Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Subarachnoid hemorrhage (SAH) is a neurologic emergency. Severe and sudden headache is the most common presentation, but patients can experience loss of consciousness or seizures at onset.
Most patients with nontraumatic SAH will harbor a ruptured cerebral aneurysm.
Patients with SAH need to be evaluated immediately and admitted to an intensive care unit environment.
The most frequent and dreaded neurologic complications associated with SAH include hydrocephalus, delayed cerebral ischemia, rebleeding, seizures, and hyponatremia. Practitioners must be aware and monitor and treat patients for these complications.
SAH outcome is still dismal with a high mortality rate and dependency in one-third of survivors.
A woman, of forty years of age, and much given to drinking, was seiz’d with an apoplexy. From this she became paralytic in both sides, and was brought into the hospital at Padua, and there she soon died…. The vessels of the pia mater were so distended with blood, that the larger ones were almost black; and the smallest made a very beautiful appearance, as if injected with red wax…. [T]he trunk of that artery into which the vertebrals are conjoin’d, exhibited a small white elliptical spot…. I found it was not of that kind, which is generally us’d to be the beginning of an ossification, as I had thought; but somewhat soft in the parietes of the artery itself, and rather in the interior coat . G. Morgagni, De Sedibus et Causis Morborum (1761)
Subarachnoid hemorrhage (SAH) refers to the extravasation of blood into the spaces filled with cerebrospinal fluid (CSF). In the absence of trauma, aneurysmal rupture is the most common cause of SAH. Often aneurysms remain silent until the cataclysmic rupture. Our understanding of the machinations underlying this condition has evolved; however, many issues remain unresolved. Despite advances in diagnosis and management, the mortality rate for SAH remains unacceptably high. The future holds promise, but there are obstacles to overcome.
Our concept of aneurysms began in ancient times. The Egyptian Papyrus of Ebers (c.1550 BC) contains an early description of an arterial aneurysm ; however, the first definite report of an intracranial aneurysm came later. Exactly when the first definite case report of SAH appeared in text is debated, although a vague description may have been referenced in Biblical writings. Our modern understanding that the anatomic framework gives rise to intracranial aneurysms began in 1664 when Sir Christopher Wren, under the direction of Thomas Willis, illustrated the collateral arterial circulation at the base of the brain (circle of Willis). In 1761, Morgagni published De sedibus et causis morborum (The Seats and Causes of Diseases), a collection of detailed clinicopathologic observations that may have included the earliest pathologically confirmed case of aneurysmal SAH. Approximately 50 years later, in 1812, Cheyne published Cases of Apoplexy and Lethargy …, which included an illustration of SAH presumably due to a ruptured aneurysm at the intracranial carotid artery bifurcation. In 1813, Blackall reported a clinical case of aneurysmal SAH in Observations on the Nature and Cure of Dropsies, which vividly described the pathologic appearance of a top-of-the-basilar artery aneurysm as a “horse-bean.” In 1859, William Gull detailed a series of 62 cases of intracranial aneurysms that included thorough clinicopathologic descriptions. He astutely suggested that the presence of an acute severe headache should raise the suspicion of SAH; he also provided a possible description of delayed ischemic deficit related to vasospasm in a patient with a ruptured left middle cerebral artery (MCA) aneurysm. The promulgation of lumbar puncture (LP) by Quincke in 1891 paved the way for the routine premortem diagnosis of SAH. A major breakthrough came in 1927, when Moniz first applied cerebral angiography to a living person to diagnose tumor; in 1933 he reported the successful visualization of an intracranial internal carotid aneurysm. The successful clipping of an intracranial aneurysm by Dandy in 1937 marked the beginning of an era in which active intervention for SAH superseded the nihilistic approach. The history of SAH continues with the advent of sophisticated neuroimaging techniques, multimodality brain monitoring, and endovascular therapy (EVT).
Aneurysmal SAHs cause 2%–7% of all strokes but disproportionately account for 27% of stroke-related years of life lost before age 65. The incidence of SAH varies substantially by region, ranging from 2 per 100,000 population per year in Beijing, China, up to 27 per 100,000 population per year in Japan. , Finland has a relatively high incidence of SAH, 22 per 100,000 population per year. The worldwide aggregate incidence of SAH is 10.5 per 100,000 per year, which is approximately the same as the incidence in North America (United States and Canada). The incidence of SAH has remained stable over approximately 40 years. , There is ethnic variability in the incidence of SAH, with a higher age-adjusted incidence among Mexican-Americans than in non-Hispanic whites. The incidence of SAH increases with age. The mean age at presentation is 49–55 years. , There is a relative risk (RR) of SAH of 1.6:1 for women in comparison with men. , In North America, the RR of SAH is 2.1:1 for black in comparison with white persons.
The prevalence of intracranial aneurysms in the general population is between 1% and 6% on the basis of autopsy studies, and 0.5%–1% on the basis of angiographic data. Of these, approximately 20%–50% rupture during the person’s lifetime. Approximately 12%–45% of patients with aneurysmal SAH have multiple aneurysms. The risk for development of a new (de novo) aneurysm after diagnosis of SAH is approximately 2% per year, according to results of a study using serial angiographic screenings.
The case fatality rate for SAH has been gradually decreasing, by approximately 0.5% per year. Between 1945 and 1974 in Rochester, Minnesota, the case fatality rate was 57%; it declined to 42% between 1974 and 1984. The case fatality rate varies by region, with a 28-day age-adjusted mortality of 23% reported in Beijing, China, to 62% in former Yugoslavia. An analysis of the Nationwide Inpatient Sample showed that between 2004 and 2008, the case fatality rate decreased to 20%, compared with 30% in the years from 1979 to 1983. Overall, approximately 10%–20% of patients with SAH die before reaching the hospital, and approximately 25% die within 24 hours of the ictus. , ,
There are diurnal and seasonal variations in the occurrence of SAH. , , In two Japanese studies, peaks of SAH occurred from 7 to 10 a.m. and from 5 to 8 p.m., an effect attributed to circadian changes in blood pressure (BP). The nadir diurnal occurrence was between 10 p.m. and 6 a.m. A seasonal variation in the incidence of SAH is not consistent in all studies. , , A Japanese study found a greater predominance in spring than in summer for both sexes ; however, this pattern was not seen in another Japanese study. A Danish study found a modest statistically significant increase in SAH hospitalization rates in the month of January over those in July. Other factors that may influence SAH occurrence include changes in the ambient temperature, barometric pressure, and humidity. ,
Many potential risk factors for SAH have been studied, but only a few have been convincingly identified. , , Risk factors are categorized as modifiable or nonmodifiable. The most important modifiable risk factors are cigarette smoking and hypertension; other modifiable risk factors for SAH include heavy alcohol use, cocaine abuse, caffeine and nicotine intake in pharmaceutical products, and use of nonsteroidal antiinflammatory drugs, but these are less well established. , , Contrary to traditional beliefs, oral contraceptive use, hypercholesterolemia, and exercise are probably not associated with an increased risk of SAH. ,
Cigarette smoking is a robust modifiable risk factor for aneurysmal SAH. In 1996, Teunissen and colleagues systematically reviewed studies evaluating risk factors for aneurysmal SAH. Among two longitudinal and seven case-control studies that evaluated smoking as a possible risk factor, , , the aggregate RR and odds ratio (OR) for SAH were 1.9 and 3.5, respectively, with smoking. At least five subsequent retrospective studies found that active cigarette smoking was an independent risk factor for SAH. , , In addition, in one of these studies, previous smoking was also a potent independent risk factor for SAH, with an OR of 4.1.
Hypertension is another robust modifiable risk factor for SAH. , , , In the review by Teunissen and colleagues of seven case-control studies a
a References 45, 46, 48, 49, 51, 62, 63.
and three longitudinal studies, , , hypertension had an aggregate OR of 2.9 and an RR of 2.8. Furthermore, four subsequent retrospective studies demonstrated that hypertension was an independent risk factor for SAH. , , ,
Heavy alcohol use is an inconsistently reported risk factor for SAH. , , , In the pooled analysis of SAH risk factors by Teunissen and colleagues, which included two longitudinal series , and three case-control series, , , heavy alcohol use (>150 g/day) was a statistically significant risk factor, with an RR of 4.7 and an OR of 1.5. In contrast, several other studies found that heavy alcohol use was not an independent predictor for SAH. , Broderick and associates found that consumption of more than two alcoholic drinks per day was not an independent risk factor for SAH. Qureshi and coworkers reported that alcohol use, defined as more than one alcoholic drink per day, was not an independent risk factor for SAH.
Nonmodifiable risk factors for SAH include a family history of SAH in a first-degree relative, female sex, low educational achievement, low body mass index, and undetermined genetic factors. b
b References 14, 25, 40, 42, 59, 60.
Some known inherited conditions associated with SAH and/or intracranial aneurysms include adult dominant polycystic kidney disease (ADPKD), Ehlers-Danlos disease (type IV), α 1 -antitrypsin deficiency, sickle cell disease, pseudoxanthoma elasticum, hereditary hemorrhagic telangiectasia, neurofibromatosis type I, tuberous sclerosis, fibromuscular dysplasia, and coarctation of the aorta. , , ,
A family history of SAH in a close relative is an important nonmodifiable risk factor. , , , , People with a first-degree relative with SAH have an RR of 3–7 for SAH in comparison with the general population ; however, having a second-degree relative with SAH does not significantly increase the risk over that of the general population. A maternal history of SAH may portend higher risk than a paternal history. Regarding the increased risk of SAH in patients with a family history of SAH, the clinical implications for screening are controversial. For example, a prospective observational study found that routine screening for aneurysms in first-degree relatives of patients with SAH did not translate into a hypothetical clinical benefit, owing to the anticipated postoperative disability that was expected to outweigh the reduction in mortality from the repair of asymptomatic aneurysms at low risk for rupture.
Female sex is another important nonmodifiable risk factor for SAH. Women have 1.6 times the risk for SAH that men have, and the higher risk in women may increase further with advancing age. The reasons for sex-related differences in SAH may be related to menstrual and hormonal influences. , , , , An earlier age of menarche (<13 years old) and null gravidity are associated with significantly increased ORs of 3.2 and 4.2, respectively, for SAH. Two studies found an increased risk of SAH with delayed age of initial parity. , Higher parity may reduce the risk of SAH. Furthermore, a retrospective study reported a reduced risk of SAH in postmenopausal women taking hormone replacement therapy.
ADPKD is a known risk factor for SAH. Intracranial aneurysms are present in 5%–40% of patients. Patients with the disease who also have SAH tend to be younger and male and have a higher proportion of MCA aneurysms than the general population of patients with SAH.
Excluding trauma, rupture of a saccular (berry) aneurysm is the most common cause (85%) of SAH. Perimesencephalic hemorrhage (10%) is the next most common cause, followed by myriad uncommon etiologies, including arteriovenous malformations (AVMs), intracranial arterial dissections, and others (5%) ( Table 29.1 ).
| Category | Cause |
|---|---|
| Inflammatory | Vasculitis |
| Vascular | Perimesencephalic hemorrhage, cerebral arteriovenous malformation (AVM), intracranial arterial dissection, carotid-cavernous fistula, cerebral sinus venous thrombosis, eclampsia, spinal AVM, spinal artery aneurysms, moyamoya disease , a |
| Infectious | Mycotic aneurysms, gnathostomiasis (parasitic), Lyme vasculitis |
| Neoplastic | Pituitary apoplexy (adenoma), carcinomatous meningitis |
| Hematologic | Coagulopathy, thrombocytopenia, sickle cell disease |
| Drugs | Cocaine, amphetamines |
| Other | Eclampsia |
a Mechanism thought to be rupture of transdural anastomotic vessels.
Perimesencephalic nonaneurysmal subarachnoid hemorrhage (PNSH) is a distinct form of nonaneurysmal SAH. The pathophysiology is not well known but may be associated with venous anomalies. , Clinically, patients present similarly to those with aneurysmal SAH; however, patients with PNSH are generally alert, without loss of consciousness at onset, and have no risk factors for aneurysms (i.e., hypertension and smoking). The standard computed tomography (CT) definition of PNSH dictates that the blood is located mainly anterior to midbrain, in the interpeduncular cistern, or anterior to the pons, in the prepontine cistern, but may extend to the anterior ambient cistern, quadrigeminal cistern, or basal sylvian fissure. In addition, there should be no involvement of the anterior hemispheric fissure, lateral sylvian fissure, or intraventricular hemorrhage (IVH). Head CT is not sufficient by itself to diagnose PNSH because posterior circulation aneurysms may produce a similar pattern of hemorrhage. By definition, angiography reveals no aneurysm. Angiographic vasospasm is not uncommon; however, delayed cerebral ischemia (DCI) is rare. Some patients may experience hydrocephalus and may rarely require a permanent shunt. The prognosis is usually excellent, and the risk of rebleeding is extremely low.
The processes preceding catastrophic aneurysm rupture occur on a continuum that involves aneurysm formation, growth, and ultimate rupture. The dynamic interactions among inflammatory, hemodynamic, hormonal, and genetic contributors that drive this insidious process are gradually being elucidated.
The saccular (berry) aneurysm is responsible for 85% of cases of aneurysmal SAH. Less commonly, aneurysms may be fusiform or mycotic. Fusiform aneurysms are dilatations of the entire arterial circumference, usually related to atherosclerosis. Mycotic (infectious) aneurysms are rare, may be saccular or fusiform, are usually associated with bacteremia, are typically seen in distal branches of the anterior circulation, and are found in the systemic arterial circulation. The best treatment for mycotic aneurysms is not well established but may involve antibiotics alone for aneurysms or adjunctive surgical or endovascular aneurysm repair.
Intracranial aneurysms are usually solitary (70%–75%) but may be multiple in some patients (25%–50%). The majority of saccular aneurysms arise from the circle of Willis and occur in the anterior circulation. The most common distribution for intracranial aneurysms includes the following arteries: anterior communicating (ACOM) (30%), posterior communicating (PCOM) (25%), middle cerebral (20%), internal carotid bifurcation (7.5%), and top of the basilar artery (7%). Other possible locations for aneurysms are the ophthalmic, anterior choroidal, anterior cerebral, pericallosal, superior cerebellar, anterior inferior cerebellar, posterior inferior cerebellar, posterior cerebral, basilar, and cavernous internal carotid arteries.
The origin of intracranial aneurysms stems from insidious and dynamic processes that slowly erode the structure of the arterial wall. Traditionally, it was believed that congenital defects in the tunica media at arterial bifurcations gave rise to intracranial aneurysms; however, this hypothesis has been largely refuted. First, because aneurysms form during life, it is likely that acquired factors rather than congenital abnormalities are implicated in the pathogenesis. , Next, the distribution of saccular aneurysms, predominantly in the anterior circulation, does not correlate well with the distribution of tunica media defects in the posterior circulation ; tunica media defects may be present without evidence of aneurysms. In addition, pathologic specimens of aneurysms show a distinct pattern of sclerosis, ischemia, and degenerative changes, which suggests that atherosclerotic processes are involved. , Finally, the site of rupture of aneurysms on pathologic studies occurs distal to the supposed defects.
During cerebral aneurysm pathogenesis, the arterial wall undergoes characteristic changes. Early on, hemodynamic factors such as hypertension result in endothelial cell injury and associated histopathologic findings of balloon-like protrusions and crater-like concavities, cytoplasmic swelling, and subendothelial fibrin and cellular infiltration. Other changes include degeneration of the arterial basement membrane and internal elastic lamina. Eventually, degeneration of the muscular tunica media occurs, possibly mediated by an apoptotic mechanism. Hemodynamic stressors may occlude the vasa vasorum, resulting in smooth muscle ischemia. Alternatively, impaired diffusion of nutrients across a damaged internal elastic lamina and basement membrane may result in tunica media degeneration.
Hemodynamic stress is considered a key mediator of aneurysm development. Contributors to hemodynamic stress include hypertension, abnormal anatomy of the cerebral vasculature, and abnormal blood flow associated with certain conditions (i.e., AVMs, sickle cell disease). It is demonstrated in animal models that the combination of hypertension with unilateral carotid artery ligation consistently produces intracranial aneurysms. Furthermore, a high prevalence of intracranial aneurysms is observed in patients with aplasia or hypoplasia of the internal carotid artery. There is a growing awareness that hemodynamic conditions producing certain patterns of arterial wall shear stress result in degradation of the arterial wall. Interestingly, areas of the arterial wall exposed to very low shear stress are most prone to growth.
In addition to hemodynamic factors, defects in arterial connective tissue structure (i.e., elastin and collagen) via either inherited or acquired conditions may predispose one to aneurysm development. Some evidence supporting this is the known association between certain diseases that affect connective tissue and aneurysms (i.e., collagen type III deficiency, α 1 -antitrypsin). ,
Inflammatory processes may also contribute to aneurysm development; however, it is unclear whether the inflammation is a primary cause of or a compensatory response to arterial wall stress. Chyatte and associates found higher levels of complement, T lymphocytes, macrophages, monocytes, immunoglobulins, and vascular cell adhesion molecule 1 in the walls of unruptured aneurysms than in normal control vessels.
Cigarette smoking may induce a proteolytic state by increasing the ratio of plasma and arterial wall elastase levels to α 1 -antitrypsin activity. In rabbits, the application of topical elastase to the arterial wall resulted in saccular aneurysm formation, growth, and rupture.
Hormonal factors such as estrogen are implicated in aneurysm development and SAH. Estrogen may exert beneficial effects on the cerebral blood vessels through mechanisms such as increased endothelial nitric oxide, mitochondrial production, and collagen strengthening. , Therefore the decrease in estrogen levels in postmenopausal women may have an adverse effect on the vasculature and possibly increase the risk for SAH; however, this issue requires further study.
The pathophysiology associated with aneurysm rupture is still being elucidated. Some clinical factors that may be useful to determine the risk of rupture of an unruptured aneurysm are the size and location of the aneurysm and a previous history of SAH ( Table 29.2 ).
| Size of Aneurysm (mm) | No History of SAH and Anterior Circulation Aneurysm | No History of SAH and Posterior Circulation or PCOM Aneurysm | History of SAH and Incidental Aneurysm |
|---|---|---|---|
| <7 | 0 | 2.5 | 1.5 anterior circulation; 3.5 posterior circulation (including PCOM) |
| 7–12 | 2.6 | 14.5 | n/a |
| 13–24 | 14.5 | 18.4 | n/a |
| >25 | 40 | 50 | n/a |
Several investigators have evaluated the histology of saccular aneurysms to identify features that may be associated with rupture. Frösen and colleagues evaluated the histology of 66 saccular aneurysms, both ruptured and unruptured, and found distinct patterns associated with ruptured aneurysms. Some of these were decellularization, apoptosis, matrix degeneration, loss of endothelialization, thrombus formation, and inflammatory infiltration. Inflammatory cells consisted mainly of T lymphocytes and macrophages. Similarly, Kataoka and associates found that ruptured aneurysms had significant inflammatory infiltration with macrophages and fragility of the wall compared with unruptured aneurysms.
Some anatomic features of the aneurysm may increase the risk of rupture; they include a smaller neck-to-body ratio and smaller caliber of associated draining arteries.
There may be specific genetic factors predisposing an aneurysm to rupture. In one study, a specific polymorphism in the endothelial nitric oxide synthase gene was found significantly more often in patients with aneurysmal SAH than in community controls and patients with unruptured aneurysms.
The classic presentation of SAH is an acute and severe headache, often described as the “worst ever.” The time to peak headache intensity is usually seconds. However, occasionally the headache may be mild and may respond to over-the-counter analgesics. Only a minority of patients have a warning “sentinel” headache days to weeks before an aneurysmal SAH, which probably represents a small aneurysmal leak. Unfortunately, this history is usually obtained retrospectively, because the headache may be transient, and even if a head CT is performed during the headache, its findings will be negative about half the time.
Syncope occurs in 50% of patients, a phenomenon that may be due to an abrupt rise in intracranial pressure (ICP), which exceeds the mean arterial pressure (MAP), thus resulting in a critically low cerebral perfusion pressure (CPP) and global cerebral ischemia. Seizures occur acutely in 6%–16% of patients. , ,
Other common manifestations are nausea, vomiting, neck stiffness, photophobia, and phonophobia. Retinal or preretinal hemorrhages (Terson syndrome) are seen on funduscopy in about 17% of patients, in relation to an acute rise in ICP; preretinal hemorrhages are associated with poor outcome. Occasionally, meningeal signs are present owing to the chemical meningitis associated with SAH. A depressed level of consciousness (LOC) is very common and ranges from mild drowsiness to coma.
Focal neurologic deficits related to aneurysm rupture may include intracerebral hemorrhage (ICH), aneurysmal mass effect, and postictal paralysis after complex partial seizures. Classically, one may observe a third nerve palsy with pupillary dilation, which is related to extrinsic compression of the oculomotor nerve by a PCOM artery aneurysm. The pupil is characteristically large and poorly reactive to light owing to the interruption of the parasympathetic fibers traversing the outside of the oculomotor nerve. This syndrome may also be seen with posterior cerebral artery and superior cerebellar artery aneurysms. The finding of sixth nerve palsy may be a “false localizing sign,” representing elevated ICP. In addition, one may observe impaired upgaze, which is seen in association with hydrocephalus and is related to pressure on the dorsal midbrain vertical gaze centers. Other focal neurologic deficits may be related to ACOM artery or MCA aneurysms ( Table 29.3 ).
| Location | Syndrome |
|---|---|
| Posterior communicating artery | Complete 3rd nerve palsy |
| Anterior communicating artery | Bilateral leg weakness, abulia |
| Middle cerebral artery | Contralateral hemiparesis, and aphasia or visuospatial defects |
| Internal carotid artery | Ophthalmoplegia, visual disturbances a |
| Basilar artery | Brainstem compression |
a Ophthalmoplegia related to internal carotid artery aneurysms is due to cavernous sinus involvement. Related visual disturbances are due to either optic nerve or optic chiasm compression and manifest as unilateral visual loss or bitemporal hemianopia, respectively.
Acute cardiac abnormalities associated with SAH are discussed in more detail later in this chapter; however, several key points are addressed now. First, cardiac arrhythmias are very prevalent in aneurysmal SAH, occurring in up to 91% of patients, and may be life threatening. Furthermore, electrocardiographic changes are very common, occurring in up to 100% of patients, and may even mimic acute myocardial infarction. Some abnormalities include peaked P waves, a shortened PR interval, a prolonged QT interval, inverted T waves, prominent U waves, Q waves, and elevation or depression of the ST segment.
The clinical presentation of aneurysmal SAH is usually straightforward; however, in the community, initial misdiagnosis occurs in 23%–51% of patients. Occasionally patients present with seizure, acute confusional state, subdural hematoma, or head trauma, making the underlying diagnosis of aneurysmal SAH more elusive. A delayed diagnosis often has a disastrous outcome. , In one study, rebleeding occurred in 65% of patients who were initially misdiagnosed and was associated with high mortality. Another study reported that of patients with an initial good clinical SAH grade, 91% had good or excellent outcome at 6 weeks when diagnosed correctly versus only 53% when the diagnosis was delayed. The most common misdiagnoses are migraine headache and headache of unknown cause ; other misdiagnoses include meningitis, influenza, hypertensive crisis, myocardial infarction, arthritis, and psychiatric disease. The most common reasons for misdiagnoses of SAH are failure to obtain appropriate imaging study and misinterpretation of or failure to perform an LP.
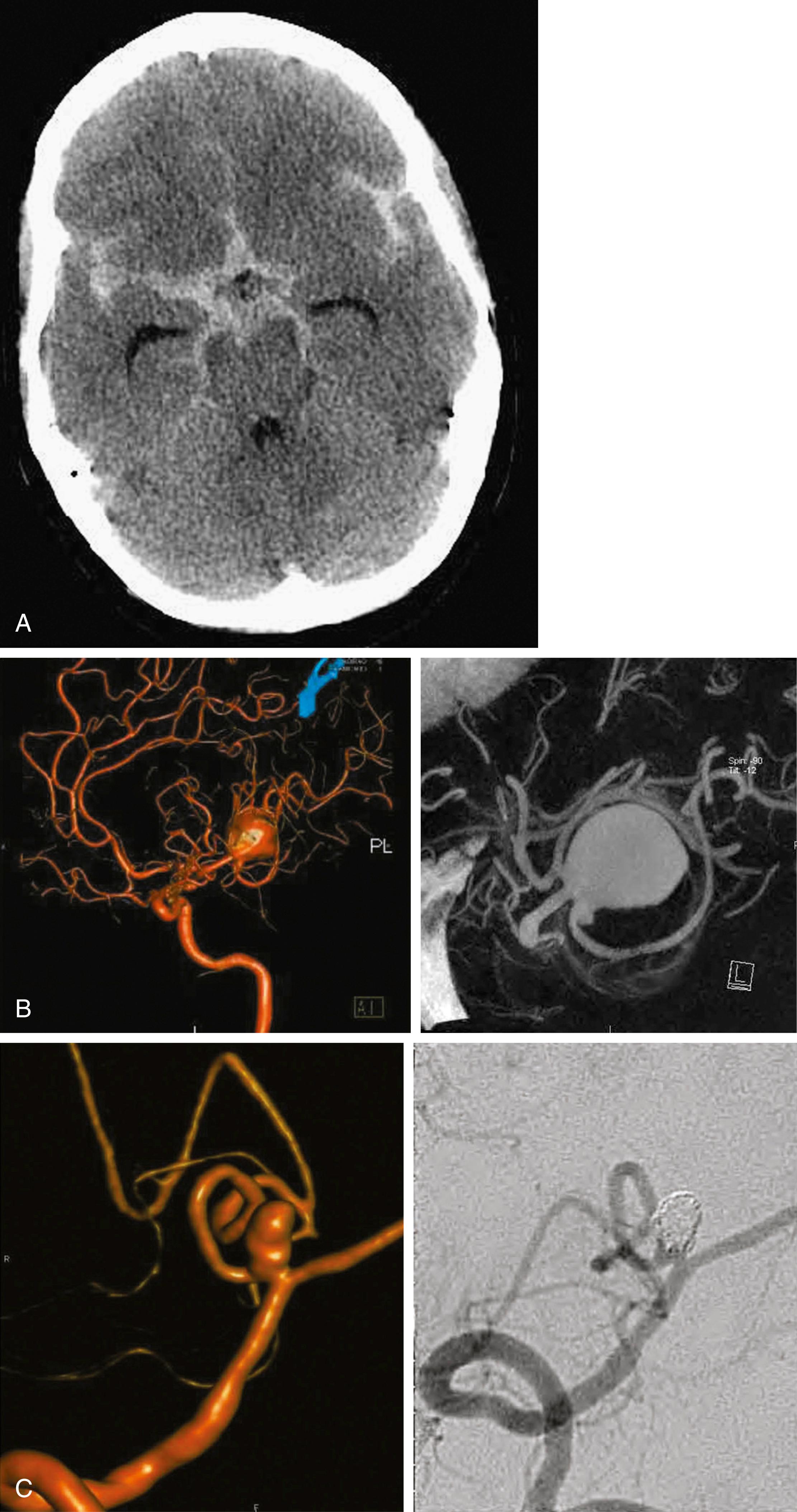
The most appropriate initial diagnostic test for SAH is a non–contrast-enhanced head CT scan. The sensitivity of CT for acute SAH is very high initially but diminishes with increasing delay between the onset of symptoms and imaging. Newer CT scanners (third-generation or later) are reported to be 98%–100% sensitive for detecting subarachnoid blood within 12 hours of onset of symptoms when compared with the “gold standard,” LP. However, the sensitivity of CT drops to 93% at 24 hours and 50% at 7 days. The accuracy of CT for SAH also depends on expert interpretation from a radiologist, because a preliminary reading by a nonexpert interpreter may be inaccurate. Classically, the head CT reveals hyperintense signal in the basal subarachnoid cisterns. Other locations include the sylvian fissures, interhemispheric fissure, interpeduncular fossa, and suprasellar, ambient, and quadrigeminal cisterns. There may also be associated ICH, IVH, subdural hematoma, cerebral edema, and hydrocephalus ( Fig. 29.1A ). The pattern of blood seen on head CT scans may suggest a certain location of the underlying aneurysm; however, the accuracy of this approach is poor and thus has little diagnostic value. The amount and location of blood on head CT scans remains one of the most important predictors of DCI (see Table 29.5 ).
| Grade | Fisher Scale | Modified Fisher Scale |
|---|---|---|
| 1 | No visualization of blood | Diffuse thin SAH with no IVH |
| 2 | Diffuse thin layer <1 mm thick in vertical layers | Any IVH with thin or no visualized SAH |
| 3 | Localized clots and/or vertical layers <1 mm thick | Diffuse or localized thick SAH without IVH |
| 4 | Diffuse blood, but intraventricular or intracerebral clots | Diffuse or localized thick SAH with IVH |
LP is considered the gold standard for detection of SAH. In patients with suspected SAH in whom head CT scan results are nondefinitive, it is absolutely essential to obtain an LP. LP may be contraindicated in patients suspected to have focal mass lesions, elevated ICP, and herniation. The presence of xanthochromia in the CSF strongly suggests SAH. In a retrospective study of patients with confirmed aneurysmal SAH ( n = 111), xanthochromia was present in 100% of patients when the CSF was collected 12 hours to 2 weeks after onset of symptoms and analyzed by spectrophotometry. Interestingly, xanthochromia was still present at 4 weeks after onset of symptoms in some (10 of 14) patients. Several hours after SAH, the lysing of red blood cells releases oxyhemoglobin, with subsequent formation of bilirubin, which has a yellowish appearance. This process may take up to 12 hours, so results of an LP performed too early after SAH may be falsely negative. One should evaluate CSF for xanthochromia with spectrophotometry rather than visual examination, which is not as sensitive. Most institutions rely on visual inspection of CSF. Other findings of CSF analysis that are suggestive of but not definitive for the diagnosis of SAH include elevated protein, elevated D-dimer levels, presence of coagulation, crenated erythrocytes, and elevated opening pressure. It may be difficult to differentiate a “traumatic tap” from SAH. The finding of a serially decreasing red blood cell count from the first to the last CSF tube may suggest a traumatic tap, but because this decrement may also be seen with aneurysmal SAH, it is not definitive. , In contrast, xanthochromia is usually absent with a traumatic tap if the CSF is promptly analyzed.
Magnetic resonance imaging (MRI) is not indicated as an initial diagnostic test for SAH; however, it may be useful if the head CT findings are negative but the results of the LP are abnormal. Mitchell and coworkers found that gradient echo T2-weighted MRI was 94% and 100% sensitive for detecting subarachnoid blood in the acute and subacute phases, respectively. In addition, Noguchi and associates , reported that the fluid-attenuated inversion recovery (FLAIR) MRI sequence detected SAH as well as non–contrast-enhanced head CT in the acute setting and better in the subacute to chronic phase.
The combination of normal CT and LP findings safely rules out SAH and thus should obviate cerebral angiography. In a prospective cohort study of patients with suspected SAH in whom CT and LP findings were negative, no SAH was observed in any patient during a 3-year follow-up. Similarly, four other studies, one retrospective and three prospective, , , that evaluated patients with acute severe headache found no evidence of subsequent SAH or sudden death on long-term follow-up in patients with initially negative head CT and LP findings. Consequently, angiography is not routinely indicated in these patients, because the possibility of SAH is remote. Furthermore, the finding of a small incidental intracranial aneurysm does not imply that it has ruptured and may lead to unnecessary surgical or endovascular interventions that may result in perioperative morbidity and mortality. Applying clinical rules may supplement the imaging in patients suspected of SAH. Accordingly, Perry et al. JAMA 2013 reported clinical decision-making rules developed in a large Canadian cohort study that had 100% sensitivity to detect SAH, which includes any of the following: age older than 40, neck pain or stiffness, witnessed loss of consciousness, or onset during exertion PLUS thunderclap headache (instant headache) and pain on neck flexion.
After diagnosis of SAH, the next step is to identify and aneurysm promptly. Current practice dictates the early treatment of ruptured intracranial aneurysms. Delayed treatment of aneurysms increases the risk of rebleeding and may prohibit aggressive hemodynamic management of DCI.
Since its introduction in 1927 by Moniz, cerebral angiography has remained the gold standard for diagnosis of intracranial aneurysms. , Advances in this technique have improved the diagnostic accuracy and decreased procedural morbidity. A “four-vessel” evaluation of the bilateral internal carotid arteries and vertebral arteries is necessary. The standard, digital subtraction angiography (DSA), may be supplemented by three-dimensional rotational angiography (3DRA) (see Fig. 29.1B and C). Van Rooij and colleagues retrospectively reviewed the ability of this modality to detect aneurysms in the setting of a negative DSA result in patients with SAH. Remarkably, 3DRA revealed small (<5 mm) ruptured intracranial aneurysms in 18 of 23 (78%) of patients with negative DSA results; 16 of the 18 underwent subsequent treatment, either surgical ( n = 7) or endovascular ( n = 9).
Catheter angiography carries significant risks. Neurologic complications may include arterial dissection or rupture, ischemic stroke, and seizures. Nonneurologic complications include groin or retroperitoneal hematoma, contrast agent nephropathy, allergic reaction to contrast agent, and femoral artery dissection. With an experienced operator, there is approximately a 1.0%–2.5% risk of neurologic complications and 0.1%–0.5% risk of permanent neurologic injury. , , , These complication rates apply to patients with various neurologic indications; patients who specifically have an angiogram for SAH may have different rates of complications. Interestingly, a study by Cloft and associates, who reviewed the incidence of angiographic complications in patients with SAH, unruptured intracranial aneurysms, or AVMs, found a combined rate of neurologic complications of 1.8%; however, the risk of permanent neurologic deficit was very low (0.07%).
CT angiography (CTA) is emerging as an alternative diagnostic test for SAH; however, the diagnostic accuracy may be less than that of standard angiography. , The accuracy of CTA for detecting aneurysms varies widely in different studies. The sensitivity and specificity of CTA in comparison with those for DSA range from 77% to 100% and 87% to 100%, respectively (see Fig. 29.1B ). , ,
Advantages of CTA include a quick procedural time, excellent anatomic rendering, and a low risk of complications. CTA reconstructed in 3D provides anatomically accurate relationships between the vascular structures and bone, which may be useful for surgical planning. , CTA poses less risk for iatrogenic complications than DSA because contrast agent is given intravenously for CTA, thus obviating the need for arterial manipulation. Interestingly, there are reported cases in which CTA revealed an aneurysm that was not seen with DSA.
Some important limitations of CTA include a lower sensitivity for smaller aneurysms (<4 mm) and posterior circulation distribution aneurysms in comparison with DSA. , Furthermore, the large bolus of contrast agent needed for CTA may increase the risk of contrast-induced nephropathy, especially if performed in conjunction with DSA.
Magnetic resonance angiography (MRA) is not appropriate to diagnose aneurysms in patients with suspected aneurysmal SAH. The sensitivity and specificity value for MRA for aneurysms in comparison with that for DSA ranges from 69% to 99% , ; however, these studies were performed mostly in patients with unruptured aneurysms. The sensitivity for detecting small (<4 mm) aneurysms is poor. Some other limitations of MRA are the prolonged time required for scanning, susceptibility to motion artifact, and inability to be performed in patients who carry metallic objects such as pacemakers.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here