Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
For the purposes of planning a strategy for controlling intracranial pressure (ICP), the four subcompartments of the intracranial space should be considered: cells, interstitial and intracellular fluid, cerebrospinal fluid (CSF), and blood.
The clinician should make a preoperative assessment of the probable intracranial compliance reserve as the basis for selection of appropriate anesthetic drugs and techniques.
The venous side of the cerebral circulation is a largely passive compartment that is often the cause of increased ICP, or “tightness,” of the surgical field.
Cerebral perfusion pressure (CPP) should be supported at or near normal waking levels in patients with recent cerebral injuries (e.g., traumatic brain injury [TBI], subarachnoid hemorrhage [SAH], and spinal cord injury [SCI]) because of low resting cerebral blood flow and impaired autoregulation.
When neurosurgical procedures are performed in the sitting position, blood pressure should be corrected to the level of the external auditory meatus and mean arterial pressure (MAP) should be maintained at 60 mm Hg in normotensive adults.
Monitoring for venous air embolism (VAE) in at-risk situations includes the precordial Doppler and end-tidal carbon dioxide analysis.
Despite encouraging preclinical data, therapeutic mild hypothermia cannot be advocated in the care of head-injured patients in the intensive care unit (ICU) or during the operative management of patients with intracranial aneurysms because of negative human trial results for those patient groups.
The most important consideration in the anesthetic management of patients undergoing clipping or coiling after acute SAH is the prevention of paroxysmal hypertension with its attendant risk of aneurysm rerupture. Nonetheless, adequate perfusion pressure is needed if temporary clips are used during management of a cerebral aneursym.
Although induced hypotension is rarely used electively in aneurysm surgery, the clinician must be ready to reduce blood pressure immediately and accurately in the event of aneurysm rupture.
Tracheal intubation of a head-injured patient with an undefined cervical spine injury can be accomplished using rapid sequence induction with manual in-line stabilization (the occiput held rigidly to the backboard), with only a very small risk of injury to the spinal cord.
CPP (CPP = MAP - ICP) should be supported to a target range of 60 to 70 mm Hg in the first 48 to 72 hours after TBI in adults.
Hypocapnia has the potential to cause cerebral ischemia, particularly in a recently injured brain and in a brain beneath retractors; it should be used only when absolutely necessary for the control of critically increased or uncertain ICP.
This chapter provides guidelines for the management of common situations in neurosurgical anesthesia. Issues that arise in connection with a wide variety of neurosurgical procedures—those constituting a checklist that the practitioner should review before undertaking anesthesia for any neurosurgical procedure—are reviewed first, followed by procedure-specific discussions. This chapter assumes familiarity with the cerebral physiology and effects of anesthetics as described in Chapter 11 , and with neurologic monitoring as described in Chapter 39 . Carotid endarterectomy (CEA) and carotid angioplasty and stenting are discussed in Chapter 56 .
Several basic elements of neurosurgical and neuroanesthetic management are recurrent and, in the absence of an established understanding between surgeon and anesthesiologist, should be discussed and agreed upon at the outset of every neurosurgical procedure ( Box 57.1 ). The list varies by procedure and may include the intended surgical position and requisite positioning aids; intentions with respect to the use of steroids, osmotic agents/diuretics, anticonvulsants, and antibiotics; the surgeon’s perception of the “tightness” of the intracranial space and the remaining intracranial compliance reserve; appropriate objectives for the management of blood pressure, carbon dioxide tension, and body temperature; anticipated blood loss; the intended use of neurophysiologic monitoring (which may impose constraints on the use of anesthetics or muscle relaxants, or both); and, sometimes, the perceived risk of air embolism. The considerations driving the decisions made about these issues are presented in this section. One additional recurrent issue, brain protection, is discussed briefly in the section on aneurysms and arteriovenous malformations (AVMs) and in detail in Chapter 11 .
Control of intracranial pressure/brain relaxation
Management of Pa CO 2
Management of arterial blood pressure
Use of steroids
Use of osmotherapy
Use of diuretics
Use of anticonvulsants
Patient positioning
Pneumocephalus
Venous air embolism
Monitoring
Intravenous fluid management
Hypothermia
Glucose management
Emergence from anesthesia
The necessity of preventing increases in intracranial pressure (ICP) or reducing ICP that is already increased is recurrent in neuroanesthesia. When the cranium is closed, the objectives are to maintain adequate cerebral perfusion pressure (CPP) (CPP = mean arterial pressure [MAP] − ICP) and prevent the herniation of brain tissue between intracranial compartments or through the foramen magnum ( Fig. 57.1 ). When the cranium is open, the issue may be to provide relaxation of the intracranial contents to facilitate surgical access or, in extreme circumstances, reverse ongoing brain herniation through the craniotomy site. The principles that apply are similar whether the cranium is open or closed.
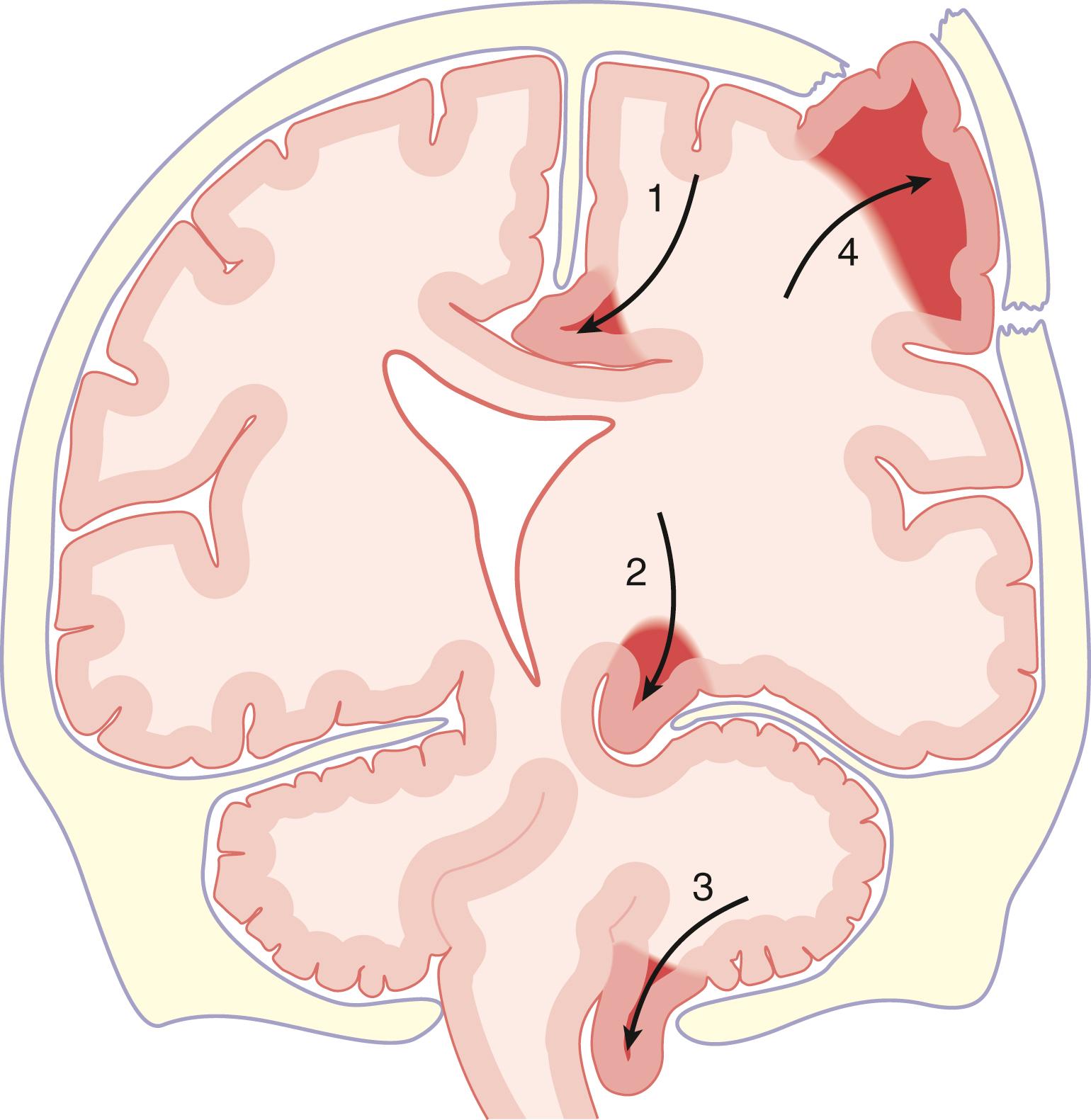
The various clinical indicators of increased ICP include headache (particularly headache that awakens the patient at night), nausea and vomiting, blurred vision, somnolence, and papilledema. Computed tomography (CT) findings suggestive of either increased ICP or reduced intracranial compliance reserve include midline shift, obliteration of the basal cisterns, loss of sulci, ventricular effacement (or enlarged ventricles in the event of hydrocephalus or ventricular trapping), and edema. Edema appears on a CT scan as a region of hypodensity. The basal cisterns appear on CT as a dark (hypodense fluid) halo around the upper end of the brainstem ( Fig. 57.2 ). They include the interpeduncular cistern, which lies between the two cerebral peduncles, the quadrigeminal cistern, which overlies the four colliculi, and the ambient cisterns, which lie lateral to the cerebral peduncles.
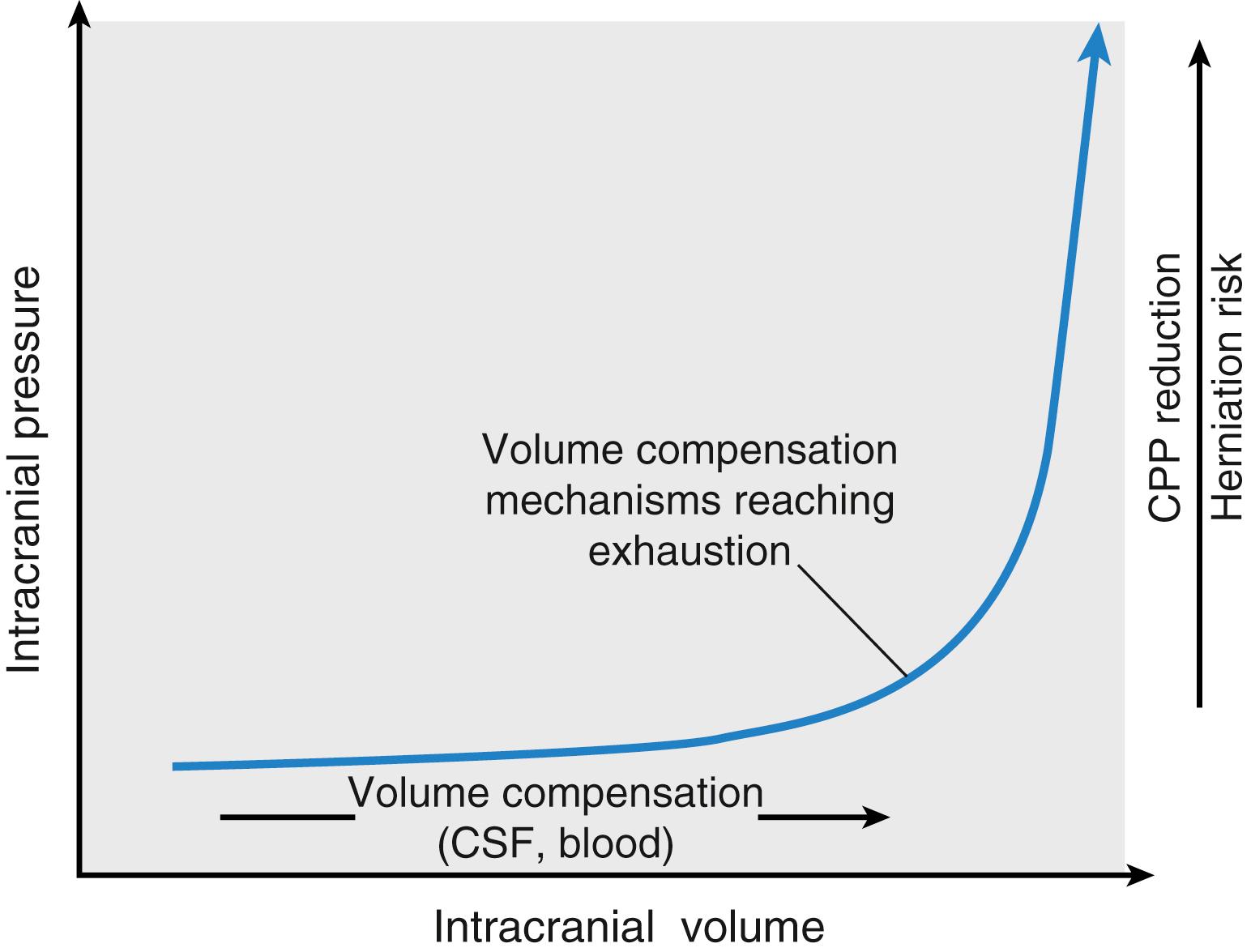
Fig. 57.3 presents the volume-pressure relationship of the intracranial space. The plateau phase occurring at low volumes reveals that the intracranial space is not completely closed, which confers some compensatory latitude. Compensation is accomplished principally by the translocation of cerebrospinal fluid (CSF) and venous blood to the spinal CSF space and the extracranial veins, respectively. Ultimately, when the compensatory potential is exhausted, even tiny incremental increases in volume can substantially increase ICP. These increases have the potential to result in either herniation of brain tissue from one compartment to another (or into the surgical field) (see Fig. 57.1 ), with resultant mechanical injury to brain tissue, or in reduction of perfusion pressure, leading to ischemic injury.
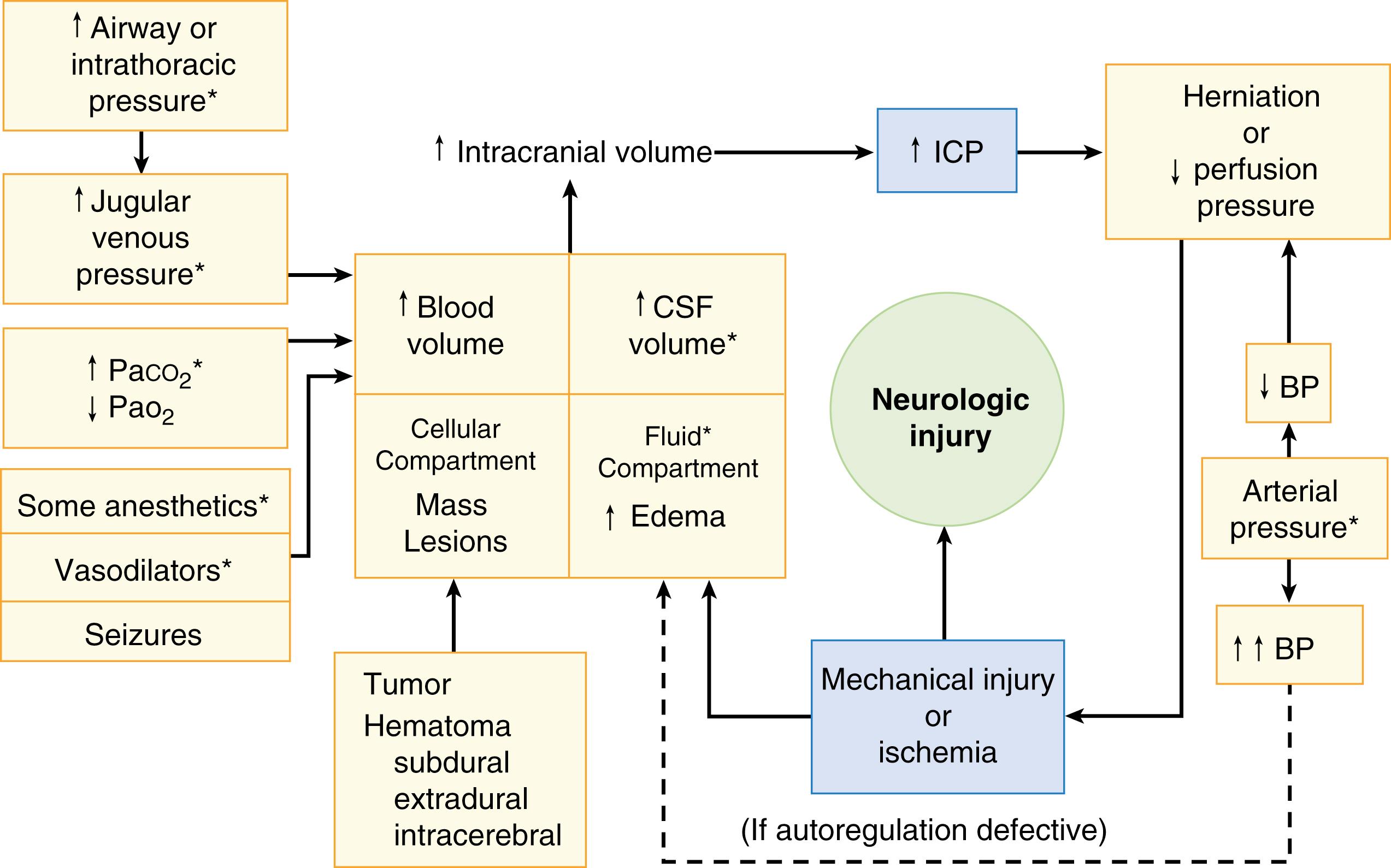
Several variables can interact to cause or aggravate intracranial hypertension ( Fig. 57.4 ). For clinicians faced with the problem of managing increased ICP, the objective is, broadly speaking, to reduce the volume of the intracranial contents. For mnemonic purposes, the clinician can divide the intracranial space into four subcompartments ( Table 57.1 ): cells (including neurons, glia, tumors, and extravasated collections of blood), fluid (intracellular and interstitial), CSF, and blood.
The cellular compartment. This compartment is largely the province of the surgeon. However, it may be the anesthesiologist’s responsibility to pose a well-placed diagnostic question. When the brain is bulging into the surgical field at the conclusion of evacuation of an extra-axial hematoma, the clinician should ask whether a subdural or extradural hematoma is present on the contralateral side that warrants either immediate burr holes or immediate postprocedure radiologic evaluation.
The CSF compartment. There is no pharmacologic manipulation of the CSF space with a time course and magnitude that is relevant to the neurosurgical operating room. The only practical means of manipulating the size of this compartment is by drainage. A tight surgical field can sometimes be improved by passage of a brain needle by the surgeon into a lateral ventricle to drain CSF. Lumbar CSF drainage can be used to improve surgical exposure in situations with no substantial hazard of uncal or transforamenal magnum herniation.
The fluid compartment. This compartment can be addressed with steroids and osmotic/diuretic agents. The use of these agents is discussed later.
The blood compartment. This compartment receives the anesthesiologist’s greatest attention because it is the most amenable to rapid alteration. The blood compartment should be viewed as having two separate components: venous and arterial.
| Compartment | Volume Control Methods |
|---|---|
|
Surgical removal |
|
Diuretics osmotic/diuretic agents Steroids (principally tumors) |
|
Drainage |
|
|
|
Decrease cerebral blood flow |
|
Improve cerebral venous drainage |
With respect to the blood compartment, the venous side of the circulation should initially be considered. It is largely a passive compartment and is often overlooked. Despite this passivity, engorgement of this compartment is a common cause of increased ICP or poor conditions in the surgical field ( Fig. 57.5 ). A head-up posture to ensure good venous drainage is the standard in neurosurgical anesthesia and critical care. Obstruction of cerebral venous drainage by extremes of head position or circumferential pressure (cervical collars, endotracheal tube ties) should be avoided. Anything that causes increased intrathoracic pressure can also result in obstruction of cerebral venous drainage. Relevant phenomena include kinking or partial obstruction of endotracheal tubes, tension pneumothorax, coughing or straining against the endotracheal tube, or gas trapping as a result of bronchospasm. Neuromuscular blockade is usually induced during craniotomies unless a contraindication is present. Such a blockade would prevent a sudden cough that can cause a dramatic herniation of cerebral structures through the craniotomy.
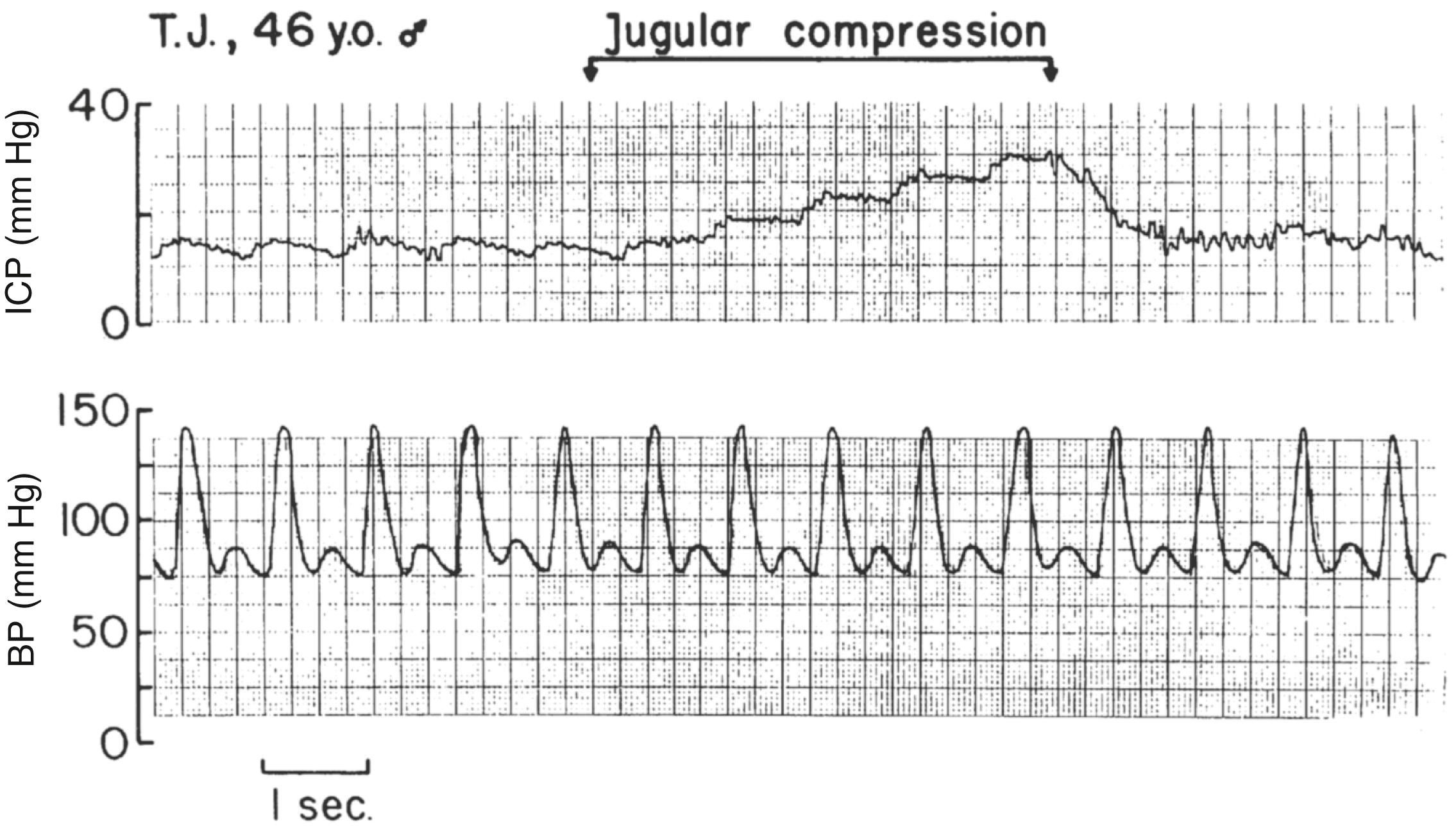
Thereafter, the arterial side of the circulation should be considered. Attention to the effect of anesthetic drugs and techniques on cerebral blood flow (CBF) (see Chapter 11 ) is an established part of neuroanesthesia because, in general, increases in CBF are associated with increases in cerebral blood volume (CBV). The notable exception to this rule occurs in the context of cerebral ischemia caused by hypotension or vessel occlusion, at which times CBV may increase as the cerebral vasculature dilates in response to a sudden reduction in CBF. However, the relationship generally applies, and attention to the control of CBF is relevant in situations in which intracranial volume compensation mechanisms are exhausted or ICP is already increased. The general approach is to select anesthetics and to control physiologic variables in a manner that avoids unnecessary increases in CBF. The variables that influence CBF are listed in Box 57.2 and are discussed in Chapter 11 .
See effects of anesthetics on cerebral blood flow and cerebral metabolic rate in Chapter 11 for detailed discussion.
Pa O 2
Pa CO 2
Cerebral metabolic rate
Arousal/pain
Seizures
Temperature
Anesthetics
Blood pressure/status of autoregulation
Vasoactive agents
Anesthetics
Pressors
Inotropes
Vasodilators
Blood viscosity
Neurogenic pathways (intra- and extra-axial)
The question of which anesthetics are appropriate, especially in the context of unstable ICP, arises frequently. Chapter 11 provides relevant information in detail, and only broad generalizations are described here.
In general, intravenous anesthetic, analgesic, and sedative drugs are associated with parallel reductions in CBF and cerebral metabolic rate (CMR) and consequently will not have adverse effects on ICP. Ketamine, given in large doses to patients with a generally normal level of consciousness before anesthesia, is the exception. Autoregulation and carbon dioxide (CO 2 ) responsiveness are generally preserved during the administration of intravenous anesthetics (see Chapter 11 ).
By contrast, all the volatile anesthetics can be, depending on physiologic and pharmacologic circumstances, dose-dependent cerebral vasodilators. The order of vasodilating potency is approximately halothane → enflurane → desflurane → isoflurane → sevoflurane. As noted in Chapter 11 , the CBF differences among desflurane, isoflurane, and sevoflurane are unlikely to be clinically significant. The net CBF effect of a volatile anesthetic depends on the interaction of several factors: the concentration of the anesthetic, the extent of previous CMR depression, simultaneous blood pressure changes acting in conjunction with previous or anesthetic-induced autoregulation abnormalities, and simultaneous changes in partial pressure of carbon dioxide in the arterial blood (Pa CO 2 ) acting in conjunction with any disease-related impairment in CO 2 responsiveness.
Nitrous oxide (N 2 O) can also be a cerebral vasodilator. The CBF effect of N 2 O is greatest when it is administered as a sole anesthetic; least when it is administered against a background of narcotics, propofol, or benzodiazepines; and intermediate when it is administered in conjunction with volatile anesthetics (see Chapter 11 ).
Despite the vasodilatory potential of both N 2 O and volatile anesthetics, experience dictates that both, with the latter in concentrations less than the minimum alveolar concentration (MAC), can be used in most elective and many emergent neurosurgical procedures when administered as part of a balanced anesthetic technique in combination with opioids. However, there are exceptions. Because both N 2 O and volatile anesthetics can be vasodilators in some circumstances, when the compensatory latitude of the intracranial space has been exhausted and physiology is abnormal, omitting them on a just-in-case basis may be prudent. In a somnolent, vomiting patient with papilledema, a large tumor mass, and compressed basal cisterns; or in a traumatic brain injury (TBI) victim with an expanding mass lesion or obliterated cisterns and sulci on CT, a predominantly intravenous technique should be used until the cranium and dura are open. Thereafter, the effect of the anesthetic technique can be assessed by direct observation of the surgical field. Although inhaled anesthetics are entirely acceptable components of most anesthetics for neurosurgery, in circumstances in which ICP is persistently increased or the surgical field is persistently “tight,” N 2 O and volatile anesthetics should be replaced by intravenous anesthetics.
Neuromuscular blockers that can release histamine (e.g., atracurium) should be given in small, divided doses. Although succinylcholine can increase ICP, the increases are small and transient. Moreover, the increases can be blocked by a preceding dose of nondepolarizing neuromuscular blocking drugs and, in at least some instances, are not evident in patients with common emergency neurosurgical conditions (TBI, SAH). Succinylcholine in conjunction with proper management of the airway and MAP can be used when rapid endotracheal intubation is needed.
From the material just presented and from the discussion of cerebral physiology in Chapter 11 , a systematic clinical approach should follow easily. A schema for approaching the problem of an acute increase in ICP or acute deterioration in conditions in the surgical field is presented in Box 57.3 .
Are the relevant pressures controlled?
Jugular venous pressure
Extreme head rotation or neck flexion?
Direct jugular compression?
Head-up posture?
Airway pressure
Airway obstruction?
Bronchospasm?
Straining, coughing; adequately relaxed?
Pneumothorax?
Excessive PEEP or APR ventilation?
Partial pressure of CO 2 and O 2 (Pa CO 2 , PaO 2 )
Arterial pressure
Is the metabolic rate controlled?
Pain/arousal?
Seizures?
Febrile?
Are any potential vasodilators in use?
N 2 O, volatile agents, nitroprusside, calcium channel blockers?
Are there any unrecognized mass lesions?
Hematoma
Air ± N 2 O
CSF (clamped ventricular drain)
APR, Airway pressure release; CSF, cerebrospinal fluid; PEEP, positive end-expiratory pressure.
If the problem has not resolved satisfactorily after following the approach in Box 57.3 , Box 57.4 presents options for resolution. CSF drainage was discussed earlier. Additional hyperosmolar solutions are frequently used (see the subsequent section Osmotherapy and Diuretics). Barbiturates have long been the most widely used drugs for inducing reduction in CMR, with the objective of causing a coupled reduction in CBF and CBV. Propofol has gained popularity for this application. However, although the use of barbiturates is supported by ICU experience demonstrating efficacy in ICP control (if not outcome), little such evidence has been accumulated for propofol. Furthermore, a frequently fatal syndrome of metabolic acidosis and rhabdomyolysis has been recognized in patients who have received prolonged propofol infusions in the ICU setting.
Further reduction of Pa CO 2 (to not <23-25 mm Hg)
CSF drainage (ventriculostomy, brain needle)
Diuresis (usually mannitol)
CMR suppression (barbiturates, propofol)
MAP reduction (if dysautoregulation)
Surgical control (i.e., lobectomy or removal of bone flap)
CMR , Cerebral metabolic rate; MAP , mean arterial pressure.
The anesthesiologist and the surgeon should agree on the objectives with respect to Pa co 2 . Induction of hypocapnia was once a routine part of the management of intracranial neurosurgical procedures. The rationale is principally that the concomitant reduction in CBF (see Chapter 11 , Fig. 11.9) and CBV will result in a reduction in ICP, or “brain relaxation.” The rationale is valid. However, two considerations should influence the clinician’s use of hyperventilation. First, the vasoconstrictive effect of hypocapnia can cause ischemia in certain situations. Second, the CBF-lowering and ICP-lowering effect is not sustained for prolonged time periods.
A normal brain is unlikely to be damaged by the typical clinical use of hyperventilation. However, this may not be the case in certain pathologic conditions.
Available data indicate that in normal subjects, ischemia will not occur at a Pa CO 2 greater than 20 mm Hg. However, in one investigation, electroencephalogram (EEG) abnormalities and paresthesia occurred in volunteers hyperventilating to Pa CO 2 values less than 20 mm Hg, and these effects were reversed by hyperbaric oxygenation, suggesting that they may truly have been caused by ischemia. Accordingly, given that a Pa CO 2 of less than 20 to 25 mm Hg offers very little additional benefit in terms of improvement in intracranial compliance, acute reduction of Pa CO 2 should be no more than 22 to 25 mm Hg in previously normocapnic persons.
Preventing herniation, maintaining ICP less than 20 mm Hg, minimizing retractor pressure, and facilitating surgical access remain priorities that may justify hypocapnia. Yet hyperventilation is potentially deleterious and should not be overused. Hyperventilation can result in ischemia, especially when baseline CBF is low, as is commonly the case in the first 24 hours after injury. An increased frequency of brain regions with very low CBF has been demonstrated in head-injured patients who were acutely hyperventilated. In addition, low levels of jugular venous oxygen saturation (SjvO 2 ) can be increased by reducing the degree of hyperventilation.
The deleterious effect of hyperventilation is difficult to prove. Muizelaar and colleagues performed a study that included a near normocapnic group in which Pa CO 2 was maintained at approximately 35 mm Hg, and a hypocapnic group in which Pa CO 2 was maintained in the vicinity of 25 mm Hg. Outcomes at 3 and 6 months after injury were not different. However, in a subset of patients with the best initial motor scores, outcome was better in the normocapnic group. These patients with good motor scores probably represented a subgroup in which the severity of injury was such that, although they needed tracheal intubation, hyperventilation was not necessarily required for control of ICP and who therefore had little to gain from hyperventilation. Thus, prophylactic hyperventilation seemed inadvisable. That conclusion has been extrapolated far beyond the circumstances of relatively mild TBI.
Hyperventilation should not be an automatic component of every neuroanesthetic. There should be an indication for its institution (usually increased or uncertain ICP or the need to improve conditions in the surgical field, or both). Hyperventilation has the potential to cause an adverse effect and should be withdrawn as the indication for it subsides. The concern regarding the hazards of hypocapnia, which evolved in the context of TBI, has influenced all of neurosurgery. In particular, it is now widely avoided in the management of SAH because of the low CBF state that is known to occur. In addition, brain tissue beneath retractors can have a similarly reduced CBF. However, hyperventilation, used as briefly as possible, is still very much a component of rescue therapy, when herniation is imminent or in progress, or when conditions in the surgical field are too difficult to allow surgery to proceed.
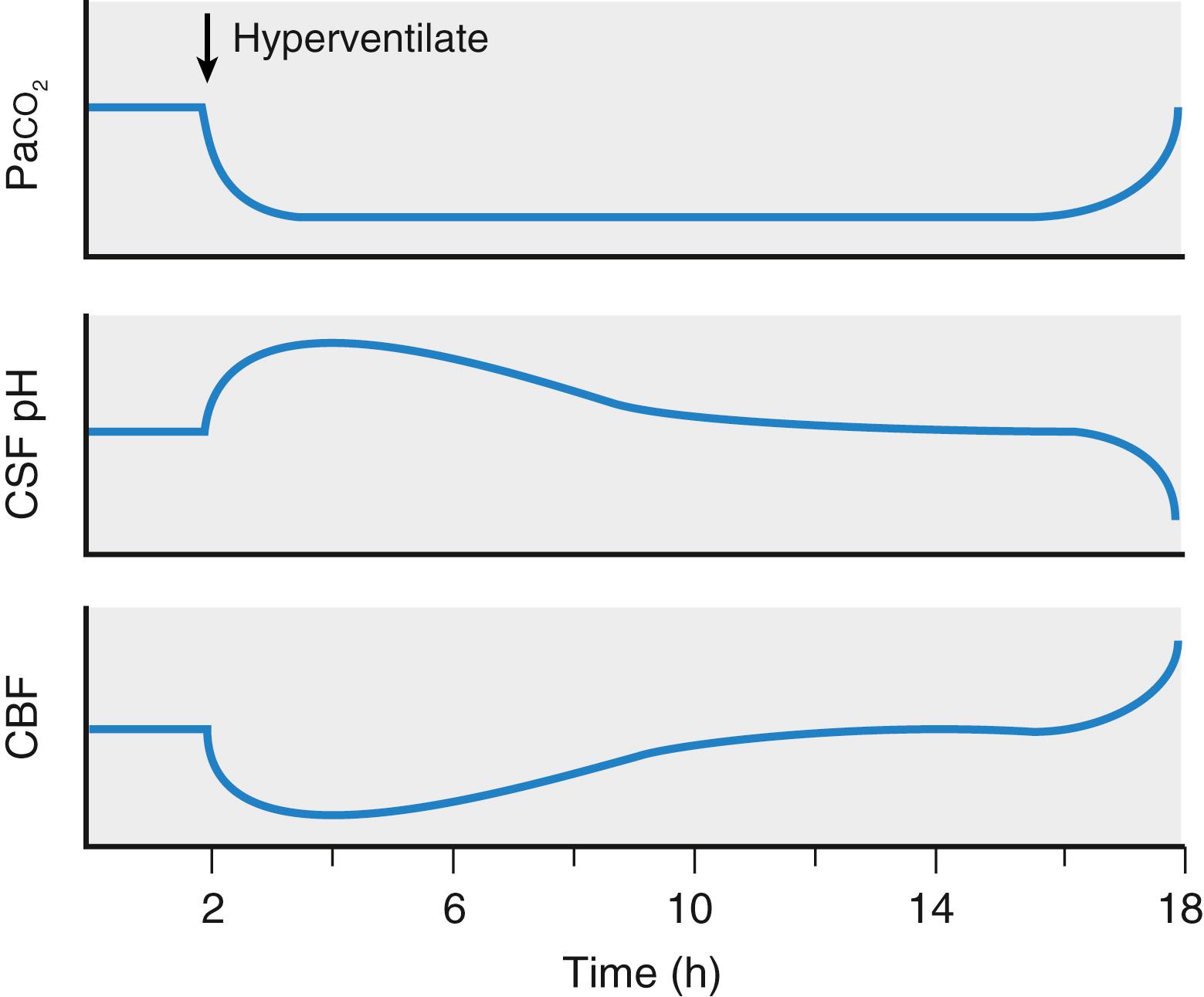
The effect of hypocapnia on CBF is not sustained. Fig. 57.6 is a nonquantitative representation of changes in CBF and CSF pH occurring in association with a sustained period of hyperventilation. With the onset of hyperventilation, the pH of both CSF and the brain’s extracellular fluid space increases, and CBF decreases abruptly. However, the cerebral alkalosis is not sustained. By alterations in function of the enzyme carbonic anhydrase, the concentration of bicarbonate in CSF and the brain’s extracellular fluid space is reduced, and with a time course of 8 to 12 hours, the pH of these compartments returns to normal. Simultaneously, CBF returns toward normal levels. The implications are twofold. First, patients should be hyperventilated for only as long as a reduction in brain volume is required. Prolonged, unnecessary hyperventilation can lead to a circumstance wherein subsequent events call for additional maneuvers to reduce the volume of the intracranial contents, and then incremental hyperventilation is not efficacious. If, after adaptation, Pa CO 2 is already in the 23 to 25 mm Hg range, additional hyperventilation may risk pulmonary barotrauma. Second, in a patient who has been hyperventilated for a sustained period (e.g., 2 days in an ICU setting), restoration of Pa CO 2 from values in the vicinity of 25 mm Hg to typical normal values (e.g., 40 mm Hg) should ideally be accomplished slowly. A sudden increase in Pa CO 2 from 25 to 40 mm Hg in a patient who has been chronically hyperventilated will have the same physiologic effect that a rapid change from 40 to 55 mm Hg would have in a previously normocapnic patient.
If hypocapnia has been required as an adjunct to brain relaxation during craniotomy, Pa CO 2 should also be allowed to increase once the retractors are removed (if dural closure requirements permit) to minimize the residual intracranial pneumatocele (see the section on Pneumocephalus).
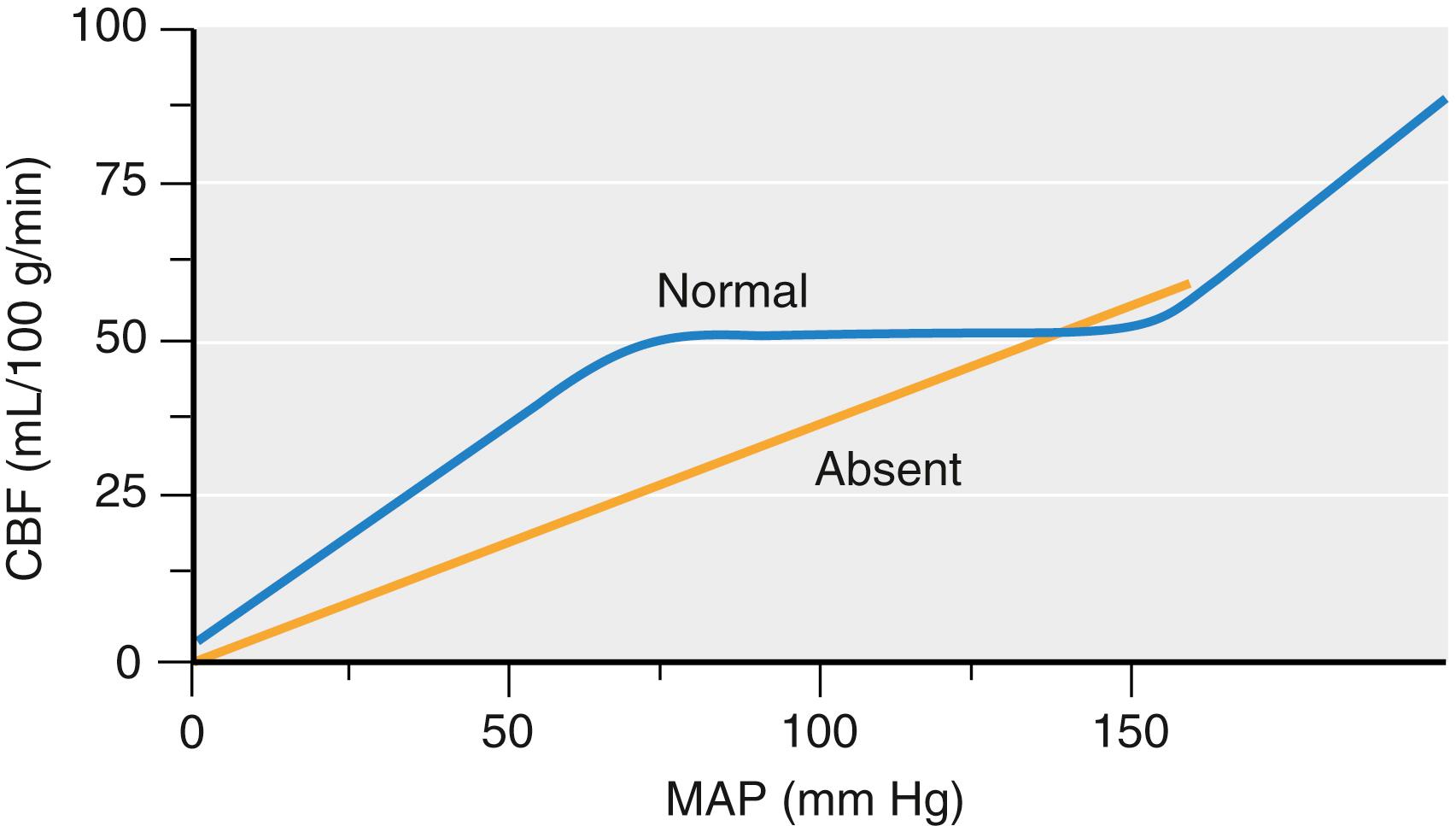
Acceptable arterial blood pressure limits should similarly be agreed on at the beginning of a neurosurgical procedure. One of the prominent themes of contemporary neurosurgery is that CPP should be maintained at normal or even high-normal levels after acute central nervous system insults and during most intracranial neurosurgical procedures. This concept has evolved from the growing appreciation that CBF is frequently very low in some brain regions after acute neurologic insults, in particular TBI and SAH. Two additional factors should be considered. The first is that the autoregulatory response to decreasing blood pressure may not be intact throughout the brain. Fig. 57.7 depicts the ischemic hazard that attends the circumstance of a low resting CBF and absent autoregulation even at blood pressure levels considered safe when autoregulation is intact. In addition, maintenance of arterial pressure is also relevant to brain compressed under retractors because the effective perfusion pressure is lowered by increased local tissue pressure.
Although the only supportive data are anecdotal, we believe that an aggressive attitude toward arterial blood pressure support should also be given to patients who have sustained a recent spinal cord injury (SCI). This also applies to a spinal cord that is under compression, at risk for compression or vascular compromise because of a disease process (most commonly cervical spinal stenosis with or without ossification of the posterior longitudinal ligament) or an intended surgical procedure, and to those patients undergoing surgery involving retraction of the spinal cord. We believe that arterial blood pressure during anesthesia in these patients should be maintained as closely as possible to, and certainly within, 10% of average awake values.
The administration of steroids for the purpose of reducing or limiting the formation of edema has a well-established place in neurosurgery. The efficacy of steroids in reducing edema associated with tumors and radiation-induced necrosis is well confirmed, but not the edema associated with any other intracranial pathology. Although the time course of this effect is relatively rapid, it is too slow for the management of acute intraoperative events. However, administration beginning 48 hours before an elective surgical procedure has the potential to reduce edema formation and improve the clinical condition by the time of craniotomy. Although clinical improvement occurs within 24 hours, a reduction in ICP may not occur for 48 to 72 hours after the initiation of therapy. Steroids somehow improve the viscoelastic properties of the intracranial space before a reduction in edema occurs, although the mechanism remains undefined. The practice of administering steroids to patients with TBI has been abandoned as a result of controlled trials that demonstrated either no benefit or deleterious effects.
Hyperosmolar agents and diuretics are used widely in neurosurgery and neurocritical care to reduce the volume of the brain’s intracellular and extracellular fluid compartments. Both osmotic and loop diuretics have been used. Although loop diuretics can be effective, hyperosmolar agents are more widely used.
Mannitol is used most commonly intraoperatively because of its long history of rapid and effective reduction of brain volume. The doses vary from 0.25 g/kg to 100 g, with 1.0 g/kg the most common dose. A systematic study in TBI demonstrated that an equivalent initial ICP-reducing effect can be achieved with 0.25 g/kg, although the duration of effect is reduced compared to larger doses. More recent studies reported better surgical brain relaxation scores with higher doses (1-1.5 g/kg) of mannitol compared to a dose of 0.25 g/kg. Mannitol should be administered by infusion (e.g., over 10-15 minutes). Sudden exposure of the cerebral circulation to extreme hyperosmolarity can have a vasodilatory effect, which can produce brain engorgement and increased ICP, both of which do not occur with slower administration.
Mannitol enters the brain and, over a reasonably short time course, appears in the CSF space. The possibility that the mannitol that gains access to the parenchyma aggravates swelling has resulted in varying degrees of reluctance among clinicians to administer mannitol. Most clinicians nonetheless find it to be a mainstay of ICP management. There is the concern that it will only be effective when some degree of blood-brain barrier (BBB) integrity is preserved in a significant portion of the brain. Clinicians respond to this concern by making empiric use of this agent; that is, if it is effective in reducing ICP or improving conditions in the surgical field, repeated doses are administered. The use of hyperosmolar agents is theoretically limited by an upper acceptable osmolarity limit of approximately 320 mOsm/L (although the data supporting the validity of that limit are soft ). However, in life-threatening situations, the use is frequently empiric, and incremental doses (e.g., 12.5 g of mannitol) are administered until a clinical response is no longer observed.
In the critical care environment, the use of hypertonic saline (HTS) in place of mannitol is increasing. Although the initial ICP effects of equiosmolar doses of mannitol and HTS are very similar, HTS may have some advantages in the ICU, where repeated administration makes the adverse effects (e.g., diuresis, renal injury) more likely to have clinical significance. In addition, there are anecdotal reports of HTS being effective in patients who were refractory to mannitol. Although there is enthusiasm for HTS, supporting data are limited. Furthermore, because of the variations in HTS concentrations (3%, 7.5%, 15%, 23.4%) and osmolar loads in the various studies, it is difficult to make specific recommendations.
The combination of a loop diuretic (usually furosemide) and an osmotic diuretic is sometimes used. The superficial rationale is that mannitol establishes an osmotic gradient that draws fluid out of brain parenchyma and that the furosemide, by hastening excretion of water from the intravascular space, facilitates the maintenance of that gradient. A second mechanism may add additional justification for the practice of combining the two diuretics. Neurons and glia have homeostatic mechanisms to ensure regulation of cell volume. Neurons and glia that shrink in response to an increased osmolarity in the external environment recover their volume rapidly as a consequence of the accumulation of so-called idiogenic osmoles , which serve to minimize the gradient between the internal and external environments. One of those idiogenic osmoles is chloride. Loop diuretics inhibit the chloride channel through which this ion must pass and thereby retard the normal volume-restoring mechanism. These diuretic combinations may cause hypovolemia and electrolyte disturbances.
The normal volume regulatory mechanisms of neurons and glia may also be relevant to the phenomenon of rebound swelling. Rebound is commonly attributed to the prior use of mannitol and assumed to be a function of the accumulation of mannitol in cerebral tissue. Although possible, the rebound may in fact be hypertonic rebound rather than mannitol rebound. After a sustained period of hyperosmolarity of any etiology, rebound swelling of neurons and glia (which have accumulated idiogenic osmoles) may occur in the event that systemic osmolarity decreases rapidly toward normal levels. Rebound cerebral swelling can certainly occur after an episode of extreme increase in blood glucose concentration. The use of HTS rather than mannitol will not obviate this phenomenon.
The general principle is that any acute irritation of the cortical surface has the potential to result in seizures. This includes acute neurologic events such as TBI and SAH. Cortical incisions and brain surface irritation by retractors may similarly be potential foci. Given the relatively benign nature of contemporary anticonvulsants (e.g., levetiracetam), routine administration to patients undergoing most supratentorial craniotomies seems appropriate in the absence of a contraindication. There is no necessity for rapid administration because the intention is to prevent seizures during the postoperative period.
The intended surgical position and the necessary positioning aids should be agreed upon at the outset. The commonly used positions and positioning aids and supports are listed in Box 57.5 (see Chapter 34 ).
Supine
Lateral (park bench)
Semi-lateral (Jannetta)
Prone
Sitting
Pin (“Mayfield”) head holder
Radiolucent pin head holder
Horseshoe head rest
Foam head support (e.g., Voss, O.S.I., Prone-View)
Vacuum mattress (“bean bag”)
Wilson-type frame
Andrews (“hinder binder”)-type frame
Relton-Hall (four-poster) frame
The prolonged duration of many neurosurgical procedures should be taken into account in all positions. Pressure points should be identified and padded carefully. Pressure and traction on nerves must be avoided. Given the high risk of thromboembolic complications in neurosurgical patients, precautions including graduated compression stockings and sequential compression devices are warranted. For cranial procedures, some component of head-up posturing (e.g., 15-20 degrees) is used to ensure optimal venous drainage. The conspicuous exception occurs with evacuation of a chronic subdural hemorrhage, after which patients are usually nursed flat to discourage reaccumulation of fluid. Patients are occasionally also maintained flat after CSF shunting to avoid overly rapid collapse of the ventricles.
The supine position is used with the head neutral or rotated for frontal, temporal, or parietal access. Extremes of head rotation can obstruct the jugular venous drainage, and a shoulder roll can attenuate this problem. The head is usually in a neutral position for bifrontal craniotomies and transsphenoidal approaches to the pituitary. The head-up posture is best accomplished by adjusting the operating table to a chaise longue (lawn chair) position (hip flexion, pillows under the knees, slight reverse Trendelenburg). This orientation, in addition to promoting cerebral venous drainage, decreases back strain.
The semilateral position, also known as the Jannetta position, named after the neurosurgeon who popularized its use for microvascular decompression of the fifth cranial nerve, is used for retromastoid access. It is achieved by lateral tilting of the table 10 to 20 degrees combined with a generous shoulder roll. Again, extreme head rotation, sufficient to cause compression of the contralateral jugular vein by the chin, should be avoided.
The lateral position can be used for access to the posterior parietal and occipital lobes and the lateral posterior fossa including tumors at the cerebellopontine angle and aneurysms of the vertebral and basilar arteries. An axillary roll is important for preventing brachial plexus injury.
The prone position is used for spinal cord, occipital lobe, craniosynostosis, and posterior fossa procedures. For cervical spine and posterior fossa procedures, the final position commonly entails neck flexion, reverse Trendelenburg, and elevation of the legs. This orientation serves to bring the surgical field to a horizontal position. There should be a plan for detaching and reattaching monitors in an orderly manner to prevent an excessive monitoring window. Awake tracheal intubation and prone positioning may be warranted in patients with an unstable cervical spine in whom an unchanged neurologic status should be confirmed before induction of anesthesia in the final surgical position. This approach is also sometimes performed in obese patients.
The head can be secured in a pin head holder (applied before the turn) or positioned on a disposable foam head rest or, less frequently, a horseshoe head rest. A complication of the prone position, which requires constant attention, is retinal ischemia and blindness caused by orbital compression causing central retinal vessel occlusion. It must be intermittently confirmed (e.g., every 15 minutes) and after any surgery-related head or neck movement that pressure has not come to bear on the eye. However, not all postoperative vision loss (POVL) is a result of direct orbital compression. Ischemic optic neuropathy actually appears to be a more frequent cause of POVL than pressure-causing occlusion of central retinal vessels. The cause-and-effect relationships associated with ischemic optic neuropathy are uncertain, but low arterial pressure, low hematocrit level, lengthy surgical procedures, and large intravascular volume fluid administration are statistically associated with the phenomenon .
Direct pressure can also result in various degrees of pressure necrosis of the forehead, maxillae, and chin, especially with prolonged spinal procedures. Pressure should be distributed as evenly as possible over facial structures. Other pressure points to check include the axillae, breasts, iliac crests, femoral canals, genitalia, knees, and heels. Traction on the brachial plexus must be avoided and can usually be accomplished by not exceeding a “90-90” position (arms abducted not >90 degrees; elbows extended not >90 degrees) with care taken to ensure that the elbow is anterior to the shoulder to prevent wrapping of the brachial plexus around the head of the humerus. An antisialagogue (e.g., glycopyrrolate) and an adhesive (e.g., benzoin) may help reduce loosening of the tape used to secure the endotracheal tube.
An objective during prone positioning, especially for lumbar spine surgery, is the avoidance of compression of the inferior vena cava. Impairment of vena cava return diverts blood to the epidural plexus and increases the potential for bleeding during spinal surgery. Minimizing vena cava pressure is an objective of all spinal surgery frames and is accomplished effectively by the Wilson, Andrews, and Jackson variants. However, this does introduce a risk of air embolism, although severe clinical occurrences have been very infrequent.
Attention should be paid to preventing injury to the tongue in the prone position. With both cervical and posterior fossa procedures, it is frequently necessary to flex the neck substantially to facilitate surgical access. This reduces the anterior-posterior dimension of the oropharynx, and compression ischemia of the base of the tongue (as well as the soft palate and posterior wall of the pharynx) can occur in the presence of foreign bodies (endotracheal tube, esophageal stethoscope, oral airway). The consequence can be macroglossia, caused by accumulation of edema after reperfusion of the ischemic tissue causing airway obstruction of rapid onset after extubation (discussed later). Accordingly, placing unnecessary adjuncts in the oral cavity and pharynx should be avoided. Omitting the oral airway entirely is unwise because the tongue may then protrude between and be trapped by the teeth as progressive swelling of facial structures occurs during a prolonged prone procedure. A rolled gauze bite block prevents this problem without adding bulk to the oropharynx.
There have been several reviews of numerous experiences with the sitting position. All concluded that the sitting position can be used with acceptable rates of morbidity and mortality. However, these reports were prepared by groups that perform 50 to 100 or more of these procedures per year, and the hazards of the sitting position may be more frequent for teams who have fewer occasions to use it. The sitting position can be avoided by using one of its alternatives (prone, semilateral, lateral). However, this position will continue to be used because even surgeons who are inclined to use alternative positions may opt for it when access to midline structures (e.g., the quadrigeminal plate, the floor of the fourth ventricle, the pontomedullary junction, and the vermis) is required. Nonetheless, alternative positions for posterior fossa surgery are available and should be considered when contraindications to the sitting position exist.
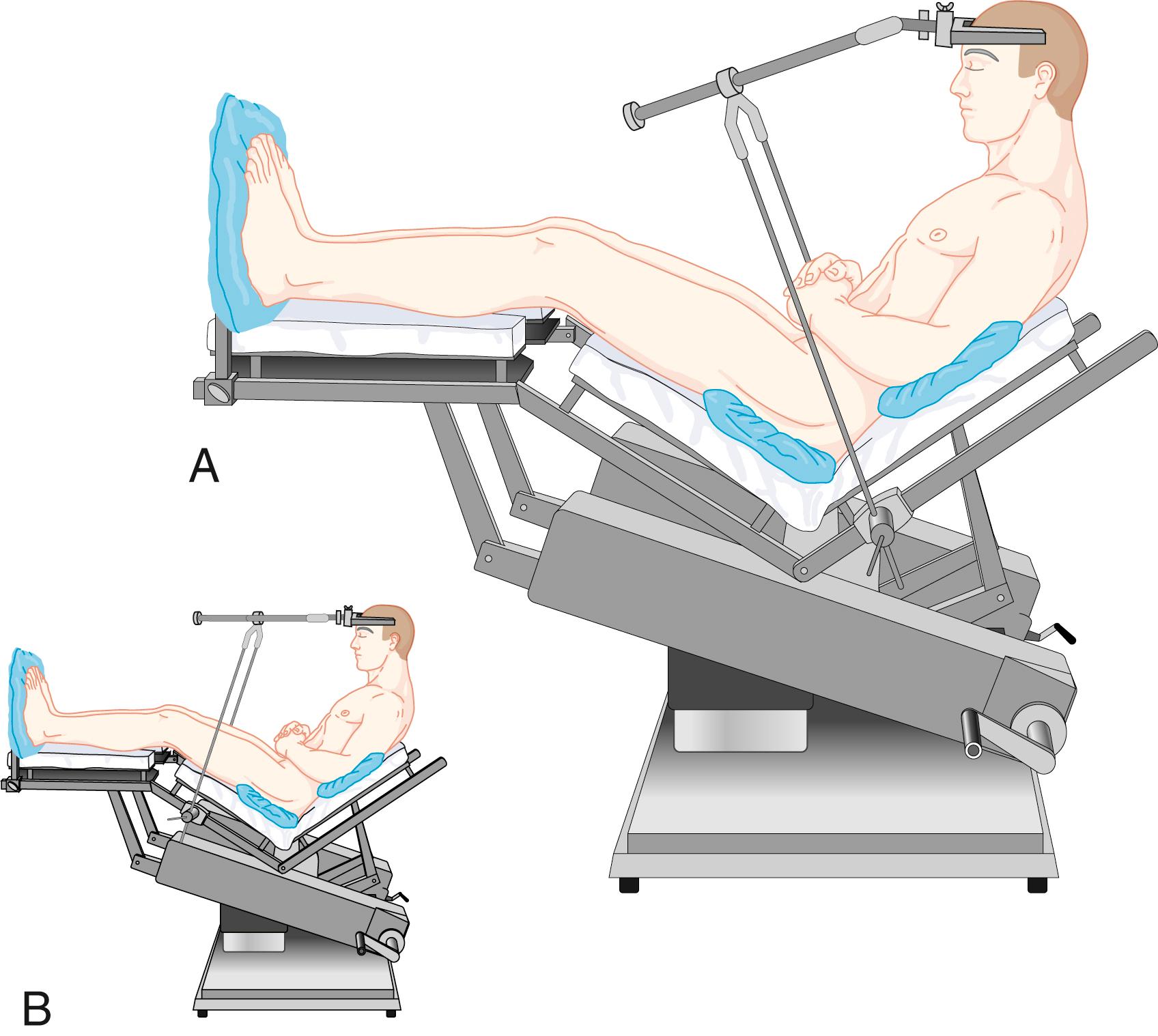
The properly positioned patient is more commonly in a modified recumbent position as shown in Fig. 57.8 rather than truly sitting. The legs should be kept as high as possible (usually with pillows under the knees) to promote venous return. The head holder should be attached to the back portion of the table (see Fig. 57.8 A ) rather than to the portions under thighs or legs (see Fig. 57.8 B ). This permits lowering of the head and closed chest compressions, if necessary, without the necessity of first taking the patient out of the head holder.
When procedures are performed in the sitting position, the clinician should think in terms of measuring and maintaining perfusion pressure at the level of the surgical field. This is best accomplished by referencing transducers to the level of the external auditory canal. If a manual blood pressure cuff on the arm is used, a correction ∗
∗ A column of blood 32 cm high exerts a pressure of 25 mm Hg.
to allow for the hydrostatic difference between the arm and the operative field should be applied.
A series of hazards are associated with the sitting position. Circulatory instability, macroglossia, and quadriplegia are discussed in this section. Pneumocephalus is discussed in its own section. Venous air embolism (VAE) and paradoxical air embolism (PAE) are discussed in the section Venous Air Embolism. Several of these hazards are also relevant when cervical spine and posterior fossa procedures are performed in non-sitting positions but occur with greater frequency in the sitting position.
Hypotension should be avoided. Prepositioning hydration, compressive stockings, and slow, incremental adjustment of table position are appropriate. Intravenous vasopressor administration may be required in some patients. However, in most healthy patients the hemodynamic changes are of a nonthreatening magnitude. In a study of healthy anesthetized adults aged 22 to 64 years old, relatively modest changes were observed. MAP was relatively unaffected, whereas wedge pressure, stroke volume, and cardiac index decreased—the latter by approximately 15%—although there was some variation with the anesthetics used. The combination of an unchanged MAP (which in general requires the use of a light, high sympathetic tone anesthetic) and a reduced cardiac index implies that systemic vascular resistance (SVR) increased. Their calculations and the observations of other investigators reveal significant increases in SVR. For patients in whom an abrupt increase in SVR may be poorly tolerated, the sitting position may represent a physiologic threat and alternative positions should be considered.
During procedures performed in the sitting position, MAP should be transduced at or corrected to head level to provide a meaningful index of CPP. Specifically, CPP (MAP – estimated ICP) should be maintained at a minimum value of 60 mm Hg in healthy patients in whom it is reasonable to assume a normal cerebral vasculature. The safe lower limit should be raised for elderly patients, for those with hypertension or known cerebral vascular disease, or for those with degenerative disease of the cervical spine or cervical spinal stenosis who may be at risk for decreased spinal cord perfusion, and in the event that substantial or sustained retractor pressure must be applied to brain or spinal cord tissue.
There have been sporadic reports of upper airway obstruction after posterior fossa procedures in which swelling of pharyngeal structures, including the soft palate, posterior pharyngeal wall, and base of the tongue, has been observed. These episodes have been attributed to edema formation at the time of reperfusion after trauma or prolonged ischemia, occurring as the result of foreign bodies (usually oral airways) causing pressure on these structures in the circumstances of lengthy procedures with sustained neck flexion (which is usually required to improve access to posterior structures). It is customary to maintain at least two fingerbreadths between the chin/mandible and the sternum/clavicle to prevent excessive reduction of the anterior-posterior diameter of the oropharynx. Consideration of the macroglossia phenomenon may also be relevant as clinicians contemplate the use of transesophageal echocardiography (TEE) in the neurosurgery suite. The centers that routinely use TEE in neurosurgery mostly use pediatric diameter probes to avoid trauma to pharyngeal and perilaryngeal structures.
The sitting position has been implicated as a cause of rare instances of unexplained postoperative quadriplegia. It has been hypothesized that neck flexion, a common concomitant of the seated position, may result in stretching or compression of the cervical spinal cord. This possibility may represent a relative contraindication to the use of this position in patients with significant degenerative disease of the cervical spine, especially when there is evidence of associated cerebral vascular disease. The arterial blood pressure management implications are mentioned in the preceding section on cardiovascular effects. It may also represent a justification for evoked response monitoring during the positioning phase of a sitting procedure for patients perceived to be at high risk (also see Chapter 39 ).
The issue of pneumocephalus arises most often in connection with posterior fossa craniotomies performed with a head-up posture. During these procedures, air may enter the supratentorial space, much as air enters an inverted pop bottle. Depending on the relationship of the brainstem and temporal lobes to the incisura, the pressure in the air collection may or may not be able to equilibrate with atmospheric pressure. This phenomenon has relevance to the use of N 2 O because any N 2 O that enters a trapped gas space augments the volume of that space. In those (probably uncommon) intraoperative circumstances where there is, in fact, a completely closed intracranial gas space, the use of N 2 O may result in an effect comparable with that of an expanding mass lesion. We do not view N 2 O as absolutely contraindicated because, before dural closure, intracranial gas is probably only rarely trapped. Nonetheless, attention to this possibility is important when one is presented with the problem of an increasingly tight brain during a posterior fossa craniotomy.
During a posterior fossa procedure done in a head-up posture, when surgical closure has reached a stage such that the intracranial space has been completely sealed from the atmosphere, N 2 O should be omitted because of the possibility of contributing to a tension pneumocephalus. Note that the use of N 2 O up to the point of dural closure may actually represent a clinical advantage, as in rabbits the gas pocket has been shown to shrink more rapidly because of the presence of N 2 O (because N 2 O diffuses much more quickly than nitrogen). Tension pneumocephalus is often naively viewed as exclusively a function of the use of N 2 O. However, tension pneumocephalus can most certainly occur as a complication of intracranial neurosurgery entirely unrelated to the use of N 2 O. It is one of the causes of delayed awakening or nonawakening after both posterior fossa and supratentorial procedures ( Fig. 57.9 ). It occurs because air enters the cranium when the patient is in a head-up position at a time when the volume of the intracranial contents has been reduced because of some combination of hypocapnia, good venous drainage, osmotic diuresis, and CSF loss from the operative field. When the cranium is closed and the patient is returned to the near supine position, CSF, venous blood, and extracellular fluid return or reaccumulate and the air pocket becomes an unyielding mass lesion (because of the very slow diffusion of nitrogen). It may cause delayed recovery of consciousness or severe headache. Among supratentorial craniotomies, the largest residual air spaces occur after frontal skull base procedures in which energetic brain relaxation measures are used to facilitate subfrontal access (see Fig. 57.9 ). At the end of these procedures, typically done in a supine/brow-up position, the intracranial dead space cannot be filled with normal saline as is commonly done with smaller craniotomy defects, and there may be a large residual pneumatocele. We doubt that the possible occurrence of this phenomenon represents a contraindication to N 2 O. However, withdrawal of N 2 O may be appropriate at the time of scalp closure. The diagnosis of pneumocephalus is established by a brow-up lateral radiography or, more commonly, a CT scan. The treatment is a twist-drill hole followed by needle puncture of the dura.
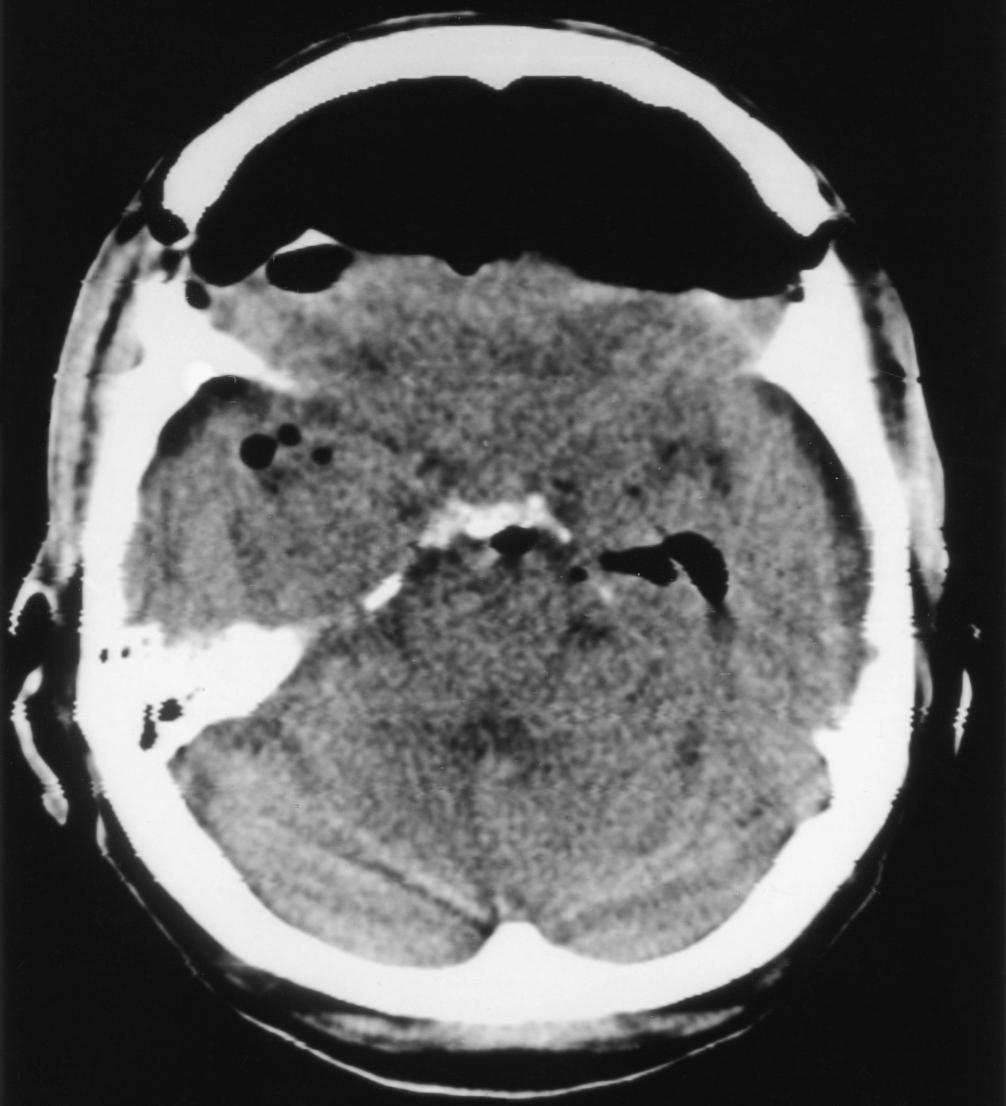
Residual intracranial air should be considered at the time of repeat anesthesia, both neurosurgical and nonneurosurgical. Air frequently remains evident on CT scan for more than 7 days after a craniotomy. Pneumocephalus can also develop de novo in the postoperative period in patients who have a residual dural defect and a communication between the nasal sinuses and the intracranial space.
The incidence of VAE varies according to the procedure, the intraoperative position, and the detection method used. During posterior fossa procedures performed in the sitting position, VAE is detectable by precordial Doppler in approximately 40% of patients and by TEE in as many as 76%. The incidence of VAE during posterior fossa procedures performed in nonsitting positions is much less (12% using precordial Doppler in the report of Black and colleagues ), and it is probable but unproven that the average volume of air entrained per event is also smaller. The incidence of VAE is apparently lower with cervical laminectomy (25% using TEE in the sitting position versus 76% for posterior fossa procedures ). Although VAE is principally a hazard of posterior fossa and upper cervical spine procedures, especially when they are performed in the sitting position, it can occur with supratentorial procedures. The most common situations involve tumors, most often parasagittal or falcine meningiomas, that encroach on the posterior half of the sagittal sinus ( Fig. 57.10 ) and craniosynostosis procedures, typically performed in children. Pin sites can also serve as VAE access sites. Accordingly, pin head holders should be removed after the patient has been taken out of significant degrees of the head-up positioning. Spontaneous ventilation (with the attendant intermittent negative intrathoracic pressure) will increase the risk of air entrainment. A 6% incidence of Doppler-detectable VAE was reported in a series of deep-brain stimulator placement procedures performed in spontaneously breathing patients.
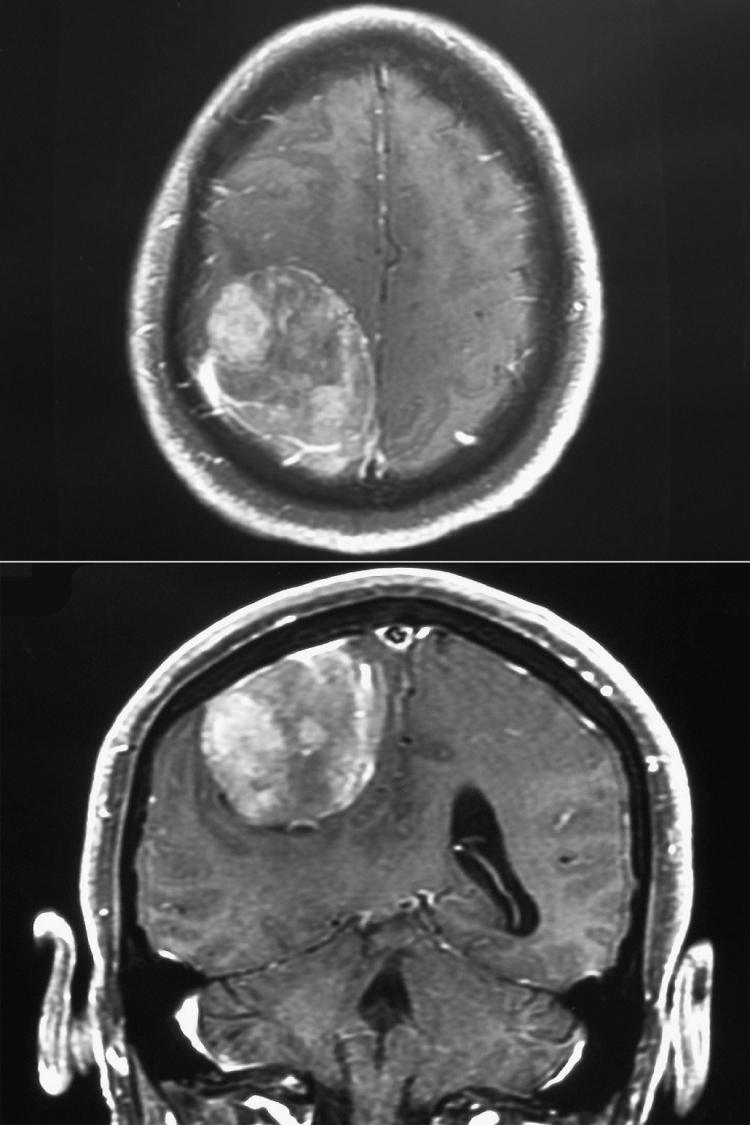
The common sources of critical VAE are the major cerebral venous sinuses, in particular the transverse, the sigmoid, and the posterior half of the sagittal sinus, all of which may be noncollapsible because of their dural attachments. Air entry may also occur via emissary veins, particularly from suboccipital musculature, via the diploic space of the skull (which can be violated by both the craniotomy and pin fixation) and the cervical epidural veins. It is believed (but not confirmed by systematic study) that the VAE risk associated with cervical laminectomy is more likely when the exposure requires dissection of suboccipital muscle with the potential to open emissary veins to the atmosphere at their point of entry into occipital bone. There is also anecdotal evidence that air under pressure in the ventricles or subdural space can occasionally enter the venous system, perhaps along the normal egress route of the CSF.
The monitors used for the detection of VAE should provide (1) a high level of sensitivity, (2) a high level of specificity, (3) a rapid response, (4) a quantitative measure of the VAE event, and (5) an indication of the course of recovery from the VAE event. The combination of a precordial Doppler and expired CO 2 monitoring meets these criteria and is the current practice in many institutions. Doppler placement in a left or right parasternal location between the second and third or third and fourth ribs has a very high detection rate for gas embolization, and when good heart tones are heard, maneuvers to confirm adequate placement appear to be unnecessary. The TEE is more sensitive than the precordial Doppler ( Fig. 57.11 ) to VAE and offers the advantage of also identifying right-to-left shunting of air. However, its safety during prolonged use (especially with pronounced neck flexion) is not well established. Expired nitrogen analysis is theoretically attractive. However, the expired nitrogen concentrations involved in anything less than catastrophic VAE are very small and push the available instrumentation to the limits of its sensitivity.
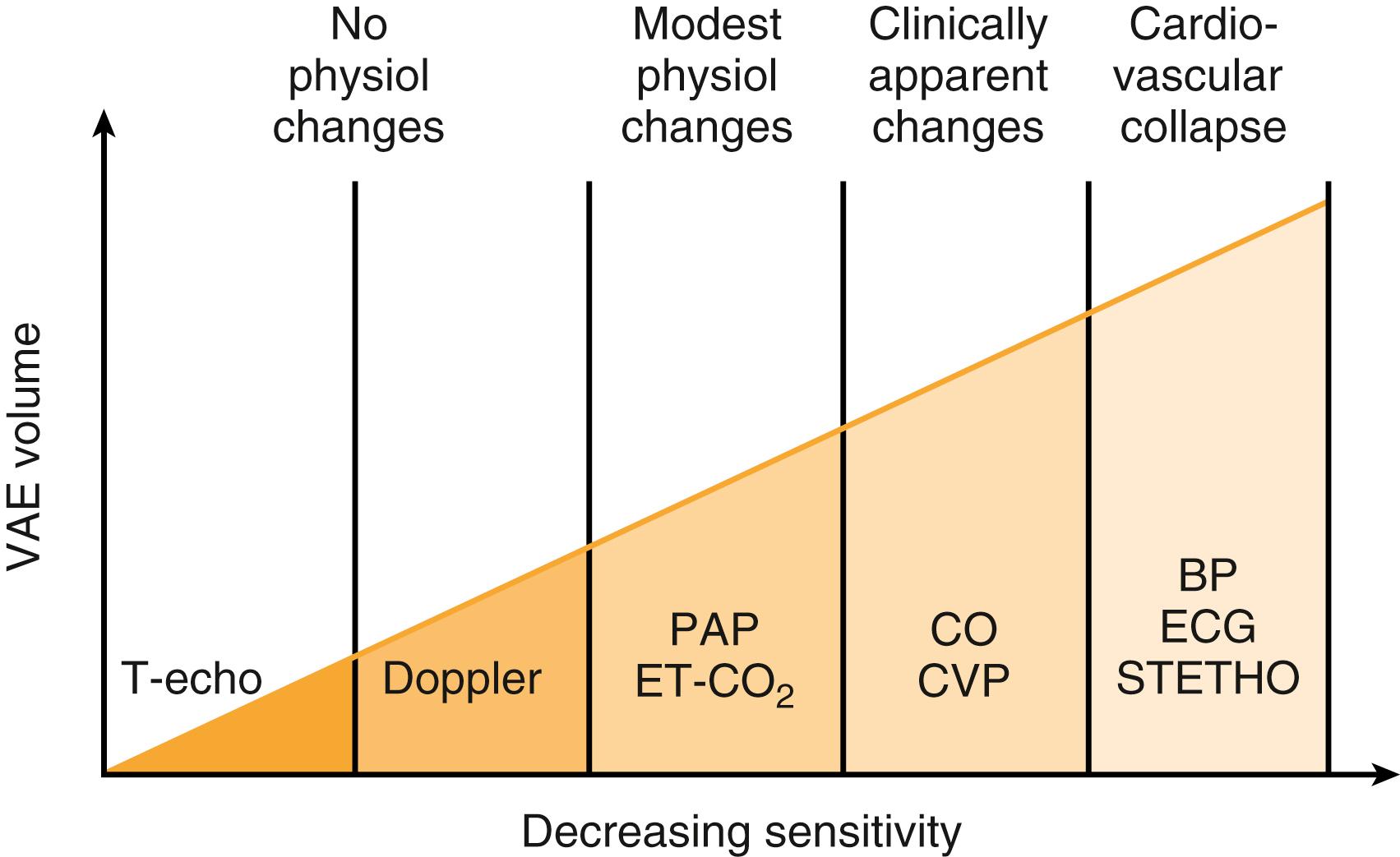
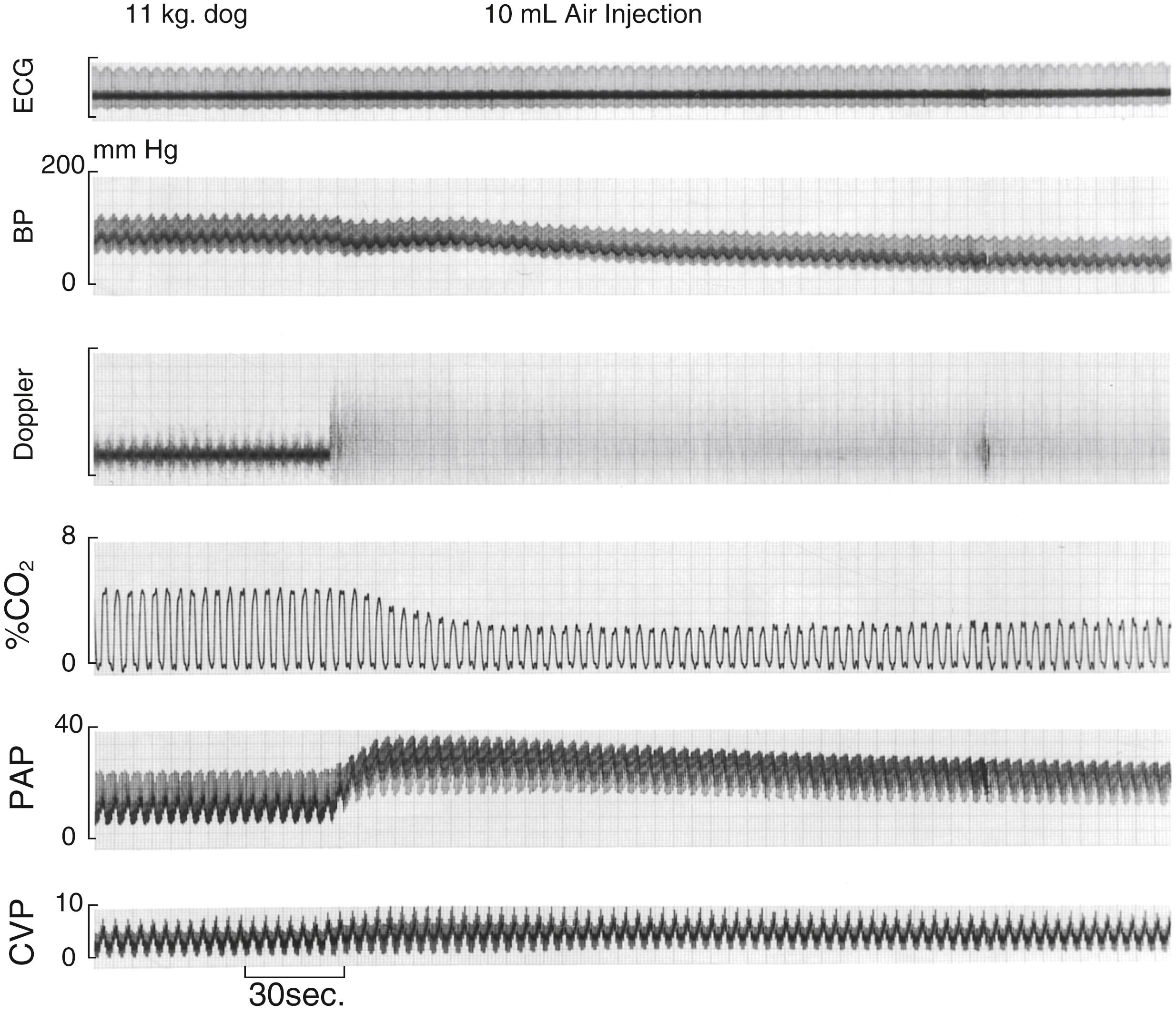
Fig. 57.12 presents the physiologic and monitor response to an air embolic event, and Box 57.6 offers an appropriate management response to such an event.
Prevent further air entry
Notify surgeon (flood or pack surgical field)
Jugular compression
Lower the head
Treat the intravascular air
Aspirate right heart catheter
Discontinue N 2 O
Fi O 2 : 1.0
Pressors, inotropes
Chest compression
All patients who undergo sitting posterior fossa procedures should have a right heart catheter placed. Although life-threatening VAE is relatively uncommon, a catheter permits immediate evacuation of an air-filled heart. With the nonsitting positions, it is frequently appropriate, after a documented discussion with the surgeon, to omit the right heart catheter. The perceived risks of VAE associated with the intended procedure and the patient’s physiologic reserve are the variables that contribute to the decision. Microvascular decompression of the fifth or seventh cranial nerves are examples of procedures for which the right heart catheter is usually omitted. The essentially horizontal semilateral position and the very limited retromastoid craniectomy that is required have resulted (at our institution) in a very low incidence of Doppler-detectable VAE. One should know the local surgical practices, particularly with respect to the degree of head-up posture, before deciding to omit a right atrial catheter. With regard to the Jannetta procedure, the necessary retromastoid craniectomy is performed in the angle between the transverse and sigmoid sinuses, and venous sinusoids and emissary veins in the suboccipital bone are common. If this procedure is performed with any degree of head-up posturing, the risk of VAE may still be substantial.
Although some surgeons may ask that neck veins not be used, a skillfully placed jugular catheter is usually acceptable. In a very limited number of patients, high ICP may make the head-down posture undesirable. In others, unfavorable anatomy with an increased likelihood of a difficult cannulation and hematoma formation may also encourage the use of alternate access sites.
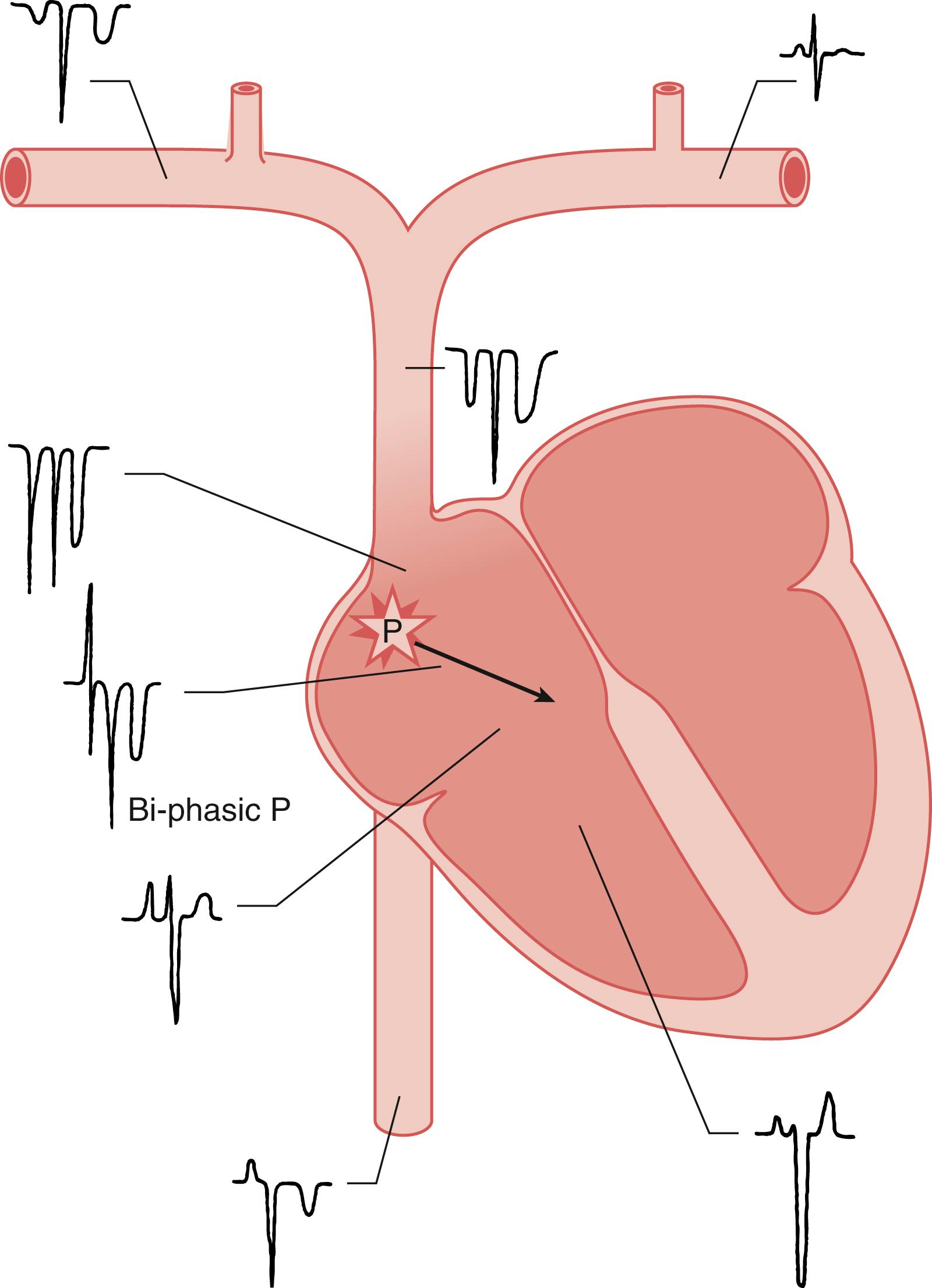
The investigation by Bunegin and colleagues suggested that a multiorificed catheter should be located with the tip 2 cm below the superior vena caval-atrial junction and a single-orificed catheter with the tip 3 cm above the superior vena caval-atrial junction. Although these small distinctions in location may be relevant for optimal recovery of small volumes of air when cardiac output is well maintained, for the recovery of massive volumes of air in the face of cardiovascular collapse, anywhere in the right atrium should suffice. Confirmation of right heart placement can be accomplished by (1) radiography, (2) intravascular electrocardiography (ECG), or (3) TEE. Although there is no literature to support the practice, with catheter access via the right internal jugular vein, a measured placement to the level of the second or third right intercostal space should suffice when the catheter passes readily. The intravascular electrocardiography technique makes use of the fact that an ECG “electrode” placed in the middle of the right atrium will initially “see” an increasing positivity as the developing P-wave vector approaches it ( Fig. 57.13 ), and then an increasing negativity as the wave of atrial depolarization passes and moves away from it. The resultant biphasic P wave is characteristic of an intraatrial electrode position. The technique requires that the central venous pressure (CVP) catheter become an exploring ECG electrode. This is accomplished by filling the catheter with an electrolyte solution (bicarbonate is best) and attaching an ECG lead (the leg lead if lead II is selected) to the hub of the CVP catheter. Commercial CVP kits with an ECG adapter are available. The ECG configurations that will be observed at various intravascular locations are shown in Fig. 57.13 . To minimize the microshock hazard, a battery-operated ECG unit is preferable, and any unnecessary electrical apparatus should be detached from the patient during catheter placement.
The possibility of the passage of air across the interatrial septum via a patent foramen ovale (PFO), which is known to be present in approximately 25% of adults, is a concern. The risk is major cerebral and coronary morbidity. However, the precise definition of the morbidity that can actually be attributed to PAE is not clear. Although the minimal pressure required to open a probe PFO is not known with certainty, the necessary gradient may be as much as 5 mm Hg. In a clinical investigation, Mammoto and colleagues observed that PAE occurred only in the context of major air embolic events, suggesting that significant increases in right heart pressures are an important predisposing factor of the occurrence of PAE. Several clinical investigations have examined factors that influence the right atrial pressure (RAP) to left atrial pressure (LAP) gradient. The use of positive end-expiratory pressure (PEEP) increases the incidence of a positive RAP to pulmonary wedge pressure gradient and generous fluid administration (e.g., 2800 mL/patient vs. 1220 mL/control patient ) reduces it. As a result, the use of PEEP, which was once advocated as a means of preventing air entrainment, was abandoned. Subsequently, the practice of more generous fluid administration for patients undergoing posterior fossa procedures evolved. However, even when mean LAP exceeds mean RAP, PAE can still occur because transient reversal of the interatrial pressure gradient can occur during each cardiac cycle.
Some centers have advocated performing bubble studies preoperatively with echocardiography or transcranial Doppler (TCD), or intraoperatively using TEE prior to positioning to identify patients with a PFO with a view to using alternatives to the sitting position in this subpopulation. Some centers thereafter advocate the use of TEE to identify paradoxical embolization intraoperatively. However, none of these practices has become a community-wide standard of care. Furthermore, because the morbid events attributable to PAE have been relatively infrequent, surgeons who are convinced that the sitting position is optimal for a given procedure are loath to be dissuaded from using it on the basis of what may seem like the very minor possibility of an injury to the patient occurring by this mechanism.
Air can sometimes traverse the pulmonary vascular bed to reach the systemic circulation. Transpulmonary passage is more likely to occur when large volumes of air are presented to the pulmonary vascular filter. In addition, pulmonary vasodilators, including volatile anesthetics, may decrease the threshold for transpulmonary passage. The magnitude of differences among anesthetics does not appear to mandate any related “tailoring” of anesthetic techniques. However, N 2 O should be discontinued promptly after even apparently minor VAE events because of the possibility that air may reach the left-sided circulation either via a PFO or the pulmonary vascular bed.
Box 57.6 presents an approach for responding to an acute VAE event. It includes raising venous pressure by direct compression of the jugular veins. PEEP and the Valsalva maneuver were once advocated. However, both PEEP and the release of a Valsalva maneuver increase the risk of PAE, and the relative superiority of jugular venous compression in raising cerebral venous pressures has been confirmed. Furthermore, the impairment of systemic venous return caused by the sudden application of substantial PEEP may be undesirable in the face of the cardiovascular dysfunction already caused by the VAE event.
It has been recommended that a patient who has sustained a hemodynamically significant VAE should be placed in a lateral position with the right side up. The rationale is that air will remain in the right atrium, where it will not contribute to an air lock in the right ventricle and where it will remain amenable to recovery via a right atrial catheter. The first difficulty is that this repositioning is all but impossible with a patient in a pin head holder. In addition, the only systematic attempt to examine the efficacy of this maneuver, albeit performed in dogs, failed to identify any hemodynamic benefit.
Nitrous oxide diffuses into air bubbles trapped in the vascular tree and, accordingly, N 2 O should be eliminated after a clinical VAE event to avoid aggravating the cardiovascular impact. As noted earlier, the PAE phenomenon adds an additional reason for eliminating N 2 O after the occurrence of VAE. When major VAE occurs, no matter how the RAP-LAP gradient was manipulated before the event, RAP increases abruptly with respect to LAP, and major VAE results in an acutely increased risk of PAE in patients with a PFO. Should N 2 O be used in patients at risk for VAE? Some clinicians may decide to simply avoid it. However, N 2 O can be used with the knowledge that it neither increases the incidence of VAE nor aggravates the hemodynamic response to VAE provided that it is eliminated when VAE occurs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here