Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anesthesiologists have long been at the forefront of patient monitoring. This has been of necessity because we are responsible for continuously assessing the patient's physiologic status and the effects of surgery and anesthetic agents. This chapter provides an introduction to the basic function and utility of the wide array of monitors employed in modern anesthesia care. Monitoring devices will be organized by organ system, not by physical property or technique on which that monitor derives its information. A detailed review of monitoring principles is available in a comprehensive text.
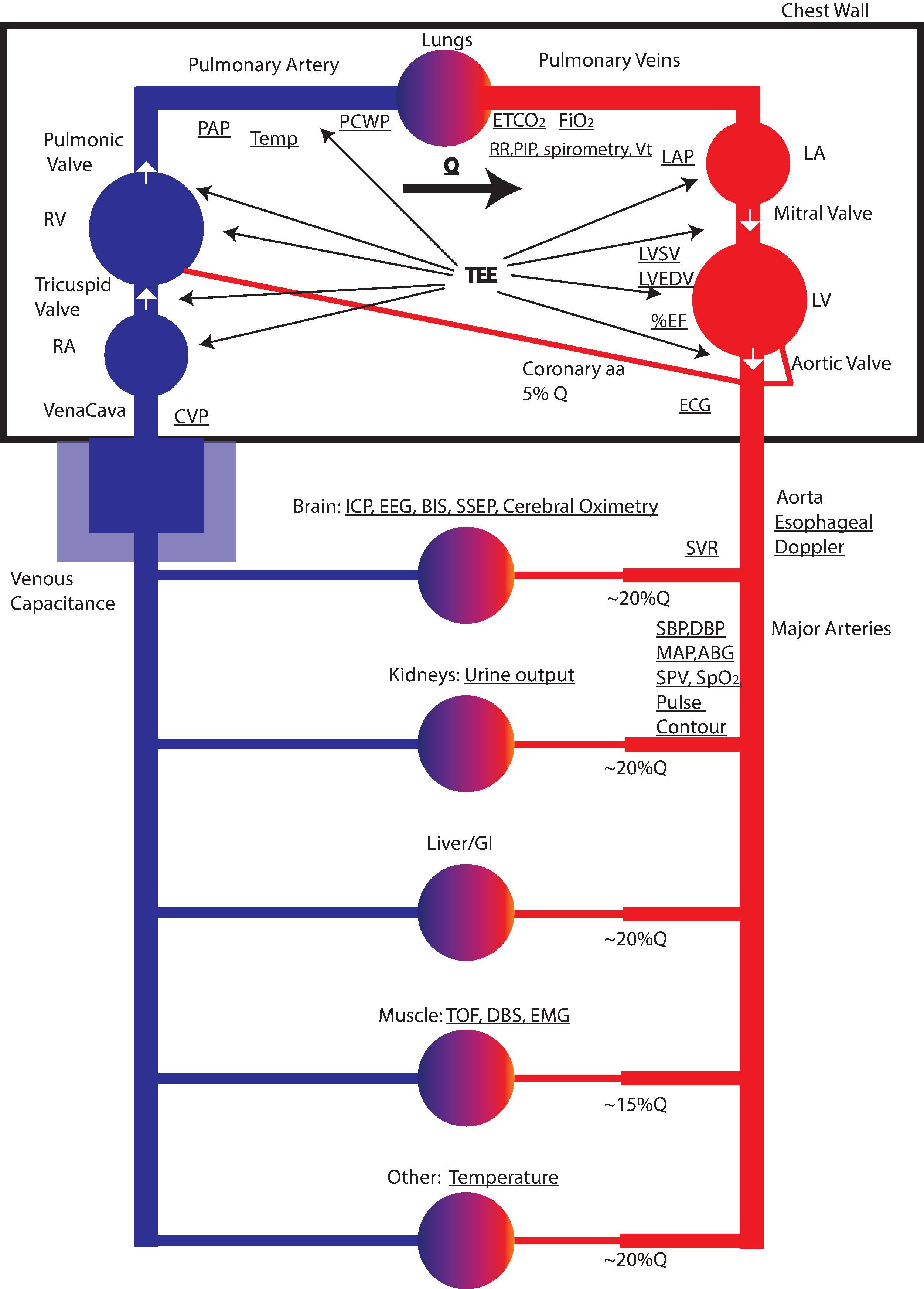
In 1986 the American Society of Anesthesiologists established a set of basic monitoring standards, stating that the patient's oxygenation, ventilation, circulation, and temperature shall be continually evaluated. These standards are periodically reviewed, affirmed, or updated, most recently in 2020. Many clinical situations will require additional monitoring. All of the organ systems monitored are perfused by the circulatory system ( Fig. 20.1 ). Our monitoring of the patient attempts to continuously assess if the patient's state is “normal” or “abnormal” and to correct the cause of the abnormality, or at least treat the abnormal number generated by our monitor. We must understand the limitations of our monitors and how to use data from multiple devices to confirm our diagnosis and follow our treatment.
Oxygen (O 2 ) is a colorless, odorless gas critical for cellular respiration. Lack of delivery of oxygen to tissues will result in cellular death. Carbon dioxide (CO 2 ) is a consequence of cellular metabolism and must be removed from the tissues to maintain acid–base homeostasis. This section will review monitors of patient oxygenation and ventilation.
Inspired Oxygen Inspired oxygen content, or fraction of inspired O 2 (FiO 2 ), can be measured by a variety of methods. Anesthesia machines most commonly use an amperometric sensor to measure O 2 in the fresh gas flow. Calibration is recommended, as the sensor, which is basically a fuel cell that consumes oxygen and generates current, has “drift” (i.e., the readings in a constant concentration of oxygen will not be constant). It is a slow responding device, meaning that it cannot be used to measure inspired/expired oxygen, as this rapidly changes. An alternative method of measuring inspired oxygen uses the fact that oxygen is paramagnetic. A paramagnetic oxygen sensor can be auto-calibrating, using room air as a source of 21% O 2 . The gradient between the sample and the room air can be measured by a pressure transducer or a torsion wire. The fast response time allows the measurement of both inspired and expired oxygen content. Measuring expired O 2 (FeO 2 ) concentration during preinduction/preoxygenation also allows the determination of complete preoxygenation/denitrogenation, aiming for FeO 2 >85%, in addition to a rough estimate of O 2 consumption.
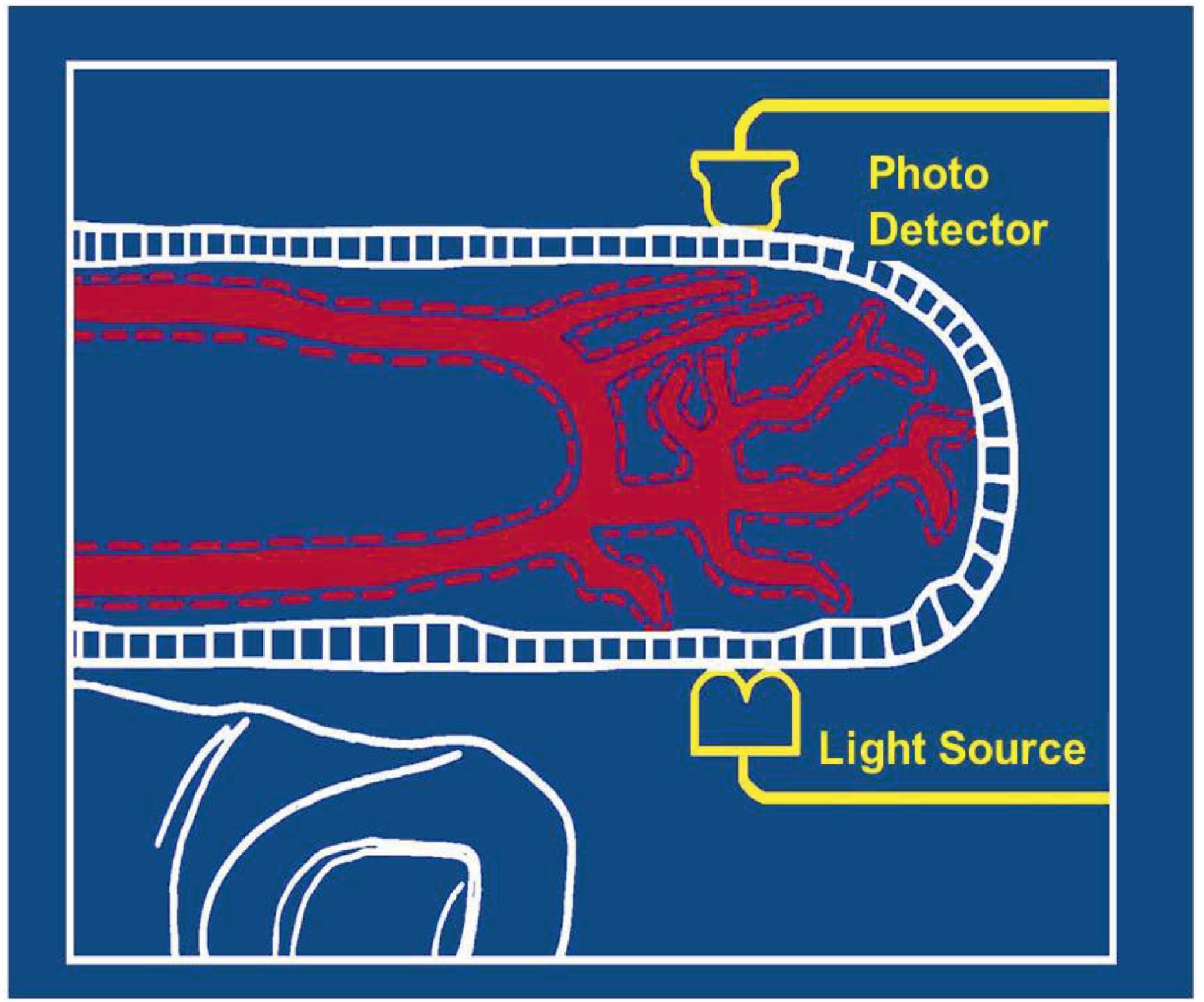
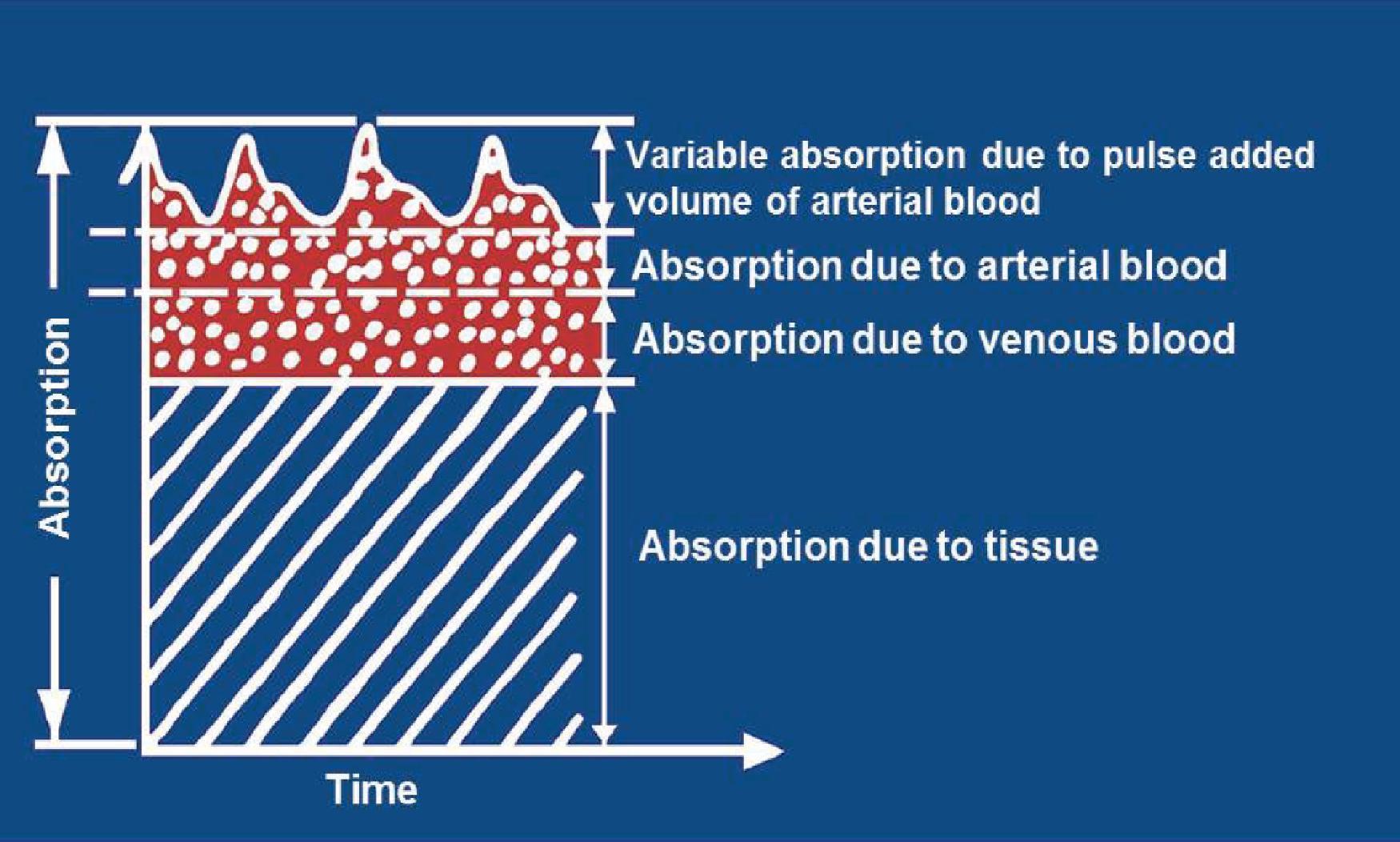
Pulse Oximetry The pulse oximeter provides a continuous noninvasive estimate of arterial hemoglobin saturation (SaO 2 ) by analyzing light transmitted through living tissue, most commonly the finger or ear ( Fig. 20.2 ). The physical principle known as Beer's law relates the concentration of a dissolved substance to the log of the ratio of the incident and transmitted light intensity through a known distance. Oxyhemoglobin and reduced (deoxy)hemoglobin absorb red and infrared light differently. The pulse oximeter contains a light-emitting diode (LED) that emits two wavelengths of light (red, 660 nanometers, and infrared, 940 nanometers) and a photodiode that measures light transmission. The device determines the signal related to arterial hemoglobin saturation by analyzing the pulsatile component of the light absorption tracing, hence the name pulse oximeter ( Fig. 20.3 ). The following equation is calculated continuously by the device to determine the ratio of pulse-added red to pulse-added infrared light absorbance:
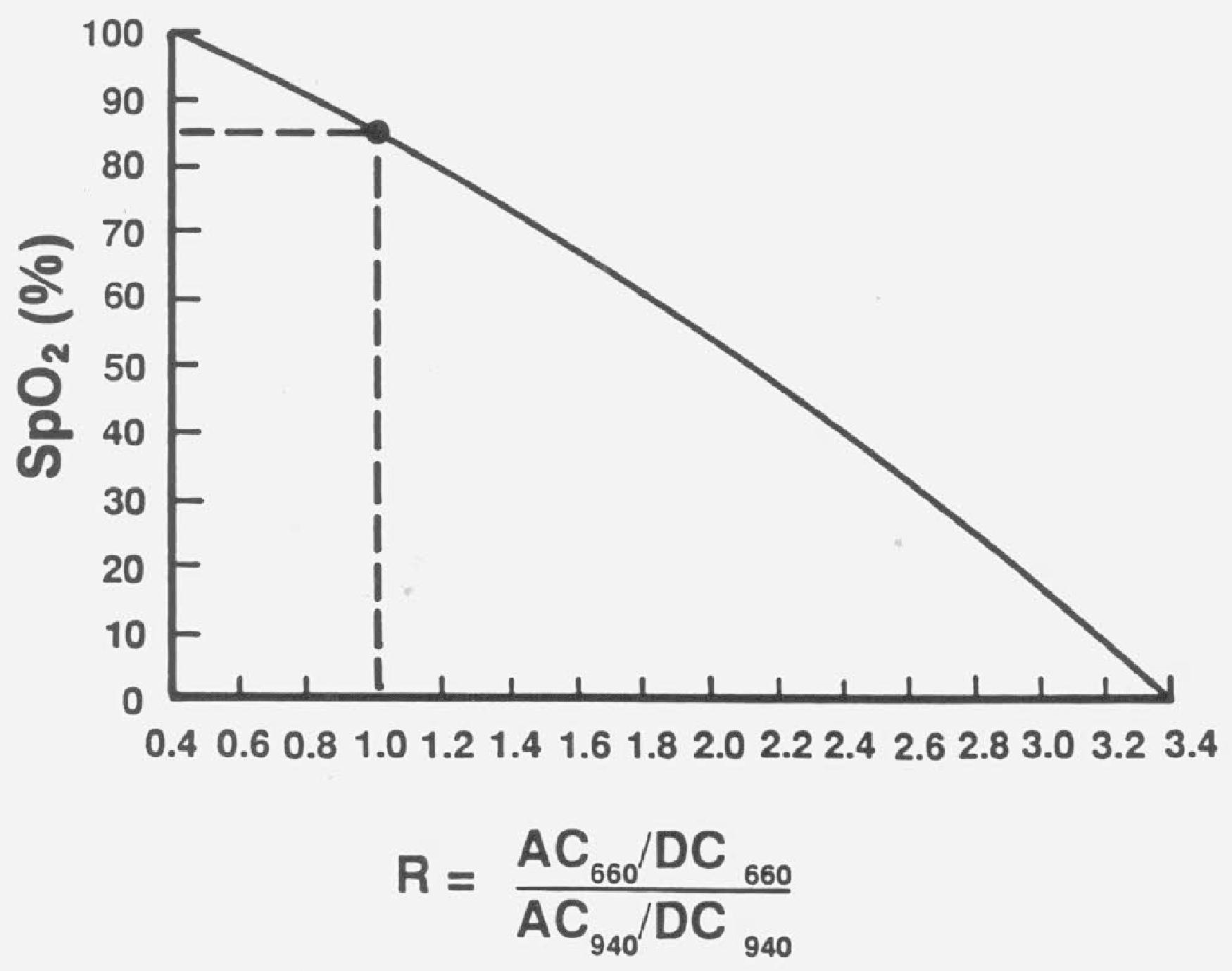
This ratio (R) of absorbance is empirically calibrated to estimate SaO 2 . That is, the device uses SaO 2 data derived from human volunteers to determine the relationship between the pulse oximeter saturation (SpO 2 ) and the ratio of light absorbance, R ( Fig. 20.4 ).
Dyes and Dyshemoglobins Standard pulse oximeters using two wavelengths of light can determine functional saturation, that is, the percentage of oxyhemoglobin, HbO 2 over HbO 2 plus reduced hemoglobin, Hb (two equations for two unknowns), using two equations:
Because pulse oximeters are calibrated using human volunteers who have little carboxyhemoglobin or methemoglobin, these forms of hemoglobin are not accounted for in a standard pulse oximeter's calculated SaO 2 ( Eq. 2 ). Therefore if either carboxyhemoglobin (e.g., from carbon monoxide poisoning) or methemoglobin (e.g., from benzocaine or prilocaine toxicity) is present, the devices will produce an erroneous saturation value. These abnormal hemoglobins can be measured by use of a multiwavelength blood gas cooximeter, which requires a blood sample for accurate measurement ( Eq. 3 ). Dyshemoglobinemias should be considered in patients with a discrepancy between PaO 2 on blood gas and SpO 2 or in specific clinical settings. Carboxyhemoglobin absorbs red light similarly to oxyhemoglobin, causing the pulse oximeter reading to equal the sum of carboxyhemoglobin and oxyhemoglobin, thus giving the impression the patient is adequately saturated with oxyhemoglobin even in the presence of severe carboxyhemoglobin toxicity. Methemoglobin has a dark appearance and absorbs both red and infrared light to a high degree, which causes the absorbance ratio R to tend toward 1. From the calibration curve, a ratio of 1 will produce an SpO 2 of 85% (see Fig. 20.4 ). Therefore an increasing amount of methemoglobin (especially >20%) will cause the pulse oximeter to display readings closer to 85%. That is, it will produce falsely low values when the patient has high SaO 2 and falsely high values of 85% when the patient is severely hypoxemic. Dyes produce similar errors as does methemoglobin (i.e., force the saturation towards 85%), although most are cleared from the circulation quickly and the error is only transient. Newer eight-wavelength pulse oximeters are available which can detect three saturations (oxy-, carboxy-, and methemoglobin). Motion artifact will also cause the SpO 2 value to tend toward 85% because the motion artifact produces noise in the numerator and denominator of Equation 1 . The ratio R is forced toward 1.0 like methemoglobin. In fact, any situation that results in a small signal-to-noise ratio may cause the SpO 2 to trend toward 85%.
The respiratory rate, pattern, and depth are all important descriptors of ventilation. Qualitatively, ventilation depth and pattern can be observed by chest rise, auscultation, or reexpansion of the rebreathing bag on the anesthesia machine. In any acute situation where the adequacy of ventilation is an issue eliminating monitoring devices altogether and going to the source by listening for bilateral clear breath sounds with a stethoscope should be done immediately. This may rule out tension pneumothorax, acute bronchospasm, endobronchial intubation, pulmonary edema, or absence of ventilation altogether.
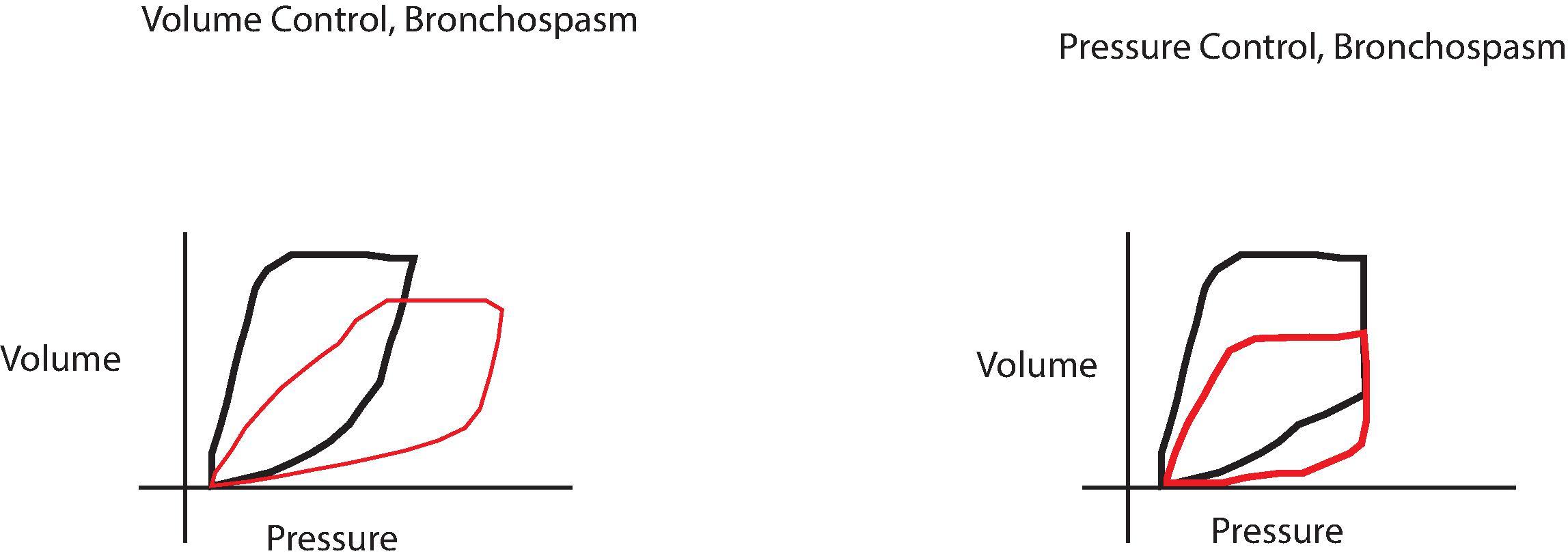
Airway Pressures Increases in peak airway pressure merit investigation, as they imply an acute increase in airflow resistance, either in the circuit or the patient (e.g., from endotracheal tube [ETT] obstruction or bronchospasm). Increased peak airway pressure can also be caused by decreases in lung or chest wall compliance. If peak airway pressure is increased and simultaneously positive end-expiratory pressure (PEEP) is increased, this may signify a tension pneumothorax, especially if associated with arterial hypotension. In this circumstance the hypotension is caused by high intrathoracic pressure impeding venous return. The mechanical ventilator can be set to produce a brief pause at end inspiration, allowing the plateau pressure to be measured. External obstruction of an ETT (e.g., from a patient biting on the tube or tube kinking) can cause an increase in peak inspiratory pressure (PIP) with minimal or no increase in the plateau pressure ( Fig. 20.5 ). This can be easily ruled out by passing a suction catheter down the ETT. A loss of or abrupt decrease in airway pressure is not specific, but can indicate a variety of major problems, including circuit disconnections, leaks, extubation, failure to deliver fresh gases, failure to set the ventilator properly, excess scavenging, and other anesthesia machine issues. Airway pressure can be measured with analog gauges or electronic pressure transducers, analogous to those used for blood pressure measurements.
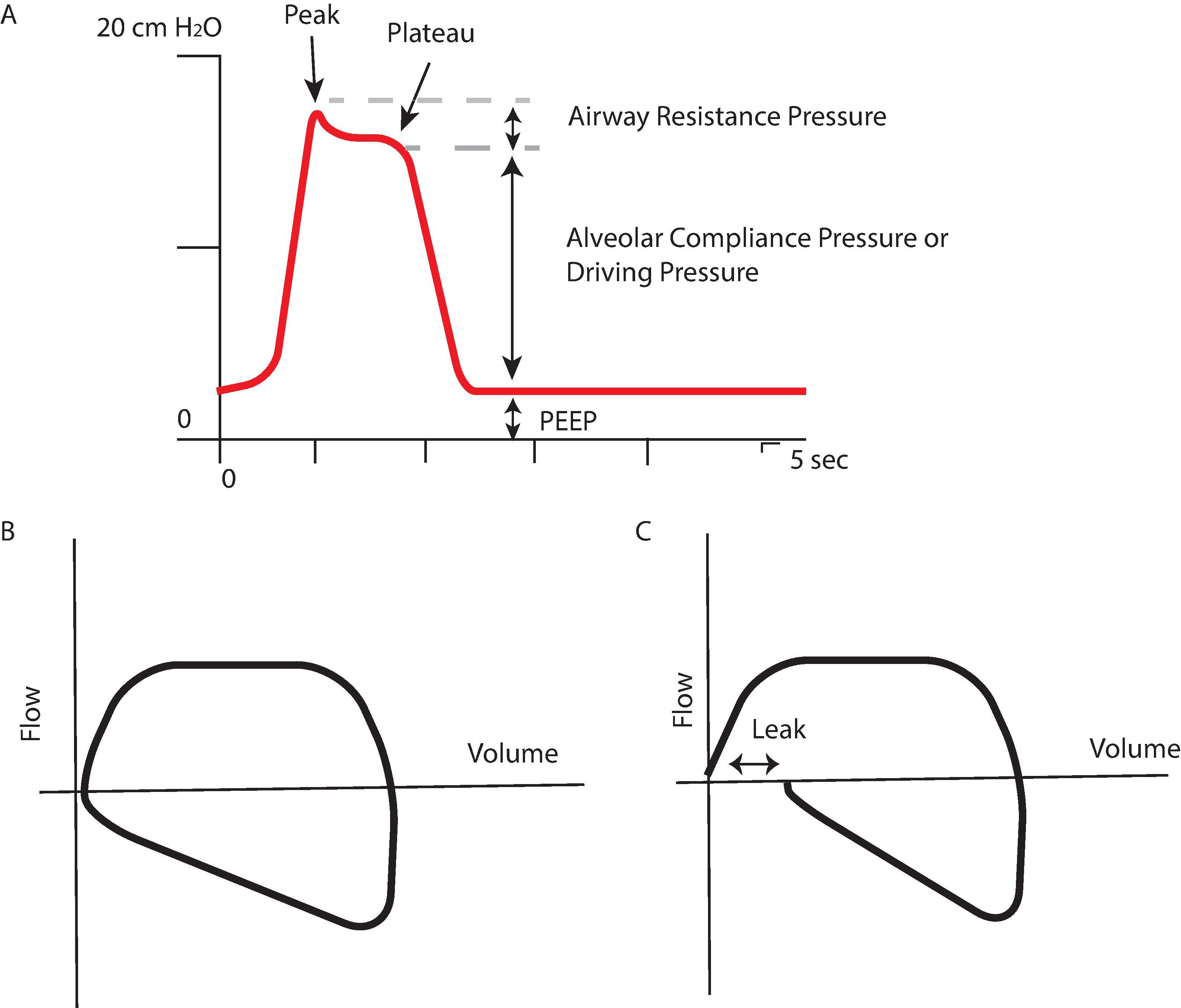
The difference between plateau pressure and PEEP is referred to as the “driving pressure.” The static respiratory system compliance equals tidal volume divided by driving pressure. Increased focus on driving pressure is relevant to the treatment of acute respiratory distress syndrome (ARDS), as increases in driving pressure are associated with increased mortality. In the operating room a metaanalysis of 17 randomized controlled trials of protective ventilation showed that increased driving pressure was associated with the development of postoperative pulmonary complications, whereas tidal volume and PEEP were not. Other studies of mechanical ventilation in the operating room include titration of “best PEEP” in patients undergoing elective thoracic surgery and open abdominal surgery. In these studies the optimum PEEP is defined as the PEEP level resulting in the greatest respiratory system compliance or lowest driving pressure. Although driving pressure has shown promise as a variable to monitor and titrate ventilator settings, it cannot be viewed in isolation, given the multiple factors that affect the value, interpretation, and changes with time. Driving pressure will remain a focus of interest in the management of mechanically ventilated patients inside and outside of the operating room; however, ventilator settings should be individualized to the patient.
Tidal Volume A 2013 randomized trial of low tidal volume in adult patients undergoing abdominal surgery found better pulmonary outcomes associated with setting tidal volume at 6 to 8 cc/kg of ideal body weight, in addition to intraoperative recruitment maneuvers and PEEP. This ventilation strategy is similar to that used in patients with ARDS (also see Chapter 41 ). Once these tidal volumes are set, the respiratory rate should be adjusted to maintain an end-tidal CO 2 (ETCO 2 ) in the normal range of 35 to 40 mm Hg. Modern ventilators use a variety of modes to achieve this tidal volume ( Fig. 20.6 ). Most ventilators have pressure limits that will alert when peak pressures are exceeded because of increased airway resistance in the circuit or in the patient ( Fig. 20.7 ). Monitoring the tidal volume and peak airway pressure together will enable the practitioner to quickly detect any changes in resistance to airflow because of resistance in the system or decreased compliance in the lung or chest wall ( Fig. 20.8 ). Tidal volumes can be measured by mechanical vanes rotating in the gas stream, pressure gradients across a flow restriction (fixed or variable), and hot wire anemometers.
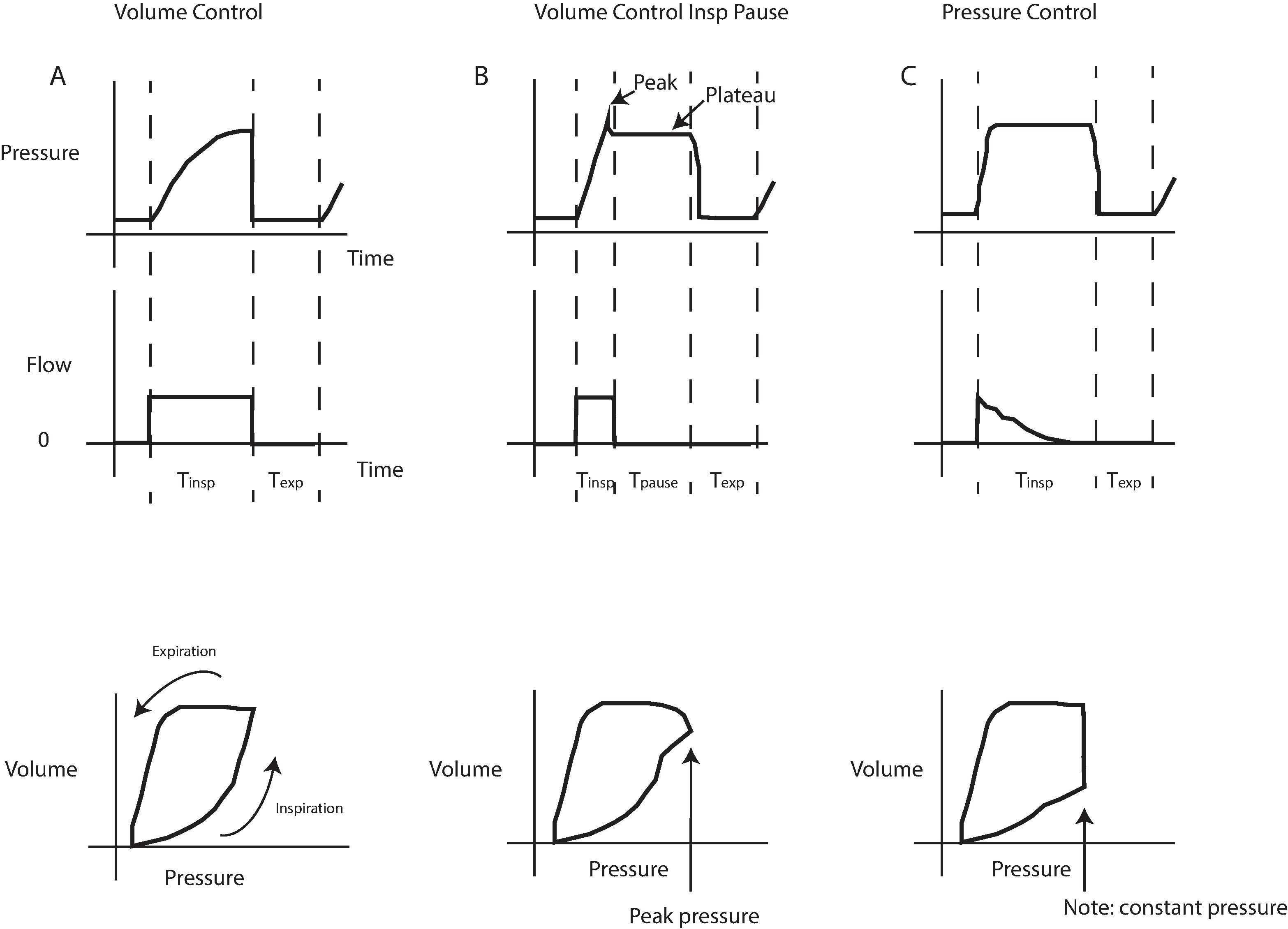
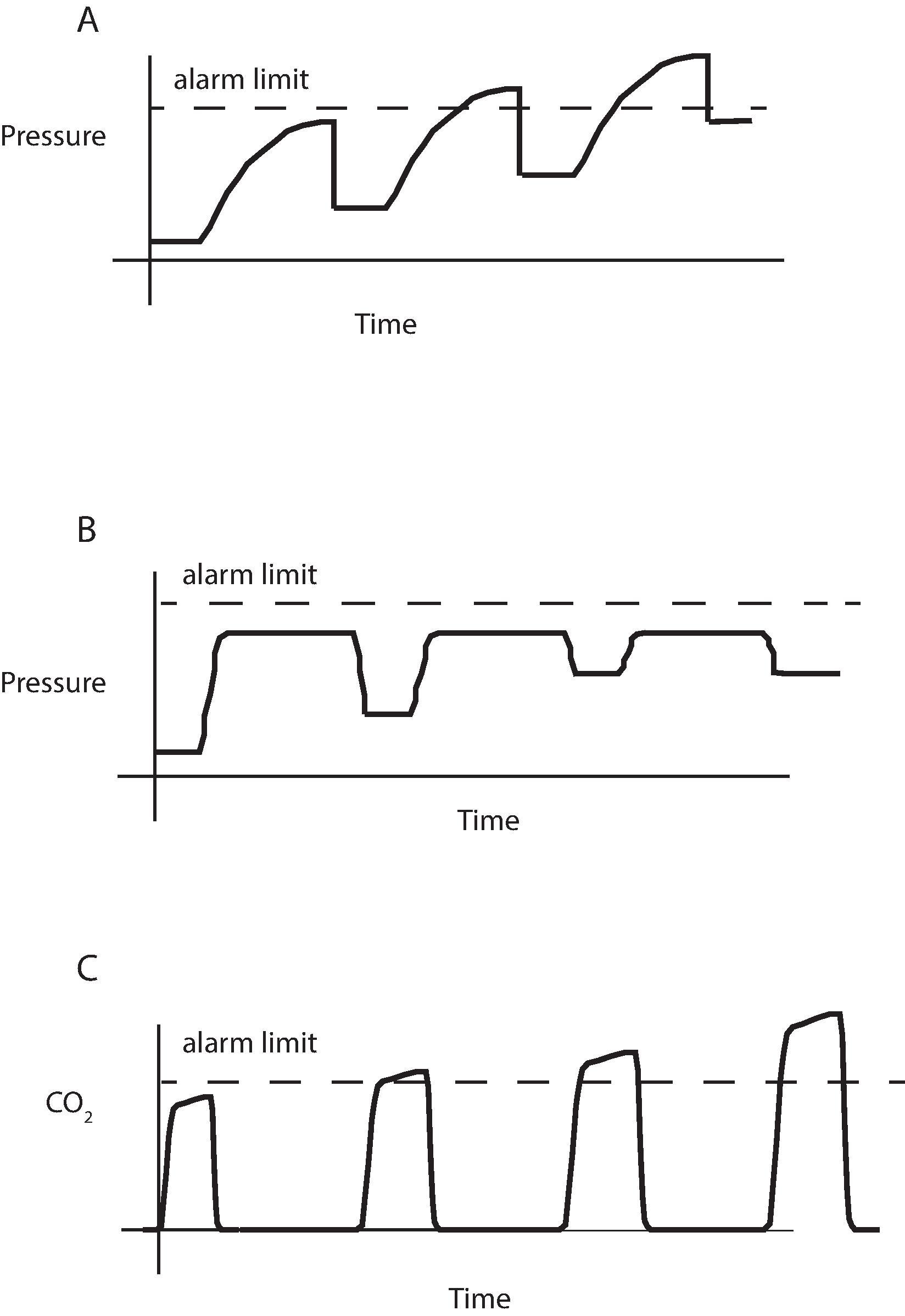
All anesthesia ventilators require a “disconnect” alarm, usually tied to the airway pressure reading. Inadequate ventilation can occur despite a nominally normal pressure. When using pressure-controlled ventilation, a significant change in ventilator volume can occur without an alarm condition occurring. Mechanical alarms and indicators of ventilation do not ensure tracheal intubation. An esophageal intubation can return “adequate” pressures and volumes, and with transmission of sounds, appear to have bilateral breath sounds. With an intact circulation, measurement of expired CO 2 is the best monitor of ventilation, discussed in detail in the next section.
Capnography/End-Tidal CO 2 Capnography is the analysis of the continuous waveform of expired CO 2 . Gas is continuously sampled from the ventilator circuit just on the patient side of the “Y” connector through a small tube into an infrared analyzer (also using Beer's law), and the CO 2 waveform is displayed on the physiologic monitor ( Figs. 20.9 and 20.10 ). Carbon dioxide generated in the tissues is delivered to the right heart through the venous system into the lungs via the pulmonary arteries. Exchange of the CO 2 into the alveolar space is fairly efficient because CO 2 has 20 times the solubility in water as does oxygen. Therefore well-perfused alveoli achieve equilibrium with CO 2 in the blood. During expiration, alveolar gas leaves the lungs, exiting the trachea through the ETT, where the aspirated gas is sampled by the capnometer, producing a peak expired CO 2 close to the arterial carbon dioxide tension (PaC o 2 ). In healthy patients ETCO 2 is usually 3 to 5 mm Hg less than PaC o 2 during general anesthesia.
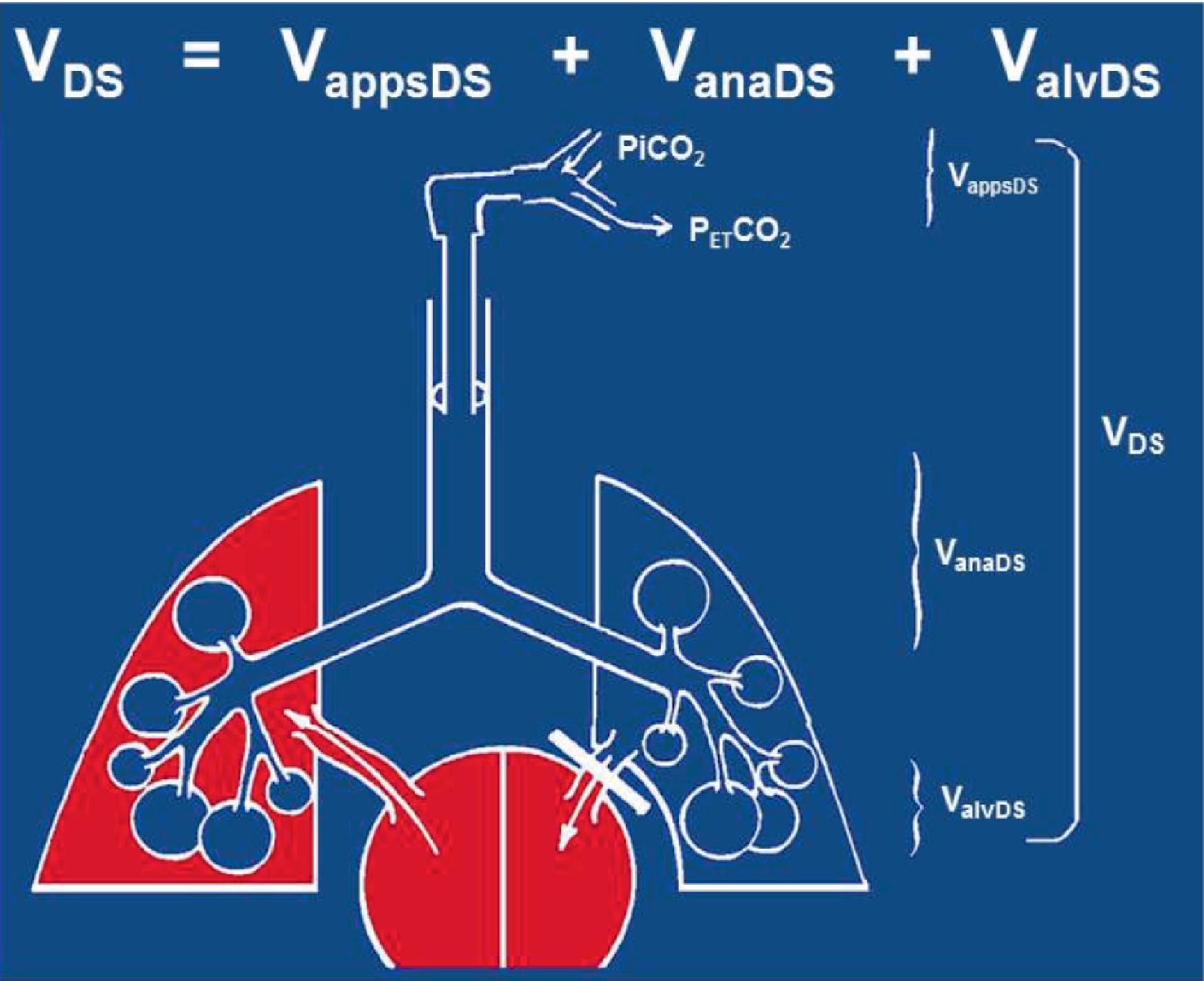
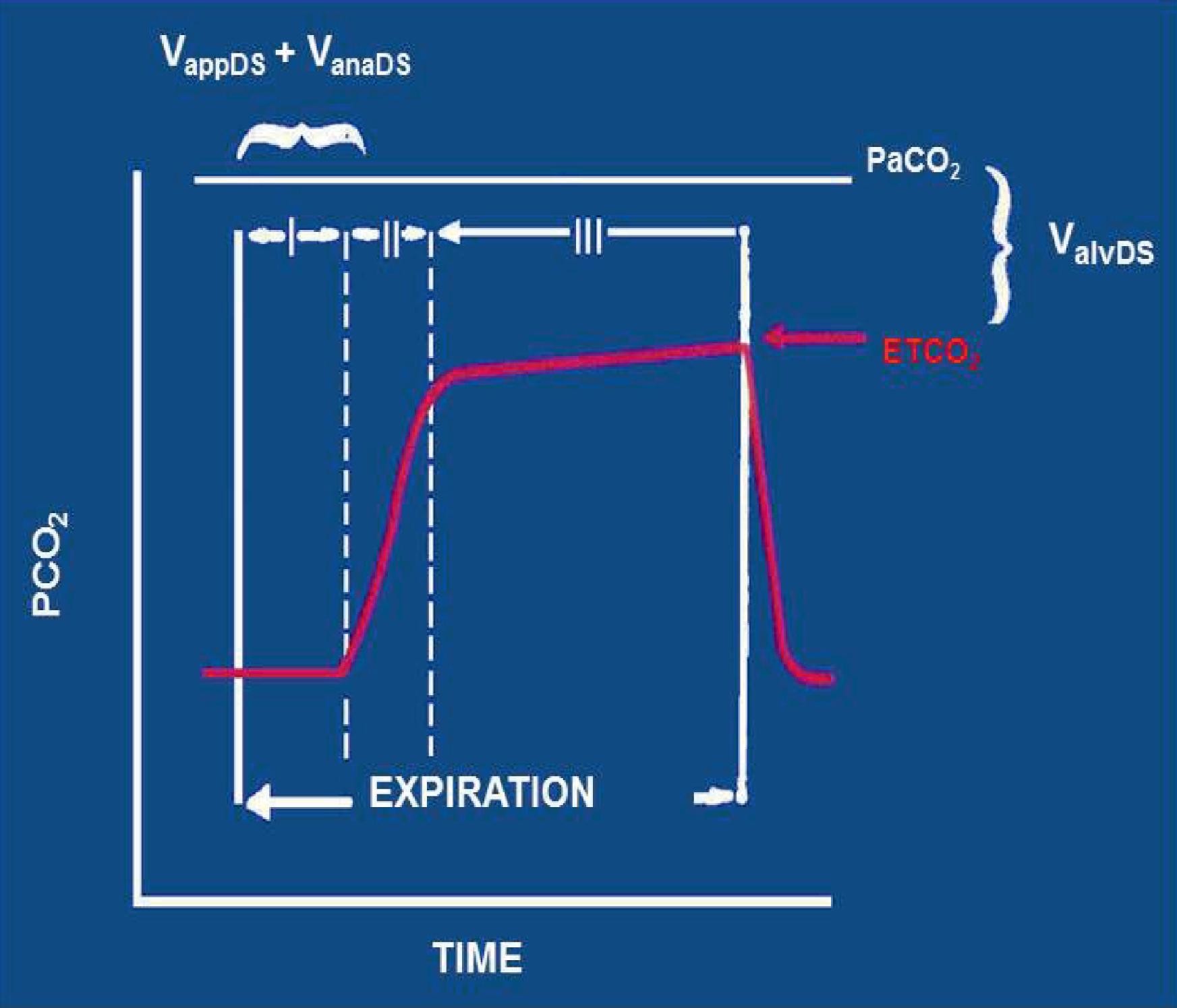
A patient's respiratory tidal volume is composed of alveolar gas volume and dead space volume. Dead space is defined as any portion of the tidal volume that does not participate in gas exchange. In healthy patients dead space is approximately one-third of the tidal volume. Dead space is composed of three subvolumes: apparatus dead space, anatomic dead space, and alveolar dead space ( Fig. 20.9 ). Because the inspired gas contains no CO 2 (unless the CO 2 absorber is malfunctioning and allowing rebreathing of CO 2 to occur), all these dead space gases will not contain CO 2 . When expiration begins in the respiratory cycle, the sampling tube will first detect the apparatus dead space, followed by the anatomic dead space. Neither of these contains CO 2 , so the capnogram will remain at zero during this initial phase I of the capnogram ( Fig. 20.10 ). As the gas from the alveolar space (well-perfused with a CO 2 tension roughly the same as PaCO 2 ) and the alveolar dead space (with zero CO 2 ) mix and are detected at the sampling tube, the CO 2 waveform will rise from zero up to a plateau value, producing a rough square wave until inspiration begins and the CO 2 waveform immediately returns to zero. The final plateau value of the capnogram is the ETCO 2 , which will approximately equal the PaCO 2 value if there is no alveolar dead space. The ETCO 2 value will almost always be lower than the PaCO 2 value. The size of this gradient will be in direct proportion to the amount of alveolar dead space in the expired volume, relative to the alveolar gas. The greater proportion of dead space, the lower the ETCO 2 value. For example, if the PaCO 2 was 40 mm Hg and half of the alveoli that were ventilated were not perfused, the ETCO 2 would be 20 mm Hg. The phase II or upsloping portion of the capnogram, seen in Figs. 20.9 and 20.11B , can be slanted rightwards when the alveoli are emptying irregularly. This can occur in patients with chronic obstructive pulmonary disease or asthma, both of which are associated with obstruction to expired gas flow. In general, the increased slope of this phase of the capnogram is associated with greater airway resistance to expired gas flow.
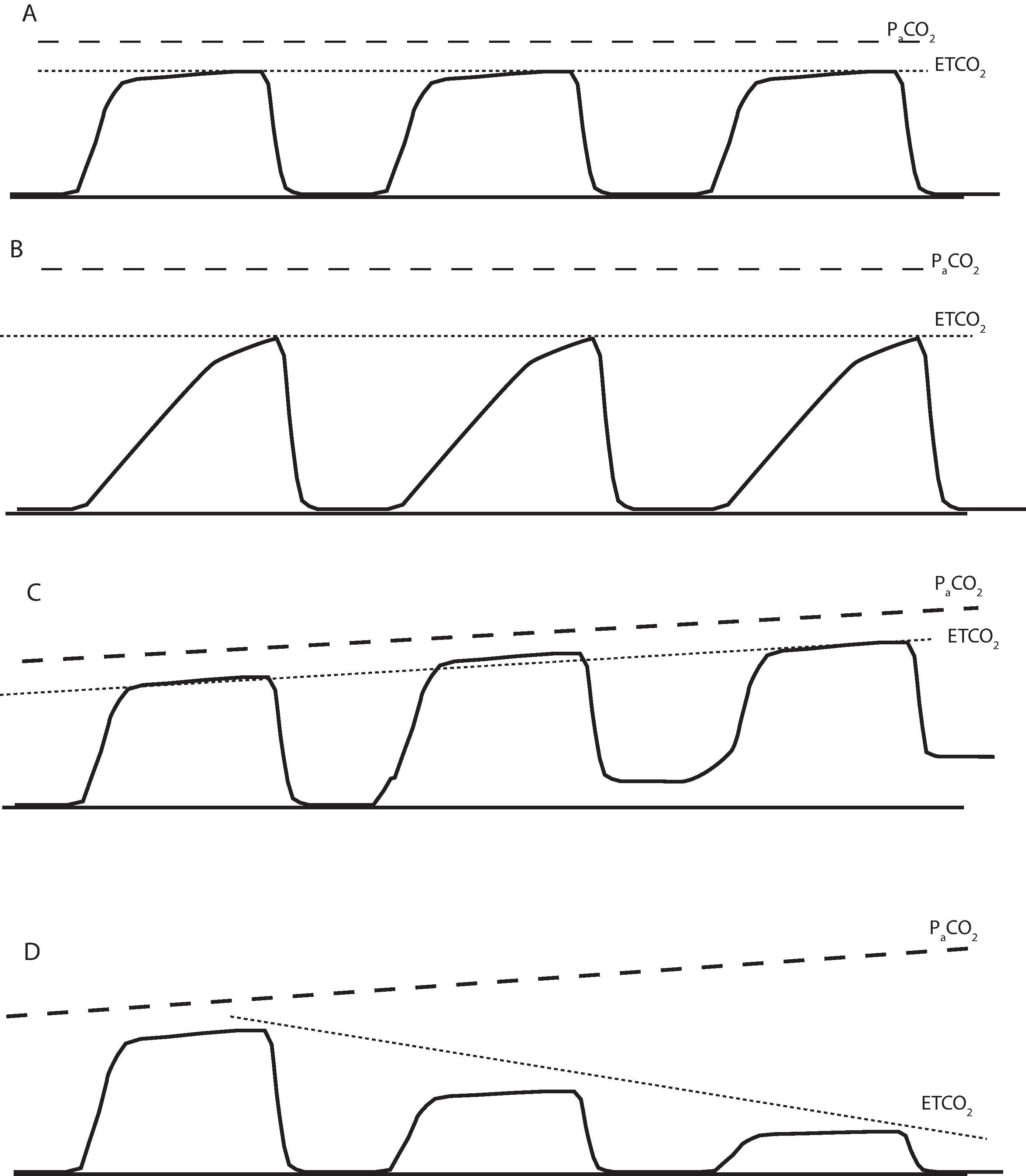
Alveolar dead space may be increased in patients with chronic lung disease who have large emphysematous areas of the lung that increase alveolar dead space and produce a large gradient between the ETCO 2 and PaCO 2 . Acute changes in alveolar dead space occur in specific clinical situations. The classic case is pulmonary embolism, in which blood flow to some capillaries is completely obstructed by the emboli, causing an acute increase in alveolar dead space and an acute drop in the ETCO 2 value (see Fig. 20.11D ). Increased dead space can also occur if there is a ventilation–perfusion mismatch causing less perfusion to well-ventilated areas of the lung. For example, when a patient is placed in the lateral position, the dependent lung is well perfused and ventilated but the nondependent lung is less well perfused and therefore has increased alveolar dead space. This combination can result in a decrease in the ETCO 2 value compared with the PaCO 2 . Finally, there may be a progressive increase in alveolar dead space because of a global lack of lung perfusion when the cardiac output (CO) decreases (see Fig. 20.11D ). For example, if a patient's CO is 5 L/min and for some reason acutely decreases to 2.5 L/min with unchanged alveolar ventilation, there will be less blood flowing per unit time to perfuse the same number of ventilated alveoli. The result is an increase in alveolar dead space and a drop in ETCO 2 . For this reason, the ETCO 2 capnogram is often referred to as the “poor man's measure of cardiac output.” Any significant decrease in CO will be associated with a drop in ETCO 2 (see Fig. 20.11D ). In a patient with cardiac arrest the most important monitor of adequacy of chest compressions during cardiopulmonary resuscitation (CPR) is the capnogram. The presence of a capnogram tracing with every ventilated breath during CPR ensures there is both ventilation and perfusion of the lung. If there is no capnogram tracing during chest compressions (or if ETCO 2 <10 mm Hg), then there is no effective CO perfusing the patient's vital organs (also see Chapter 45 ). The other advantage of monitoring the capnogram during CPR is the lack of motion artifact, unlike other monitors used during CPR such as the electrocardiogram (ECG) and pulse oximeter. Because a normal continuous capnogram waveform reflects adequate ventilation and perfusion, one can argue that the capnogram is the most important monitor used during general anesthesia.
Although the sampling tube can be placed on nasal cannula or around the mouth in nonintubated patients, a reliable capnographic waveform is only achieved in an intubated patient or with a secure supraglottic airway. In nonclosed systems (where the sampling tube is placed by the airway under a mask or a nasal cannula) there may be aspiration of room air (which has no CO 2 ), which will dilute the capnographic sample.
Multiple characteristics of the circulation can be measured, including the heart rate, ECG, blood pressure, urine output, central venous pressures, pulmonary artery pressures, CO, and systolic pressure variation (SPV) ( Table 20.1 ). Some of these are difficult to measure, and all require interpretation. Many important variables cannot be measured, such as venous capacitance, organ blood flow/perfusion, and circulating blood volume. Other values are derived from combinations of measured values (e.g., stroke volume, vascular resistance). No single characteristic determines adequacy of perfusion, and a solid understanding of the underlying physiology is necessary to interpret even the simplest monitor.
| Measured Variable (Abbreviation) | Value (Units) |
|---|---|
| Systolic blood pressure (SBP) | 90–140 mm Hg |
| Diastolic blood pressure (DBP) | 60–90 mm Hg |
| Mean blood pressure (MAP) | 70–105 mm Hg |
| Systolic pressure variation (SPV) a | 3.9–6.0 mm Hg |
| Pulse pressure variation (PPV) a | 5.0%–9.0% |
| Central venous pressure (CVP) | 2–6 mm Hg |
| Right ventricular pressure | 15–25/0–8 mm Hg |
| Pulmonary artery pressure (PAP) | 15–25/8–15 mm Hg |
| Mean pulmonary artery pressure | 10–20 mm Hg |
| Pulmonary capillary wedge pressure (PCWP) | 6–12 mm Hg |
| Left atrial pressure (LAP) | 6–12 mm Hg |
| Heart rate (HR) | 60–90 beats/min |
| Arterial O 2 saturation (SpO 2 ) | 95%–100% |
| Cardiac output (Q or CO) | 4–8 L/m |
| Cardiac index (CI) | 2.5–4.0 L/m/m 2 |
| Ejection fraction (EF) | 55%–70% |
| End diastolic volume | 65–240 mL |
| Calculated Values | |
| Stroke volume (SV), stroke volume index (SVI) | 60–100 mL/beat, 33–47 mL/m 2 /beat |
| Systemic vascular resistance (SVR) | 800–1200 dynes*sec/cm 5 |
| Pulmonary vascular resistance (PVR) | <250 dynes*sec/cm 5 |
| Respiratory Parameters | |
| Respiratory rate (RR) | 12–20 breaths/min |
| Peak inspiratory pressure (PIP) | 15–20 cm H 2 O |
| Tidal volume (Vt) | 6–8 mL/kg ideal body weight |
| End-tidal CO 2 (ETCO 2 ) | 35–40 mm Hg |
| Cerebral Parameters | |
| Intracranial pressure (ICP) | 5–15 mm Hg |
| Electroencephalogram (EEG) | Waveform varies by state of consciousness |
| Somatosensory evoked potential (SSEP) | Normal amplitude and latency |
| Bispectral index scale (BIS) | 80–100 awake |
| Muscle Parameters | |
| Train-of-four (TOF) | 4 twitches present |
| TOF ratio | >0.9 |
| Double burst stimulation (DBS) | No fade |
| Tetany | No fade |
| Electromyography (EMG) | Depends on stimulus |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here