers published a comparative study on previously published isometric points for the ACL and concluded that the ACL isometric zone was high and proximal in the ACL attachment, close to the posterior end of the Blumensaat line. These data, along with other studies, focused on placing grafts in the proximal aspect of the ACL attachment, in a so-called isometric position. The problem with applying published isometric data to the clinical situation is that the data are valid only for knee flexion-extension and do not indicate the most effective ACL graft position for controlling knee rotational loading as occurs during the pivot shift test.
The length-tension behavior of ACL fibers is primarily controlled by the femoral attachment in reference to the center of femoral rotation, the coupled motions applied, the resting length of ACL fibers, and tibial attachment locations. In Chapter 3 , the concept of an isometric transition zone or contour plot is presented. The zone represents a central transition zone in which fibers undergo 2 mm of length change during knee flexion and extension. This zone is not an isometric zone because the length change is not zero. All ACL fibers anterior to the zone lengthen with knee flexion, whereas the posterior fibers lengthen with knee extension. Under loading conditions, fibers in both the AM and PL divisions contribute to resist tibial displacements. ACL fibers in the PL division attach in part posterior to the transitional zone and lengthen with knee extension. Note that the function of the ACL fibers is determined by the anterior-to-posterior direction (knee at extension) as well as the proximal-to-distal femoral attachment. Placement of a graft in an anterior or posterior position has a large effect in producing deleterious lengthening and graft failure. The obvious example is a graft placed anterior to the “resident's ridge,” which is anterior to the femoral ACL attachment. The femoral proximal-to-distal division of the ACL used in two-bundle descriptions of fiber length in knee flexion and extension oversimplifies the functional behavior of the ACL fibers, which are more dependent on their anterior and posterior femoral attachments.
The division of the ACL into two bundles was historically based on the tibial attachment site and not on a corresponding femoral attachment site. Recent studies project the tibial bundles onto two corresponding femoral sites. In these studies, to be described later, we usually divide the ACL femoral attachment site at a midpoint into a corresponding AM and PL bundle. In our opinion, there is not at present convincing anatomic data to support a division of the ACL into two separate bundles, although one study reported a bony ridge between the two femoral attachment bundles. Because of the lack of a clear anatomic division of the ACL into two bundles, there is discrepancy among authors on anatomic descriptions of the ACL bundles and recommendations for the surgical technique on tibial and femoral tunnels for two bundle graft reconstructions.
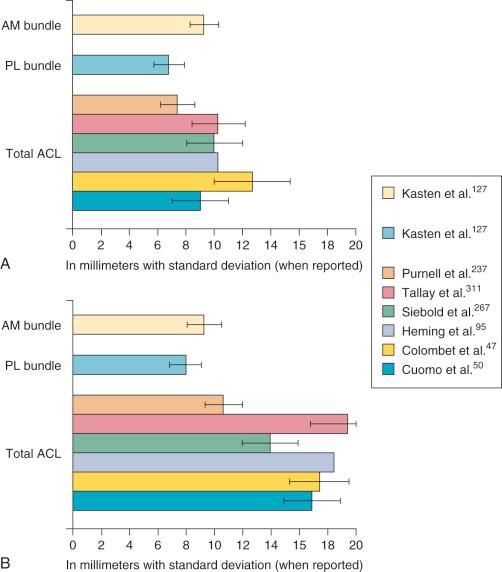
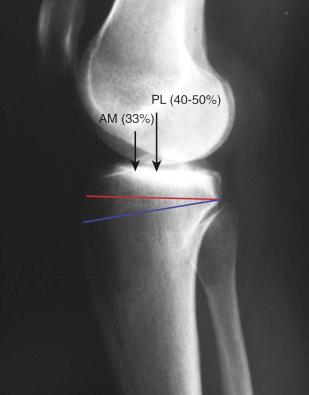
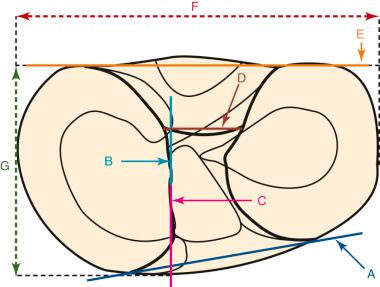
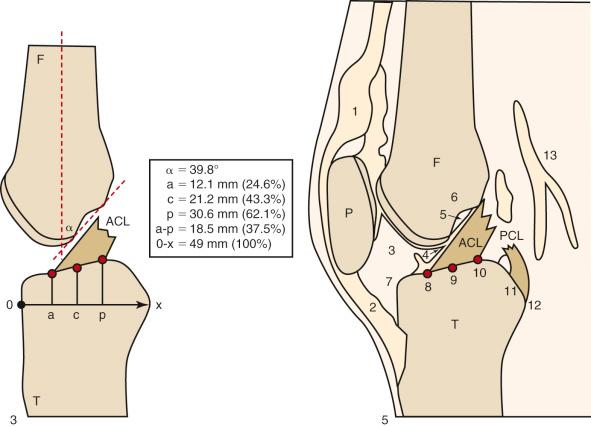
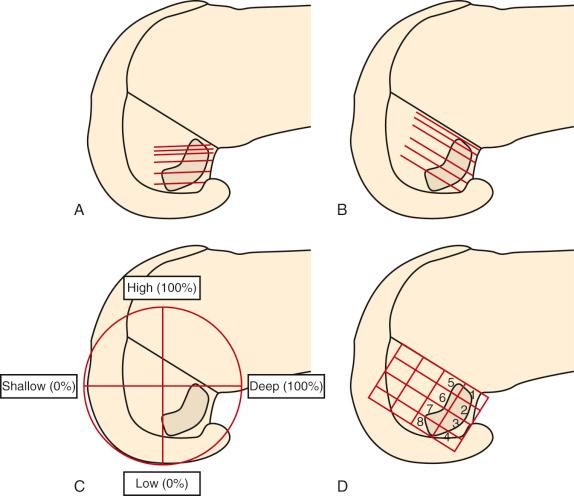
Hwang and associates performed a systematic review of the ACL tibial footprint in which 19 studies were analyzed. The authors noted wide variability in the reported size and shape of the native tibial footprint ( Fig. 7-8 ). As a general guideline, on a lateral radiograph, the AM bundle was located at one third the anterior-to-posterior distance, the PL bundle was located at one half this distance, and the ACL center was located midway between ( Fig. 7-9 and 7-10 ). The authors mentioned that although the line described by Amis and Jakob was parallel to the medial plateau and the line described by Staubli and Rauschning was perpendicular to the tibial axis, both lines yielded approximately the same results ( Table 7-1 ). The approximate arthroscopic landmarks of the reviewed studies are shown in Table 7-2 . These measurements need to be adjusted according to the relative size of the knee joint and potential anatomic variations. Even so, these measurements allow identification of the anterior two thirds of the ACL footprint for a 10-mm tunnel that avoids any portion of the tunnel occupying the posterior one third (PL bundle portion), ensuring that the tunnel remains anterior to the posterior lateral meniscus attachment.
| Reference | Center of AM Bundle (Mean ± SD [Range]) |
Center of PL Bundle (Mean ± SD [Range]) |
|---|---|---|
| Amis & Jakob | ||
| Kasten et al. (2010) | 35% ± 4% (23%-42%) | 48% ± 4% (39%-58%) |
| Doi et al. (2009) | 34.6% ± 4.3% (24.0%-42.9%) | 38.5% ± 4.3% (28.6%-48.1%) |
| Colombet et al. (2006) | 36% ± 3.8% (NA) | 52% ± 3.4% (NA) |
| Staubli & Rauschning | ||
| Iriuchishima et al. (2010) | 31% ± 3% (NA) | 50% ± 3% (NA) |
| Zantop et al. (2008) | 30% (NA) | 44% (NA) |
| Reference | Key Findings |
|---|---|
| Iriuchishima et al. (2010) | Distance between center of AM and PL footprints and anterior margin of PCL is 23 ± 4 mm and 12 ± 3 mm, respectively. |
| Purnell et al. (2008) | Distance from posterior fibers of ACL to anterior margin of PCL is 16.5 ± 2 mm (range, 12.7-19.1 mm). |
| Siebold et al. (2008) | Posterior horn of lateral meniscus is adjacent to posterior border of PL footprint; centrum of PL footprint is 5 mm anterior. |
| Heming et al. (2007) | Centrum of tibial footprint is 15.0 mm anterior to tibial notch of PCL. |
| Edwards et al. (2007) | Centrum of tibial footprint is 15 ± 2 mm (range, 11-18 mm) from anterior PCL; center of PL bundle is 10 ± 1 mm (range, 8-13 mm) and AM is 17 ± 2 mm (range, 13-19 mm) from anterior PCL. |
| Luites et al. (2007) | Centrum of AM bundle is one fourth interspinous distance from ML intercondylar eminence; PL bundle centrum is 4 mm more lateral, approximately halfway between spines; tibial footprint centrum as a whole is two fifths ML (interspinous distance). |
| Cuomo et al. (2006) | Anterior border of ACL tibial footprint is 22 ± 3 mm (range, 16-27 mm) anterior to “over the back position” (ridge just anterior to PCL); posterior border is 6 ± 2 mm (range, 2-8 mm) anterior to ridge. |
| Colombet et al. (2006) | Mean distance between AM bundle and anterior border of PCL is 17.5 ± 1.7 mm. |
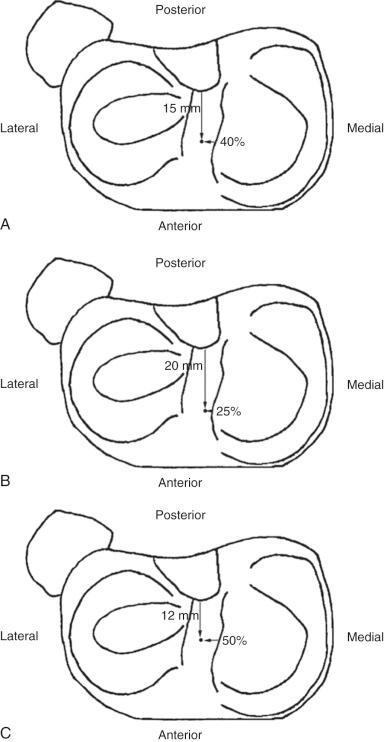
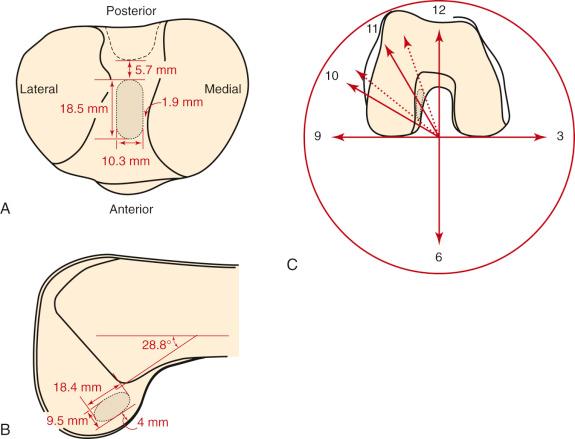
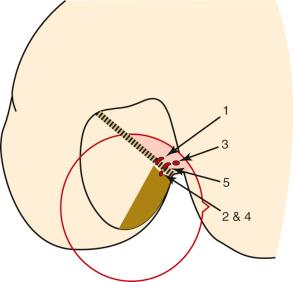
Edwards and coworkers defined the ACL tibial attachment in 55 cadaveric specimens. In Figure 7-11 , the tibial bony landmarks are depicted. The “over-the-back” ridge (posterior interspinous ridge [RER]) corresponds to the interspinous ridge just anterior and proximal to the posterior cruciate ligament (PCL) attachment. Figure 7-12 presents these authors' recommendations for best-fit central and two-tunnel positions. The center of the ACL attachment was 15 ± 2 mm (range, 11-18 mm) from the RER at 36% of the anteroposterior (AP) depth of the tibia. The center of the PL bundle anterior from the RER was 10 ± 1 mm (range, 8-13 mm) and the AM bundle was 17 ± 2 mm (range, 13-19 mm), corresponding to 29% and 46% of the tibial depth, respectively. These authors were not able to define two separate anatomic ACL bundles. Instead, they defined two functional bundles by observing the tightening and slackening behavior of the ACL fibers during flexion and extension.
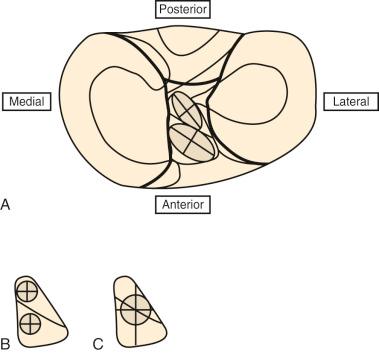
Stabuli and Rauschning reported the center of the ACL tibial attachment at 43% of the AP depth, which extended from 25% to 62% of the tibial width. These important landmarks provide a reference to measure lateral radiographs before and after ACL graft placement to avoid a posterior and vertical graft placement ( Fig. 7-13 ). Siebold and associates performed a cadaveric study in 50 knees and documented the ACL tibial insertion using a digital image analysis system and divided the ACL into AM and PL bundles. The average AP length of the ACL tibial attachment was 14 mm (range, 9-18 mm). The average male ACL tibial insertion area was 130 mm 2 versus the average female knee of 106 mm 2 . The average AP length of the ACL footprint in females was 14 mm (range, 9-18 mm) and in males, 15 mm (range, 12-18 mm). The average width of the ACL in all knees was 10 mm. The study concluded that two tibial bone tunnels cannot be placed at the anatomic centers of the AM and PL bundles because the space is too narrow and the tunnels would overlap. This highlights the difficulty of the ACL double-bundle technique in recreating the native ACL fiber attachment.
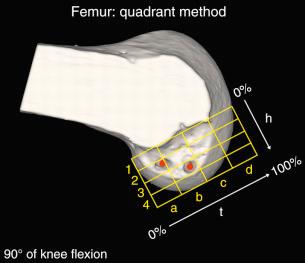
Forsythe and colleagues quantified the position of AM and PL bone tunnels in ACL reconstruction using arthroscopic dissection and computed tomography (CT) images in cadaveric knees ( Figs. 7-14 and 7-15 ). Three-dimensional models were created from the CT scans and aligned into an anatomic coordinate system. The tibial and femoral measurements were similar to those published by other studies ( Table 7-3 ).
| REFERENCE | TIBIAL MEASUREMENTS | FEMORAL MEASUREMENTS WITH QUADRANT METHOD | ||||||
|---|---|---|---|---|---|---|---|---|
| ANTERIOR TO POSTERIOR (%) | MEDIAL TO LATERAL (%) | PARALLEL TO BLUMENSAAT LINE (%) | PERPENDICULAR TO BLUMENSAAT LINE (%) | |||||
| AM | PL | AM | PL | AM | PL | AM | PL | |
| Tsukada et al. (2008) | 37.6 | 50.1 | 46.5 | 51.2 | ||||
| Forsythe et al. (2010) | 25 | 46.4 | 50.5 | 52.4 | 21.7 | 35.1 | 33.2 | 55.3 |
| Colombet et al. (2006) | 26.4 | 47.6 | 25.3 | 32.3 | ||||
| Zantop et al. (2008) | 18.5 | 29.3 | 22.3 | 53.6 | ||||
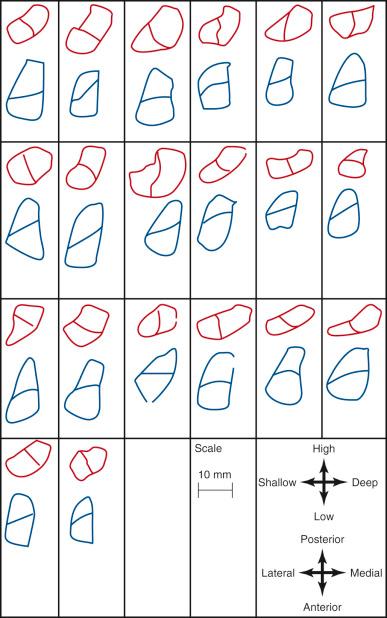
In 2012, Piefer and associates conducted a systematic review of studies that described the location of the ACL femoral footprint. Using the 20 articles selected for analysis, the authors provided general recommendations regarding the arthroscopic identification of the femoral footprint ( Table 7-4 ). Already appreciated was the ACL location, which was noted to be in the posterior third of the lateral wall, posterior to the lateral intercondylar ridge (resident's ridge). The anatomic centrum of the ACL femoral footprint was 43% of the proximal-to-distal length of the lateral femoral intercondylar notch wall and femoral socket radius plus 2.5 mm anterior to the posterior articular cartilage margin. There is a well-known anatomic variability of size and shape in the majority of knees in which the ACL fibers can be visualized ( Table 7-5 and Fig. 7-16 ).
| Reference | Bony Landmarks | Angle to Femoral Anatomic Axis (Degrees) | Distance of Centrum or Footprint to Articular Cartilage Margin |
|---|---|---|---|
| Heming et al. (2007) | NA | 28.8 | Footprint is 4 mm from posterior * |
| Purnell et al. (2008) | Lateral intercondylar resident's ridge is anterior border | 34.9 ± 3.7 | Footprint is 3.5 ± 0.9 mm from posterior * |
| Siebold et al. (2008) | AM is 3.6 ± 1.2 mm posterior to “over the top” position; distance between AM and PL centers is 7 ± 2 mm | 12 | PL centrum is 6 ± 3 mm from “shallow” (distal) articular cartilage |
| Yasuda et al. (2004) | Anatomic centrum of AM bundle is 5-6 mm distal from “back” of femur | 30 | PL centrum is 5-8 mm from posterior |
| Ferretti et al. (2007) | Lateral intercondylar ridge is anterior border; bifurcate ridge separates AM and PL bundles | NA | NA |
| Steckel et al. (2010) | Bundles are oriented more horizontally when knee is flexed past 90 degrees | 19 when knee is flexed to 90 | NA |
| Luites et al. (2007) | Centrum is 7.9 ± 1.4 mm shallow (distal) along notch roof and 4.0 ± 1.3 mm posterior to notch roof; footprint includes notch roof in 31 of 35 | NA | NA |
| Takahashi et al. (2006) | AM bundle is 4.1 ± 0.8 mm from notch roof perpendicular to Blumensaat line; PL bundle is 11.3 ± 1.8 mm from notch roof perpendicular to Blumensaat line | NA | AM bundle is 7.6 ± 1.5 mm (24.5% ± 4.7%) from posterior margin of article surface; PL bundle is 7.0 ± 1.4 mm (22.9% ± 4.7%) from posterior margin of articular surface |
| Edwards et al. (2008) | AM bundle extends to posterior proximal notch | 37 ± 16 to femoral axis | Measured parallel to femoral axis: AM centrum is 4.3 ± 1.1 mm from posterior at 10:30 o'clock position; PL centrum is 8.9 ± 2.1 mm from posterior at 10 o'clock position |
| Zantop et al. (2008) | AM bundle is 5.3 ± 0.7 mm from roof of notch | NA | AM centrum is 18.9 ± 0.5 mm from shallow cartilage margin; PL centrum is 6.5 ± 0.9 mm from shallow (distal) cartilage margin and 5.8 ± 0.6 mm from posterior cartilage margin |
| Colombet et al. (2006) | Center of AM bundle is 1.8 ± 1.3 mm from over-the-top position | NA | Footprint is 2.5 ± 1.1 mm from posterior articular cartilage * ; distal border of footprint is 2.8 ± 1.5 mm from distal margin of articular cartilage |
| Iwahashi et al. (2010) | Anterior border in line with posterior cortical border of femoral diaphysis resident's ridge | Parallel to roof of intercondylar notch | Footprint extends to posterior cartilage margin * |
| Hara et al. (2009) | Lateral intercondylar resident's ridge is anterior border | NA | NA |
* Descriptions of the distance from the posterior edge of the footprint as a whole to the posterior border of the articular cartilage.
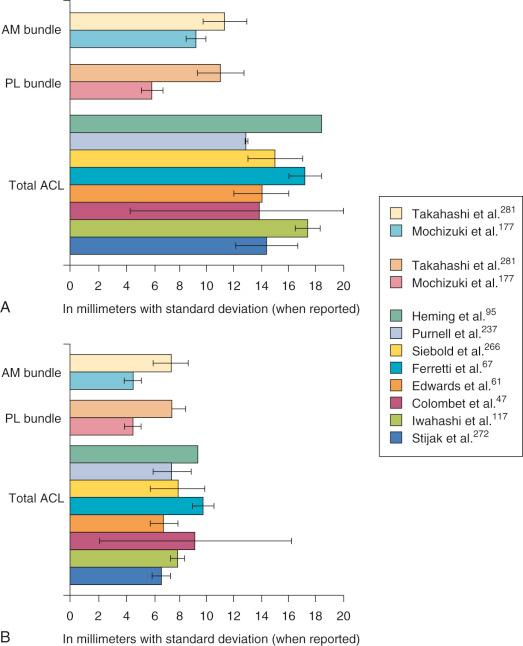
| Reference | Size (mm) | Shape |
|---|---|---|
| Heming et al. (2007) | 18.4 × 9.5 | NA |
| Purnell et al. (2008) | 12.9 ± 0.1 × 7.6 ± 1.4 | NA |
| Steckel et al. (2010) | NA | Semilunar |
| Siebold et al. (2008) | 15 ± 3 × 8 ± 2 | Long oval |
| Yasuda et al. (2004) | NA | Egg shaped |
| Ferretti et al. (2007) | 17.2 ± 1.2 × 9.9 ± 0.8 | Segment of a circle with straight anterior border and convex posterior border |
| Luites et al. (2007) | NA | Oval |
| Takahashi et al. (2006) | AM: 11.3 ± 1.6 × 7.5 ± 1.3 PL: 11 ± 1.7 × 7.6 ± 1.0 |
Elliptic |
| Edwards et al. (2008) | 14 ± 2 × 7 ± 1 | Variable |
| Mochizuki et al. (2006) | AM: 9.2 ± 0.7 × 4.7 ± 0.6 PL: 6.0 ± 0.8 × 4.7 ± 0.6 |
Oval |
| Colombet et al. (2006) | 13.9 ± 9.5 × 9.3 ± 7.1 | Variable |
| Iwahashi et al. (2010) | 17.4 ± 0.9 × 8 ± 0.5 | Oval |
| Stijak et al. (2009) | 14.4 ± 2.3 × 6.8 ± 0.7 | NA |
These data indicate that a 10-mm drill would produce a central point at the 43% proximal-to-distal line (just proximal to the midpoint). Assuming a 5-mm radius, plus a 3-mm back wall (to the articular cartilage bone margin), there would be an 8-mm point for the posterior articular cartilage margin. We prefer arthroscopic identification of the ACL femoral footprint compared with the various radiographic techniques shown in Table 7-6 . The goal is to avoid a distal ACL footprint location (thereby producing a short graft) but to place the ACL graft into the proximal two thirds of the native footprint. This means the surgeon should select a point just proximal to the 43% line. We recommended the use of standard anatomic descriptions (anterior, posterior, proximal, and distal), rather than terms such as shallow, deep, high, and low.
| Reference | Center of Anteromedial Bundle (%) | Center of Posterolateral Bundle (%) | Center of Anterior Cruciate Ligament Footprint (%) |
|---|---|---|---|
| Bernard & Hertel Method | |||
| Colombet et al. (2006) | 26.4 ± 2.6 × 25.3 ± 4.2 | 32.3 ± 3.9 × 47.6 ± 6.5 | 29.35 × 36.45 |
| Iriuchishima et al. (2010) | 15 ± 6 × 26 ± 8 | 32 ± 9 × 52 ± 5 | 23.5 × 39 |
| Forsythe et al. (2010) | 21.7 ± 2.5 × 33.2 ± 5.6 | 35.1 ± 3.5 × 55.3 ± 5.3 | 28.4 × 44.25 |
| Zantop et al. (2008) | 18.5 × 22.3 | 29.3 × 53.6 | 23.9 × 37.95 |
| Tsuda et al. (2010) | 25.9 ± 2 × 17.8 ± 2.9 | 34.8 ± 2 × 42.1 ± 3.9 | 30.35 × 29.95 |
| Pietrini et al. (2011) | 21.6 ± 5.6 × 14.2 ± 7.7 | 28.9 ± 4.6 × 42.3 ± 6 | 25.25 × 28.25 |
| Guo et al. (2009) | NA | NA | 43.1 ± 4.6 × 38.3 ± 1.3 |
| Steckel et al. (2010) | NA | NA | 26.9 ± 3.5 × 27.5 ± 3.2 |
| Mean | 21.5 × 23.1 | 32 × 48.8 | 28.5 × 35.2 |
| Mochizuki Method | |||
| Mochizuki et al. (2006) | 28.3 ± 2.1 × 28 ± 2 | 59.8 ± 4.1 × 53 ± 2 | 44.05 × 40.5 |
| Edwards Grid Method | |||
| Edwards et al. (2007) | 21 × 24 | 27 × 57 | 24 × 40.5 |
| Takahashi Method | |||
| Takahashi et al. (2006) | 31.9 × 26.9 | 39.8 × 53.2 | 35.85 × 40.05 |
Edwards and coworkers described the anatomic locations of the ACL femoral attachments of the AM and PL bundles in a companion study in 22 cadaveric knees using a measurement grid system shown in Figure 7-17 . The authors reported a wide variation in the size and shape of the ACL attachment and bundle arrangement that was selected ( Fig. 7-18 ). The femoral attachment was oriented at 37 degrees to the long axis of the femur. The authors reported that the AM bundle extended to the posterior proximal limit of the femoral notch between the 10:30 and 11:30 o'clock positions. The PL bundle was located between the 9:00 and 10:30 o'clock positions. They reported that a 6-mm graft tunnel had the best fit if the AM bundle was located at 11 o'clock, 6 mm from the posterior outlet, and the PL bundle was located at 10 o'clock, 9 mm from the posterior outlet. They noted that other studies had different recommendations for the placement of two femoral tunnels in the double-bundle technique.
Zantop and colleagues performed a cadaveric study in 20 knees that outlined the AM and PL bundle locations at the tibial and femoral sites. The authors arrived at different conclusions from those of Siebold and associates. The tibia ACL division into the AM bundle was oriented from medial lateral, occupied the anterior one half of the ACL footprint, and was aligned with the anterior horn of the lateral meniscus. The center of the PL bundle was located 11 mm posterior and 4 mm medial to the anterior insertion of the lateral meniscus. The center of the AM bundle was located at 30% and the PL bundle was located at 40% on the lateral transtibial line.
Rue and associates, in a cadaveric study, used a transtibial-drilled femoral tunnel, placed at the 10:30 position, and determined the location of a single-graft femoral tunnel in relation to the AM and PL bundles. The authors placed the guide pin for the tibial tunnel approximately 7 mm anterior to the PCL, which would represent a posterior one-third tibial attachment. They reported that the transtibial drilling of the femoral tunnel resulted in the AM bundle occupying an average of 32% (range, 3%-49%) of the ACL attachment and the PL bundle occupying an average of 26% (range, 7%-41%) of the ACL attachment. In addition, the remainder of the area of the 10-mm tunnel did not overlap the ACL footprint. The wide ranges reported in this study for the location of the femoral tunnel in terms of the native ACL femoral attachment highlight the problems of using the tibial tunnel to drill the femoral tunnel.
Heming and colleagues reported the ACL tibial and femoral footprints in 12 cadaveric knees ( Fig. 7-19 ). Note in Figure 7-19, C , the orientation of the clock face places the 9 to 3 o'clock horizontal line at the base of the femoral condyles and not within the center of the notch, which is typically used by other authors. A guide pin placed between the ACL anatomic femoral and tibial attachment centers (as in a transtibial drilling technique) was only possible if the tibial tunnel started in a medial position close to the joint line that would be too short to be functional for ACL grafts. The study concluded that transtibial drilling techniques resulted in a more vertical graft orientation.
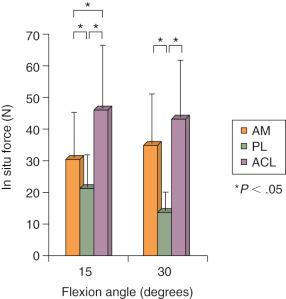
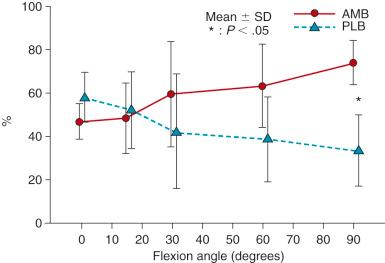
Biomechanical studies have reported marked differences in the function of the AM and PL bundles. Sakane and coworkers, in one of the first cadaveric robotic studies, reported the PL bundle provided the primary restraint to anterior tibial translation at low flexion angles. Increasing forces were reported in the AM bundle with knee flexion; these remained lower than the calculated in situ forces of the PL bundle. However, subsequent studies have shown an increased role of the AM bundle and decreased role of the PL bundle in limiting anterior tibial translation.
The role of the ACL bundles in restraining rotational motions was examined by Zantop and associates in a robotic study. A 134-N anterior tibial load or a combined 10 N-m valgus and 4 N-m internal tibial torque were applied to cadaveric knees. Sectioning the PL bundle at 30 degrees of flexion resulted in an approximate 7-mm increase in anterior tibial translation, whereas sectioning the AM bundle produced at 60 degrees an approximate 9-mm increase in anterior translation. Similar data were reported at low flexion angles for the combined rotational loads. These data imply a near-absence of function of the remaining “intact” bundle that is not supported by other studies. For example, Markolf and colleagues, in a cadaveric study, reported that cutting the PL bundle resulted in an increase of only 1.1 mm at 10 degrees of flexion and 0.5 mm at 30 degrees of flexion. These authors questioned the need to reconstruct the PL bundle in ACL reconstructions.
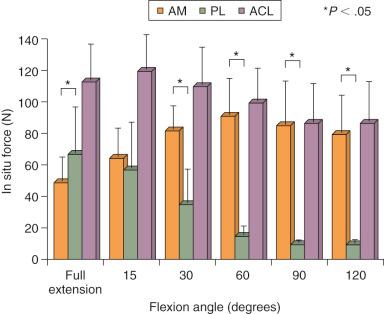
Gabriel and coworkers, in a robotic cadaveric experiment, removed all of the soft tissues to produce a femur-ACL-tibia specimen. For anterior tibial loading ( Fig. 7-20 ), the AM bundle was reported to be nearly equal to the PL bundle at 15 degrees of flexion, with increasing AM forces with knee flexion. Under the rotation loads of valgus and internal tibial rotation, the forces in the AM bundle exceeded those of the PL bundle ( Fig. 7-21 ).
Li and associates used MRI and fluoroscopic images of subjects performing a lunge to create 3-dimensional models in which the AM and PL attachments were outlined. The study reported that the AM and PL bundles reached their maximum elongation between full extension and 30 degrees of flexion and did not demonstrate a reciprocal behavior with knee flexion-extension.
Giron and colleagues, in a cadaveric experiment, determined that a “deep” (proximal) femoral tunnel position could be achieved with either one of three techniques (double incision, transtibial, or AM portal). However, Arnold and coworkers showed in cadaveric experiments that the ACL is entirely attached to the lateral femoral wall and that this attachment position was not able to be accessed through a transtibial tunnel (40% AP tibial tunnel measurement). The transtibial tunnel method resulted in the guide pin and drill hole placed at the junction of the proximal ACL attachment and femoral roof ( Fig. 7-22 ).
Mae and associates, in a cadaveric knee study, used a robotic simulator to study the effect of single- versus two-femoral tunnel ACL graft reconstruction. A single tibial tunnel was placed in the center of the ACL tibial attachment. The locations of the single and two tunnels were described at the 1 o'clock and 2:30 positions. The data for AP translation and in situ graft forces at 44-N graft pretension showed little difference between the single- and double-bundle grafts, both of which produced a mild to moderate overconstraint that limited anterior tibial translation. The study reported on the load-sharing between the normal ACL bundles ( Fig. 7-23 ), which demonstrated the PL bundle functioned more at low flexion angles, equal to the AM at 10 degrees of flexion, with a further increase in AM bundle function with increasing knee flexion. A study of load-sharing between the two bundles of a simulated pivot shift type of loading was not performed.
Yagi and colleagues compared single- and double-bundle ACL hamstring reconstructions. A double-looped single-bundle hamstring graft was placed at approximately the 11 o'clock position and tensioned to 44 N, whereas each bundle of the double-bundle graft was tensioned to 44 N. The double-bundle graft had a total of 88 N of tension versus 44 N for the single-graft reconstruction. In response to a 134-N anterior load at 30 degrees of flexion, the data showed an unexplained residual anterior tibial translation (intact, 6.4 mm ± 2.4 mm: single bundle, 10.2 ± 2.5 mm), which would not be anticipated from a single graft in terms of resisting translation. The finding of a lax single graft most likely explains the reported decreased ability of the single graft to resist the combined rotation-translation motions.
Petersen and coworkers concluded that the location of the ACL double-bundle grafts in clinical studies varied markedly at both the femoral and tibial sites. In a cadaveric study, Petersen et al. found that a two-tunnel tibial technique provided an advantage in resisting combined rotatory loads, simulating the pivot shift (anterior tibial translation, 7.5 mm versus 9.5 mm, 30 degrees of flexion, 5 N-m internal rotation, 10 N-m valgus torque). Of note, the single tibial tunnel and graft were placed 7 to 8 mm anterior to the PCL; the authors stated that this was a position recommended by some surgeons. As previously discussed, the posterior tibial tunnel position results in a more vertical ACL graft, with portions of the graft possibly even posterior to the native ACL tibial attachment. The addition of a second graft placed in a more anterior tibial position within the ACL footprint would be expected to provide better resistance to the loading conditions. Although the data do not provide evidence for a double-bundle ACL procedure, it does provide evidence to avoid placement of a single ACL graft in the posterior one third of the ACL tibial attachment.
Yamamoto and associates conducted a cadaveric robotic study that compared a single ACL graft placed in the 10 o'clock position on the lateral wall with an anatomic double-bundle reconstruction. This is one of the few published studies in which a single-graft lateral wall placement was used, avoiding a more proximal graft placement. Similar loading conditions were used as in the other robotic studies (anterior tibial load 134 N, combined rotatory load 10 N-m valgus and 5 N-m internal tibial torque). There was no statistical difference in the anterior tibial translation or in situ force at 30 degrees of knee flexion between the intact ACL, the single bundle, or the anatomic double-bundle reconstructions. Under the combined rotatory loading conditions, there were no differences in anterior tibial translation, internal tibial rotation, and ACL in situ graft forces, which is in direct contrast to other robotic experiments.
Markolf and colleagues measured in cadaver knees a simulated pivot shift in the intact knee and then after single-bundle and double-bundle ACL reconstruction. The single-bundle reconstruction (placed at the anatomic AM bundle site) restored mean tibial rotations and lateral plateau displacements to levels similar to those of the intact knee, whereas the double-bundle reconstructions reduced coupled rotations and displacements to levels less than those in the intact knee. The authors concluded that the overconstraint induced by the double-bundle reconstructions has unknown clinical consequences and that the need for the added complexity of this procedure is questionable.
Cuomo and coworkers reported on the effects of tensioning single- and double-bundle ACL reconstructions in cadaveric knees instrumented with a six-DOF system under defined loading conditions (anterior translation, 90 N; 5 N-m internal rotation torque). Different flexion positions were used for tensioning each of the grafts in the double-bundle construct. The single graft was tensioned at 20 degrees of knee flexion. All grafts had sufficient tension applied to restore normal intact knee translation. Tensioning each of the grafts individually (AM bundle at 90 degrees, PL bundle at 20 degrees, varying which was tensioned first) resulted in overconstraining AP translation. In contrast, tensioning both the AM and PL grafts simultaneously at 20 degrees provided the best match for the intact knee AP translation throughout knee flexion. The data show the difficulty in tensioning two ACL grafts at the time of surgery in terms of matching the intact ACL load-sharing among fibers. With anterior loading, both the AM and PL fibers participate in load-sharing, but at different percentages as already discussed. For single-bundle ACL grafts placed within the center of the femoral and tibial attachments, higher overall graft tension occurred under anterior loading compared with the two graft bundles tensioned at 20 degrees of flexion. Importantly, the data show that tensioning of the AM and PL grafts under low tensions (20 N) provided the best results. The single graft required 38 ± 27 N to restore the native knee laxity.
Scopp and associates, in cadaveric experiments, reported that a more oblique femoral tunnel placement (60 degrees from vertical) was more effective in resisting internal rotational torques (difference 4.4 degrees, 6.5 N-m) than a “standard” tunnel placement (30 degrees from vertical). The femoral tunnel was drilled using a transtibial technique and, most likely, a posterior tibial tunnel placement. Anterior tibial translation values were not restored to normal (increased 2.5 mm and 2.2 mm, standard and oblique femoral tunnels).
Simmons and colleagues, in cadaver experiments, showed the deleterious effects of a more vertical tibial tunnel (70 degrees and 80 degrees) with impingement against the PCL and higher graft tension. A more oblique tibial tunnel and femoral tunnel in the 60-degree coronal plane did not impinge against the PCL. The study recommended a posterior tibial tunnel placement and more proximal ACL femoral attachment with use of a transtibial drilling technique, again showing from a historical perspective the studies in the literature in which a more vertical graft orientation results.
Arnold and coworkers showed in cadavers that the passive ACL tension flexion-extension tension curve was not reproduced by femoral tunnels placed at the 10 and 11 o'clock positions, but was reproduced by grafts in the 9 o'clock positioned tunnels. However, a less ideal posterior ACL tibial tunnel was used in this experiment.
In summary, a majority of in vitro robotic studies compare a double-bundle graft construct with a hybrid proximal femoral and posterior tibial orientation of a more vertical single-bundle graft construct and not a centrally placed femoral and tibial graft construct. Therefore, the published data apply specifically to this type of single-graft construct and show as expected that a vertical single graft is not positioned to resist rotational loading. It may be concluded that there is sufficient experimental data to recommend this hybrid graft orientation be avoided in clinical practice.
There are incomplete in vitro data on the comparison of an anatomic single-graft construct (within central tibial and femoral tunnels) with a double-bundle construct. There are also incomplete experimental data on the function of individual regions of ACL fibers in resisting coupled motions as occurs in the pivot shift phenomena, which is an important area for future study ( Fig. 7-24 ). Other biomechanical studies reviewed show that a single ACL graft placed within the anatomic femoral and tibial attachments (avoiding a high femoral and posterior tibial position) restore rotatory stability and question the need for a double-bundle procedure.
One theoretical advantage of a double-graft construct tensioned appropriately is that there is initially less graft tension in each of the graft strands compared with the overall tension in a single graft. Stated differently, a single graft will always exhibit much higher graft tension to resist anterior loading than two graft arms in which load-sharing occurs. There is also the theoretical advantage of tensioning the two graft strands at different knee flexion positions to exhibit a different percent sharing of the overall tension than a single graft. From an experimental standpoint, either a single-graft or double-graft construct can be tensioned to restore normal motion limits; however, this may occur at the expense of high graft tensions, particularly in a single-graft construct. Thus, the advantage of a double-graft construct (either ACL or PCL) is to restore normal knee motion limits under the lowest graft tensile loads, which is advantageous during graft healing and remodeling. A graft under lower loads has the theoretical advantage of less risk in stretching out with return of the abnormal motion limits. In addition, an ACL or PCL graft under higher tension, under cyclic loading conditions, is at high risk of graft stretching and failure. Again, it should be noted that either one or two ACL grafts does not restore native ACL fiber length-tension properties.
We conducted a series of robotic cadaveric in vitro studies on the kinematic function of the AM and PL bundles of the ACL. The studies involved a six-DOF robotic testing protocol on intact knees, knees in which either the AM or PL bundle was sectioned, and knees in which complete ACL sectioning was performed. As previously described, the robotic testing protocol involved, for the first time, the simulation of the pivot shift event using four DOF; namely, anterior tibial translation with internal tibial rotation with valgus loading of the limb during knee flexion-extension to induce maximum anterior tibial subluxation. The studies also involved, for the first time, digitization of the tibial plateau to determine medial and lateral tibiofemoral compartment translations and centers of tibial rotation in the simulated pivot shift test.
The results showed both ACL bundles functioned synergistically to resist medial and lateral compartment subluxations during the simulated Lachman and pivot shift tests. The AM bundle provided more restraint to anterior tibial translation under both tests than the PL bundle. Both ACL bundles had to be sectioned to produce tibiofemoral compartment subluxations during pivot shift loading ( Figs. 7-25, 7-26, and 7-27 ). Neither bundle contributed to resisting internal rotation during pivot shift loading tests. We concluded that an ACL graft designed to duplicate the AM bundle restores medial and lateral compartment translations under multiple loading conditions; however, the PL bundle only provides a back-up restraint at low flexion angles. Neither ACL bundle resists internal tibial rotation or, in its absence, allows a positive pivot shift subluxation. Furthermore, the PL bundle is not the primary restraint for providing rotational knee stability, as has been quoted in the literature.
We conducted a robotic cadaveric in vitro study that found that a single ACL graft placed into the anatomic center of the femoral and tibial attachment sites restored normal tibiofemoral compartment translations and rotations under simulated Lachman and pivot shift testing conditions. The study involved a six-DOF robotic testing protocol on intact knees, ACL-sectioned knees, and ACL-reconstructed knees. The reconstruction restored rotational stability as defined by normal motion limits and normal medial and lateral compartment translations under simulated pivot shift loading conditions ( Fig. 7-28 ).
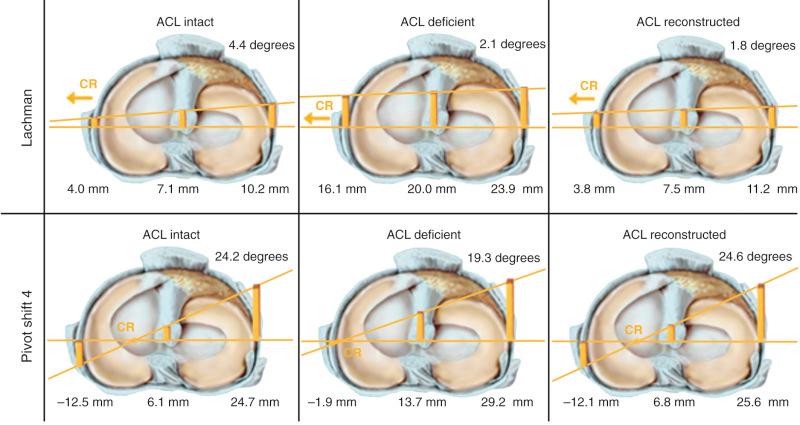
The results of this study support the recommendations in this chapter on the use of a single ACL graft instead of a double-bundle ACL graft construct. This finding is supported by Markolf and associates who studied the effect of single- and double-bundle ACL grafts in cadaveric knees. As described in this chapter, prior robotic pivot shift studies on double-bundle ACL reconstructions used loading profiles that did not involve a coupled anterior tibial translation and internal rotation to induce maximum anterior tibial subluxations, and, in addition, did not measure resulting tibiofemoral compartment translations or subluxations. We question the validity of these studies in terms of the conclusions that a single ACL graft does not restore knee rotational stability or that a double-bundle ACL construct is required.
The assessment of ACL graft function must take into account the restoration of the normal coupled motion limits of anterior tibial translation and internal tibial rotation during the pivot shift phenomenon, which strongly correlates with patient giving-way symptoms. During KT-2000 (MEDmetric) testing, only anterior tibial translation is assessed. If the knee joint has a 3-mm or lower increase in central anterior tibial translation over the opposite knee, it can be assumed that there is no positive pivot shift because this amount of constraint to anterior tibial translation also limits anterior subluxation of the lateral tibial plateau. Conversely, if a greater than 5-mm increased anterior tibial translation exists, there may occur a positive pivot shift test and patient complaints of giving-way. The problem is in knees that demonstrate 3 to 5 mm of increased anterior tibial translation, which may represent 20% to 30% of patients in clinical investigations, especially when allografts are used. This mild to moderate increase in anterior tibial translation results in a mildly positive Lachman test with a hard endpoint. However, these knees may demonstrate a positive pivot shift and giving-way symptoms. Since the pivot shift test is highly subjective and variable among examiners (see Chapter 3 ), an author may report a successful result based on the KT-2000 (anterior tibial translation) or a pivot shift test, whereas another examiner may grade the knee as a failure, based on the method in which the pivot shift test is performed. There is a pressing need for clinical examination methods that simulate the pivot shift phenomenon and the resultant subluxations (in millimeters) of the medial and lateral tibiofemoral compartments.
Bull and associates were among the first authors to report intraoperative measurement of tibial translations and rotations using a 3-dimensional motion analysis system. In the operative setting, there was a marked variability in knees in the amount of medial and lateral tibial plateau displacements during the pivot shift test. Robinson and colleagues performed an ACL double-bundle reconstruction using computerized navigation techniques in 22 patients. The exact location of ACL grafts and the loads applied by the examiner are variables still to be determined.
In a frequently quoted study, Tashman and coworkers devised a unique methodology of dynamic in vivo measurements of patients who had undergone ACL reconstruction. The investigators used a biplanar radiographic system to measure tibiofemoral displacements while the patients ran downhill on a treadmill. The reconstructed knees showed an increase in mean values of external tibial rotation (3.8 ± 2.3 degrees) and adduction (2.8 ± 1.6 degrees). The effect of these small differences from a clinical standpoint is unknown and future studies are required that involve more dynamic rotational movements in comparison to straight-ahead running to measure the rotational kinematics of the knee joint. The specific ACL graft femoral and tibial tunnel placement was not identified in this study.
Ristanis and associates assessed 11 patients who had undergone B-PT-B reconstruction using kinematic data from a six-camera optoelectronic system obtained during a jump landing and pivoting maneuver. The ACL reconstruction did not restore tibial rotation to normal values of the opposite uninvolved extremity or controls. The femoral tunnel was placed through an AM approach with the knee joint flexed to 120 degrees to achieve a 10 to 11 o'clock lateral wall position. The tibial tunnel was placed into the center of the ACL footprint. The authors noted that a limitation of the study was the movement of skin markers that may not have accurately represented true tibiofemoral joint translations.
Monaco and colleagues evaluated the effect, on internal tibial rotation, of a single-bundle ACL reconstruction combined with an extraarticular procedure compared with a double-bundle ACL reconstruction. Computerized navigation instrumentation was used in the operating room before and after the ACL procedures. The addition of the PL bundle after the AM bundle did not have an effect in reducing AP translation or the limit of internal tibial rotation, and there were no significant differences between the single- and double-bundle reconstructions. The extraarticular reconstruction significantly reduced the internal tibial rotation limit, as would be expected. The tibial placement of the graft was performed with the guide pin 7 mm anterior to the PCL insertion into the posterior ACL attachment. The authors did not determine the effect of either graft configuration on the coupled motions of the pivot shift test but only on the limit of internal tibial rotation alone.
Our conclusions of single-graft versus double-bundle graft ACL studies are summarized in Table 7-7 . The hypothesis presented is that an ideally placed single-graft construct (central femoral and tibial ACL attachments, avoiding a vertical graft orientation) provides control of the abnormal pivot shift motions, avoiding the necessity and added complexity of a double-bundle graft reconstruction.
|
|
|
|
|
|
|
|
|
Given the variation in ACL anatomic shapes among specimens, it is important during surgery to outline the individual size and shape of the ACL attachment for each knee. This can be done in a primary ACL reconstruction, but usually not in revisions. The cadaveric studies provide important anatomic reference landmarks. Because of the variation in ACL attachment shapes, it would be expected that there would exist variation among surgeons on the locations chosen for both single- and double-bundle ACL graft locations. Newer computer navigation techniques have been studied to aid in the placement of ACL grafts; however, it is still unknown at present which anatomic points to select for graft tunnels that would correlate with clinical stability results.
The important landmarks for the ACL tibial attachments are the medial tibial spine, RER of the proximal PCL fossa, and the attachment of the lateral meniscus. The PCL is a poor soft tissue landmark for the posterior extent of the native ACL attachment. It should be noted that some authors have advocated a guide pin and tibial tunnel placement 6 to 8 mm from the PCL, which would place the tibial tunnel in the posterior portion of the ACL. In some knees, the tibial tunnel would be posterior to the native ACL attachment and just a few millimeters from the RER or interspinous ridge. This posterior tibial tunnel produces a near-vertical ACL graft that would not be expected to resist rotational loads in obliterating the pivot shift phenomenon, as already discussed.
The ACL tibial attachment location we recommend for a single graft is directly adjacent to the lateral meniscus anterior horn attachment as shown in Figure 7-29 . The ACL attachment can be easily mapped at surgery based on the anatomic references provided. In some knees, the anterior extent of the ACL attachment may be obscured by soft tissues, and in these cases, the RER or posterior interspinous ridge of the PCL fossa is an important landmark. The center of the ACL will be 16 to 20 mm anterior to the RER. The guide pin is most ideally placed eccentric and 2 to 3 mm anterior and medial to the true ACL center, because the ACL graft displaces to the posterior and lateral aspect of the tibial tunnel. The tunnel places the majority of the graft within the central tibial attachment and avoids the posterior attachment location. It is important that graft impingement against the anterior intercondylar notch does not occur because the circular graft may occupy a portion of the native flattened ACL tibial attachment. An anterior notchplasty is required, particularly in knees with an A -shaped notch. In many ACL revision knees, the bony ridge posterior to the ACL attachment (“no-man's land,” Fig. 7-30 ) has been disrupted with a prior graft tunnel that extends 1 to 2 mm from the RER and requires a bone graft before the revision procedure. To avoid a vertical graft orientation, it is important that during the ACL tibial tunnel preparation (primary or revision surgery), the tibial drill does not inadvertently penetrate into or beyond the posterior one-third ACL attachment and adjacent RER.
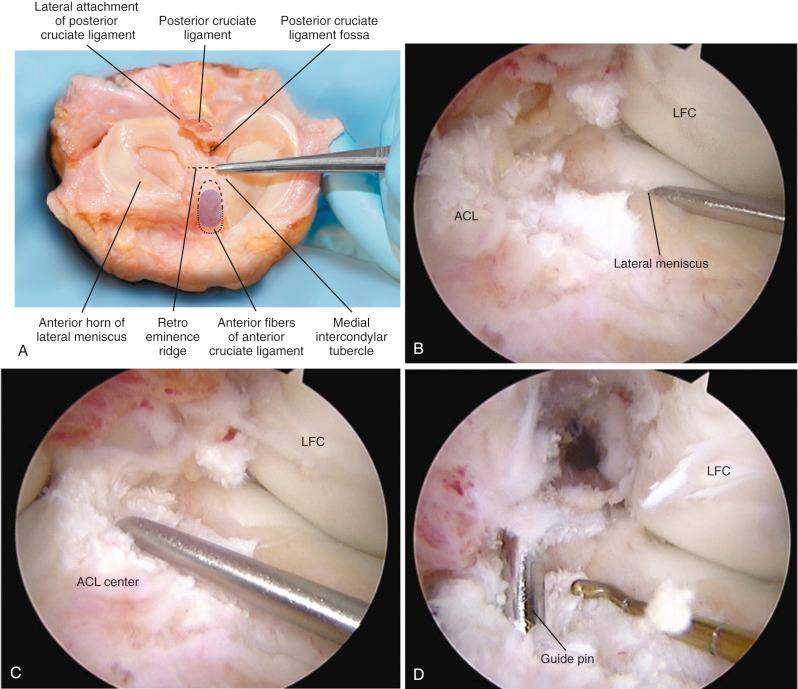
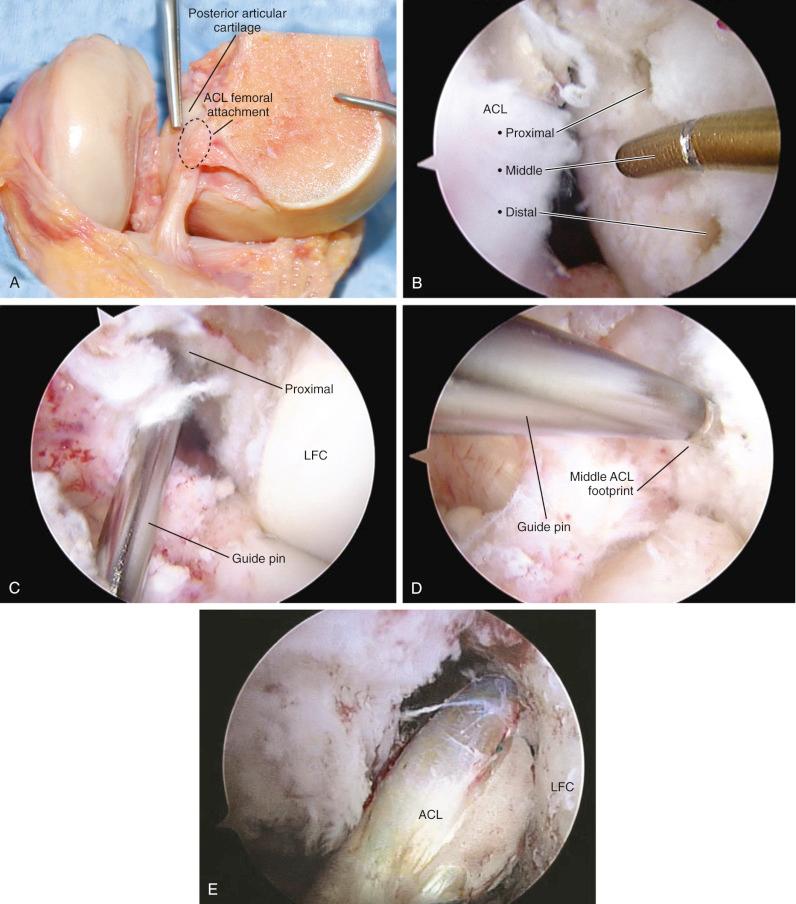
Important landmarks for the femoral attachment are the posterior articular cartilage, the Blumenstaat line, and identification of the ACL attachment on the lateral femoral wall of the notch (in the regions outlined on the grid systems). The goal is to locate the tunnel in the central to proximal thirds to maintain ACL graft length, and within the central direct ACL native insertion that occurs through fibrocartilage and not within the posterior indirect insertion of fibers adjacent to the femoral articular cartilage edge. A tunnel distance (posterior or deep) from the femoral condyle articular cartilage of 3 mm prevents an excessive posterior graft placement that would be slightly lax with increasing knee flexion (flexion laxity). Again, the emphasis is made that no ACL fibers extend to the intercondylar roof; all of the fibers are on the lateral wall. Tsukada and coworkers reported that the osseous ridge on the lateral femoral wall of the intercondylar notch (resident's ridge) had major positional and dimensional variations and should not be used as a reference for femoral tunnel creation.
Bird and associates reported in a cadaveric study that, with the knee at 90 degrees of flexion, the central location of the ACL attachment was 50% of the AP depth of the femoral condyle (junction articular cartilage) along the lateral bifurcate ridge. The possible error in this technique is the distance that the central point is measured along the bifurcate ridge to avoid a tunnel adjacent to the femoral articular cartilage. Piefer and colleagues, in a systematic review of 20 ACL femoral footprint publication, noted differences in techniques among studies; however, these investigators arrived at recommended arthroscopic osseous landmarks on the lateral wall of the intercondylar notch ( Fig. 7-31 ) which are very useful for the arthroscopic surgeon. These include, when possible, identification of the native ACL attachment, the resident's ridge or intercondylar ridge, the bifurcate ridge, the notch roof where no ACL fibers attach, and the articular cartilage junction of the lateral femoral condyle. We first place the knee at 30 degrees (see Fig. 7-30 ), making sure the radius of the tunnel plus 3 mm is followed. For example, a 10 mm drill hole would have a radius of 5 mm plus 3 mm, or 8 mm from the articular cartilage. We measure tunnel placement at both 30 degrees and 90 degrees of knee flexion and each method is different in terms of measurements as discussed. The question regarding the ideal femoral graft placement at time zero (for example, proximal two thirds, central two thirds) and its effects on subsequent biologic remodeling cannot be answered and still requires more kinematic and clinical studies. It is our impression based on current knowledge that there would be no difference between these two femoral placements that would affect final clinical results. With femoral tunnel placement try to ensure the placement is not too anterior or posterior or distal in the femoral footprint.
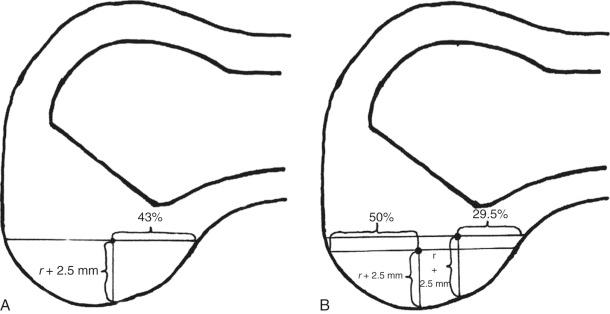
Hensler and associates conducted a study on the ACL femoral tunnel morphology induced by use of an AM portal drilling technique with knee hyperflexion. From a review of the literature, the authors determined that the average femoral ACL insertion size was 8.9 mm wide, 16.3 mm long, and 136 mm 2 in area. The femoral tunnel aperture was influenced by drill-bit diameter, transverse drill angle, and knee flexion angle ( Fig. 7-32 ). The femoral tunnel aperture best matched the native ACL footprint at 102 degrees of knee flexion, whereas at 130 degrees of flexion, a mismatch in area of 13.5% was calculated ( Fig. 7-33 ). Even so, the higher knee flexion angle of 115 degrees to 120 degrees lessens the chance of a posterior femoral wall blow-out. This study shows how the AM accessory portal drilling of the femoral ACL tunnel requires clinical experience and precise guide pin placement. The surgeon must determine that the exiting guide pin position is in the midlateral plane, above the femoral epicondyle, and maintain a 3- to 4-mm osseous posterior rim from the tunnel wall to the femoral articular cartilage. An angled flexible guide pin and drill are useful in larger patients when the AM portal drilling technique is selected because it is difficult to achieve 120 degrees of knee hyperflexion.
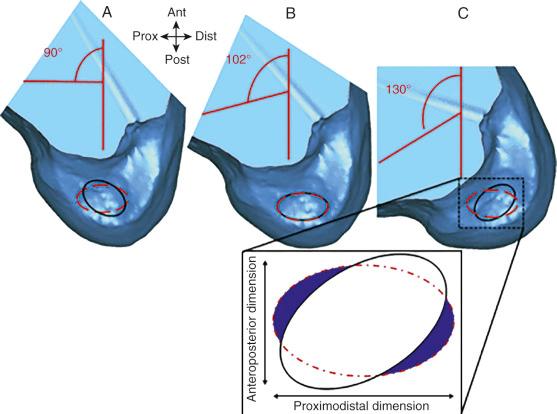
For single grafts, we recommend a central anatomic ACL placement with the femoral guide pin 2 to 3 mm above the midpoint of the proximal-to-distal length of the ACL attachment (30 degrees of knee flexion) and 8 mm from the posterior articular cartilage edge (see Fig. 7-30 ). This will produce a 10-mm tunnel, leaving a 3-mm thick posterior tunnel wall. The senior author prefers to define the ACL attachment at 20 degrees to 30 degrees of flexion with the arthroscope in the AM portal. After the femoral site is marked, the knee can be placed in 120 degrees of flexion if an AM portal arthroscopic drilling technique is selected. A 9- to 10-mm diameter tunnel occupies the central ACL attachment, leaving only the most proximal and distal few millimeters of the attachment not occupied by a graft. It is important that the ACL femoral tunnel not be placed too far posteriorly because this produces excessive graft tension with knee extension. In addition, the graft should not be too distal at its femoral attachment because this shortens the intraarticular graft tibiofemoral length. The location for a single-tunnel ACL reconstruction is more distal than recommendations for a 1 o'clock tunnel that replaces only the proximal portion of the ACL femoral attachment. However, it has not been determined in clinical studies if a difference exists between the 1 o'clock and central femoral graft placement sites.
In Figure 7-34, A , the hybrid graft position of a proximal femoral and posterior tibial position is shown, which we do not recommend. The preferred central anatomic tibial and femoral position is presented in Figure 7-34, B , and the femoral position is presented in Figure 7-34, C . In our opinion, it would not be possible from a clinical standpoint to examine the difference in knee stability (pivot shift and Lachman tests and subjective symptoms) between a central femoral ACL graft location (that occupies the central two thirds, single graft) and a proximal femoral ACL graft location (that occupies the proximal two thirds, single graft). Both graft locations are described as anatomic and avoid placing the graft in the distal two thirds that would represent a shortened ACL construct. A recent study suggested that a central femoral anatomic placement, achieved using an AM approach, was associated with a higher failure rate compared with grafts placed through a transtibial approach (5.16% and 3.20%, respectively). However, this study only used revision surgery in the calculation of failure and patients were followed only for a mean period of 22 months postoperatively.
The process of biologic healing of ACL grafts is discussed in detail in Chapter 5 . The potential use of MRI to document healing of the collagenous portion of the graft, the osseous tunnels, and the bone-ligament insertion sites after surgery is well known. However, standard MRI techniques that use echo times longer than a few milliseconds acquire very low signal from the tendon graft, which is subsequently viewed through negative contrast with surrounding tissues. In addition, fixation materials cause local signal loss that results in low contrast between the graft and hardware.
In an effort to improve these problems, contrast-enhanced MRI was used to evaluate ACL grafts serially over the first postoperative year by Muramatsu and colleagues and Ntoulia and associates. In 2008, Muramatsu and colleagues compared the signal/noise quotient (SNQ) of 20 ACL B-PT-B autografts with 24 fresh-frozen B-PT-B allografts at 1, 4, 6, and 12 months postoperatively. The center of the intraarticular portion of the ACL graft was evaluated before and after contrast enhancement. The authors reported statistically significant differences at all time periods, with the autografts demonstrating higher SNQ values. They did not provide any clinical data in regard to knee stability or sports activities resumed postoperatively.
In 2011, Ntoulia and associates described the use of contrast-enhanced MRI to study changes in signal intensity in three distinct graft sites over time. The sites were (1) the intraarticular portion of the graft, (2) the site of the graft location inside the osseous tunnels, and (3) site of the graft that was adjacent to the fixation material inside the tunnels. The cohort included 32 males who underwent ACL B-PT-B reconstruction and MRI evaluations at the third postoperative day and 6 and 12 months postoperatively. All patients had a clinically stable knee postoperatively (negative Lachman and pivot shift tests) and resumed their preinjury sports activity level by 12 months. Distinct signal changes were noted between 3 days postoperative and 6 and 12 months later. The intraarticular site was the first and fastest to achieve satisfactory contrast medium uptake, which was noted at the 6-month test with no further alteration found at the 12-month test. The other sites showed a slower progression of remodeling that was still in progress at the 12-month test.
Other studies have more recently advocated the use of ultrashort echo-time (UTE) MRI techniques to study ACL grafts after implantation. Three-dimensional UTE techniques allow visualization of short-T 2 components in tendons, ligaments, and menisci that provide superior contrast between ACL grafts and fixation material. At the time of writing, only one in vivo study documented the use of 3-dimensional UTE in three asymptomatic men who had undergone semitendinosus-gracilis (STG) autograft reconstruction 3 to 8 years previously. The tests demonstrated a high graft signal that allowed visualization of the graft's internal structure (allowing homogeneity of the graft to be judged) and superior depiction of the fixation material. The authors commented that the addition of contrast agents may even further enhance the image contrast.
In 2014, Biercevicz and colleagues further improved UTE MRI techniques by using high-resolution 3-dimensional images with no slice gap, volume and relaxation time (instead of signal intensity), and T 2 mapping in a porcine model. The authors reported that a multiple regression model based on ligament volume and its corresponding T 2 values significantly predicted the ligament's maximum load, yield load, and linear stiffness (that were determined with tensile testing) after 52 weeks of healing ( R 2 = 0.92, 0.82, 0.33, respectively; P < .001). The T 2 relaxation time was believed to represent an MRI parameter that would be applicable across scanners of the same strength and among institutions. Further studies are required to evaluate the efficacy of using this method to predict structural ACL properties in vivo for longitudinal analysis.
In 2015, Ge and associates used MRI to obtain 3-dimensional dual-echo steady-state images that were imported into solid modeling software that created 3-dimensional model reconstructions of bone tunnels in 36 patients. Eighteen patients had autograft and 18 had allograft ACL reconstructions and all were at least 2 years postoperative at the time of the study. The study found SNQs of the allograft group were consistently higher than those in the autograft group in the tibial tunnel. The authors concluded that the allograft tendons may have inferior remodeling in the bone tunnel; however, all knees had bone tunnel enlargement at the femoral aperture most likely because of the biomechanical effect of graft motion (sagittal and longitudinal graft-tunnel motion).
Perform thorough history.
Comprehensive physical examination assesses knee flexion and extension, patellofemoral indices, tibiofemoral crepitus, tibiofemoral joint line pain, muscle strength, gait abnormalities.
Perform all stability tests to document abnormal tibial translation, rotations, and tibiofemoral compartment translation.
Knee arthrometer test: 134 N.
Radiographs: standing anteroposterior at 0 degree, lateral at 30 degrees; weight-bearing posteroanterior at 45 degrees; patellofemoral axial views; double-stance hip-knee-ankle alignment; lateral and posterior stress views as indicated.
MRI, including articular cartilage image techniques.
Cincinnati Knee Rating System for subjective, objective, functional scoring.
A thorough history is performed, including a detailed account of all knee injuries and the operative procedures that have been performed. A comprehensive physical examination involving assessment of knee flexion and extension, patellofemoral indices, tibiofemoral crepitus, tibiofemoral joint line pain, muscle strength, and gait abnormalities is also completed. The surgeon must determine all of the abnormal translations and rotations in the knee joint. The medial posterior tibiofemoral step-off on the posterior drawer test is done at 90 degrees of flexion. Quantification of posterior tibial translation may be performed using stress radiography in knees with PCL ruptures to determine if the rupture is partial or complete (see Chapter 16 ). The appropriate tests to determine the integrity of the ACL are performed, including KT-2000 arthrometer testing at 20 degrees of flexion (134-N force) to quantify total AP displacement. The pivot shift test is recorded on a scale of 0 to III, with a grade of 0 indicating no pivot shift; grade I, a slip or glide; grade II, a jerk with gross subluxation or clunk; and grade III, gross subluxation with impingement of the posterior aspect of the lateral side of the tibial plateau against the femoral condyle.
Insufficiency of the PLS and medial ligament structures are determined by varus and valgus stress testing at 0 and 30 degrees of knee flexion. The surgeon estimates the amount of joint opening (in millimeters) between the initial closed contact position of each tibiofemoral compartment, performed in a constrained manner avoiding internal or external tibial rotation, to the maximal opened position. The result is recorded according to the increase in the tibiofemoral compartment of the affected knee compared with that of the opposite normal knee. Abnormal medial or lateral joint opening may be measured with stress radiographs. The tibiofemoral rotation dial test at 30 and 90 degrees detects increases in external tibial rotation with posterior subluxation of the lateral tibial plateau or anterior subluxation of the medial tibial plateau because of medial ligament injury. A word of caution is necessary; it is possible to confuse increased external tibial rotation for a PL injury and miss the medial side ligament injury that requires correction. The presence of a varus recurvatum in both the supine and standing positions is carefully assessed. Gait analysis is performed to detect a varus or valgus thrust or hyperextension thrust. Patients can often demonstrate their knee instability on standing, with a few degrees of knee flexion, by producing an external femoral rotation, which reproduces the pivot shift phenomena. Abnormal varus or valgus tibiofemoral joint opening may also be demonstrated by the patient.
Radiographs taken during the initial examination include standing AP at 0 degrees, lateral at 30 degrees of knee flexion, weight-bearing physical activity (PA) at 45 degrees of knee flexion, and patellofemoral axial views. Double-stance full-standing radiographs of both lower extremities, from the femoral heads to the ankle joints, are obtained in knees in which varus or valgus lower extremity alignment is detected on clinical examination. The mechanical axis and weight-bearing line are measured as discussed in Chapter 26 . MRI is performed to provide further details of the condition of the articular cartilage and menisci. Fast-spin-echo techniques and 3-Telsa articular cartilage T 2 mapping are available when deemed necessary to obtain superior-quality articular cartilage images.
At our center, patients complete questionnaires and are interviewed for the assessment of symptoms, functional limitations, sports and occupational activity levels, and patient perception of the overall knee condition according to the Cincinnati Knee Rating System (see Chapter 41 ). The forms are available online for the patient to obtain before their consultation.
Obtain full knee motion, muscle reeducation, and return of function before surgery.
Address patient expectation and goals of surgery.
Determine need for concomitant procedures, extraarticular procedures, and other ligament reconstructions to correct all instabilities.
All abnormalities or potential problems are addressed preoperatively, including patient expectation issues, muscular weakness, painful neuromas, residual CRPS, and anterior knee pain caused by patellofemoral cartilage damage. A loss of full knee extension creates problems. It is important to obtain full knee motion before surgery. The exception is a bucket-handle meniscus tear that produces a mechanical block to full extension or flexion.
Considerable counseling and patient education are required on the expected results and outcomes from the reconstruction, as previously discussed. This is especially important in knees with preexisting arthritis or loss of meniscal function or those that require additional major operative procedures. A surgeon-rehabilitation team is required to provide instruction on rehabilitation to ensure that the postoperative exercise program will be successfully followed by the patient. The patient and family consult with the physical therapy team before surgery to ensure that the postoperative rehabilitation requirements are thoroughly understood. We have written specific patient-oriented electronic books (eBooks) for ACL surgery that provide extensive preoperative information and counseling.
Knees with grossly positive clinical laxity tests have involvement of the secondary ligament restraints, primarily the lateral structures. Associated medial or lateral ligament laxity is an indication for medial or lateral ligament reconstruction. In the ACL Reconstruction Clinical Studies section that follows, the results of autografts and allografts are discussed in further detail.
All knee ligament subluxation tests are performed after the induction of anesthesia in both the injured and contralateral limbs. The amount of increased anterior tibial translation, posterior tibial translation, lateral and medial joint opening, and external tibial rotation are documented. A thorough arthroscopic examination is conducted, noting articular cartilage surface abnormalities and the condition of the menisci. Appropriate debridement and meniscus repair or partial excision are performed as necessary.
The lateral and medial gap tests are done during the arthroscopic examination (see Chapter 17 ). The knee is flexed to 30 degrees and a varus load of approximately 89 N applied. A calibrated nerve hook is used to measure the amount of tibiofemoral compartment opening. Knees that have 12 mm or more of joint opening at the periphery or 10 mm at the midpoint of the tibiofemoral compartment require a PL or medial ligament reconstructive procedure.
Primary knees: Prefer B-PT-B autograft in athletes, STG in recreational, more sedentary patients or those with patellofemoral problems.
Allografts reserved for multiligament procedures, special cases where graft harvest is to be avoided.
Patient uses chlorhexidine wash operative limb three days before surgery.
Antibiotic infusion 1 hour before surgery.
Nonsteroidal antiinflammatory medication morning of surgery, continued 72 hours postoperative.
Knee to be reconstructed initialed by surgeon, time-out in operating room.
Patient supine, place tourniquet middle thigh for use only as necessary.
Use leg holder for initial arthroscopy, meniscus repairs.
Knee portion bed flexed 60 to 90 degrees, bed midportion retroflexed 15 degrees.
Uninvolved extremity placed in well-cushioned padded holder.
After initial arthroscopy, adjust bed to allow 120 degrees of knee flexion.
Inflate tourniquet 275-mm pressure.
3- to 4-cm incision adjacent medial border patellar tendon, medial to inferior pole of patella, mobilize skin flaps for cosmetic approach.
Retinaculum middle patellar tendon incised, limited dissection only for width of graft to be removed.
50% of patients will have small area of numbness just lateral to the patellar tendon counsel preoperatively.
Use precut 10-mm and 22-mm paper ruler to define graft dimensions.
Patellar tendon incised in midportion.
Trapezoidal bone block graft from patella removed with fine saw cuts, osteotome, and similar procedure for tibial bone block.
Sutures placed each bone block, prepared for passage.
Graft wrapped in blood-soaked sponge.
Diameter of tunnels 1 mm larger than diameter of bone block.
End of procedure, loosely approximate tendon graft harvest site with sutures.
Meticulous bone graft from core reamer patella, tibia defects. Place two horizontal mattress sutures inferior pole patella, superior tibial tendon attachment to hold bone grafts in defects, close anterior tissues.
5- to 6-cm longitudinal incision from superior pole patella, extending proximally.
Graft harvest: 10 mm wide through all 3 layers, length 60 to 70 cm.
Bone graft sized 9- to 10-mm diameter.
Close quadriceps tendon defect with sutures.
Meticulous bone grafting patellar defect, closure soft tissues
3- to 4-cm oblique incision over pes tendons.
Identify, palpate STG tendons.
Turn down confluent tibial attachment.
Grasp each tendon 90-degree angle distal end, roll two to three times about straight hemostat.
Superficial tissues removed, overlying sartorius fascia protected.
Proximal fascia bluntly dissected, ST tendon attachment medial gastrocnemius fascia incised, avoid saphenous nerve.
Displace each tendon 10 cm in push-pull maneuver.
Pass graft harvester, transect each tendon 20 cm for a four-strand graft or 24 cm for a six-strand graft.
Prepare, wrap in blood-soaked sponge.
Six-strand graft used in women and patients of small stature.
Goal is to reconstruct the posterior femoral tibial ITB attachment, which is an important restraint to abnormal internal tibial rotation.
Procedure is described in detail in Chapter 8 .
The principles of ACL surgery are summarized in Table 7-8 . Currently, there is no standard graft choice for ACL reconstruction. Autograft tissue sources include B-PT-B, quadriceps tendon-patellar bone (QT-PB), and STG tendons. We prefer B-PT-B autogenous grafts in athletes, a recommendation supported by several long-term studies and in investigations showing a higher rate of ACL primary reconstruction failure after allografts compared with autografts, especially in younger patients. A B-PT-B autograft is not recommended if there is associated patellofemoral arthritis (grade 2B classification, see Chapter 44 ), anterior knee pain, or history of patellar subluxation or dislocation. A B-PT-B autograft is not performed when patient issues suggest a decreased ability to follow the more involved rehabilitation program and cope with the initial pain related to this graft. In recreational athletes and more sedentary patients, a four-strand or six-strand STG autograft is recommended. Modern STG graft fixation methods have increased the success rate, and the rehabilitation postoperatively is easier on the patient. There is a suggestion in the literature of increased STG failure rates with associated physiologic posterolateral laxity. Although allografts offer technical ease and reduced donor site pain, there are risks of disease transmission, a biomechanically inferior graft, and biologic reaction to irradiation and chemical sterilization processing. A recent meta-analysis demonstrated that, when compared with low-dose irradiated allografts (<2.5 mrad), nonirradiated allografts produced superior stability (Lachman, pivot shift, KT-2000 tests), had a lower risk of revision, and higher Lysholm scores. The orthopedic surgeon should understand the sterilization techniques used by the tissue bank and use grafts supplied by banks accredited by the American Association of Tissue Banks.
|
|
|
|
|
|
|
|
|
|
|
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here