Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Perioperative management of patients undergoing vascular surgery requires an understanding of the underlying pathophysiology of the specific vascular lesion.
Major vascular surgery is particularly challenging to the anesthesiologist because these are high-risk operations in a patient population with a high prevalence of either overt or occult coronary artery disease, which is the leading cause of perioperative and long-term mortality after vascular surgery.
Accurate clinical assessment of the pretest probability of significant coronary artery disease is necessary for prudent use and rational interpretation of preoperative cardiac testing.
Guidelines on perioperative cardiovascular evaluation and care suggest that coronary intervention is rarely necessary to simply decrease the risk for surgery unless such intervention is indicated, irrespective of the preoperative context. Prophylactic coronary revascularization has not been shown to reduce perioperative or long-term morbidity after major vascular surgery. Medical therapy is the cornerstone for the management of coronary artery disease.
Patients should take their usual cardiovascular medications throughout the perioperative period. Antiplatelet therapy requires special consideration and must be individualized to each patient.
Prevention and treatment of perioperative myocardial ischemia require careful control of the determinants of myocardial oxygen supply and demand. ST-segment monitoring, particularly with computerized ST-segment analysis, should be used to detect myocardial ischemia during the perioperative period.
Initiation of perioperative β-adrenergic blocker therapy has potential benefits and risks.
All patients who require statin therapy on an ongoing basis should also receive statins in the perioperative period.
The clinical usefulness of any intraoperative monitoring technique ultimately depends on patient selection, accurate interpretation of data, and appropriate therapeutic intervention.
Maintenance of vital organ perfusion and function by the provision of stable perioperative hemodynamics is more important to overall outcome after aortic surgery than is the choice of anesthetic drug or technique.
The pathophysiology of aortic cross-clamping and unclamping is complex and depends on many factors, including the level of the cross-clamp, the extent of coronary artery disease and myocardial dysfunction, intravascular blood volume and distribution, activation of the sympathetic nervous system, and the anesthetic drugs and techniques.
The degree of preoperative renal insufficiency is the strongest predictor of postoperative renal dysfunction.
Endovascular aortic surgery has become an established, less invasive alternative to conventional open aortic repair. Endoleak, or the inability to obtain or maintain complete exclusion of the aneurysm sac from arterial blood flow, is a complication specific to endovascular aortic repair.
The primary clinical utility of cerebral monitoring during carotid endarterectomy is to identify patients in need of carotid artery shunting; second, such monitoring is used to identify patients who may benefit from an increase in arterial blood pressure or change in surgical technique.
Postoperative hypothermia is associated with many undesirable physiologic effects and may contribute to adverse cardiac outcome.
The editors, publisher, and the authors of this chapter would like to thank Dr. Edward J. Norris for contributing a chapter on this topic to the prior edition of this work. It has served as the foundation for the current chapter.
Patients undergoing vascular surgery have a high incidence of coexisting disease, including diabetes mellitus, hypertension, renal impairment, and pulmonary disease, all of which should be assessed and, if possible, optimized before surgery. Because of the systemic nature of atherosclerotic disease, patients with vascular disease frequently have arterial disease affecting multiple vascular territories. Coronary artery disease (CAD) is the leading cause of perioperative mortality at the time of vascular surgery, and long-term survival after vascular procedures is significantly limited by the frequent occurrence of morbid cardiac events. Less than 10% of patients who undergo vascular surgery have normal coronary arteries, and more than 50% have advanced or severe CAD. Unrecognized myocardial infarction (MI) (determined by wall motion abnormalities at rest in the absence of a history of MI) and silent myocardial ischemia (determined by stress-induced wall motion abnormalities in the absence of angina) often occur in vascular surgery patients (23% and 28%, respectively) and are associated with increased long-term mortality and adverse cardiac events. Left ventricular systolic dysfunction (LVSD) is five times more common in patients with vascular disease than in matched controls. It is not clear whether any specific category of vascular disease is associated with a greater likelihood of coexisting CAD. Some investigators have shown a similar incidence and severity of CAD in patients with aortic, lower extremity, and carotid disease. Others have shown that patients with lower extremity vascular disease are more likely to have significant CAD and to experience perioperative morbidity. Medical therapy is the cornerstone of the management of CAD.
Preoperatively, the potential for MI and death in patients undergoing vascular surgery must be considered ( Table 56.1 ). Nonfatal and fatal MIs are the most important and specific outcomes that determine perioperative cardiac morbidity. When multiple recent studies are pooled, the overall prevalence of perioperative MI and death is 4.9% and 2.4%, respectively. When outcomes are assessed over the long term (2 to 5 years), the prevalence of MI and death is 8.9% and 11.2%, respectively. This perioperative and long-term morbidity and mortality persist despite aggressive medical and surgical therapy.
| Study | MI (%) | Death (%) | Comments |
|---|---|---|---|
| Short-Term Follow-Up (in Hospital or 30-Day) | |||
| Ouyang et al. | 8 | 0 | Small study |
| Raby et al. | 2.3 | 0.06 | Aortic, lower extremity, carotid |
| Mangano et al. | 4.1 | 2.3 | Vascular patient only reported |
| Bode et al. | 4.5 | 3.1 | All lower extremity |
| Christopherson et al. | 4.0 | 2.0 | All lower extremity |
| Mangano et al. | 5.0 | 0 | Vascular patient only reported |
| Fleisher et al. | 6.0 | 3.0 | Vascular patient only reported |
| Pasternack et al. | 4.5 | 1.0 | Aortic, lower extremity, carotid |
| Krupski et al. | 2.1 | 2.9 | Aortic, lower extremity |
| Baron et al. | 5.9 | 4.0 | All aortic |
| Norris et al. | 3.3 | 5.4 | All aortic |
| Fleron et al. | 5.5 | 4.1 | All aortic |
| McFalls et al. | 8.4 | 3.2 | Aortic, lower extremity |
| Average | 4.9 | 2.4 | |
| Long-Term Follow-Up (in Hospital and After Discharge) | |||
| Raby et al | 7.4 | 5.1 | 20-mo follow-up |
| Mangano et al. | 4.7 | 3.5 | 15-mo follow-up |
| Mangano et al. | 19.4 | 13.5 | 24-mo follow-up |
| Hertzer et al. | 12 | 60-mo follow-up | |
| Krupski et al. | 3.9 | 11.2 | 24-mo follow-up |
| McFalls et al. | 22 | 30-mo follow-up | |
| Average | 8.9 | 11.2 | |
A guideline-based approach to health care is relatively new and originated primarily in the United States. The American College of Cardiology (ACC) Foundation and the American Heart Association (AHA) jointly produced guidelines in the area of cardiovascular disease for more than 2 decades. The ACC/AHA Task Force on Practice Guidelines published “Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery” in 1996. This evidence-based approach to perioperative evaluation and management was updated in 2002, 2007, 2009, and 2014. A stepwise approach (simplified from the 2007 guidelines) to perioperative cardiac evaluation and care for noncardiac surgery is provided in Chapter 31 . The authors emphasize that the purpose of the preoperative evaluation is not to give medical clearance but rather to perform an evaluation of the patient’s current medical status; make recommendations concerning the evaluation, management, and risk for cardiac problems; and provide a clinical risk profile that the patient and caregivers can use in making treatment decisions that may influence perioperative and longer-term cardiac outcomes. The overriding theme of the perioperative guidelines is that intervention is rarely necessary to simply lower the risk associated with surgery unless such intervention is indicated, irrespective of the preoperative context. Thus, preoperative testing should not be performed unless it is likely to influence patient care. The particular challenge that the vascular surgery patient presents is emphasized throughout the document. Aspects of the updated guidelines and their evidence-based approach will be discussed throughout this chapter.
The preoperative cardiac assessment presents an opportunity to initiate and optimize pharmacologic management, perform appropriate diagnostic and therapeutic interventions, and adjust overall care to decrease not only perioperative risk but also long-term risks from cardiovascular events. The challenge for clinicians is to accurately assess risk for cardiac morbidity while maintaining a cost-effective, clinically relevant, and evidence-based strategy. The ACC/AHA stepwise approach considers vascular surgery distinct from other noncardiac surgical procedures and is reviewed in detail in Chapter 31 . Only issues specific to vascular surgery are reviewed in this chapter.
After assessment of cardiac risk, the additional challenge exists of modifying perioperative management to reduce risk by adjusting or adding cardiac medications (e.g., β-adrenergic blocker), direct coronary intervention (e.g., percutaneous coronary intervention [PCI] or coronary artery bypass grafting [CABG]), modifying or intensifying perioperative management (e.g., invasive hemodynamic monitoring), or changing preoperative plans (e.g., performing endovascular aneurysm repair [EVAR] rather than open aortic repair). Coordination is essential among surgeons, anesthesiologists, and cardiologists, each of whom may have different criteria for risk assessment and different objectives for risk modification.
Assessing cardiac risk in patients before vascular surgery is a controversial and difficult task. Although risk indices are a cost-effective screening method for determining which patients may require further cardiac evaluation (i.e., additional risk stratification with noninvasive technologies), the high pretest probability of CAD in vascular surgery patients makes the risk index somewhat less useful. Vascular surgery-specific indices have been recently developed to optimize the prediction of perioperative mortality and cardiac morbidity in patients undergoing elective and urgent vascular surgery. Risk indices do not provide specific risk prediction for individuals, but rather place patients in general risk categories, most commonly designated as low (cardiac risk generally <1%), intermediate (cardiac risk of 1% to 5%), or high (cardiac risk often >5%). Clinical risk variables identified by logistic regression in vascular surgery cohorts can be used along with noninvasive cardiac testing to optimize preoperative assessment of cardiac risk before vascular surgery. From the registry of the Coronary Artery Revascularization Prophylaxis (CARP) trial, the absence of multiple preoperative cardiac risk variables identifies patients with the best long-term survival after elective vascular surgery.
Accurate clinical assessment of the pretest probability of significant CAD is extremely important. In general, noninvasive cardiac testing before vascular surgery is best directed at patients considered to be at intermediate clinical risk. Such testing should not be undertaken if it is unlikely to alter patient management and should not be considered as a preliminary step leading to coronary revascularization. A revascularization procedure is rarely needed solely for the purpose of getting a patient through the perioperative period. Extensive cardiac evaluation before vascular operations can result in morbidity, delays, and patient refusal to undergo vascular surgery. A complete review of this subject is found in Chapter 31 .
The largest series on outcome in vascular surgery patients is that of Hertzer and colleagues from the Cleveland Clinic. These investigators performed cardiac catheterization in 1000 consecutive patients scheduled to undergo peripheral vascular surgery (aortic aneurysm resection, carotid endarterectomy, and lower extremity revascularization). The incidence and severity of CAD were assessed according to the following classification: normal coronary arteries; mild-to-moderate CAD with no lesion exceeding 70% stenosis; advanced, compensated CAD with one or more lesions exceeding 70% stenosis but with adequate collateral circulation; severe, correctable CAD with more than 70% stenosis in one or more coronary arteries; and severe inoperable CAD with greater than 70% stenosis in one or more coronary arteries and severe distal disease or poor ventricular function. The most remarkable findings were that only 8.5% of patients had normal coronary arteries and 60% had advanced or severe coronary lesions (>70% stenosis). Even when CAD was not suspected by the clinical history, more than a third of patients had advanced or severe coronary lesions ( Table 56.2 ).
| Clinical CAD | ||||||
|---|---|---|---|---|---|---|
| None | Suspected | Total | ||||
| Angiographic Classification | No. | % | No. | % | No. | % |
| Normal coronary arteries | 64 | 14 | 21 | 4 | 85 | 8.5 |
| Mild‐to‐moderate CAD | 218 | 49 | 99 | 18 | 317 | 32 |
| Advanced, compensated CAD | 97 | 22 | 192 | 34 | 289 | 29 |
| Severe, correctable CAD | 63 | 14 | 188 | 34 | 251 | 25 |
| Severe, inoperable CAD | 4 | 1 | 54 | 10 | 58 | 5.8 |
In the Hertzer series, patients with severe correctable CAD were offered CABG before their vascular surgery, patients with normal or mild-to-moderate CAD went directly to vascular surgery, and those with severe inoperable CAD were treated on an individual basis. Combined mortality rates over the immediate- and long-term (4.6-year follow-up) postoperative period are shown in Table 56.3 . Of the 216 patients who underwent coronary revascularization (CABG), 12 (5.5%) died after this surgery. This mortality rate is higher than that reported for patients undergoing CABG surgery without peripheral vascular disease (1% to 2%). Perhaps the risks associated with CABG should be seriously considered as part of the preoperative evaluation of these patients. When overall early and late mortality (>5 years) is considered, death occurred in 12% versus 26% of patients who did or did not undergo CABG. Although these data appear to support the beneficial effect of CABG on outcome, the mortality from CABG itself (5.5%) reduces its apparent benefits.
| Total No. of Patients | Normal or Mild-to- Moderate CAD | Advanced, Compensated CAD | Severe, Correctable CAD | Severe, Inoperable CAD | Total Cardiac Deaths | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With CABG | No CABG | ||||||||||||
| Clinical Features | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Men | 685 | 10/242 | 4.1 | 33/204 | 16 | 13/174 | 7.5 | 6/24 | 25 | 14/41 | 34 | 76 | 11 |
| Women | 315 | 5/160 | 3.1 | 12/85 | 14 | 12/42 | 29 | 3/11 | 27 | 8/17 | 47 | 40 | 13 |
| Age <70 yr | 722 | 10/328 | 3.0 | 29/198 | 15 | 19/148 | 13 | 3/20 | 15 | 13/28 | 46 | 74 | 10 |
| Age >70 yr | 278 | 5/74 | 6.8 | 16/91 | 18 | 6/68 | 8.8 | 6/15 | 40 | 9/30 | 30 | 42 | 15 |
| Normotensive | 403 | 7/185 | 3.8 | 15/102 | 15 | 8/82 | 9.8 | 2/15 | 13 | 8/19 | 42 | 40 | 9.9 |
| Hypertensive | 597 | 8/217 | 3.4 | 30/187 | 16 | 17/134 | 13 | 7/20 | 35 | 14/39 | 36 | 76 | 13 |
| Nondiabetic | 830 | 12/348 | 3.4 | 28/232 | 12 | 17/183 | 9.3 | 8/30 | 27 | 13/37 | 35 | 78 | 9.4 |
| Diabetic | 170 | 3/54 | 5.5 | 17/57 | 30 | 8/33 | 24 | 1/5 | 20 | 9/21 | 43 | 38 | 22 |
| Total | 1000 | 15/402 | 3.7 | 45/289 | 16 | 25/216 | 12 | 9/35 | 26 | 22/58 | 38 | 116 | 12 |
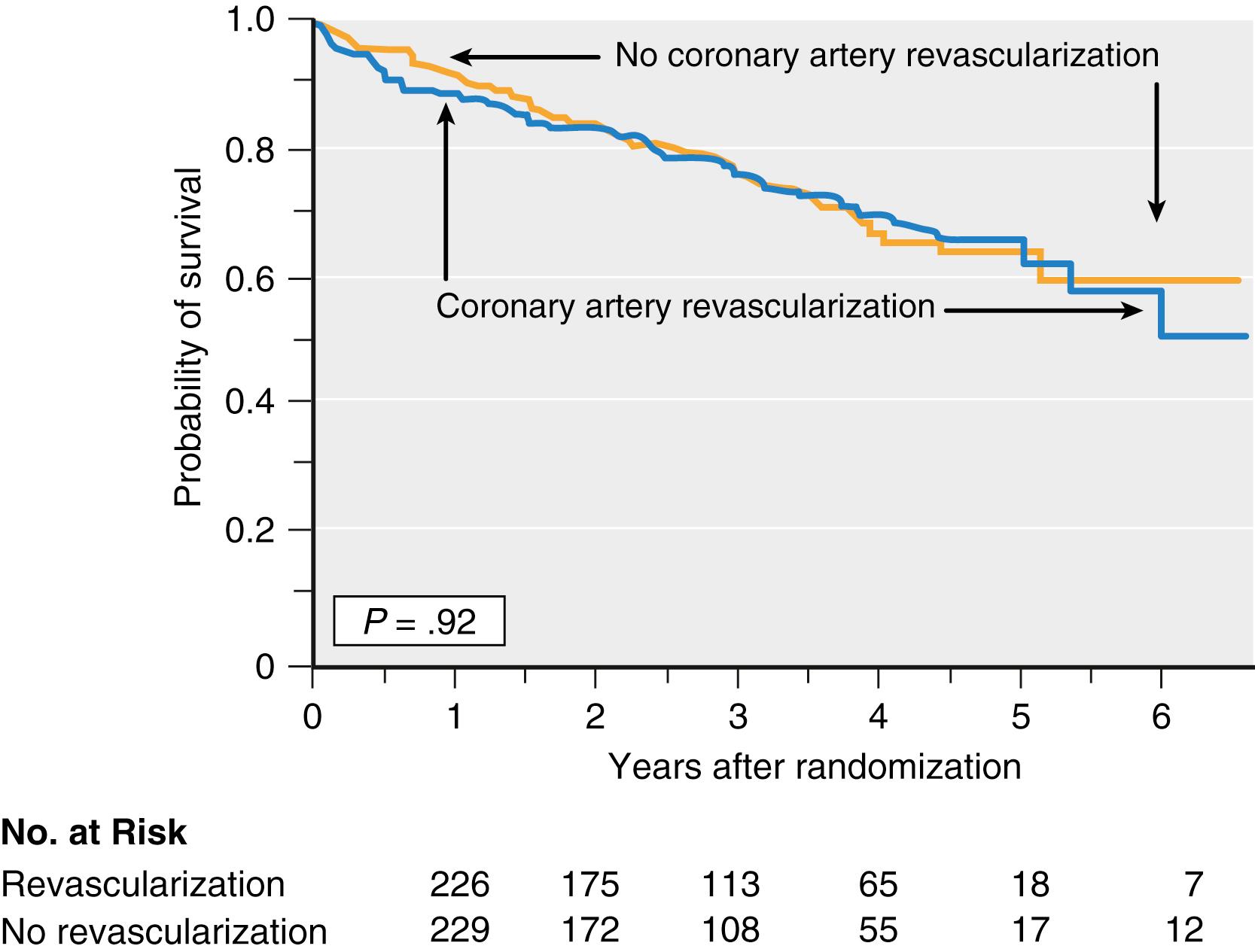
Two randomized clinical trials have been performed to determine the impact of prophylactic coronary artery revascularization on outcome after open aortic and lower extremity arterial vascular surgery. Of the 5859 patients screened in the CARP trial, 41190 underwent coronary angiography based on a combination of clinical risk factors and noninvasive stress imaging data. The incidence and severity of CAD on these angiograms were 43% of patients had one or more major coronary arteries with at least a 70% stenosis suitable for revascularization (and were randomized to either revascularization or no revascularization before vascular surgery); 31% had nonobstructed coronary arteries; 18% had coronary stenosis considered unsuitable for revascularization; and 5% had left main coronary artery stenosis of 50% or more. The CARP trial showed that prophylactic revascularization (by CABG or PCI) was generally safe but did not improve long-term outcome after vascular surgery. Long-term mortality (2.7 years) was 22% in the revascularization group and 23% in the group considered inappropriate for revascularization ( Fig. 56.1 ). Although the trial was not designed to test the short-term benefit of prophylactic revascularization, perioperative outcomes were not decreased, including death (3.1% vs. 3.4%) and MI (12% vs. 14%). The CARP trial results can be applied to most of the vascular surgery patients; however, they cannot be extrapolated to patients with unstable cardiac symptoms, left main coronary artery disease, aortic stenosis, or severe left ventricular dysfunction because these conditions excluded patients from study participation. The DECREASE-V trial 26 screened 1880 vascular surgery patients, 430 of whom with three or more clinical risk factors underwent noninvasive stress testing using stress-echo or perfusion imaging. Patients with extensive stress-induced ischemia (26%) were randomly assigned to revascularization or no revascularization. Coronary angiography showed two-vessel disease in 24%, three-vessel disease in 67%, and left main coronary artery disease in 8%. Prophylactic coronary revascularization (CABG or PCI) did not improve perioperative or long-term outcome ( Table 56.4 ). The incidence of all-cause death or nonfatal MI at 30 days in patients who underwent revascularization or who did not was 43% versus 33%, respectively. The incidence of the composite end point at 1 year was similar, 49% versus 44%, respectively. As noted previously, this trial has come under scrutiny based on concerns of scientific misconduct by the principal investigator.
| Revascularization No. (%) | No Revascularization No. (%) | HR (95% CI) | P -value | |
|---|---|---|---|---|
| No. of Patients | 49 | 52 | ||
| Events before surgery All-cause mortality Myocardial infarction Composite |
2 (4.1) 1 (2.1) 3 (6.1) |
0 0 0 |
0.23 0.11 |
|
| Events up to 30 days after surgery All-cause mortality Myocardial infarction Composite |
11 (22.5) 17 (34.7) 21 (42.9) |
6 (11.5) 16 (30.8) 17 (32.7) |
2.2 (0.74-6.6) 1.4 (0.73-2.8) |
0.14 0.30 |
| Events up to 365 days after surgery All-cause mortality Myocardial infarction Composite |
13 (26.5) 18 (36.7) 24 (49.0) |
12 (23.1) 19 (36.5) 23 (44.2) |
1.3 (0.55-2.9) 1.2 (0.68-2.3) |
0.58 0.48 |
The lack of benefit of prophylactic coronary revascularization in the CARP and DECREASE-V trials is difficult to reconcile with the more favorable data from Hertzer and co-workers 25 and other studies (Coronary Artery Surgery Study [CASS] and Bypass Angioplasty Revascularization Investigation [BARI] ). Clearly, issues are involved that go beyond critical coronary lesions; perhaps the current understanding of the pathophysiology of perioperative MI is incomplete. For example, perioperative MI may be caused by culprit lesions (i.e., vulnerable plaques with high likelihood of thrombotic complications) often located in coronary vessels without critical stenosis. For this type of MI (atherothrombotic), perioperative strategies aimed at reducing potential triggers of coronary plaque destabilization and rupture may be more appropriate than those leading to coronary revascularization. Demand ischemia is likely the predominant cause of perioperative MI, which has been confirmed by a recent angiographic study.
Postoperative pulmonary complications are potentially serious in patients undergoing vascular surgery, with the most significant morbidity seen in patients undergoing open aortic procedures (see also Chapter 54 ). The most important pulmonary complications are atelectasis, pneumonia, respiratory failure, and exacerbation of underlying chronic disease. Given the prevalence of cigarette smoking in this population, chronic obstructive pulmonary disease (COPD) and chronic bronchitis are common and, when present, place the patient at increased risk for postoperative pulmonary complications. When clinical assessment suggests severe pulmonary compromise, pulmonary function tests may be useful in evaluating and optimizing respiratory function (see also Chapter 39, Chapter 51 ). Preoperative analysis of arterial blood gases should be used to establish a baseline for postoperative comparison. Baseline hypercapnia (partial pressure of arterial carbon dioxide >45 mm Hg) indicates a more frequent risk for postoperative morbidity. Bronchodilator therapy may be indicated on the basis of results of pulmonary function tests, although the risk for β-adrenergic agonist–induced arrhythmia or myocardial ischemia also must be considered. Preoperative treatment with a short course of glucocorticoids (prednisone 40 mg/day for 2 days) may be helpful for patients with significant COPD or asthma. Evidence of pulmonary infection should be treated with appropriate antibiotics. Although improved pulmonary outcome with regional anesthesia is not clear, patients with significant pulmonary disease may benefit from epidural techniques. Use of these techniques in the postoperative period helps to avoid respiratory depression from systemic opiates (see also Chapter 81 ). Pulmonary complications in the postoperative period are difficult to avoid. Incentive spirometry and continuous positive airway pressure (CPAP) do provide benefit. Given proper pulmonary care, even patients with severe pulmonary insufficiency, however, may undergo aortic surgery with acceptable morbidity and mortality outcomes.
Chronic renal disease is common in vascular surgery patients and is associated with an increased risk for death and cardiovascular disease (see also Chapter 30, Chapter 42 ). Chronic renal disease strongly predicts long-term mortality in patients with symptomatic lower extremity arterial occlusive disease irrespective of disease severity, cardiovascular risk, and concomitant treatment. Cardiovascular disease is independently associated with a decline in renal function and the development of kidney disease. Serum creatinine and creatinine clearance are used to assess renal function perioperatively. A preoperative serum creatinine level more than 2 mg/dL is an independent risk factor for cardiac complications after major noncardiac surgery. Preoperative creatinine clearance less than 60 mL/min is an independent predictor of both short-term and long-term mortality after elective vascular surgery. Perioperative β-adrenergic blocker and statin administration decrease risk for death in vascular surgery patients with renal impairment. Atherosclerotic disease in the abdominal aorta or renal arteries may compromise renal blood flow and renal function. Conversely, renal artery stenosis causes hypertension through renin-induced and angiotensin-induced vasoconstriction. Hypertension itself may cause renal insufficiency or failure. Diabetic nephropathy is also common (see also Chapter 32 ). Superimposed on baseline abnormalities in renal function are the preoperatively and intraoperatively administered angiographic dyes, which are directly nephrotoxic. Renal ischemia occurs with interruption of renal blood flow from aortic cross-clamping. Even with infrarenal aortic cross-clamps, renal blood flow may decrease despite normal systemic arterial blood pressure and cardiac output. Embolic plaque can be showered into the renal arteries, especially when suprarenal aortic cross-clamps are applied and released. Fluctuations in intravascular volume and cardiac output can compromise renal perfusion during the intraoperative and postoperative periods. In one series of more than 500 patients, the prevalence of acute renal failure was 7% after abdominal aortic reconstruction.
Perioperative β-adrenergic blocker therapy is an important and controversial topic, particularly in patients undergoing vascular surgery, and is reviewed more fully in Chapter 38, Chapter 39 . Patients receiving chronic β-adrenergic blocker therapy should continue taking β-adrenergic blockers throughout the perioperative period. However, β-adrenergic blockers should not be used as the initial or primary treatment of tachycardia caused by perioperative events, such as hypovolemia, anemia, pain, or infection, because these conditions require prompt treatment of the underlying cause. Treatment of tachycardia caused by the sympathetic stimulation associated with surgical stress should be considered in high-risk patients, particularly those with known ischemic potential (i.e., ischemia on preoperative testing). Hypotension and bradycardia should be avoided. Acute initiation of large-dose β-adrenergic blockade in the perioperative period should be avoided. If a decision is made to initiate β-blocker treatment in the perioperative period to reduce cardiac risk, the safest approach may be to initiate therapy with a small dose and titrate to effect over a 7- to 10-day period before the planned surgery. Perioperative β-adrenergic blocker therapy can decrease the number of patients referred for preoperative cardiac testing. However, such testing should not be eliminated, and its risk-to-benefit ratio should be carefully assessed.
In addition to their lipid-lowering properties, statins have beneficial anti-inflammatory, plaque-stabilizing, and antioxidant effects (see also Chapter 32 ). Over the last decade, statin use has emerged as a promising strategy for the prevention of perioperative cardiovascular complications in patients undergoing vascular surgery. This approach is supported by the double-blind, placebo-controlled DECREASE-III trial. Unfortunately, controversy exists regarding this trial because of scientific misconduct identified by a recent investigation by Erasmus University. Statin use can help preserve renal function after aortic surgery and improve graft patency after lower extremity bypass surgery. Although current guidelines recommend the use of statins in all patients with peripheral arterial disease, the optimal timing and dosing of statins for perioperative use have not been established.
The timing of noncardiac surgery in patients treated with coronary stents with the risk of stent thrombosis if the dual antiplatelet therapy (DAPT) is discontinued versus the risk of increased intraoperative bleeding if DAPT is continued. Previous recommendations regarding the duration of DAPT and timing of noncardiac surgeries were based on observations of those treated with first-generation stents. Currently, used newer generations of stents, particularly the newer drug eluting stents (DES), have lower risk of in-stent thrombosis and require a shorter minimum duration of DAPT. The safety of treating patients with newer generation DES treated for shorter durations of DAPT (3 to 6 months) has been demonstrated in a meta-analysis of four trials. Also, in the PARIS (Patterns of Nonadherence to Antiplatelet Regimens in Stented Patients) registry, interruption of DAPT based on the physician judgment in patients undergoing surgery at any point did not affect the risk of major cardiac events. Hence, the ACC/AHA guidelines were changed in 2016 to reflect those changes (see Chapter 31, Chapter 32 ).
Anesthesia for conventional abdominal aortic reconstruction requires an understanding of the pathophysiology, extensive knowledge of the surgical procedure, the ability to interpret sophisticated hemodynamic data, and skillful pharmacologic control and manipulation of hemodynamics. Preoperative and intraoperative communication with the surgical team is essential. All open operative procedures on the abdominal aorta and its major branches require large incisions and extensive dissection, clamping and unclamping of the aorta or its major branches, varying duration of organ ischemia-reperfusion, significant fluid shifts and temperature fluctuations, and activation of neurohumoral and inflammatory responses. The major objectives of surgical treatment of the aorta are to relieve symptoms, reduce the frequency of associated complications, and in the case of aortic aneurysm, prevent rupture. Over the last two decades, the growth and development of catheter-based technology for the treatment of peripheral arterial disease have generated tremendous interest for less invasive methods to treat aortic disease.
Endovascular aortic aneurysm repair (discussed later) has become an established, less invasive alternative to conventional open repair, and its use has expanded to more than 75% of elective repairs and 30% of rupture repairs. The endovascular field continues to evolve rapidly with new devices, innovations, and indications for aortic disease.
Abdominal aortic aneurysms (AAAs) occur frequently in elderly men, with an incidence approaching 8% (see also Chapter 65 ). Increasing age, smoking, family history of AAA, and atherosclerotic disease are established risk factors. Although the prevalence is less frequent in women, the risk factors for AAA resemble those in men. More than 30,000 deaths result from rupture of AAAs each year in the United States. The number of hospital discharges each year with the first diagnosis of aortic aneurysm is nearly 70,000. Approximately 40,000 patients undergo repair of AAA each year in the United States, at a cost likely to exceed a billion dollars. The incidence of AAA appears to be increasing and is age- and gender-dependent.
AAA is a multifactorial disease associated with aortic aging and atherosclerosis. Although no unified concept of pathogenesis exists, genetic, biochemical, metabolic, infectious, mechanical, and hemodynamic factors may contribute to the development of AAA disease. Adventitial elastin degradation (elastolysis), a hallmark of AAA formation, may be the primary event. Chronic inflammation plays a fundamental role in the destruction of connective tissue in the aortic wall. Concomitant aortoiliac occlusive disease is present in approximately 20% to 25% of patients with AAA. Approximately 5% of patients undergoing abdominal aortic resection have inflammatory aneurysms. Rare causes of AAA disease include trauma, mycotic infection, syphilis, and Marfan syndrome.
Most AAAs are detected incidentally when imaging is performed for other reasons or through screening programs. The natural history of AAA disease is progressive enlargement and ultimate rupture and death. The diameter and rate of expansion of asymptomatic AAAs are the best predictors of the risk for rupture. Current guidelines emphasize that it is not possible to recommend a single threshold diameter for operative intervention that can be generalized to all patients. Yet, elective repair should be undertaken in all patients with AAA 6 cm or larger in diameter. Although some controversy exists regarding elective AAA repair when its diameter is in the 5.5- to 5.9-cm range, the risk for rupture of a 5.5-cm aneurysm (per year) is equal to or greater than the risk for perioperative mortality, and thus surgical repair is indicated. The 1-year incidence of probable rupture in patients refusing or unfit for elective repair is 9.4%, 10.2%, and 32.5% for aneurysms 5.5 to 5.9 cm, 6.0 to 6.9 cm, and 7.0 cm or greater, respectively. Over 90% of AAAs are less than the current threshold (5.5 cm) for surgical repair at the time of detection. Randomized controlled trials in patients with AAAs 4.0 to 5.5 cm in diameter have provided important insight into the natural history of small asymptomatic aortic aneurysms. Four trials have demonstrated that surveillance of small aneurysms (4.0 to 5.5 cm) is a safe management option and that early repair (open or endovascular surgery) did not result in any long-term survival benefit. Surgical repair is often considered if small aneurysms become symptomatic or expand more than 0.5 cm in a 6-month period. Although significant interest exists in medical treatment (e.g., antibiotics, β-adrenergic blockers, statins) to delay or reverse expansion of small aneurysms, evidence for a protective effect is limited. Aneurysms less than 4.0 cm in diameter are thought to be relatively benign in terms of rupture and expansion.
Perioperative mortality from elective resection of infrarenal AAAs has progressively declined from 18% to 20% during the 1950s, 6% to 8% in the mid-1960s, 5% to 6% in the early 1970s, and 2% to 4% in the 1980s, at which time it plateaued. A publication of data from 1000 consecutive elective open infrarenal abdominal aneurysm repairs over a 15-year period reported a perioperative mortality rate of 2.4%. Hertzer and co-workers reported a mortality rate of 1.2% for 1135 consecutive elective open infrarenal abdominal aortic repairs at the Cleveland Clinic. This single-center mortality rate is considerably less than the mortality rates of 5.6% to 8.4% reported from large national data sets. These more frequent mortality rates on the national level suggest that all the technologic and treatment advances over the last 2 decades have not had an impact on outcomes of patients requiring open AAA repair. Regionalization of patient care and endovascular treatments currently hold the most promise for improvement in operative mortality.
For ruptured AAAs, perioperative mortality has not changed significantly over the last 4 decades and remains nearly 50%, with few exceptions. Including patients with rupture who die before reaching a hospital, the overall mortality rate after rupture may very well exceed 90%.
The long-term durability of open infrarenal AAA repair is excellent and well established. The incidence of late graft complications is infrequent (0.4% to 2.3%). Postoperative survival rates after repair of non-ruptured AAA are 92% at 1 year and 67% at 5 years.
The infrarenal aorta and the iliac arteries are two of the most common sites of chronic atherosclerosis. Because of the diffuse and progressive nature of aortoiliac atherosclerosis, plaque enlargement may reduce blood flow to the lower extremities below a critical level and result in symptoms of ischemia. Unlike patients with aortic aneurysmal disease, patients undergo surgery for aortoiliac occlusive disease only if they are symptomatic. Surgical intervention is indicated for disabling intermittent claudication and limb-threatening ischemia. Intervention is directed toward restoring peripheral pulsatile circulation to relieve claudication and toward preventing amputation. Patients with localized aortoiliac occlusive disease typically have claudication because collateral circulation adequate to prevent critical lower extremity ischemia usually exists. Perioperative mortality is lower in patients undergoing aortoiliac reconstruction than in those undergoing abdominal aortic surgery.
Therapeutic options for managing aortoiliac occlusive disease include anatomic or direct reconstruction (i.e., aortobifemoral bypass), extra-anatomic or indirect bypass grafts (i.e., axillofemoral bypass), and catheter-based endoluminal techniques (i.e., percutaneous transluminal angioplasty [PTA] with or without stent insertion). Aortobifemoral bypass is viewed as the gold standard in treating aortoiliac occlusive disease. Extra-anatomic bypass grafts are generally reserved for specific indications, usually patients with infection, failure of previous reconstruction, or prohibitive risk. Reduced long-term patency and inferior functional results are frequently the trade-off for lower perioperative morbidity and mortality. Catheter-based endoluminal techniques, such as PTA, are used for relatively localized disease and may be reasonable alternatives to aortobifemoral bypass in 10% to 15% of patients with aortoiliac occlusive disease.
Atherosclerosis is the most common cause of renal artery stenosis. Occlusive lesions are located almost exclusively in the proximal segment and orifice of the renal artery and are usually an extension of aortic atherosclerosis. Fibromuscular dysplasia is an important, but less common, cause of renal artery stenosis and most frequently involves the distal two thirds of the renal arteries. Hemodynamically significant renal artery stenosis may cause hypertension by activation of the renin-angiotensin-aldosterone system, and bilateral involvement may result in renal failure. Patients with renovascular hypertension frequently have poorly controlled hypertension despite maximal medical therapy. These patients often have severe bilateral renal artery stenosis and may have recurrent congestive heart failure or flash pulmonary edema. Indications for intervention include control of hypertension and salvage of renal function. Operative interventions include aortorenal bypass, extra-anatomic bypass (hepatorenal or splenorenal bypass), or transaortic endarterectomy. Suprarenal or supraceliac aortic cross-clamping is frequently required for open operative interventions. PTA with stenting of the renal artery is used as the first-line treatment in selected patients.
Stenosis at the origin of the celiac and mesenteric arteries occurs as a result of extension of aortic atherosclerosis. The inferior mesenteric artery is by far the most commonly involved, followed by the superior mesenteric artery and the celiac artery.
Occlusion of a single vessel rarely causes ischemic symptoms because of the extensive nature of visceral collateralization. However, occlusion or significant stenosis of any two vessels may compromise collateral flow sufficiently to give rise to chronic visceral ischemia. Operative repair of visceral artery stenosis is reserved for symptomatic patients. Operative interventions include transaortic endarterectomy and bypass grafts, which frequently require supraceliac aortic cross-clamping. Mortality rates for such procedures range from 7% to 18%. To avoid the high mortality associated with open repair, PTA with stenting has increasingly been applied in patients with chronic visceral ischemia. Acute visceral artery occlusion can be caused by an embolus or, less commonly, by thrombosis. To avoid the extremely high mortality associated with acute visceral ischemia, diagnosis and surgical intervention must occur before gangrene of the bowel develops.
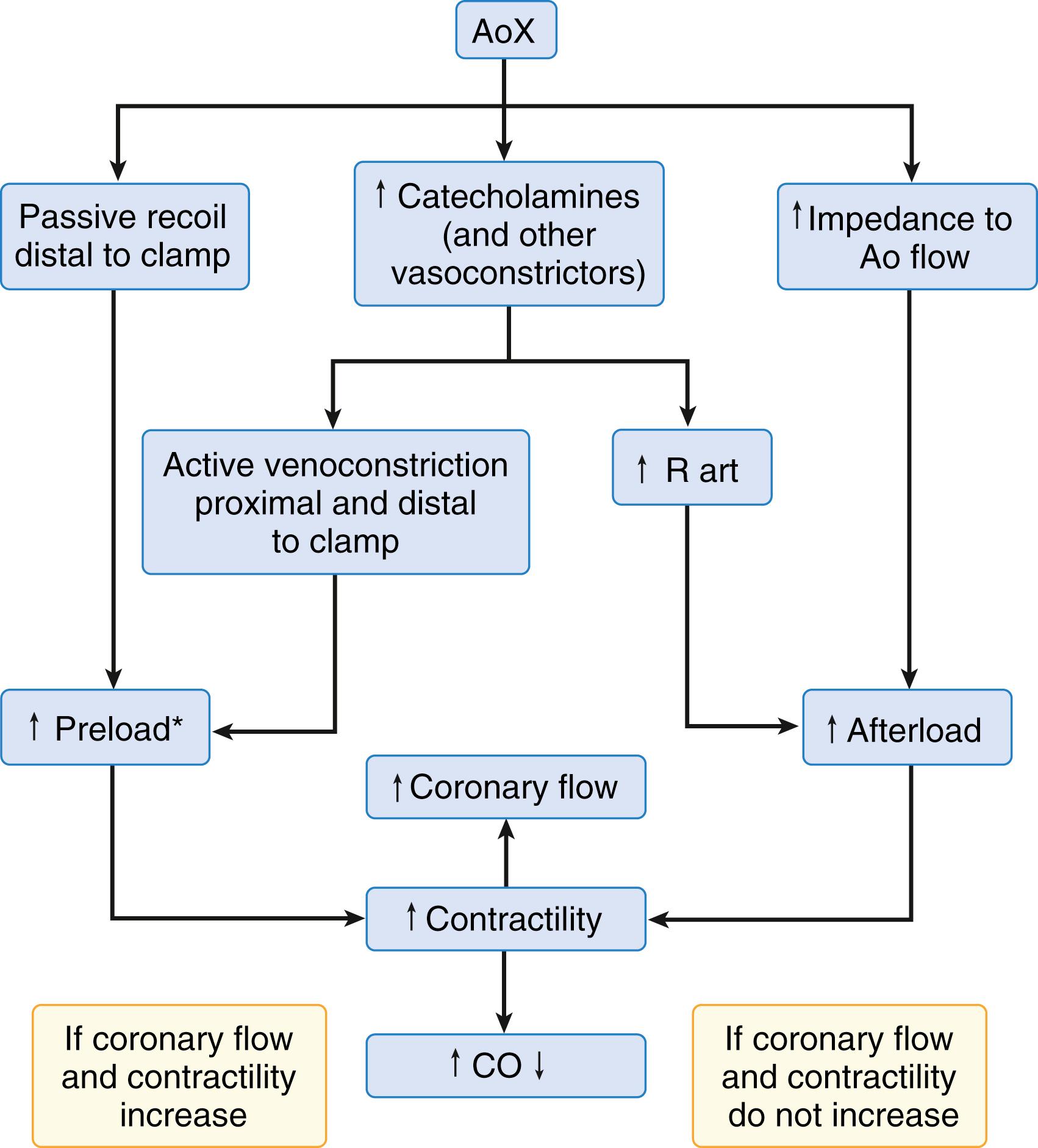
The pathophysiology of aortic cross-clamping is complex and depends on many factors, including level of the cross-clamp, status of the left ventricle, degree of periaortic collateralization, intravascular blood volume and distribution, activation of the sympathetic nervous system, and anesthetic drugs and techniques. Most abdominal aortic reconstructions require clamping at the infrarenal level. However, clamping at the suprarenal and supraceliac levels is required for suprarenal aneurysms and renal or visceral reconstructions and is frequently necessary for juxtarenal aneurysms, inflammatory aneurysms, and aortoiliac occlusive disease with proximal extension. These higher levels of aortic occlusion have a significant impact on the cardiovascular system, as well as on other vital organs rendered ischemic or hypoperfused. Ischemic complications may result in renal failure, hepatic ischemia and coagulopathy, bowel infarction, and paraplegia. With EVAR now common, an increasing proportion of patients undergoing open repair have anatomically complex aneurysms, many of which require suprarenal cross- clamping.
The hemodynamic and metabolic changes associated with aortic cross-clamping are summarized in Box 56.1 . The magnitude and direction of these changes are complex, dynamic, and vary among experimental and clinical studies. However, several important factors must be considered ( Box 56.2 ). The systemic cardiovascular consequences of aortic cross-clamping can be dramatic, depending primarily on the level at which the cross-clamp is applied. Arterial hypertension above the clamp and arterial hypotension below the clamp are the most consistent components of the hemodynamic response to aortic cross-clamping at any level. The increase in arterial blood pressure above the clamp is primarily due to the sudden increase in impedance to aortic blood flow and the resultant increase in systolic ventricular wall tension or afterload. However, factors such as myocardial contractility, preload, blood volume, and activation of the sympathetic nervous system also may be important. Cross-clamping of the aorta at or above the diaphragm results in the most profound increases in arterial blood pressure unless diverting circulatory support or IV vasodilators are used. Changes in cardiac output and filling pressure with aortic cross-clamping are not consistent and require an integrated approach in understanding the direction and magnitude of such changes ( Fig. 56.2 ). Cross-clamping of the proximal descending thoracic aorta increases mean arterial, central venous, mean pulmonary arterial, and pulmonary capillary wedge pressure by 35%, 56%, 43%, and 90%, respectively, and decreases the cardiac index by 29%. Heart rate and left ventricular stroke work are not significantly changed. Supraceliac aortic cross-clamping increases mean arterial pressure by 54% and pulmonary capillary wedge pressure by 38%. Ejection fraction, as determined by two-dimensional echocardiography, decreases by 38%. Despite normalization of systemic and pulmonary capillary wedge pressure with anesthetic agents and vasodilator therapy, supraceliac aortic cross-clamping causes significant increases in left ventricular end-systolic and end-diastolic area (69% and 28%, respectively), as well as wall motion abnormalities indicative of ischemia in 11 of 12 patients ( Table 56.5 ). Aortic cross- clamping at the suprarenal level causes similar but smaller cardiovascular changes and clamping at the infrarenal level is associated with only minimal changes and no wall motion abnormalities.
∗ These changes are of greater significance with longer duration of cross-clamping and with more proximal cross-clamping.
and Therapeutic Interventions
↑ Arterial blood pressure above the clamp
↓ Arterial blood pressure below the clamp
↑ Segmental wall motion abnormalities
↑ Left ventricular wall tension
↓ Ejection fraction
↓ Cardiac output †
‡ When ventilatory settings are unchanged from pre-clamp levels.
† Cardiac output may increase with thoracic cross-clamping.
, ‡
↓ Renal blood flow
↑ Pulmonary occlusion pressure
↑ Central venous pressure
↑ Coronary blood flow
↓ Total body oxygen consumption
↓ Total body carbon dioxide production
↑ Mixed venous oxygen saturation
↓ Total body oxygen extraction
↑ Epinephrine and norepinephrine
Respiratory alkalosis
Metabolic acidosis
Afterload reduction
Sodium nitroprusside
Inhalational anesthetics
Amrinone
Shunts and aorta-to-femoral bypass
Preload reduction
Nitroglycerin
Controlled phlebotomy
Atrial-to-femoral bypass
Renal protection
Fluid administration
Distal aortic perfusion techniques
Selective renal artery perfusion
Mannitol
Drugs to augment renal perfusion
Other
Hypothermia
↓ Minute ventilation
Sodium bicarbonate
Level of aortic cross-clamp
Species differences
Anesthetic agents and techniques
Use of vasodilator therapy
Use of diverting circulatory support
Degree of periaortic collateralization
Left ventricular function
Status of the coronary circulation
Volume status
Neuroendocrine activation
Duration of aortic cross-clamp
Body temperature
| Percent Change After Occlusion | |||
|---|---|---|---|
| Cardiovascular Variable | Supraceliac | Suprarenal-Infraceliac | Infrarenal |
| Mean arterial blood pressure | 54 | 5 ∗ | 2 ∗ |
| Pulmonary capillary wedge pressure | 38 | 10 ∗ | 0 ∗ |
| End-diastolic area | 28 | 2 ∗ | 9 ∗ |
| End-systolic area | 69 | 10 ∗ | 11 ∗ |
| Ejection fraction | −38 | −10 ∗ | −3 ∗ |
| Patients with wall motion abnormalities | 92 | 33 | 0 |
∗ Statistically different ( P < .05) from group undergoing supraceliac aortic occlusion.
The marked increases in ventricular filling pressure (preload) reported with high aortic cross-clamping have been attributed to increased afterload and redistribution of blood volume, which is of prime importance during thoracic aortic cross-clamping. The splanchnic circulation, an important source of functional blood volume reserve, is central to this hypothesis. The splanchnic organs contain nearly 25% of the total blood volume, nearly two thirds (>800 mL) of which can be autotransfused from the highly compliant venous vasculature into the systemic circulation within seconds. Primarily because of smaller splanchnic venous capacitance, blood volume is redistributed from vascular beds distal to the clamp to the relatively noncompliant vascular beds proximal to the clamp ( Fig. 56.3 ). Both passive and active mechanisms lower splanchnic venous capacitance with thoracic aortic cross-clamping. Cross-clamping the aorta above the splanchnic system dramatically reduces splanchnic arterial flow, which produces a significant reduction in pressure within the splanchnic capacitance vessels. This decreased pressure allows the splanchnic veins to passively recoil and increase venous return to the heart and blood volume proximal to the clamp. Thoracic aortic cross-clamping also results in significant increases in plasma epinephrine and norepinephrine, which may enhance venomotor tone both above and below the clamp. The splanchnic veins are highly sensitive to adrenergic stimulation. The major effect of catecholamines on the splanchnic capacitance vessels is venoconstriction, which actively forces out splanchnic blood, reduces splanchnic venous capacitance, and increases venous return to the heart.
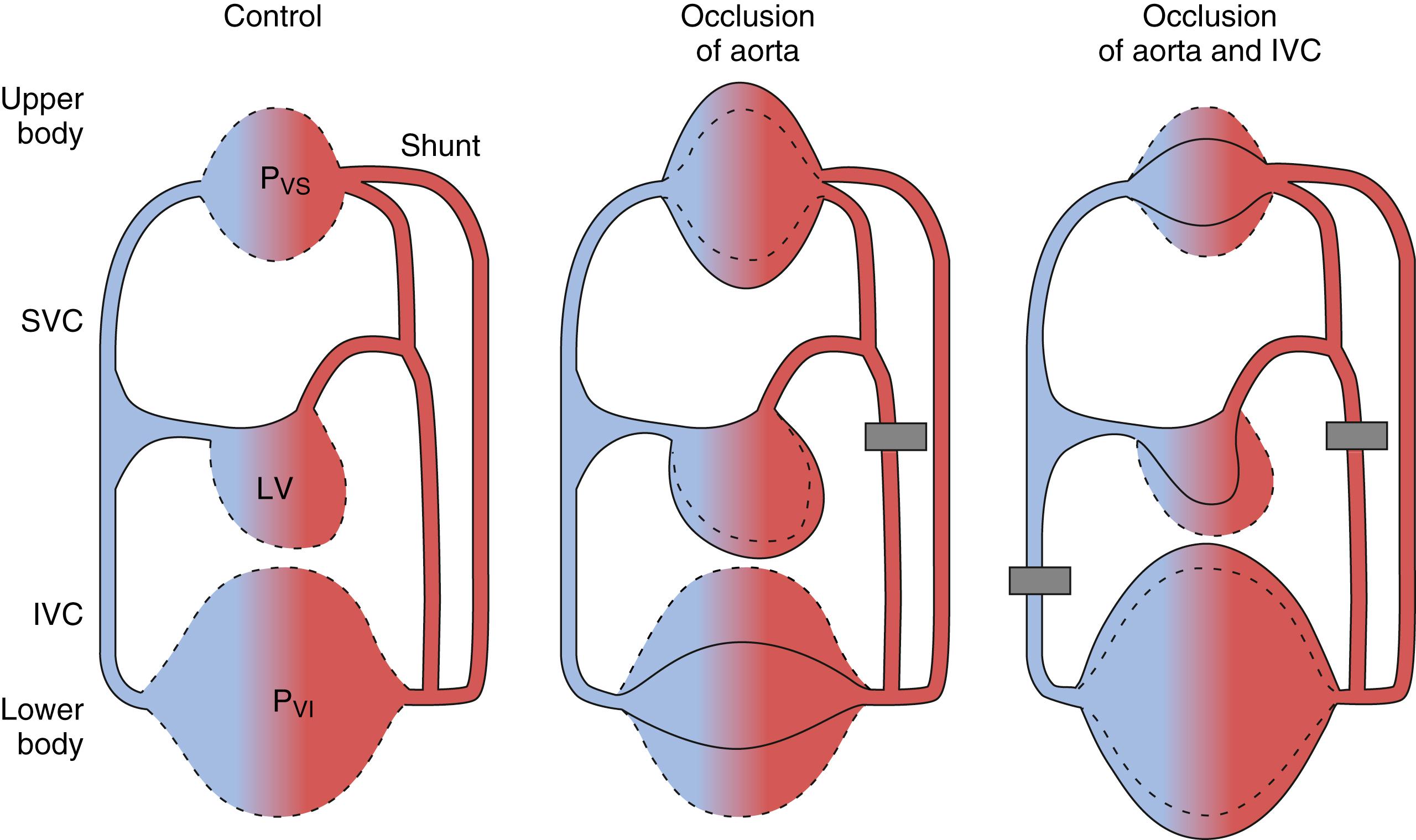
Several animal studies support the blood volume redistribution hypothesis. Cross-clamping the thoracic aorta in dogs results in marked increases in mean arterial pressure and end-diastolic left ventricular pressure (84% and 188%, respectively) and no significant change in stroke volume. In this same experimental model, simultaneous cross-clamping of the thoracic aorta and the inferior vena cava resulted in no significant change in mean arterial pressure or preload (see Fig. 56.3 ). Stroke volume was reduced by 74%. By transfusing blood (above the clamps) during this period of simultaneous clamping, the authors reproduced the hemodynamic effect of thoracic aortic cross-clamping alone. This study also demonstrated that thoracic aortic cross-clamping is associated with a significant and dramatic increase (155%) in blood flow above the level of the clamp whereas no change in blood flow occurred with simultaneous aortic and inferior vena cava clamping. In other animal models, the proximal aortic hypertension and increased central venous pressure (CVP) occurring after thoracic aortic cross-clamping were completely reversed by phlebotomy. Aortic cross-clamping at the thoracic and suprarenal levels in dogs both resulted in proximal aortic hypertension, but only occlusion at the thoracic level increased central venous pressure. In this study, thoracic aortic occlusion increased blood volume in organs and tissues proximal to the clamp whereas no such increase occurred with suprarenal aortic cross-clamping. These experimental data strongly support the hypothesis of blood volume redistribution during aortic cross-clamping and help explain the marked differences in hemodynamic responses observed after aortic cross-clamping at different levels.
Afterload-dependent increases in preload also occur with aortic cross-clamping, usually in the setting of impaired myocardial contractility and reduced coronary reserve. The impaired left ventricle may respond to increased afterload with an increase in end-systolic volume and a concomitant reduction in stroke volume (afterload mismatch). The reduction in stroke volume may be due to limited preload reserve, myocardial ischemia, or inability of the heart to generate a pressure-induced increase in contractility (the Anrep effect). If right ventricular function remains normal, the pre-clamp right ventricular stroke volume added to the increased left ventricular end-systolic volume results in left ventricular dilation and elevated end-diastolic volume. If corrective measures are not undertaken, overt left ventricular overload may result, with severe peripheral organ dysfunction and pulmonary edema.
Most clinical studies indicate that cardiac output decreases with thoracic aortic cross-clamping (without vasodilator therapy or diverting circulatory support), whereas most animal studies show no significant change or an increase in cardiac output.
However, the status of the left ventricle clearly plays a major role. Whereas a normal intact heart can withstand large increases in volume without significant ventricular distention or dysfunction, an impaired heart with reduced myocardial contractility and coronary reserve may respond to such increase in volume conditions with marked ventricular distention as a result of acute left ventricular dysfunction and myocardial ischemia. Although impaired myocardial contractility and reduced coronary reserve are rare in animal experiments, such disorders are frequent in the elderly population undergoing aortic reconstruction. The increase in ventricular loading conditions seen with thoracic and supraceliac cross-clamping in the clinical setting may increase left ventricular wall stress (afterload), with resultant acute deterioration of left ventricular function and myocardial ischemia.
Impaired subendocardial perfusion caused by high intramyocardial pressure may be the cause of wall motion abnormalities and changes in ejection fraction. Reflex mechanisms causing immediate feedback inhibition may also explain the reduction in cardiac output with aortic cross-clamping. For example, baroreceptor activation resulting from increased aortic pressure should depress the heart rate, contractility, and vascular tone. Thoracic aortic cross-clamping with the use of vasodilator therapy to normalize ventricular loading conditions maintains or increases cardiac output. The metabolic effects of aortic cross-clamping are summarized in Box 56.1 . Cross-clamping of the thoracic aorta decreases total-body O2 consumption by approximately 50%. For reasons that are unclear, O2 consumption decreases in tissues above the clamp. In clinical studies, increased mixed venous O2 saturation occurs with aortic cross-clamping above the celiac axis. This increase in mixed venous O2 saturation may be explained by a reduction in O2 consumption that exceeds the reduction in cardiac output, thus decreasing total body O2 extraction. Central hypervolemia and increased arteriovenous shunting in tissues proximal to the aortic clamp may play a role in reducing total body O2 extraction. Arterial blood pressure, blood flow, and O2 consumption distal to a thoracic aortic cross-clamp decrease by 78% to 88%, 79% to 88%, and 62%, respectively, from baseline values before clamping. Blood flow through tissues and organs below the level of aortic occlusion is dependent on perfusion pressure and is independent of cardiac output. Administration of sodium nitroprusside to maintain proximal aortic pressure above the cross-clamp at pre-clamp levels has been shown to further reduce arterial pressure distal to the clamp by 53%. As discussed later, these data have significant implications regarding vital organ protection during aortic cross-clamping.
The cardiovascular response to infrarenal aortic cross-clamping is less significant than with high aortic cross-clamping (see Table 56.5 ). Although several clinical reports have noted no significant hemodynamic response to infrarenal cross-clamping, the hemodynamic response generally consists of increases in arterial pressure (7% to 10%) and systemic vascular resistance (20% to 32%), with no significant change in heart rate. Cardiac output is most consistently decreased by 9% to 33%. Reported changes in ventricular filling pressure have been inconsistent. Blood volume redistribution may affect preload with infrarenal aortic cross-clamping (see Fig. 56.3 ). In this situation, blood volume below the clamp shifts to the compliant venous segments of the splanchnic circulation above the clamp, thereby dampening the expected increase in preload. The preload changes with infrarenal aortic cross-clamping also may depend on the status of the coronary circulation. Patients with severe ischemic heart disease responded to infrarenal aortic cross-clamping with significantly increased central venous (35%) and pulmonary capillary (50%) pressure, whereas patients without CAD had decreased filling pressure. Echocardiographically detected segmental wall motion abnormalities occur in up to 30% of patients during infrarenal aortic reconstruction, with over 60% occurring at the time of aortic cross-clamping. Patients with aortoiliac occlusive disease may have less hemodynamic response to infrarenal aortic cross-clamping than do patients with AAA disease, perhaps as a result of more extensive periaortic collateral vascularization.
Preservation of renal function is highly important during aortic reconstructive surgery. Acute renal failure occurs in approximately 3% of patients undergoing elective infrarenal aortic reconstruction, and mortality resulting from postoperative acute renal failure is more frequent than 40%. Despite significant improvements in the perioperative care of these patients, the frequent incidence of morbidity and mortality resulting from acute renal failure has remained largely unchanged over the last several decades. Most of the morbidity associated with significant postoperative renal dysfunction is nonrenal.
The adequacy of renal perfusion “cannot” be assumed by urine output. Although urine output is closely monitored and often augmented during aortic surgery, intraoperative urine output does not predict postoperative renal function. Procedures requiring aortic cross-clamping above the renal arteries dramatically reduce renal blood flow. Experimental studies report an 83% to 90% reduction in renal blood flow during thoracic aortic cross-clamping. Infrarenal aortic cross-clamping in humans is associated with a 75% increase in renal vascular resistance, a 38% decrease in renal blood flow, and a redistribution of intrarenal blood flow toward the renal cortex. These rather profound alterations in renal hemodynamics occurred despite no significant change in systemic hemodynamics, and they persisted after unclamping. The sustained deterioration in renal perfusion and function during and after infrarenal aortic cross-clamping has been attributed to renal vasoconstriction, but the specific pathophysiologic process remains unknown. Renal sympathetic blockade with epidural anesthesia to a T6 level does not prevent or modify the severe impairment in renal perfusion and function that occurs during and after infrarenal aortic cross-clamping. Although plasma renin activity is increased during aortic cross-clamping, pretreatment with converting enzyme inhibitors before infrarenal aortic cross-clamping does not attenuate the decreased renal blood flow and glomerular filtration rate. Other mediators, such as plasma endothelin, myoglobin, and prostaglandins, may contribute to the decreased renal perfusion and function after aortic cross-clamping.
Acute tubular necrosis accounts for nearly all the renal dysfunction and failure after aortic reconstruction. The degree of preoperative renal insufficiency remains the strongest predictor of postoperative renal dysfunction. In addition to aortic cross-clamping-induced reductions in renal blood flow, ischemic reperfusion injury, intravascular volume depletion, embolization of atherosclerotic debris to the kidneys, and surgical trauma to the renal arteries all contribute to renal dysfunction.
Mannitol, loop diuretics, and dopamine are used clinically to preserve renal function during aortic surgery. Significant controversy exists regarding the use of these drugs, as well as the mechanisms by which they may offer a protective effect. Although not proved, pharmacologic “protection” before aortic cross-clamping is believed to be beneficial and is therefore given. The use of mannitol 12.5 g/70 kg to induce osmotic diuresis before aortic cross-clamping is ubiquitous in clinical practice. Mannitol improves renal cortical blood flow during infrarenal aortic cross-clamping and reduces ischemia-induced renal vascular endothelial cell edema and vascular congestion. Other mechanisms by which mannitol may be beneficial include acting as a scavenger of free radicals, decreasing renin secretion, and increasing renal prostaglandin synthesis. Loop diuretics and low-dose dopamine (1 to 3 μg/kg/min) are used to protect the kidneys from aortic cross-clamp-induced injury by increasing renal blood flow and urine output intraoperatively. Routine use of these drugs is common for patients with preoperative renal insufficiency and for procedures requiring suprarenal aortic cross-clamping. Intraoperative use of these drugs requires increased surveillance of intravascular volume and electrolytes during the postoperative period. Therapy with these drugs could actually be harmful because of hypovolemia and resultant renal hypoperfusion. In addition, dopamine’s positive inotropic and chronotropic activity may cause tachycardia and increase myocardial O2 consumption in patients with limited coronary reserve.
Fenoldopam mesylate, a selective dopamine type 1 agonist that preferentially dilates the renal and splanchnic vascular beds, has shown some promise as a renoprotective drug. However, its role in the prevention of renal dysfunction after aortic surgery is not known. Statin use is associated with preserved renal function after aortic surgery requiring suprarenal aortic cross-clamping. Remote ischemic preconditioning reduces the incidence of renal impairment after open aortic surgery. Optimal systemic hemodynamics, including maintenance of intravascular volume and hematocrit, is generally considered the most effective means of renal protection during and after aortic cross-clamping. The goal is to achieve a preload adequate to allow the left ventricle to cope with cross-clamping-induced changes in contractility and afterload while maintaining cardiac output. However, in providing such therapy, excessive intravascular volume should be avoided because it may lead to inappropriate increases in preload or pulmonary edema in patients with decreased myocardial reserve.
Patients with preexisting impaired ventricular function and reduced coronary reserve are most vulnerable to the stress imposed on the cardiovascular system by aortic cross-clamping. Rational therapeutic strategies to prevent the deleterious effect of aortic cross-clamping primarily include measures to reduce afterload and maintain a normal preload and cardiac output. Vasodilators, positive and negative inotropic drugs, and controlled intravascular volume depletion (i.e., phlebotomy) may be used selectively.
Patients with impaired ventricular function requiring supraceliac aortic cross-clamping are the most challenging. Myocardial ischemia, reflecting an unfavorable balance between myocardial O2 supply and demand, may result from the hemodynamic consequences of aortic cross-clamping. Controlled (i.e., slow clamp application) supraceliac aortic cross-clamping is important to avoid abrupt and extreme stress on the heart. Both afterload and preload reduction are often required. Afterload reduction, most commonly accomplished with the use of sodium nitroprusside or clevidipine (predominantly arteriolar dilators), is necessary to unload the heart and reduce ventricular wall tension. In a large series of patients requiring cross-clamping of the descending thoracic aorta, stable left ventricular function was maintained with sodium nitroprusside during cross-clamping. Sodium nitroprusside most likely allowed adequate intravascular volume before unclamping, which resulted in stable unclamping hemodynamics. A normal preload is equally important and involves careful IV fluid titration and vasodilator administration. Nitroglycerin can be used because it increases venous capacity more than does sodium nitroprusside.
In patients without evidence of left ventricular decompensation or myocardial ischemia during supraceliac aortic cross-clamping, a proximal aortic mean arterial pressure of up to 120 mm Hg is acceptable. The surgeon may request lower proximal arterial pressure if friable aortic tissue is encountered. Blood flow below the aortic clamp depends on pressure and decreases further during therapy with vasodilators. In this setting, vital organs and tissues distal to the clamp are exposed to reduced perfusion pressure and blood flow. Though infrequent, maintenance of adequate cardiac output may require active intervention with inotropic drugs.
The hemodynamic and metabolic effects of aortic unclamping are listed in Box 56.3 . The hemodynamic response to unclamping depends on many factors, including the level of aortic occlusion, total occlusion time, use of diverting support, and intravascular volume. Hypotension, the most consistent hemodynamic response to aortic unclamping, can be profound, particularly after removal of a supraceliac cross-clamp ( Fig. 56.4 ). Reactive hyperemia in tissues and organs distal to the clamp and the resultant relative central hypovolemia are the dominant mechanisms of the hypotension. Washout of vasoactive and cardiodepressant mediators from ischemic tissues, as well as humoral factors, may also contribute to the hemodynamic responses after unclamping the aorta. These humoral factors and mediators, which may also play a role in organ dysfunction after aortic occlusion, include lactic acid, renin-angiotensin, O2 free radicals, prostaglandins, neutrophils, activated complement, cytokines, and myocardial-depressant factors.
∗ These changes are of greater significance with longer duration of cross-clamping and with more proximal cross-clamping.
and Therapeutic Intervention
↓ Myocardial contractility
↓ Arterial blood pressure
↑ Pulmonary artery pressure
↓ Central venous pressure
↓ Venous return
↓ Cardiac output
↑ Total body oxygen consumption
↑ Lactate
↓ Mixed venous oxygen saturation
↑ Prostaglandins
↑ Activated complement
↑ Myocardial-depressant factor(s)
↓ Temperature
Metabolic acidosis
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here