Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Aortic aneurysms (AAs), either of the abdominal or thoracic aorta, are important causes of morbidity and mortality in developed countries. On the basis of autopsy studies, it has been estimated that 1%–2% of the population has AAs with up to 10% prevalence in older age groups . Ruptured abdominal aortic aneurysm (AAA) alone is responsible for 1%–2% of deaths in the Western World. By definition, an aneurysm is a localized or diffuse dilatation of the vessel wall with a diameter at least 1.5 times its normal caliber . The classification of AAs is generally based on anatomic location, size, and morphologic shape (saccular or fusiform). In addition, aneurysms may be further classified as true (lined by attenuated media) or false (lined by adjacent fibrous tissue) and as dissecting or nondissecting.
AAAs are by far the most common type of AA with a prevalence of approximately 5% among men older than 65 years of age . On the other hand, thoracic aortic aneurysms (TAAs) are uncommon in comparison to AAA and the etiology varies by anatomic region. Structural and embryologic differences between the thoracic and abdominal aorta suggest that regional heterogeneity may explain differences in the development of aneurysms above and below the diaphragm. As a general principle, aneurysms affecting the ascending thoracic aorta are primarily associated with cystic medial degeneration (CMD), a term discouraged by the consensus statement on surgical pathology of aorta and is replaced by mucoid extracellular matrix accumulation (MEMA) and is further subdivided into intralamellar (MEMA-1) and translamellar (MEMA-T), which may occur as part of the normal aging process, but may be accelerated by other conditions including hypertension, bicuspid aortic valve (BAV), and genetic alterations. In contrast, AAA is associated with atherosclerotic disease, but the precise pathologic mechanisms leading to aneurysmal dilatation remain poorly understood. The feared consequences of AAs are aortic dissection (AD) or rupture, which have a high mortality if left untreated.
ADs are classified into acute and chronic types depending on the duration of the disease . Acute ADs are most often localized to the ascending aorta, and autopsy series have demonstrated a triad of predisposing factors including hypertension, BAV, and Marfan disease . In the elderly population, chronic dissections have been identified as a cause of aortic regurgitation and aneurysmal dilatation .
Inflammatory conditions of the aorta although uncommon may also be a cause of AAs and ADs. Inflammatory aneurysms were historically most frequently due to bacterial or syphilitic infections, but the latter is exceedingly rare in developed countries. The majority of inflammatory aneurysms seen today are located in the thoracic aorta and are the result of Takayasu’s aortitis in young adults, giant cell aortitis, and idiopathic in the elderly, whereas infectious (mycotic) aneurysms are generally found in the descending thoracic aorta . There is increasing recognition of “isolated aortitis” as a variant with no apparent systemic disease. Several recent studies have indicated that aortitis discovered as result of AAs is usually clinically isolated.
Since most AAs are asymptomatic until rupture, early detection and targeted treatment are crucial. Open surgical repair is the standard of care for treating most large AAs, but endovascular aneurysm repair (EVAR), a minimally invasive technique for AAs, is now the predominant form of treatment and is continually being refined. The pathologist plays a critical role in the evaluation of new technologies used to treat cardiovascular diseases.
The aorta was called “the greatest artery” by the ancients. The aorta is the largest blood vessel in the human body and absorbs the impact of 2.3–3 billion heart beats a year while delivering roughly 200 million liters of blood to the various parts of the body . The aortic wall absorbs the impact of systole and diastole by expanding and recoiling, respectively, thus helping to propel the blood distally.
The aorta is classically divided into two major anatomic segments, the thoracic and abdominal aorta. Several distinct anatomic regions form the thoracic aorta including the aortic root, ascending, arch, and the descending aorta. The aortic root is defined as the segment between the ventriculoarterial attachment (aortic annulus) and the sinotubular junction. Important structures within the aortic root include the aortic valve and aortic sinuses. The ascending aorta starts at the sinotubular junction and ends at the takeoff of the brachiocephalic artery. The aortic arch represents the curved portion of the aorta between the ascending and the descending aorta and gives rise to the brachiocephalic trunk, the left common carotid and the left subclavian arteries. Finally, the descending aorta starts after the takeoff of the subclavian artery and has both a thoracic and abdominal component with the diaphragm serving as the anatomic division between the thoracic and abdominal aorta ( Fig. 9.1 ). The aortic size decreases as the distance from the aortic valve increases. The diameter of the ascending aorta is <2.1 cm/m 2 whereas that of the abdominal aorta is <1.6 cm/m 2 , and the abdominal aortic diameter is regarded to be <3.0 cm. The mean wall thickness of the aorta as measured by CT is <4 mm .
![Figure 9.1, Anatomy of thoracic and proximal abdominal aorta (Massachusetts General Hospital Thoracic Aortic Center). [12] . Figure 9.1, Anatomy of thoracic and proximal abdominal aorta (Massachusetts General Hospital Thoracic Aortic Center). [12] .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Aneurysmsoftheaortaascendingthoracicandabdominalandtheirmanagement/0_3s20B9780128222249000098.jpg)
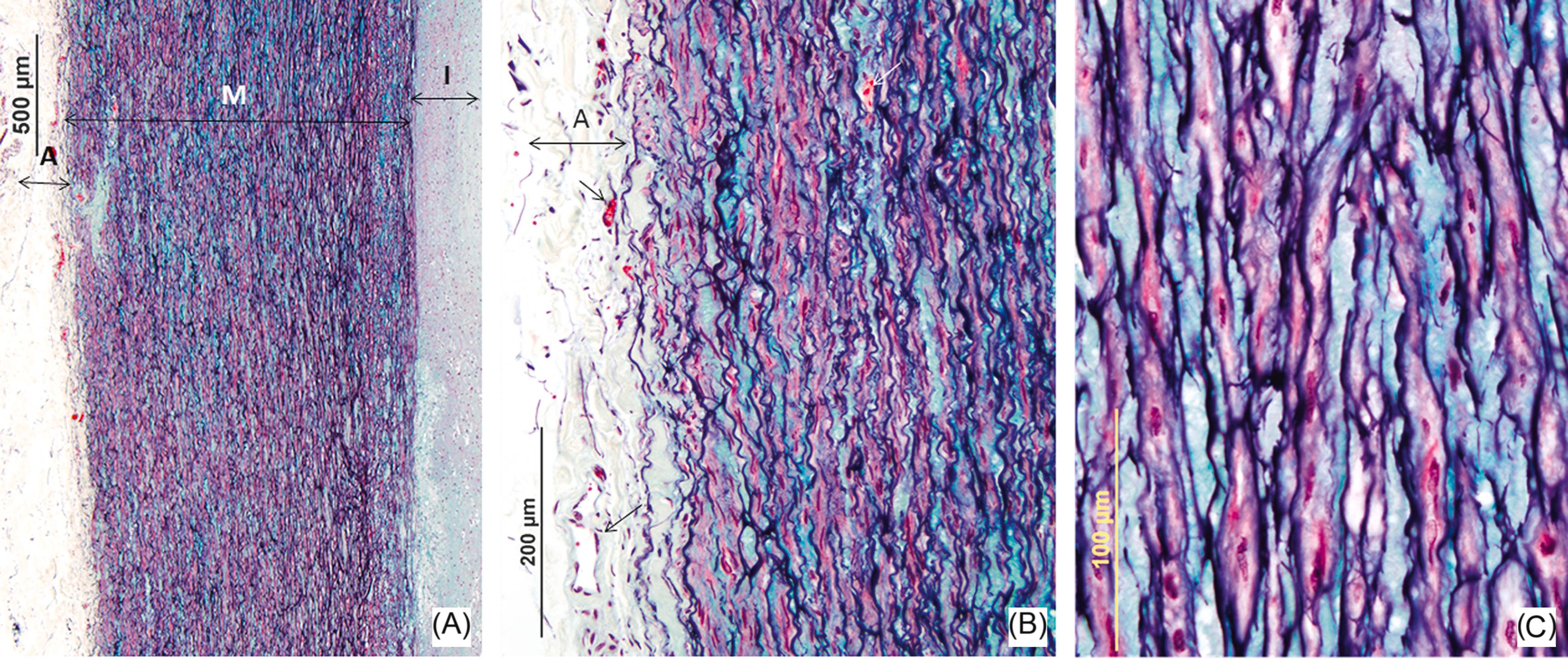
The aorta is a large elastic artery composed of three distinct histologic layers: the intima, media, and adventitia ( Fig. 9.2 ). The intima consists of an endothelial cell layer overlying a basement membrane and underlying connective tissue and smooth muscle cells, the amount of which may vary depending on the age of the patient. The border of the intima is separated by the internal elastic lamina (IEL). The IEL forms the boundary between the intima and the media, and the latter is the thickest layer of the three layers of the aortic wall. The smooth muscle layer of the media is arranged circularly along the long axis of the aorta and is separated by multiple layers of elastic lamellae. The role of the intima is to provide a smooth nonthrombogenic surface for blood flow . Medial lamellar units are defined as bands of elastin filaments with associated collagen, elastin fibers, and smooth muscle cells that work together to provide elasticity. The thoracic aorta has a greater concentration of elastin and is therefore more compliant than the abdominal aorta. The adventitia is the outermost layer and consists of collagen fibers and fibroblasts that provide support and structure to the aortic wall. Blood vessels (vasa vasorum) reside in the adventitia and in the outer medial wall. The vasa vasorum supply oxygen to the outer one-third to one-half of the thoracic aorta whereas the inner half is supplied from the lumen. The abdominal aorta has an absence of medial vasa vasorum and is thinner than the thoracic aorta, supporting less than 28 layers of lamellar units, whereas the thoracic aorta has approximately 35 layers of elastic lamellae that are avascular and another 25 that are vascular .
Vascular aging begins in infancy although the changes do not become apparent until around 40 years of age . Age-related changes demonstrate a progressive decline in the elastic properties of the aortic wall with fragmentation of elastin followed by an increase in collagenous remodeling, resulting in an increase in collagen to elastic ratio that leads to loss of aortic compliance . The elastic properties of the aorta are essential for the maintenance of the pumping function. The degeneration of elastin results in an increase in wall stiffness, and an increase in pulse wave velocity . Collagen becomes thicker and more linear in arrangement with age and tropoelastin expression, a molecule involved in elastin synthesis, shows decreased expression by 50% with each decade . It has been shown that the interruption of the vasa vasorum flow leads to an acute decrease in distensibility of the ascending aorta . The aortic changes in elastic tissue incurred by old age are reflected in further gross and microscopic abnormalities. The aorta becomes tortuous due to an increase in length, and its intimal surface doubles between the second and the sixth decade . The aortic diameter increases with age, the largest increase occurring in the ascending aorta and the smallest in the abdominal aorta . The medial thickness does not change significantly, whereas the intimal thickness increases with age (the greatest increase occurring in the abdominal aorta).
Hypertension and atherosclerosis greatly affect the extent of age-related aortic changes. Hypertension results in an accentuation of the age-related increase in the abdominal aortic circumference. Hypertension and atherosclerosis both have a marked effect that result in increases in abdominal aortic circumference and intimal thickness .
The estimated incidence of TAAs is 6–10 cases/100,000 person-years and the incidence has increased over the past several decades . A recent Swedish national healthcare registry from 1987 to 2002 showed an increase in the incidence of TAA especially in men rising to 16.3 per 100,000 per year in men and 9.1 per 100,000 per year in women . The majority of TAAs (60%) affect the aortic root and/or ascending aorta, 40% involve the descending aorta, 10% involve the arch, and 10% affect the thoracoabdominal aorta, but some may involve multiple sites . About 21% have a family history of TAA. It is estimated that the prevalence of asymptomatic TAA is between 0.16% and 0.34%, and these studies define TAA with an aortic diameter >5 cm . The etiologic types of TAAs, natural history, and treatment differ depending on the anatomic location.
At least half of the patients presenting with thoracic aneurysms are asymptomatic, although there is often a history of hypertension. Most are discovered at the time of physical examination, radiography, or CT scan. Over 25% of patients complain of back or chest pain that may be pericardial and radiate to the neck and jaw, particularly if the arch is involved. Aneurysms of the descending thoracic aorta present with back pain between the scapulae and those in the thoracoabdominal portion of the aorta, lower back pain. On imaging, TAAs are evident on radiography and demonstrate a widening of the mediastinal silhouette. Chest radiographs often demonstrate a shadow that is to the right of the cardiac silhouette in ascending aneurysms, whereas an anterior and left-sided shadow may be indicative of an aneurysm involving the descending aorta. It is not always possible on routine radiography to determine whether an enlarged aorta represents a tortuous aorta or an aneurysm; therefore, it is recommended that tomographic imaging such as CT scanning, transthoracic, or MR angiography be performed, as these modalities can detect and size TAAs . However, while transthoracic echocardiography is ideal for imaging the ascending aorta in Marfan’s, it does not image the middle and descending aorta.
Aneurysms of the ascending thoracic aorta exhibit a variety of underlying pathologies including annuloaortic ectasia, pseudoaneurysms, noninfectious/infectious aortitis, inherited connective tissue disorders, atherosclerosis, aneurysms of the sinus of Valsalva, and dissections. The majority of these entities are associated with MEMA of the aortic wall. In fact, 21% of patients with TAA have at least one family member with a known aneurysm. Reported risk factors for MEMA include Marfan syndrome (MFS) (most common), Ehlers–Danlos syndrome (EDS), Loeys–Dietz syndrome (LDS), and Turner syndrome (TS). Other risk factors include increasing age, aortic valve disease, and systemic hypertension. In a pathology series of 513 resected ascending aortas from the Mayo Clinic, MEMA was the most common finding, present in 41% of resections. Other pathologic findings included aortitis (11%), AD (21%), histologically normal aorta (18%), and other (9%) ( Table 9.1 ) . In the same study, BAV was present in 25% of patients and 13% had inherited connective tissue diseases. The relationship between the extent of MEMA and the etiology of TAA has been evaluated in several pathology series. The Mayo Clinic study showed that patients with inherited connective tissue diseases were at greatest risk for MEMA with an age-dependent increase in severity ( Table 9.2 ) . Roberts et al. showed severe elastic fiber loss and MEMA essentially limited to patients with aortic regurgitation and congenitally malformed aortic valves, whereas in patients with aortic stenosis this finding was unusual .
| Clinical feature | CMD | AD | Normal | Aortitis | CMD_GCA | Other | Total | P |
|---|---|---|---|---|---|---|---|---|
| Patients | ||||||||
| Number | 209 | 109 | 90 | 43 | 14 | 48 | 513 | |
| Percentage | 40.7 | 21.2 | 17.5 | 8.4 | 2.8 | 9.4 | 100 | |
| Age (years) | ||||||||
| Mean (SD) | 59.6 (17.5) | 63.1 (14.1) | 53.5 (16.5) | 62.1 (19.3) | 71.2 (7.6) | 52.9 (19.1) | 59.2 (17.2) | <0.0001 |
| Median | 65.0 | 67.0 | 53.5 | 70.0 | 72.0 | 56.5 | 64.0 | |
| Range | 6.0–89.0 | 22.0–87.0 | 5.0–82.0 | 16.0–85.0 | 50.0–84.0 | 2.0–80.0 | 2.0–89.0 | |
| Sex | ||||||||
| F (%) | 79 (37.8) | 40 (36.7) | 31 (34.4) | 31 (72.1) | 11 (78.6) | 18 (37.5) | 210 (40.9) | <0.0001 |
| M (%) | 130 (62.2) | 69 (63.3) | 59 (65.6) | 12 (27.9) | 3 (21.4) | 30 (62.5) | 303 (59.1) | |
| F:M ratio | 1:1.7 | 1:1.7 | 1:1.9 | 2.6:1 | 3.7:1 | 1:1.7 | 1:1.4 | |
| Concomitant surgery | ||||||||
| N (%) | 182 (87.1) | 61 (56) | 87 (96.7) | 38 (88.4) | 6 (42.9) | 43 (89.6) | 417 (81.3) | <0.0001 |
| AV surgery | 169 (80.9) | 50 (45.9) | 73 (81.1) | 30 (69.8) | 5 (35.7) | 33 (68.8) | 360 (70.2) | <0.0001 |
| CABG | 34 (16.3) | 13 (11.9) | 24 (26.7) | 16 (37.2) | 2 (14.3) | 10 (20.8) | 99 (19.3) | 0.0039 |
| Medical history | ||||||||
| ACVD | 181 (86.6) | 94 (86.2) | 86 (95.6) | 39 (90.7) | 12 (85.7) | 48 (100) | 460 (89.7) | 0.001 |
| Hypertension | 118 (56.5) | 70 (64.2) | 33 (36.6) | 26 (60.5) | 9 (64.3) | 23 (47.9) | 279 (54.4) | 0.0029 |
| CAD | 40 (19.1) | 33 (30.3) | 27 (30.0) | 15 (34.9) | 2 (14.3) | 12 (25.0) | 129 (25.1) | 0.0842 |
| CHF | 31 (14.8) | 11 (10.1) | 8 (8.9) | 8 (18.6) | 1 (7.1) | 6 (12.5) | 65 (12.7) | 0.4856 |
| MI | 15 (7.2) | 17 (15.6) | 9 (10.0) | 3 (7.0) | 0 (0) | 2 (4.2) | 46 (9.0) | 0.0827 |
| CHD | 58 (27.8) | 14 (12.8) | 59 (65.6) | 3 (7.0) | 0 (0) | 21 (43.8) | 155 (30.2) | <0.0001 |
| Bicuspid AV | 47 (22.5) | 12 (11.0) | 51 (56.7) | 3 (7.0) | 0 (0) | 14 (29.2) | 127 (24.8) | <0.0001 |
| Other CHD | 18 (8.6) | 4 (3.7) | 14 (15.6) | 0 (0) | 0 (0) | 8 (16.7) | 44 (8.6) | 0.004 |
| CTD | ||||||||
| Inherited | 42 (20.1) | 18 (16.5) | 3 (3.3) | 0 (0) | 0 (0) | 4 (8.3) | 67 (13.1) | 0.0002 |
| Acquired | 7 (3.3) | 2 (1.8) | 3 (3.3) | 2 (4.7) | 2 (14.3) | 0 (0) | 16 (3) | 0.132 |
| Arteritis | 9 (4) | 4 (4) | 1 (1) | 14 (33) | 5 (36) | 0 (0) | 33 (6) | <0.0001 |
| No CMD | Mild CMD | Moderate CMD | Severe CMD | ||
|---|---|---|---|---|---|
| N =223 | N =69 | N =61 | N =160 | P | |
| Age (years) | 0.0977 | ||||
| Mean (SD) | 57.5 (17.1) | 59.4 (17.1) | 59.6 (17.6) | 61.3 (17.1) | |
| Median | 60.0 | 63.0 | 64.0 | 67.5 | |
| Range | 2.0–82.0 | 6.0–87.0 | 9.0–83.0 | 14.0–89.0 | |
| Sex | 0.1722 | ||||
| F (%) | 94 (42.2) | 20 (29.0) | 25 (41.0) | 71 (44.4) | |
| M (%) | 129 (57.8) | 41 (71.0) | 36 (59.0) | 89 (55.6) | |
| Concomitant surgery | 184 (82.5) | 58 (84.1) | 46 (75.4) | 129 (80.6) | 0.5726 |
| AV surgery | 146 (65.5) | 53 (76.8) | 41 (67.2) | 120 (75.0) | 0.1194 |
| CABG | 54 (24.2) | 9 (13.0) | 12 (19.7) | 24 (15) | 0.0688 |
| ACVD | 210 (94.2) | 64 (92.8) | 55 (90.2) | 131 (81.9) | 0.0011 |
| Hypertension | 113 (50.7) | 40 (58.0) | 42 (68.9) | 84 (52.5) | 0.0728 |
| CAD | 74 (33.2) | 12 (17.4) | 15 (24.6) | 28 (17.5) | 0.0020 |
| CHF | 26 (11.7) | 8 (11.6) | 11 (18) | 20 (12.5) | 0.6003 |
| MI | 23 (10.3) | 5 (7.2) | 7 (11.5) | 11 (6.9) | 0.5572 |
| Congenital HD | 88 (39.5) | 31 (44.9) | 14 (23) | 22 (13.8) | <0.0001 |
| BAV | 73 (32.7) | 25 (36.2) | 12 (19.7) | 17 (10.6) | <0.0001 |
| Other | 23 (10.3) | 10 (14.5) | 2 (3.3) | 9 (5.6) | 0.0481 |
| ICTD | 10 (4.5) | 5 (7.2) | 13 (21.3) | 39 (24.4) | <0.0001 |
| ACTD | 4 (1.8) | 3 (4.3) | 4 (6.6) | 5 (3.1) | 0.2585 |
A detailed discussion of annuloaortic ectasia and sinus of Valsalva aneurysms is presented below, as these are distinct types of TAA with notable pathologic findings.
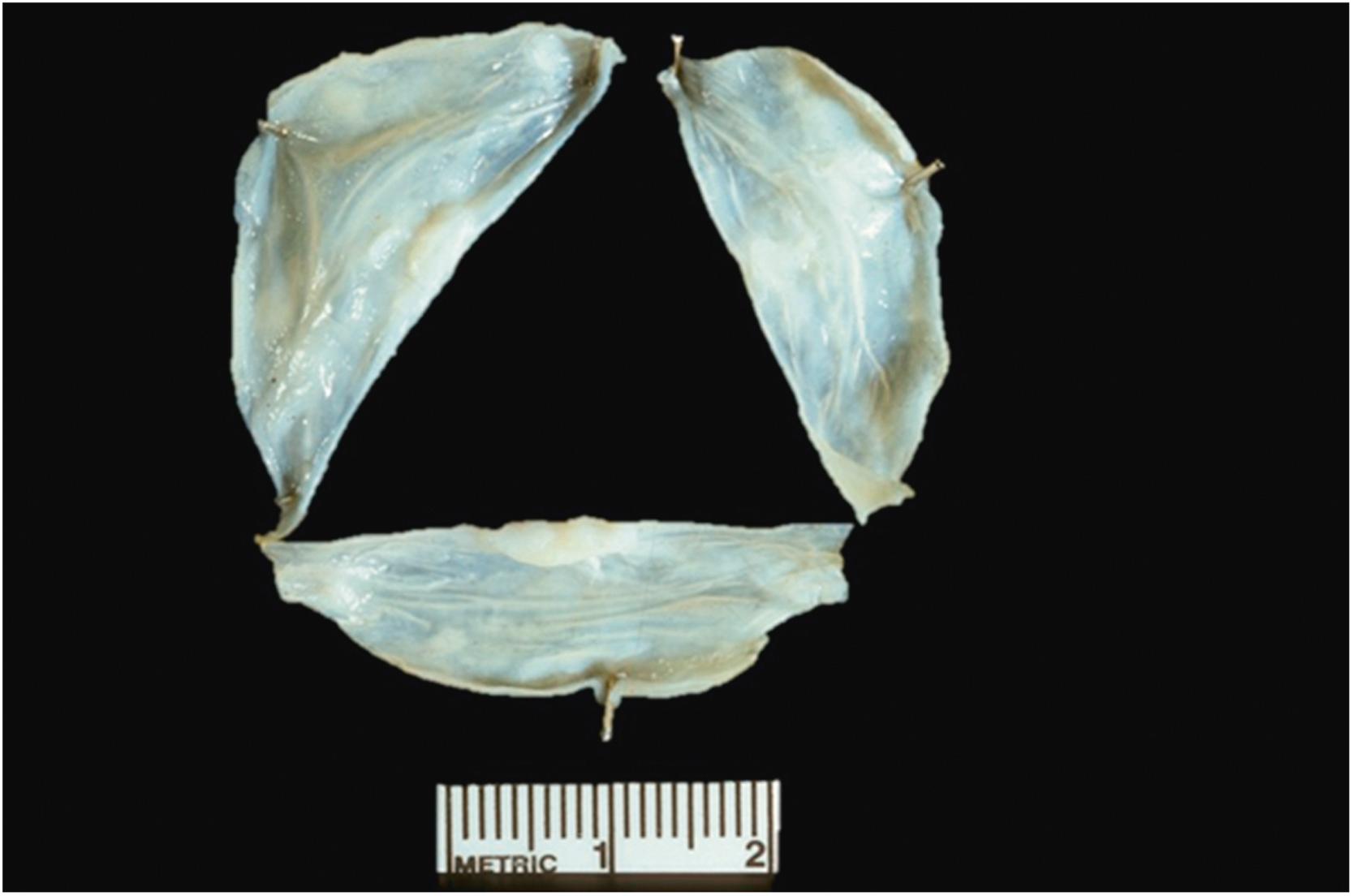
Annuloaortic ectasia is a term that was introduced in 1961 to denote aneurysmal dilatation of the proximal ascending aorta and aortic annulus . In its broadest sense, the term has been used when specific conditions, such as Takayasu’s disease, result in AA with insufficiency . The term “annuloaortic ectasia” is generally restricted to cases of idiopathic MEMA of the aorta many patients have MFS or have a forme fruste of the disease. Medial degeneration leads to weakening of the aortic root, which results in dilatation and aneurysm formation ( Fig. 9.3 ) . The proportion of patients with extra cardiac manifestations of MFS varies from 16% to 22% to over 50% . Idiopathic annuloaortic ectasia is at least twice as common in men than women and typically presents in the fourth, fifth, and sixth decades. In the past, the tendency to group together idiopathic annuloaortic ectasia and MFS came from the difficulty of clearly separating the two entities clinically. Today, many genetic forms of TAA besides MFS and LDS are recognized, as well as the culprit mutations that produce both syndromic and nonsyndromic diseases. Molecular genetic testing, in combination with clinical findings, is helpful in rendering a more specific diagnosis. To date, more than a dozen genes are available for clinical molecular testing .
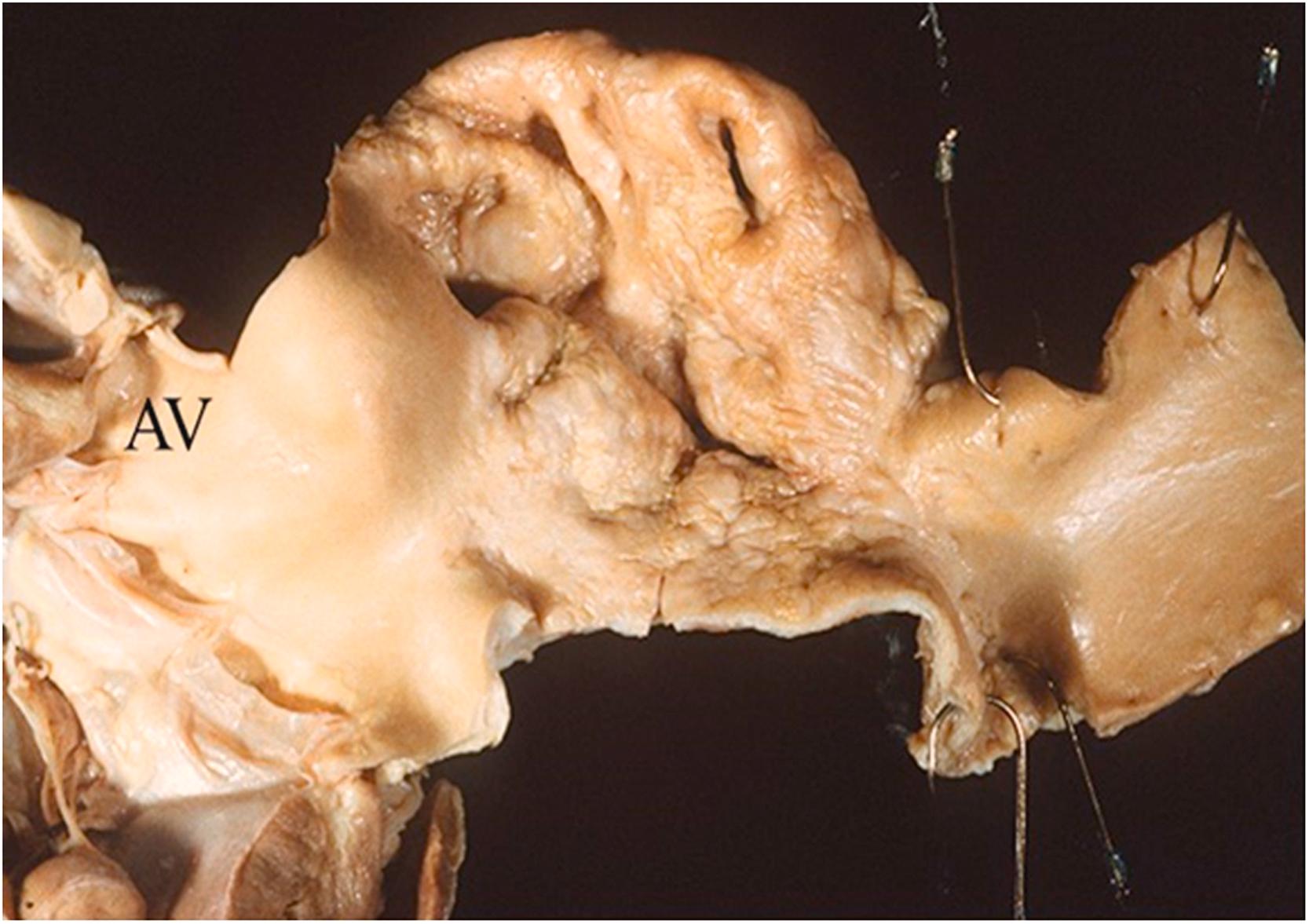
![Figure 9.3, Marfan syndrome with annuloaortic ectasia in the absence of dissection. This 32-year-old man died suddenly. At autopsy, there were classic features of Marfan syndrome, and the aortic root was markedly dilated with involvement of all three aortic sinuses ( arrowheads ), the annulus, and the ascending aorta. The aortic root was 55 mm in diameter, and the aortic valve was insufficient. [34] . Figure 9.3, Marfan syndrome with annuloaortic ectasia in the absence of dissection. This 32-year-old man died suddenly. At autopsy, there were classic features of Marfan syndrome, and the aortic root was markedly dilated with involvement of all three aortic sinuses ( arrowheads ), the annulus, and the ascending aorta. The aortic root was 55 mm in diameter, and the aortic valve was insufficient. [34] .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Aneurysmsoftheaortaascendingthoracicandabdominalandtheirmanagement/2_3s20B9780128222249000098.jpg)
Aortic root dilatation has also been associated with BAV and aortic coarctation. It has been shown that the aortic expansion rate is higher in BAV (0.19 cm/year) than in tricuspid aortic valve (0.13 cm/year). It is also possible that the aortic root may dilate in the absence of significant aortic stenosis or regurgitation ( Fig. 9.4 ) . Besides congenital connective tissue disease, systemic hypertension is an important contributor of aortic dilatation due to higher wall stress.
Other causes to consider are inflammatory diseases such as Takayasu’s aortitis and giant cell aortitis, or bacterial and fungal aortitis. Aneurysms from atherosclerosis have been described in the ascending aorta but are, however, uncommon as compared to those of the abdominal aorta. Nevertheless, aneurysms may occur from chronic dissection, trauma, aortic surgery, false aneurysm, and resuscitation.
ADs or rupture is related to the diameter of the aorta. At one time, Davies et al. showed that in patients with ADs or rupture, the aortic diameter measured 6.0 cm, however, today it is recommended to offer prophylactic surgery for patients without predisposing conditions other than hypertension when an aorta reaches 5.5 cm, if the growth exceeds 0.5 cm/year, or if the patient is undergoing major cardiac surgery with an ascending aorta over 4.5 cm . Svensson et al. . showed that 12.5% of MFS patients with ADs had an aortic diameter of <5.0 cm, and 12.5% of patients with BAV and dissection had an aortic diameter similar to patients with MFS. In patients with tricuspid valve and type A dissection, the diameter of the aorta was <5.0 cm in 42% of patients . On the other hand, patients with LDS or a family history of TAA leading to acute aortic dissection (TAADs) experience ADs with minimum aortic enlargement, and it is recommended that patients with a transforming growth factor β receptor type II (TGFBR2) mutation undergo surgical repair of AA when the diameter reaches 4.2 cm (see below) .
The specific cardiovascular features of MFS and other ascending aneurysm etiologies are discussed later, as is AD, the second most common complication of annuloaortic ectasia after aortic insufficiency.
Aneurysm of the sinus of Valsalva was first described in 1839 . The aneurysm results from a weakness in the wall of the sinus leading to the dilatation or blind pouch (diverticulum) in one of the aortic sinuses, usually the right. Sinus of Valsalva aneurysms is rare and accounts for 0.14% prevalence in a surgical series of a Western population men are affected more often than women (male to female ratio, 4:1). This entity is more common in the Eastern as compared to the Western population.
Most aneurysms of the sinus of Valsalva are congenital lesions with a strong association with ventricular septal defect (VSD). Other congenital anomalies that have been associated with sinus of Valsalva aneurysm are MFS and EDS, or other disorders such as coarctation of the aorta and BAV. The congenital defect may result from a defect of fusion between the aortic media and the heart at the fibrous annulus of the aortic valve. Infective endocarditis and syphilis currently account for the majority of acquired aneurysms of the sinus of Valsalva, representing fewer than 20% of total cases.
The age at presentation of congenital aneurysms ranges from 11 to 67 years, with a mean of 34 years . In a clinical review of 377 cases, the vast majority were located in the right sinus (81%) and were equally divided between those associated with VSD (46%) and those without VSD (54%) ( Fig. 9.5A and B ). The next most frequent site is the noncoronary sinus (17%); at this site, only 6% of lesions are associated with VSD. Only 2% have been reported in the left coronary sinus . Dilatation of all three sinuses is unusual. When it occurs, it is associated with MFS and connective tissue diseases, and is more appropriately called aortic root aneurysm . The clinical course before rupture is that of a silent lesion. In patients with VSD, heart failure and mitral regurgitation may occur. In some patients, cardiovascular collapse and even sudden death may occur. In patients who survive the initial rupture, cardiomegaly is usually present . Aortic regurgitation is present in one-third of patients and right- or left-sided heart failure is present in 80% of patients with ruptured sinus of Valsalva aneurysm. Chest pain is usually absent. The aneurismal sac may result in obstruction of the right ventricular tract and (rarely) coronary occlusion; conduction disturbances have also been reported.
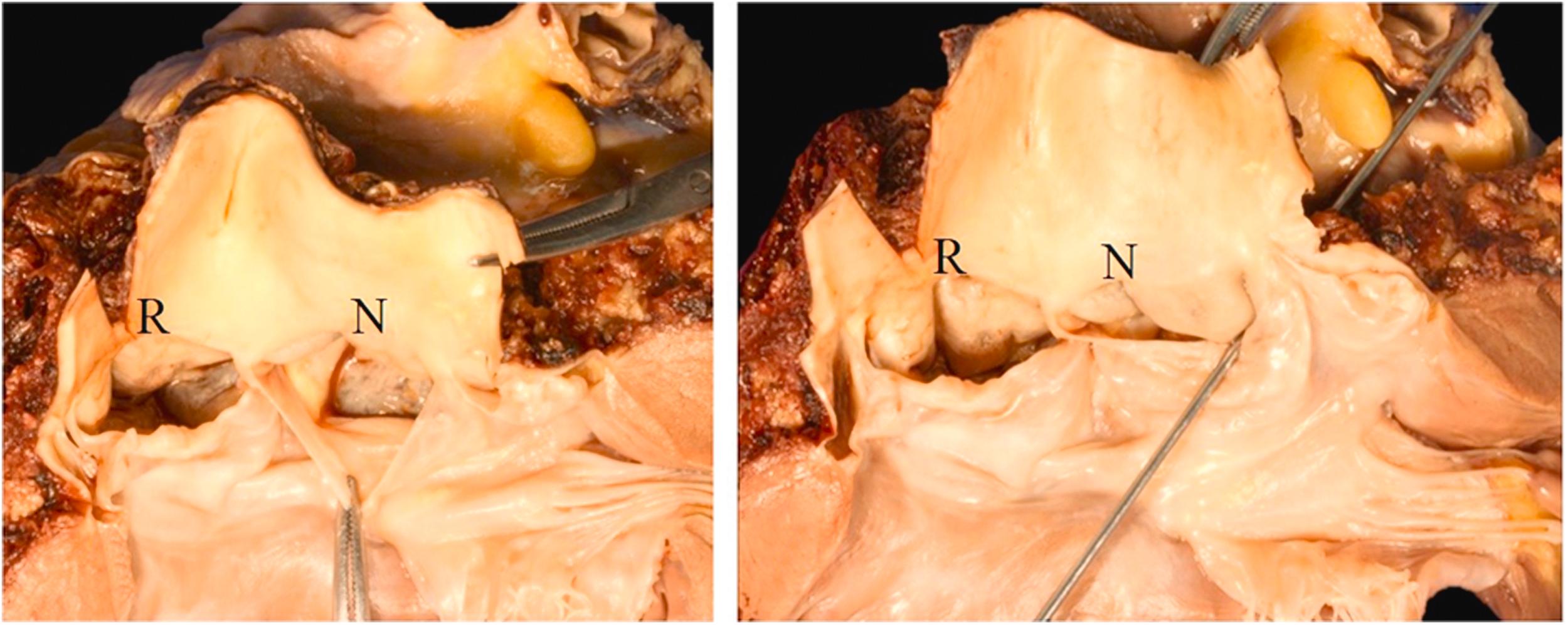
The location within the right sinus of Valsalva is predictive of the site of rupture: those in the left portion of the right sinus of Valsalva protrude toward and rupture into the right ventricular outflow tract near the pulmonary valve. A VSD is frequently associated with this particular anomaly. Those that arise in the middle of the right sinus of Valsalva protrude and rupture into the body of the right ventricle; VSDs are uncommon in this manifestation of the disease. When the aneurysm originates from the posterior portion of the right sinus of Valsalva, it protrudes toward the plane of the tricuspid valve, rupture occurring mostly into the right atrium . Other reported sites of rupture of aneurysms located in the right or left sinuses are the left ventricle and atrium, pericardium, pulmonary artery, and superior vena cava.
The diameter of the rupture site at the base of the sinus of Valsalva aneurysm ranges from 0.4 to 1.1 cm (mean 0.7 cm). The wall of the sinus of Valsalva is largely made up of the aorta and consists of elastic lamellae with interspersed smooth muscle cells, whereas the base at the ventricular attachment is thin and consists of smooth muscle cells in a collagenous matrix; usually, no elastic fibers are identified .
The causes of AA of the transverse or descending aorta include dissections, atherosclerosis, noninfectious aortitis, medial degeneration, with or without MFS and rarely, syphilis or other infections. Atherosclerotic lesions are most frequent in the descending aorta and aortic arch where they account for 90% of descending TAA , whereas atherosclerosis accounts for only 1% of ascending TAAs. Atherosclerotic aneurysms are frequently (30%–40%) associated with noncontiguous aneurysms in the abdominal, aorta, iliac, or femoral arteries .
MFS is the classic syndromic form of TAA and has served as the model for many studies of pathogenesis and treatment of TAA. MFS is a heritable autosomal dominant disorder caused by mutations in the FBN1 gene that encodes fibrillin-1, a protein found in the extracellular matrix (ECM) . Fibrillin-1 is an important component of elastic fibers; mutations in the FBN1 gene result in both a decrease in the amount of elastin and loss of elastin structure. Studies have suggested that fibrillin-1 regulates TGFβ type II receptors and that some disease features may be related to increased TGFβ signaling . Classically, the diagnosis of MFS was made based on clinical criteria, including a close family relative with the syndrome, ocular changes, and skeletal changes. Ghent criteria for diagnosis were proposed in 1996 and revised in 2010; MFS is diagnosed if ectopia lentis or aortic dilatation is present in addition to an FBN1 gene mutation . Currently, molecular techniques are helpful in the diagnosis. In approximately 75% of cases, mutations in fibrillin-1 are inherited while in the remaining 25% of cases, de novo mutations are implicated . Cardiovascular abnormalities are detected in the majority of patients with the syndrome; the exact proportion depends on the criteria for diagnosis. The cardiovascular involvement is manifested by aortic root dilatation (39%), ADs (36%), mitral valve prolapse (without other cardiac involvement) (21%), and miscellaneous cardiovascular manifestations ( Fig. 9.6 ). These unusual abnormalities include aneurysms of the sinus of Valsalva and peripheral aneurysms. Isolated aneurysms of Valsalva are relatively uncommon but have been described and involve equally all three sinuses and may manifest soon after birth .
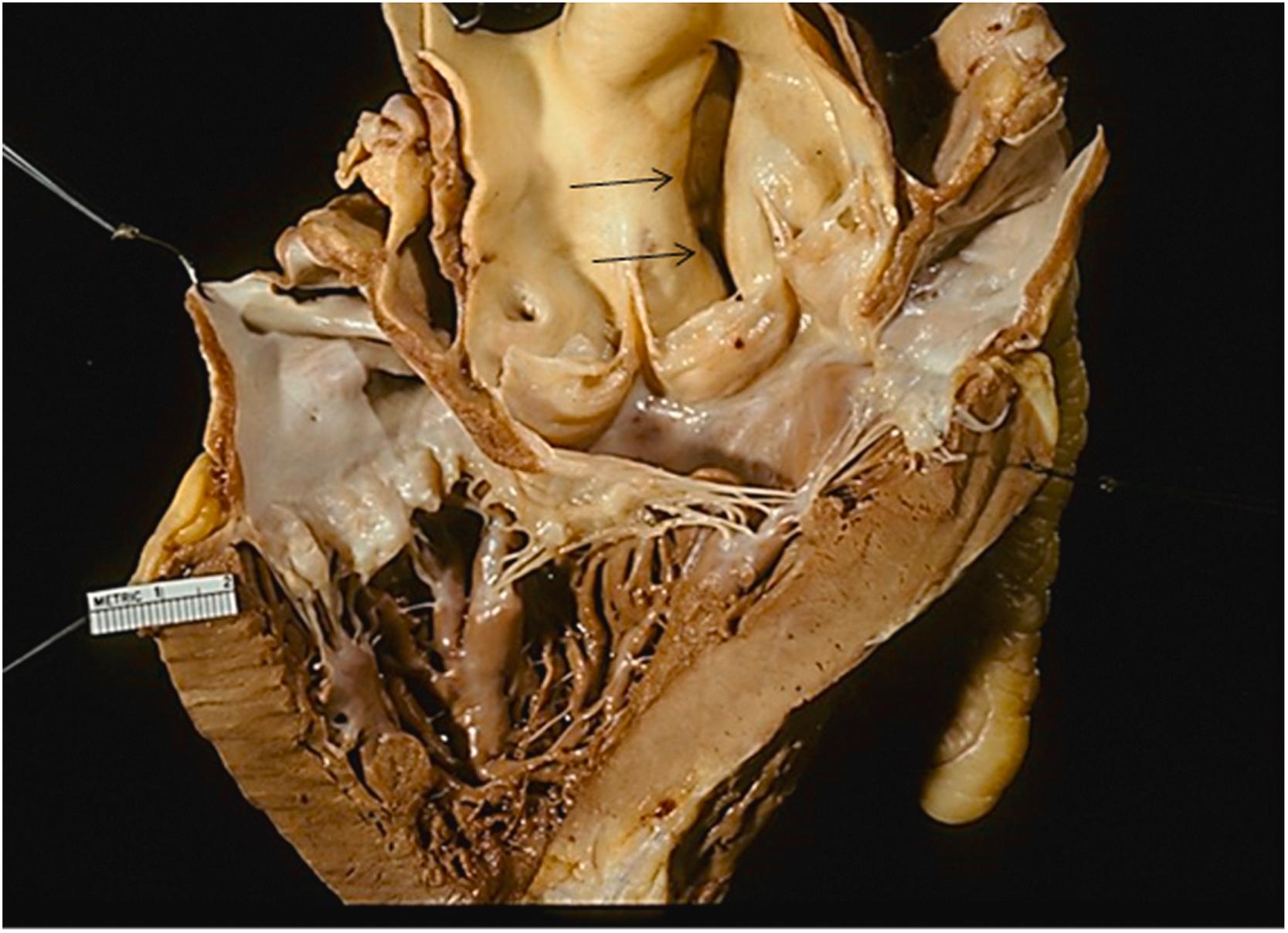
Patients with MFS are much younger at diagnosis with symptoms of aortic root dilatation often occurring in the third and fourth decades. They are also much more likely to have a family history of aortic disease, however, in one-fourth of patients, no family history can be elicited. Although CMD tends to be very pronounced in these patients, medial degeneration is not specific for the disease . Because of the complications of AD and aortic insufficiency, patients with MFS should be followed for progressive aortic root dilatation, which is the most important determining factor for prognosis . Risk of sudden death or AD is low in patients with MFS with aortic root diameter between 45 and 49 mm, however, an aortic diameter of 50 mm is recommended as an appropriate threshold for prophylactic surgery . Moreover, even at diameters of <50 mm, progressive dilatation of the aorta can lead to AD and/or rupture, therefore careful close observation for the consideration for the timing of invasive surgical treatment is recommended .
Rapid advances are being made in the understanding of TAA at the molecular genetic level and a great deal of research is aimed at identifying new genes responsible for TAA. There is strong evidence that inherited genetic defects play a causative role as demonstrated by the presence of aortic root dilatation in first-degree relatives of over 50% of patients with TAA . Familial thoracic aortic aneurysms and dissections (FTAADs) occur as part of known syndromes such as MFS but can also be inherited in families in an autosomal dominant manner or as an isolated condition (nonsyndromic) . Analysis of several large databases of patients with TAA showed that approximately 20% of non-Marfan patients had an inherited pattern of TAA . These patients not only presented at a younger age than the sporadic group, but the familial aneurysms also grow at a higher rate suggesting a more aggressive course .
A new classification system of familial forms of thoracic aneurysm has been proposed by the Montalcino Aortic Consortium ( Table 9.3 ) . The genes underlying familial forms of AA code for proteins can be grouped according to function including (1) regulation of ECM (FBN1, COL3A1, EFEMP2), (2) participation in the TGFβ pathway (TGFB2, TGFBR1, TGFBR2, and SMAD3), and (3) involvement with the contractile apparatus of smooth muscle cells (ACTA2, MYH11, MYLK, and PRKG1). The vascular form of Ehlers–Danlos is caused by genes coding for collagen (COL3A1) and will be discussed in more detail below. SMAD3 mutations have recently been associated with AAs and early-onset osteoarthritis as well as diffuse arterial aneurysms including cerebral, pulmonary, mesenteric, splenic, and iliac arteries. ACTA2 encodes the smooth muscle cell isoform of alpha actin; these mutations are responsible for up to 10% of FTAAD.
| Gene | Protein | Human aneurysmal syndrome | Syndromic disorders | Nonsyndromic disorders | Clinical characteristics and phenotype | Inheritance | OMIM | Associated histologic findings in aorta | |
|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular manifestation | Systemic manifestations | ||||||||
| Extra cellular matrix proteins | |||||||||
| ARIH1 | Ariadne RBR E3 ubiquitin protein ligase 1 | FTAA | + | + | Aortic and intracranial aneurysm | Atrophy scar, pes planus | Unknown | 605624 | |
| COL3A1 | Collagen 3 α1 chain | EDS vascular type (EDS type IV) | + | − | TAAD, MVP, visceral arterial dissection, AVMs (celiac and mesenteric vessels), pulmonary artery aneurysms, IA | Thin bruisable skin, acrogeria, fragile tissue prone to rupture, joint laxity, characteristic facial features (pinched nose, thin lips, prominent ears) | AD | 130050 | MD+, MEMA-T+ |
| COL5A1 | Collagen 5 α1 chain | EDS classic type (EDS type I) | + | − | ARD, rupture/dissection of medium-sized arteries | Thin bruisable skin, acrogeria, fragile tissue prone to rupture, Joint laxity, characteristic facial features | AD | 130000 | |
| COL5A2 | Collagen 5 α2 chain | EDS classic type (EDS type II) | + | − | ARD | Thin bruisable skin, acrogeria, fragile tissue prone to rupture, Joint laxity, characteristic facial features | AD | 130000 | |
| EFEMP2 | Fibulin-4 | Cutis laxa, AR type Ib | + | − | Ascending aortic aneurysms, other arterial aneurysms, arterial tortuosity, and stenosis | Emphysema, birth fractures, arachnodactyly, mild joint hypermobility | AR | 614437 | |
| ELN | Elastin | Cutis laxa, AD | + | − | ARD, ascending aortic aneurysm and dissection, BAV, IA possibly associated with SVAS | Loose and/or wrinkled skin, gastrointestinal diverticula, hernia, genital prolapse, rarely bronchiectasis and emphysema | AD | 123700 185500 | |
| FBN1 | Fibrillin-1 | Marfan syndrome | + | + | ARD, TAAD, AAA, MVP, aortic regurgitation, other aortic aneurysms, pulmonary artery dilatation, arterial tortuosity | Joint laxity, scoliosis, pectus abnormalities, myopia and actopia lentis, high arched palate, pes planus, pneumothorax, dural ectasia | AD | 154700 | MD+++, MEMA-T+++, SMCL+, EFF+++ |
| FBN2 | Fibrillin-2 | Contractual arachnodactyly | + | − | Rare ARD and aortic dissection, BAV, PDA | Contractures, arachnodactyly, scoliosis, crumpled ears | AD | 121050 | |
| LOX | Lysyl oxidase | AAT10 | − | + | TAAD, AAA, hepatic artery aneurysm, BAV, CAD | AD | 617168 | ||
| MFAP5 | Microfibril-associated glycoprotein 2 | AAT9 | − | + | ARD, TAAD | AD | 616166 | ||
| PLOD1 | Lysyl hydroxylase 1 | EDS kyphoscoliotic type (EDS type VI) | + | − | TAAD, MVP, aortic regurgitation, intra cranial hemorrhage | Joint laxity, thin and translucent skin, scoliosis, myopia, glaucoma | AR | 225400 | |
| ROBO4 | Roundabout guidance receptor 4 | BAV | − | + | BAV/TAA | AD | 607528 | ||
| TIMP1 | Tissue inhibitors of matrix metalloproteinase 1 | AOVD in Turner syndrome | + | − | BAV/TAA | Short stature, gonadal dysgenesis, with infertility, low-set posteriorly rotated ears, low posterior hairline, recurrent ear infections and hearing loss | XLD | 305370 | MD++, MEMA-T+++ |
| TIMP3 | Tissue inhibitors of matrix metalloproteinase 3 | AOVD in Turner syndrome | + | − | BAV/TAA | Short stature, gonadal dysgenesis, with infertility, low-set posteriorly rotated ears, low posterior hairline, recurrent ear infections and hearing loss | XLD | 188826 | MD++, MEMA-T+++ |
| TGF-β signaling | |||||||||
| LTBP1 | Latent TGF-β binding protein 1 | Aortic dilation with associated musculoskeletal findings | + | − | TAAD | Ptosis, malar hypoplasia, high arched palate, retrognathia, pes planus, hindfoot deformity, obstructive sleep apnea, low truncal tone and joint laxity (during childhood) | AD | 150390 | |
| LTBP3 | Latent TGF-β binding protein 3 | Dental anomalies and short stature | + | − | TAAD, AAA, visceral and peripheral arterial aneurysm | Amelogenesis imperfecta, short stature, platyspondyly | AR | 602090 | |
| SKI | Sloan Kettering proto-oncoprotein | Shprintzen–Goldberg syndrome | + | − | ARD, arterial tortuosity, pulmonary artery dilation, other (splenic) arterial aneurysms | Hypertelorism, downslanting palpebral fissures, high-arched palate, micrognathia, low-set posteriorly rotated ears, hypotonia, inguinal or umbilical hernia, arachnodactyly, joint laxity | AD | 182212 | |
| SLC2A10 | Glucose transporter 10 | Arterial tortuosity syndrome | + | − | ARD, ascending aortic aneurysms, other arterial aneurysms, arterial tortuosity, elongated arteries aortic/pulmonary artery stenosis | Hyperlaxity of the skin, joint laxity or contractures, inguinal hernias, micrognathia, elongated face, high palate, beaked nose, sliding hernia, ventricular hypertrophy | AR | 208050 | MD++, EFF+++ |
| SMAD2 | SMAD2 | LDS type VI | + | − | ARD, ascending aortic aneurysms, vertebral/carotid aneurysms and dissections, AAA | Downslanting palpebral fissures | AD | Unassigned | |
| SMAD3 | SMAD3 | LDS type III (aneurysm-osteoarthritis syndrome) | + | + | ARD, TAAD, AAA, arterial tortuosity, other arterial aneurysms/dissections, IA, MVP, BAV, congenital heart defects | Velvety skin, striae in unusual locations, recurrent umbilical/inguinal hernias, early-onset joint pain | AD | 613795 | |
| SMAD4 | SMAD4 | JP/HHT syndrome | + | − | ARD, TAAD, AVMs, IA | hamartomatous polyps (gastrointestinal tract), telangiectasia (skin, oral and nasal mucosa) | AD | 175050 | |
| SMAD6 | SMAD6 | AOVD2 | − | + | BAV/TAA | AD | 602931 | ||
| TGFB2 | TGF-β2 | LDS type IV | + | + | ARD, TAAD, arterial tortuosity, other arterial aneurysms, BAV | Mild craniofacial feature, skeletal and cutaneous anomalies | AD | 614816 | |
| TGFB3 | TGF-β3 | LDS type V | + | − | ARD, TAAD, AAA/dissection, other arterial aneurysms, IA/dissection | Cleft palate, bifid uvula, skeletal overgrowth, cervical spine instability, and clubfoot deformity | AD | 615582 | |
| TGFBR1 | TGF-β receptor type I | LDS type I, AAT5 | + | + | TAAD, AAA, arterial tortuosity, other arterial aneurysms/dissection, IA, PDA, BAV | Hypertelorism, cleft palate, craniosynostosis, food allergies, asthma, rhinitis, eczema | AD | 609192 | MD+++, MEMA-I+++, MEMA-T+, EFF+++, EFD+ |
| TGFBR2 | TGF-β receptor type II | LDS type II, AAT3 | + | + | TAAD, AAA, arterial tortuosity, other arterial aneurysms/dissection, IA, PDA, BAV | Hypertelorism, cleft palate, craniosynostosis, food allergies, asthma, rhinitis, eczema | AD | 610168 | MD+++, MEMA-I+++, MEMA-T+, EFF+++, EFD+ |
| SMC and its contraction | |||||||||
| ACTA2 | α-smooth muscle actin | AAT6, Multisystemic smooth muscle dysfunction syndrome, MYMY5 | + | + | TAAD, CAD, stroke (moyamoya disease), PDA, pulmonary artery dilation, BAV | Persistent livedo reticularis, iris flocculi, congenital mydriasis, intestinal hypoperistalsis and malrotation, hypotonic bladder | AD | 611788 613834 614042 | |
| FLNA | Filamin A | Periventricular nodular heterotopia 1, cardiac valvular dysplasia | + | − | Aortic dilatation/aneurysms, peripheral arterial dilatation, PDA, IA, BAV | Epilepsy, joint laxity, | XLD X-linked | 300049 314400 | |
| FOXE3 | Forkhead box 3 | AAT11 | − | + | TAAD (primarily type A dissection) | AD | 617349 | ||
| MYH11 | Smooth muscle myosin heavy chain | AAT4 | − | + | TAAD, PDA, CAD, peripheral vascular occlusive disease, carotid IA, PDA | Acute myeloid leukemia, intestinal pseudo-obstruction | AD | 132900 | MD++, EFF++ |
| MYLK | Myosin light chain kinase | AAT7 | − | + | TAAD | Intestinal pseudo-obstruction, polyps, diverticulosis, adenocarcinoma of the colon | AD | 613780 | |
| PRKG1 | Type I cGMP-dependent protein kinase | AAT8 | − | + | TAAD, AAA, coronary artery aneurysm/dissection, aortic tortuosity, small vessel CVD | AD | 615436 | ||
| Neural crest migration | |||||||||
| NOTCH1 | NOTCH1 | AOVD1 | − | + | BAV/TAAD | AD | 109730 | ||
| Others | |||||||||
| HCN4 | Hyperpolarization-activated cyclic nucleotide-gated potassium channel 4 | Noncompaction cardiomyopathy, bradycardia, and mitral valve disease | − | + | Ascending aorta dilation, cardiac noncompaction, sick sinus syndrome | AD | 163800 | ||
| MAT2A | Methionine adenosyltransferase II α | FTAA | − | + | Thoracic aortic aneurysms, BAV | AD | Unassigned | ||
| SLC2A10 | Glucose transporter 10 | Arterial tortuosity syndrome | + | − | ARD, ascending aortic aneurysms, other arterial aneurysms, arterial tortuosity, elongated arteries aortic/pulmonary artery stenosis, aortic regurgitation | Marfanoid skeletal features (pectus deformity, joint laxity), hyperextensible skin, cutis laxa, diaphragmatic hernia, elongated facies, blepharophimosis, beaked nose, high-arched palate, micrognathia, and downslanting palpebral fissures | AR | 208050 | MD++, EFF+++ |
Earlier studies have identified several chromosomal loci using methods of linkage analysis and gene sequencing. These loci have been mapped to the 5q13–14 (TAAD1), 11q23.2–24 (FAA1), and 3p24–25 (TAAD2) chromosome . The mutation on the 3p24.2–25 chromosome has been localized to the TGFBR2 and can cause both isolated and familial forms of the disease with histological evidence of MEMA . It has been shown that FTAAD can result from mutations in either TGFBR1 or TGFBR2. Identification of the specific gene in these patients has important implications for treatment, as individuals bearing the TGFBR2 mutation develop ADs earlier and with only minimal enlargement of the aortic root. This observation has led to current guidelines recommending prophylactic surgical repair when the ascending aorta reaches a diameter of 4.2 cm .
Another genetic disorder commonly associated with aortic root aneurysms is LDS. Two types have recently been distinguished: LDS type 1 and LDS type 2. The former has features that overlap with MFS whereas the latter shares some similarities with EDS. The syndrome results from mutations in TGFβ receptor genes (TGFBR1 and TGFBR2).
LDS predisposes patients to TAAs and dissections along with craniofacial abnormalities which may include bifid uvula, cleft palate, craniosynostosis, and hypertelorism . In addition, these patients have a strong predilection for arterial tortuosity and dilatation of the ascending aorta with aneurysms and dissections throughout the arterial tree . First-degree family members should undergo clinical and genetic screening. Similar to patients with FTAAD, the timing of surgical repair of TAA in patients with LDS can be determined based on the mutated gene causing the disease . For patients with the TGFBR2 mutation, prophylactic aortic root replacement is recommended when the ascending aorta reaches a diameter of 4.2 cm by transesophageal echocardiography (internal diameter) or 4.4–4.6 cm by CT and/or MRI (external diameter) irrespective of age . A histopathologic study has shown that the histologic characteristics of medial degeneration in LDS differ from those observed in MFS and is characterized by a more subtle but diffuse form of medial degeneration (severe intralamellar elastic fiber fragmentation), increased collagen deposition, and elastic fiber disarray .
The EDS comprises a heterogeneous group of diseases, characterized by fragility of the soft connective tissues affecting primarily the skin, ligaments, joints, blood vessels, and internal organs. It occurs primarily secondary to a collagen disorder resulting from mutations in one of the genes coding fibrillar collagens or enzymes involved in the synthesis of collagens . Typical features include skin hyperextensibility, fragile tissues prone to dystrophic scarring, easy bruising, and hypermobility of the joints. Body stature and habitus are usually normal, in contrast to MFS.
At least 10 different forms of the disease have been recognized; however, in 1997, Beighton et al . proposed a simpler classification reducing the number of major types to six (I–VI) and gave descriptive names: the arthrochalasia type, the classic type, the dermatosparaxis type, the hypermobility type, the kyphoscoliosis type, and the vascular type . The classic hypermobile and vascular types have a higher prevalence of aortic dilatation. Vascular EDS (formerly type IV) is an autosomal dominant disorder of type III collagen caused by mutations in COL3A1 (detected in 98%–99% of cases) that results in diminished arterial tensile strength due to a decreased amount of collagen III. Vascular EDS occurs in 1 in 10,000 or 200,000 patients. The pathologic manifestations of vascular EDS include arterial dissections (including aortic), rupture, and aneurysms of the aorta or large elastic arteries without dissection is more common, frequently resulting in sudden death. Characteristically, there are multiple rupture sites in the aorta ( Fig. 9.7 ). Arterial rupture is most prevalent in the third and fourth decades and mostly involves mid-sized arteries. The most frequently involved sites are the abdominal aorta and its branches, the great vessels of the aortic arch, and the large arteries of the limbs . Although, aortic root dilatation has been described as involving close to 30% of cases with classic or hypermobile form of EDS .
BAV is the most common congenital heart valve abnormality occurring in 1%–2% of the population (male-to-female ratio 2:1 to 4:1) . There is a well-known association between BAV with abnormalities of the aortic wall such as ascending AA and AD . The natural course of aortic abnormality related to BAV can vary from slow aortic diameter growth to rapid progression or early manifestation of life-threatening complications . In several large series, BAV was found to be the most common valvular abnormality associated with aneurysms of the ascending aorta . The prevalence of BAV with aneurysmal aortic dilatation reported in the literature ranges from 33% to 80% . In a comprehensive pathology study of 1025 consecutively excised aortic valves, unicuspid and bicuspid aortic valves were associated with increased replacement of the ascending aorta due to dilatation and aneurysm formation as compared to the tricuspid aortic valve (54.8%, 38.8%, 16.6%, respectively) .
Several investigators have reported histologic features of MEMA associated with BAV; in one study, 75% of those with BAV undergoing aortic valve replacement had biopsy-proven MEMA , whereas others have reported either mild MEMA or histologically normal media ( Table 9.2 ). One study found that aortic valve disease with pure aortic regurgitation in the presence of congenitally malformed aortic valves was associated with greater loss of medial elastic fibers in patients with ascending AAs, as compared to those with aortic stenosis. Patients with aortic regurgitation had a 35 times more likely significant loss of medial elastic fibers than normal controls and the majority of patients with aortic stenosis had no or minimal loss of elastic fibers suggesting that patients with aortic stenosis and ascending AA may only rarely require graft replacement .
The precise mechanisms underlying the development of TAA in BAV are not completely understood. Two general theories have been proposed and both may be operative in the pathogenesis of the disease . One theory ascribes aneurysm formation to flow-related disturbances on the proximal aorta that result in progressive aortic dilatation. It has been postulated that the position of the raphe in BAV may be partly responsible for flow patterns that lead to aneurysm development . A second theory proposes that a genetic or developmental abnormality results in histologic changes leading to weakness in the aortic wall. Several genetic variants related to either valve malformation or aortic complications have been identified (e.g., NOTCH1, TGFBR2, ACTA2, GATA5, NKX2.5, SMAD6, and ROBO4), although the prevalence of these variants was less than 5% in the study populations . A common developmental error is thought to explain this association as the aortic valve and ascending aorta share a common embryologic origin . Four types of aortic dilatation have been reported: aortic root (13%); ascending aorta alone (14%); ascending aorta and transverse arch (28%); and aortic root and tubular ascending aorta with tapering across the arch (45%) . Because nearly half of those with a BAV have some degree of aortic dilatation, cardiologists should routinely image the ascending aorta in all BAV patients.
In general, there is an under appreciation of the occurrence of ADs in patients with TS. TS is characterized by the absence of one X chromosome in a female. Individuals with TS have short stature and nonfunctioning ovaries, which result in infertility. ADs are six times more common in TS than in the general population, with an estimated incidence of 40 cases per 100,000 TS years . It is most frequently seen in women in their mid-30s but has been reported in children and adolescents. A bicuspid valve occurs in 30% of patients with TS, but of TS patients with dissections 95% have bicuspid valve. The syndrome is also associated with coarctation of the aorta; it affects 12% of patients and is observed in 89% of children and adolescents with dissection. Hypertension is present in 90% of TS with dissection. Therefore, if hypertension is observed in a patient with TS, it should be aggressively treated .
Numerous studies have documented the emerging role of aortitis in the pathogenesis of ascending AAs, particularly among the elderly . Aortitis is defined as inflammation of the wall of the aorta with or without disruption of elastic fibers, aortic wall necrosis, or fibrosis. By definition, one must exclude underlying conditions that may secondarily result in chronic inflammation. The most important of these is atherosclerosis, which is characterized by chronic inflammation of the intima, often with involvement of the media and even adventitia. In addition, because atherosclerosis may be superimposed on aortitis, the distinction between atherosclerosis and aortitis as a primary diagnosis is sometimes difficult. This distinction is usually facilitated, however, by considering the distribution of the process and clinical data.
Aortitis is classified broadly into infectious and noninfectious causes ( Table 9.4 ). The reported frequency in surgical series evaluating aortic tissues has varied greatly from 1% to 22% . In two pathology series published in the last decade, patients with aortitis were generally older than 50 years and subjects with noninfectious aortitis were more frequently women whereas infectious aortitis primarily affected men . These findings corroborated earlier studies in which similar age and sex profiles were reported . The 2012 Revised Chapel Hill Consensus Conference Nomenclature of Vasculitides recognizes two major aortitis variants: Takayasu’s arteritis (especially in younger patients) and giant cell arteritis (in older patients) . A third diagnostic category, referred to as nonspecific, isolated, or idiopathic aortitis, has also been proposed to account for aortitis without systemic involvement . Histological features of isolated aortitis are indistinguishable from Takayasu’s disease and giant cell aortitis, but these patients have no clinical evidence of autoimmune disease ( Fig. 9.8 ). The two features most helpful for distinguishing isolated aortitis from the other forms of aortitis are both clinical rather than microscopic in nature. They include the absence of signs or symptoms of extra-aortic arteritis and the absence of systemic disease. Because there are no pathognomonic histologic features for the three major types of aortitis, evaluation of clinical and pathologic data is important to arrive at a final diagnosis. Noninfectious aortitis is important to recognize as it typically involves the ascending aorta and causes aneurysms that lead to aortic root repair .
| Noninfectious |
| Inflammatory |
| Large vessel |
| Giant cell arteritis |
| Takayasu’s arteritis |
| Isolated “idiopathic” aortitis |
| Aortitis of collagen vascular disease |
| Rheumatoid arthritis |
| Ankylosing spondylitis |
| Reiter syndrome |
| Behçet’s disease |
| Systemic lupus erythematous |
| IgG4-related systemic disease |
| Granulomatosis with polyangiitis |
| Inflammatory aneurysms of the abdominal aorta |
| Sarcoidosis |
| Infectious |
| Bacterial |
| Salmonella spp. |
| Staphylococcus spp. |
| Streptococcal spp. |
| Other |
| Syphilitic aortitis |
| Mycobacterial (i.e., Mycobacterium tuberculosis ) |
Other noninfectious diseases that may involve the aorta are IgG4-related disease-associated aortitis, rheumatoid arthritis, ankylosing spondylitis, Reiter’s syndrome, Behçet’s disease, and rarely, granulomatosis with polyangiitis (GPA) (formerly Wegener’s granulomatosis). Tuberculous aortitis is currently rare in developed countries and involves the thoracic or abdominal aorta with equal frequency and is usually the result of contiguous spread from a tuberculoma from the lung or periaortic lymph node. Very rarely, the aorta may be involved by sarcoid, which tends to involve small and medium-sized arteries of the lung. Since tuberculous and sarcoid involvement of the aorta are rare, they will not be discussed further.
Takayasu’s arteritis was first described by the Japanese ophthalmologist, Takayasu, in 1908 in a 21-year-old woman with ocular changes . The incidence of Takayasu’s disease in North American and European populations is approximately 2 per million per year. The majority of the reported cases have been in Asian and African patients, but the disease has a worldwide distribution . The average age at diagnosis is 25–30 years and anywhere from 75% to 97% of patients are female . Clinical criteria for establishing the diagnosis have been in place since 1990 and include age at disease onset <40 years; claudication of extremities; decreased brachial artery pulse; blood pressure (systolic) difference (between arms) >10 mm Hg; bruit over subclavian artery or aorta; and arteriogram abnormalities. To establish the diagnosis of Takayasu’s arteritis the presence of at least three of the above six criteria are required .
The most common presentation includes symptoms from arterial occlusive disease of the aorta, aortic arch, and large vessels. In addition to stenotic lesions, aneurysmal dilatations may cause palpable pulsatile masses, embolism from mural thrombi, and rarely sudden death from rupture of a rapidly expanding aneurysm. Although stenotic lesions generally dominate, the incidence of aneurysmal lesions is estimated between 30% and 45%; indeed, aneurysms may be the sole clinical manifestation . Involvement of the aortic root and aortic valve occurs in 10%–20% of patients and may lead to aortic insufficiency ( Fig. 9.9 ) . Rapid AA expansion, aortic rupture, and the development of AA at the site of anastomosis of prior reconstructive surgery have also been reported . Pulmonary arteries may also be involved, and symptoms may mimic pulmonary embolism or pulmonary hypertension.
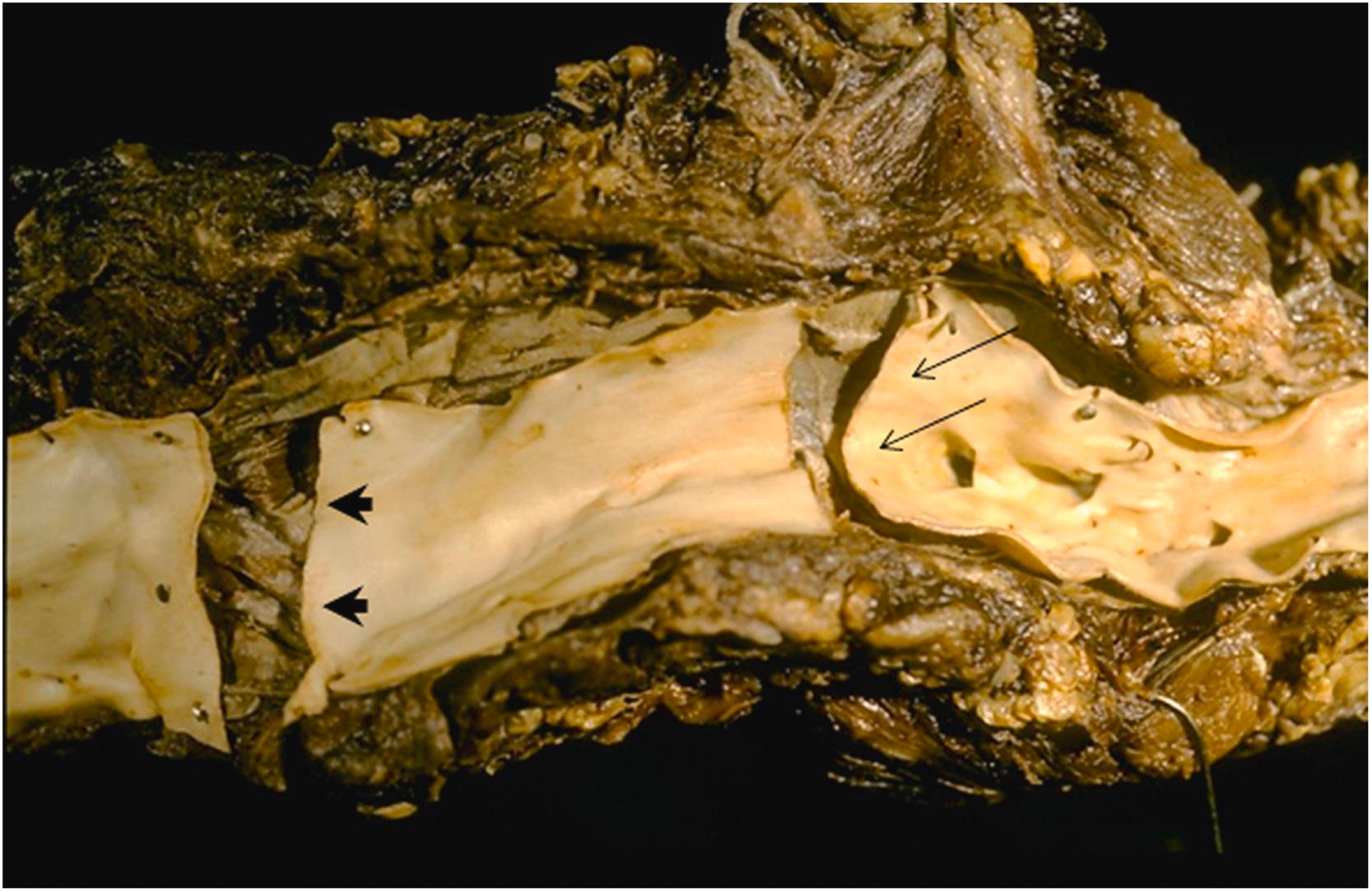
![Figure 9.9, Takayasu’s aortitis in a 51-year-old white female with severe congestive heart failure. Physical examination revealed aortic incompetence and pericarditis. Coronary angiography showed bilateral ostial stenosis. (A) At autopsy, there were thickened and fibrotic aortic valve cups but no commissural fusion. Instead, there is widening of the commissures ( arrow ). Also, note the markedly thickened ascending aortic wall. (B) A higher magnification of the anterior portion of the aortic arch and narrowing of the aortic arch vessels. [118] . Figure 9.9, Takayasu’s aortitis in a 51-year-old white female with severe congestive heart failure. Physical examination revealed aortic incompetence and pericarditis. Coronary angiography showed bilateral ostial stenosis. (A) At autopsy, there were thickened and fibrotic aortic valve cups but no commissural fusion. Instead, there is widening of the commissures ( arrow ). Also, note the markedly thickened ascending aortic wall. (B) A higher magnification of the anterior portion of the aortic arch and narrowing of the aortic arch vessels. [118] .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Aneurysmsoftheaortaascendingthoracicandabdominalandtheirmanagement/8_3s20B9780128222249000098.jpg)
The acute phase of Takayasu’s disease is characterized by edema, patchy necrosis, chronic inflammation, and scattered giant cells in the outer two-thirds of the media, adventitia, adventitial fat, and vasa vasorum ( Fig. 9.10 ). The vasa vasorum may show intimal proliferation with obliteration but no fibrinoid necrosis.
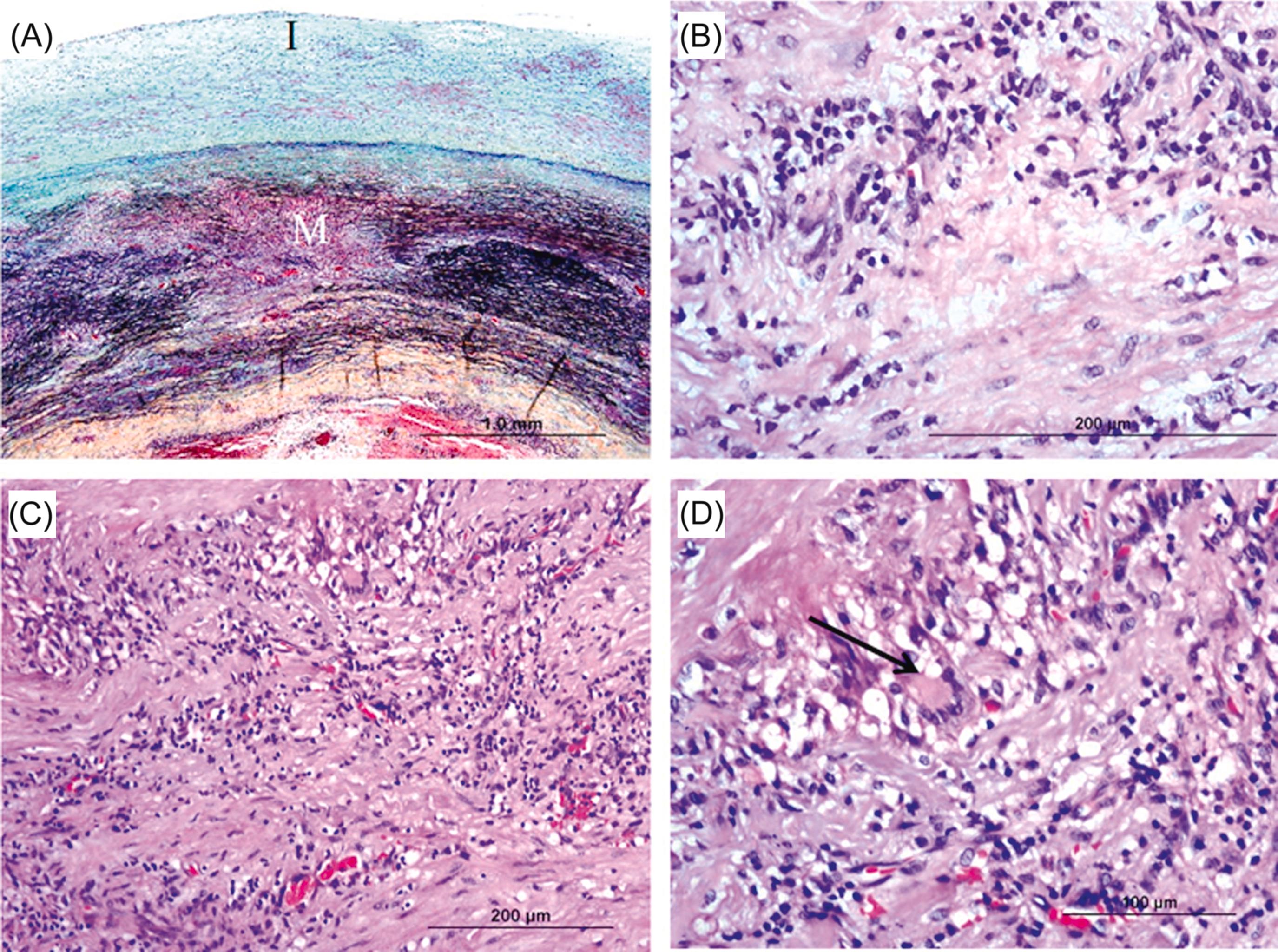
The late phase is characterized by marked intimal and adventitial thickening of the vessels. Scarring and revascularization gives rise to the tree-bark appearance characteristic of aortitis. The stenotic lesions are produced by a circumferentially thickened intima with a glossy, gray, or myxoid appearance of the cut surface. Multisegmental involvement with areas of normal between affected segments is characteristic, although there may be diffuse involvement of the aorta and isolated disease of individual arteries ( Fig. 9.11 ).
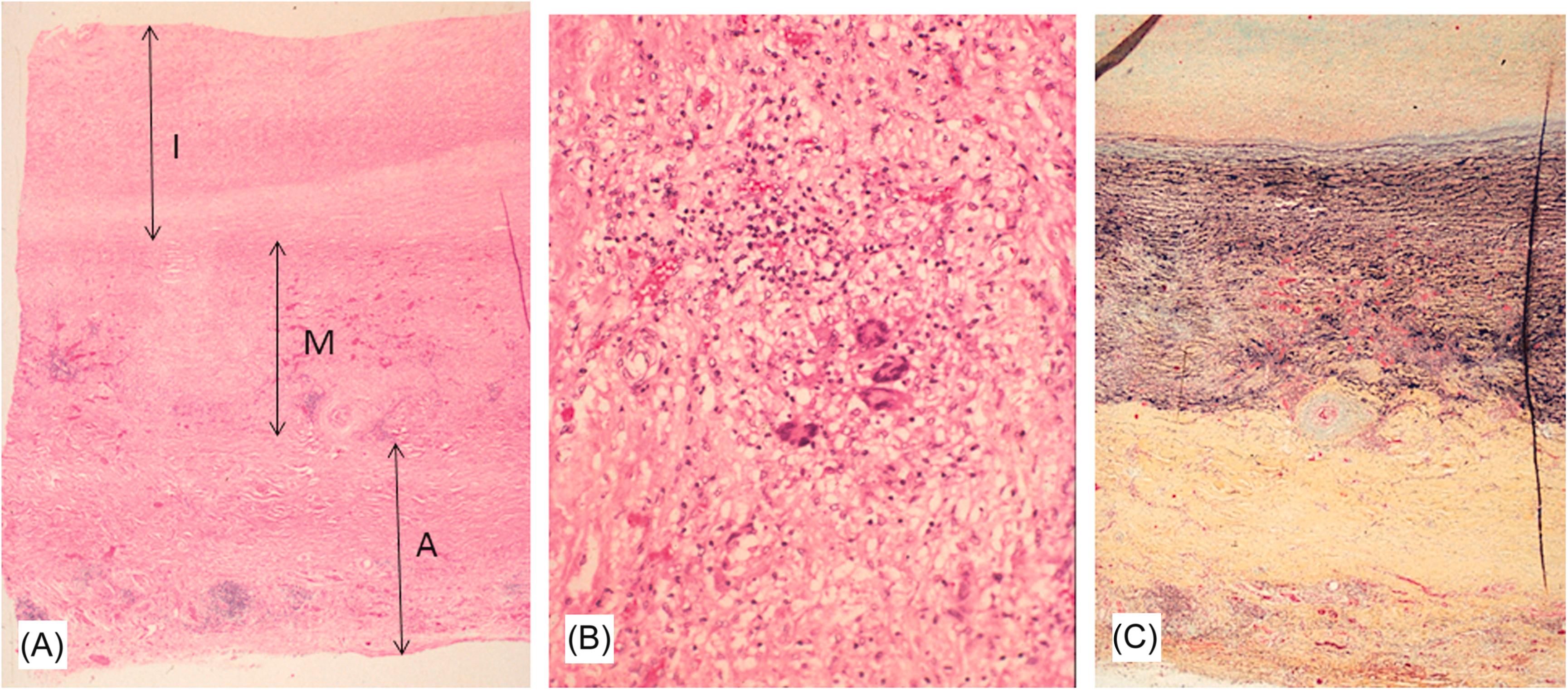
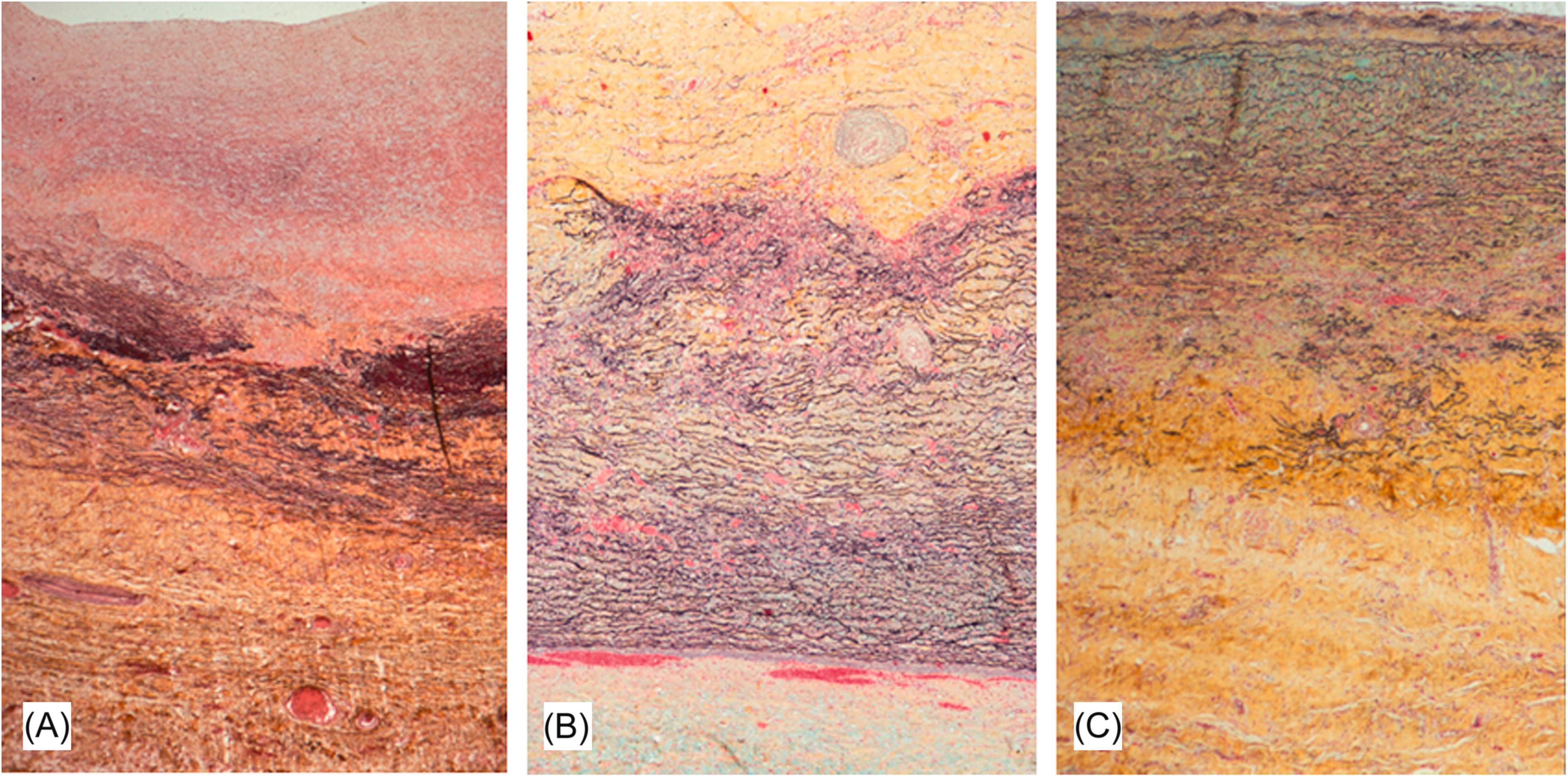
In the chronic phase, the intima is hypocellular with scattered smooth muscle cells and fibroblasts ( Fig. 9.12A ). The medial elastic laminae are disorganized or focally absent and replaced by collagen and granulation tissue ( Fig. 9.12B and C ). Areas of necrosis with giant cell infiltrates occasionally persist into the late phase. Areas of scarring may demonstrate dystrophic calcification of the media and adventitia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here