Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anemia is defined by the World Health Organization as a hemoglobin (Hb) concentration less than 13.0 g/dL in adult men and nonmenstruating women and less than 12.0 g/dL in menstruating women. The incidence of anemia in patients with chronic kidney disease (CKD) increases as the glomerular filtration rate (GFR) declines. Population studies, including the United States National Health and Nutrition Examination Survey (NHANES) and the Prevalence of Anemia in Early Renal Insufficiency study, suggest that the incidence of anemia is less than 10% in CKD stages 1 and 2, 20% to 40% in CKD stage 3, 50% to 60% in CKD stage 4, and more than 70% in CKD stage 5.
The pathogenesis of anemia in patients with CKD is multifactorial ( Box 55.1 ), but the contribution of erythropoietin (EPO) deficiency becomes greater as GFR declines. Hypoxia inducible factor (HIF), which is produced in the kidneys and other tissues, is a substance whose spontaneous degradation is retarded in the presence of decreased oxygen delivery because of anemia or hypoxemia. The sustained presence of HIF leads to signal transduction and the synthesis of EPO. In normal patients, plasma EPO levels increase dramatically in response to anemia. Because of alterations in their functioning mass, the kidneys in patients with CKD fail to increase EPO production in response to anemia or other conditions that decrease oxygen delivery. This is due both to impaired hypoxia sensing and a decreased population of EPO-producing cells.
Insufficient production of endogenous EPO
Iron deficiency
Acute and chronic inflammatory conditions
Severe hyperparathyroidism
Aluminum toxicity
Folate deficiency
Decreased survival of RBCs and RBC loss
##
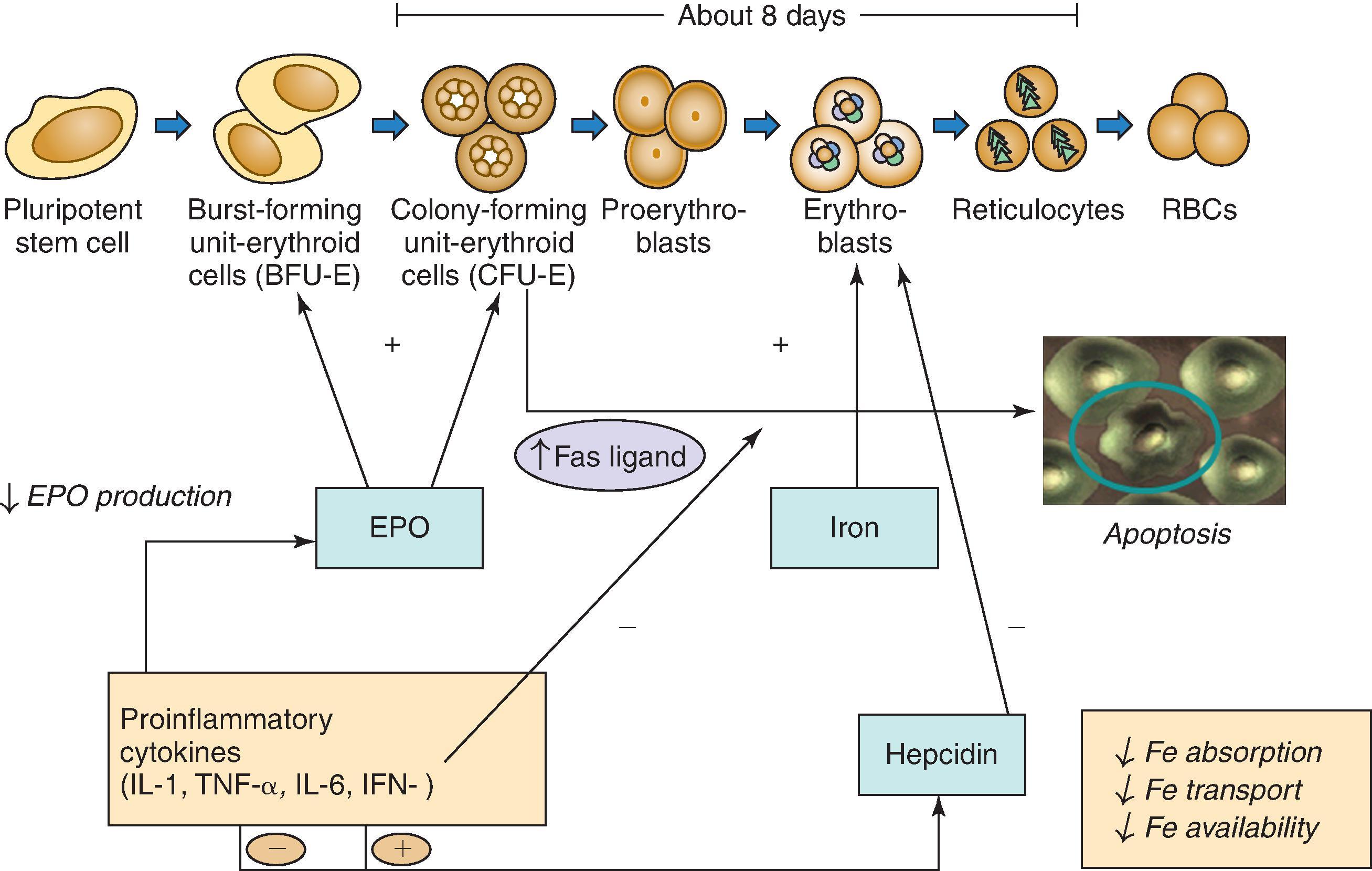
The kidneys produce about 90% of circulating EPO, and loss of EPO production in the setting of CKD is the primary cause of anemia in these patients. EPO binds to receptors on erythroid progenitor cells in the bone marrow, specifically the burst-forming units (BFU-E) and colony-forming units (CFU-E). The absence of EPO causes these cells to undergo programmed death or apoptosis, which is mediated by Fas ligand. In the presence of EPO, these erythroid progenitors differentiate into reticulocytes and red blood cells (RBCs).
Fig. 55.1 demonstrates the complex interactions among EPO; proinflammatory cytokines such as interleukin 1 (IL-1), tumor necrosis factor-α (TNF-α), IL-6, and interferon-γ (IFN-γ); hepcidin; and iron in the production of RBCs. Hepcidin is a peptide produced by the liver that interferes with RBC production by decreasing iron availability for incorporation into erythroblasts. Hepcidin gene expression is upregulated by IL-6 and iron overload and downregulated by TNF-α and iron deficiency. Hepcidin clearance is substantially reduced in CKD. At the cell surface of macrophages and proximal intestinal cells (and probably other cells), hepcidin binds to ferroportin, the membrane-embedded iron exporter, resulting in internalization and degradation of the complex. This inhibits iron transport across the cell membrane, trapping it in macrophages and preventing it from being absorbed from the intestine. Hepcidin activity is probably the basis for most “anemia of chronic disease” syndromes and contributes to the anemia in patients with CKD when inflammation and infection are present. However, in anemic CKD patients without inflammation or infection, EPO deficiency plays a much greater role than hepcidin excess.
The evidence for inhibition of RBC production by uremic toxins in patients with CKD is poor, as most CKD patients have an appropriate erythropoietic response to exogenously administered EPO if they are iron replete and free of inflammation or infection. It has been demonstrated that RBC survival is decreased from 120 days in normal individuals to 60 to 90 days in patients with CKD not yet receiving dialysis. This may be a result of RBC trauma from microvascular disease, as well as decreased resistance to oxidative stress.
The major clinical manifestations of anemia in patients with or without CKD are fatigue (both with exercise and at rest), decreased cognitive function, loss of libido, and decreased sense of well-being. These symptoms tend to occur when the Hb is less than 10 g/dL and are more severe as Hb levels fall further. More insidious are the cardiac complications of anemia, which may occur when the patient is otherwise asymptomatic and contribute to the adverse cardiovascular morbidity and mortality observed among patients with CKD. In patients with underlying coronary artery disease, anemia may lead to an exacerbation of angina because of decreased myocardial oxygen delivery. Decreased oxygen delivery to tissues because of anemia leads to peripheral vasodilation, increased sympathetic nervous system activity, increased heart rate and stroke volume, and, ultimately, left ventricular hypertrophy (LVH). LVH strongly correlates with adverse outcomes, including hospitalization and mortality, in patients with CKD. Each decrease in Hb of 0.5 g/dL is associated with a 32% increased risk of LVH over the course of a year; in contrast, each 5-mm Hg increase in systolic blood pressure correlates with only an 11% increase in LV mass. Many anemic CKD patients treated with erythropoiesis-stimulating agents (ESAs) report a decrease in subjective symptoms and improved quality of life (QoL), but evidence supporting regression of LVH, fewer clinical cardiac events, or decreased mortality with ESA treatment is not compelling (see later discussion).
Because anemia is common in CKD, the consequences of anemia are severe, and treatment is available, the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines for anemia in CKD recommended screening all patients with CKD stage 3 at least annually and more frequently in those with more advanced CKD; in those with diagnosed anemia not receiving treatment, Hb concentration should be measured at least every 3 months in patients with CKD stage 3 to 5 and monthly in patients on maintenance dialysis. If anemia is present (defined as Hb <13.0 g/dL in adult men and Hb <12.0 g/dL in adult women), further evaluation should be undertaken to determine the cause. This evaluation should include a complete blood count including RBC indices, reticulocyte count, serum ferritin concentration, and transferrin saturation (TSAT) or reticulocyte Hb content (CHr). The anemia of EPO deficiency is normocytic (normal mean corpuscular volume [MCV]) and normochromic (normal mean corpuscular Hb concentration [MCHC]). A low MCV (microcytosis) is suggestive of iron deficiency but may be seen in hemoglobinopathies such as thalassemia. A high MCV (macrocytosis) is suggestive of vitamin B 12 or folate deficiency. If the MCV is elevated, vitamin B 12 and folate levels should be assessed.
The serum ferritin level correlates with iron bound to tissue ferritin in the reticuloendothelial (RE) system. Serum ferritin does not carry or bind to iron, and its function is unknown. Serum ferritin is also an acute phase reactant that increases in the setting of acute or chronic inflammation independent of tissue iron stores. TSAT is a measure of circulating iron available for delivery to the erythroid marrow and is calculated by dividing the serum iron concentration by the total iron binding capacity (TIBC). The TIBC correlates with the serum level of transferrin, which is the major iron-carrying protein in the blood. TSAT less than 20% in an anemic patient with CKD is consistent with absolute or functional iron deficiency, both of which are characterized by decreased delivery of iron to the erythroid marrow.
Absolute iron deficiency occurs in the setting of decreased total body iron stores and is accompanied by serum ferritin level less than 25 ng/mL in men and less than 12 ng/mL in women. Functional iron deficiency is seen in patients with low TSAT and normal or elevated serum ferritin. It may be a result of the pharmacologic stimulation of RBC production by ESAs, which causes iron demand by the erythroid marrow in excess of the ability of the RE system to release iron to circulating transferrin. Functional iron deficiency may also result from the action of hepcidin in the setting of inflammation or infection. The hallmark of functional iron deficiency anemia is that it responds to the administration of intravenous (IV) iron supplements, with increase in Hb level and/or decrease in ESA requirements despite the normal or elevated serum ferritin concentration. If the anemic patient with low TSAT and normal or high serum ferritin level does not respond to IV iron, the presumptive diagnosis is RE blockade, meaning that hepcidin has completely prevented the release of iron from macrophages to circulating transferrin. It should be noted that, although the diagnosis of iron depletion is based on serum ferritin concentration less than 25 ng/mL, and that of iron-deficient erythropoiesis is based on TSAT less than 16%, anemic CKD patients with considerably higher serum ferritin and TSAT levels often respond to iron supplementation (see Iron Therapy, later).
The reticulocyte count is a useful and inexpensive test to distinguish anemia caused by underproduction of RBCs from that caused by RBC loss or destruction. In the setting of EPO deficiency, RBC production is decreased, and most anemic patients would be expected to have decreased absolute reticulocyte count (<40,000 to 50,000 cells per milliliter of whole blood). Elevated reticulocyte count is inconsistent with EPO deficiency, and an evaluation for hemolysis and blood loss should be undertaken.
Although it would seem that demonstration of decreased blood EPO level would secure the diagnosis of EPO deficiency, routine testing for EPO levels in anemic patients with CKD is not recommended. The reason is that patients who respond to exogenous ESAs may have normal or even elevated EPO concentration, which, nevertheless, may be inappropriately low for the severity of their anemia. Furthermore, the test is expensive. Therefore, it is recommended that EPO deficiency be a diagnosis of exclusion (i.e., negative evaluation for other treatable causes of anemia) in the anemic CKD patient. However, a cause other than EPO deficiency should be considered if anemia severity is disproportionate to the GFR or if leukopenia and/or thrombocytopenia are present.
After other treatable causes of anemia have been excluded and a diagnosis of EPO deficiency inferred, the treatment of choice for many anemic patients with CKD is an ESA. Recombinant human erythropoietin (rHuEPO, or epoetin) has been available since 1989 and revolutionized the treatment of anemia in patients with CKD who previously depended on blood transfusions and androgens. Although absorption of epoetin administered subcutaneously (SC) is incomplete with degradation of some of the protein before it reaches the circulation, the slower absorption and sustained serum epoetin levels may make this route of administration 20% to 30% more efficient than a comparable IV-administered dose. Nonetheless, the vast majority of patients undergoing hemodialysis (HD) in the United States receive an ESA by the IV route because of convenience of administration. One possible additional motivation for IV administration is the association between cases of pure red cell aplasia (PRCA) in Europe and SC administration of the Eprex formulation of epoetin alfa (discussed later).
Patients with nondialysis-dependent (NDD) CKD and patients undergoing peritoneal dialysis usually receive ESAs SC. The package insert for epoetin recommends thrice-weekly dosing, because the clinical trials submitted for approval by the US Food and Drug Administration (FDA) involved patients undergoing HD who received the drug with each treatment. For NDD-CKD patients and patients on peritoneal dialysis, thrice-weekly SC dosing is painful and not practical. Further, it is not necessary because clinical trials in these patients have shown epoetin administered every 1 to 2 weeks to be equally effective. Epoetin is effective in maintaining target Hb levels in 76% of NDD-CKD patients when administered as infrequently as every 4 weeks.
Darbepoetin alfa is a bioengineered epoetin molecule with two additional N-linked carbohydrate side chains. It has a longer half-life and duration of action than epoetin. As with epoetin, studies have demonstrated that darbepoetin is effective in maintaining target Hb levels when administered as infrequently as every 4 weeks in selected patients. There appears to be no difference in SC versus IV administration in terms of efficacy. The side effect profile of darbepoetin is virtually identical to that of epoetin; both agents are associated with the development or exacerbation of hypertension in 20% to 30% of patients. The mechanism for hypertension is multifactorial and related to increased RBC mass, attenuation of the peripheral vasodilation associated with anemia, and, perhaps, a direct inhibitory effect on vascular endothelial vasodilatory mediators such as nitric oxide and prostaglandins. The existence or exacerbation of hypertension is not a contraindication to ESA therapy; rather, the hypertension should be treated with more aggressive pharmacologic therapy, increased ultrafiltration on dialysis, and/or a decrease in the ESA dose to slow the rate of Hb rise and to allow for physiologic vasomotor adaptation. There is no evidence that the rate of vascular access thrombosis is increased in patients undergoing HD when ESA treatment is used to maintain Hb levels within the currently recommended target range. All other side effects reported with ESA therapy are no greater than with placebo.
Mircera (methoxy polyethylene glycol-epoetin beta) has been extensively used in other parts of the world for a number of years and was introduced into the US market following the expiration of patents on epoetin in 2014. The pegylation of the molecule retards its metabolism and allows for once-monthly IV or SC dosing. Mircera carries the same FDA warnings as epoetin and darbepoetin.
Biosimilar ESAs, which are lower-cost versions of the originator or reference ESAs, have become available in the United States after being extensively used in other parts of the world. The FDA defines a biosimilar agent as one that is “highly similar to the reference product with no clinically meaningful differences in terms of the safety profile, purity, or potency.” Because of the molecular complexity of biologic drugs, biosimilars are not exact copies of the original product, unlike generic versions of small molecule drugs. Since the clinical safety and efficacy of an originator biologic molecule have already been demonstrated, the FDA does not require sponsors of biosimilar agents to repeat these studies. Instead, the FDA requires the sponsor of a biosimilar agent to demonstrate that it is not significantly different from the reference product using smaller-scale direct comparisons and extrapolation. The sponsor of the biosimilar agent must provide evidence demonstrating that its biologic product is structurally and functionally similar to the reference product; that it uses the same mechanism of action for the proposed condition(s) of use; that the condition(s) of use proposed in labeling have been previously approved for the reference product; that it has the same route of administration, dosage form, and strength as the reference product; and that it is manufactured, processed, packed, or held in a facility that meets standards designed to assure that the biologic product continues to be safe, pure, and potent. Epoetin alfa-ebpx, a biosimilar of epoetin alfa, was first approved by the FDA in 2018 for use in the United States and is marketed under the trade name Retacrit. The pharmacologic properties of ESAs approved in the United States as of 2020 are summarized in Table 55.1 .
| Generic Name | Brand Name | Dosing Frequency | Starting Dose |
| Epoetin | Epogen, Procrit, Retacrit (biosimilar) | Three times weekly IV in HD patients; every 1–2 weeks SC in NDD-CKD and PD patients | 50 units/kg based on three times weekly dosing |
| Darbepoetin | Aranesp | Every 1–2 weeks IV or SC in ESKD patients; every 4 weeks SC in NDD-CKD patients | 0.45 µg/kg weekly or 0.75 µg/kg every 2 weeks in ESKD patients; 0.45 µg/kg every 4 weeks in ND-CKD patients |
| Methoxy polyethylene glycol-epoetin beta | Mircera | Initiation: every 2 weeks; maintenance: monthly. IV in HD patients, SC in NDD-CKD and PD patients | 0.6 µg/kg every 2 weeks; monthly when Hb is stable at twice the every-2-weeks dose |
PRCA is a form of aplastic anemia caused by the production of anti-EPO antibodies induced by administration of exogenous ESAs. The diagnosis of PRCA should be suspected in a patient with a sudden weekly drop in Hb of approximately 1 g/dL, or a weekly transfusion requirement and low reticulocyte count (<20,000 cells/µL), despite a high dose of ESA for several months. In contrast to classic aplastic anemia, the white blood cell and platelet counts are preserved in PRCA. A definitive diagnosis of PRCA is made by the demonstration of anti-EPO antibodies in the blood or a bone marrow examination showing normal cellularity and less than 4% erythroblasts. Treatment includes discontinuation of the ESA and immunosuppressive therapy (e.g., cyclophosphamide); most patients respond after several months and do not relapse after the immunosuppressive therapy is discontinued. A cluster of PRCA cases in Europe was traced almost exclusively to subcutaneous administration of a form of epoetin alfa stabilized with Tween 80. This additive was never used in the United States where PRCA has always been rare. With removal of this preparation from the European market, the incidence of PRCA fell dramatically. An additional small cluster of PRCA cases was reported with one of the biosimilar ESAs approved in Europe. That cluster was traced to interaction of the agent with tungsten used in the manufacturing of the needles of prefilled syringes. Once the root cause was identified and eliminated, no further clusters of PRCA with that agent have been reported.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here