Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In this chapter, the embryologic and anatomic characteristics of the bile ducts and gallbladder are reviewed, with a focus on information useful for diagnosing and treating biliary tract disease and understanding the anomalies and congenital malformations of these structures. Biliary tract disease in infants and children is considered because many of the disorders that occur early in life are due to abnormal morphogenesis or adversely affect the process of development.
The human liver is formed from 2 primordia ( Fig. 62.1 ): the liver diverticulum and the septum transversum (see also Chapter 71 ). Proximity of cardiac mesoderm, which expresses fibroblast growth factors (FGFs) 1, 2, and 8, and bone morphogenetic proteins cause the foregut endoderm to develop into the liver. The liver diverticulum forms through the proliferation of endodermal cells at the cranioventral junction of the yolk sac with the foregut and grows into the septum transversum in a cranioventral direction. Surrounding mesoderm and ectoderm participate in the hepatic specification of the endoderm to hepatoblasts, via signaling from mesodermal tissues, including ligands of bone morphogenic protein, Wnt, and FGF families of proteins. After their specification and migration into the septum transversum, hepatoblasts undergo proliferation and migration between 26 and 32 days of gestation in the process of forming an organ bud. The homeodomain transcription factors hematopoietically expressed homeobox (Hhex) and Prospero homeobox protein 1 (Prox1), in the anterior endoderm and hepatic diverticulum, are required for the migration of hepatoblasts into the septum transversum that precedes liver growth and morphogenesis. Another homeodomain protein, Hlx, is necessary for hepatoblast proliferation. At the 5-mm stage, a solid cranial portion (hepatic) and a hollow caudal portion of the diverticulum can be clearly distinguished. The large hepatic portion differentiates into proliferating cords of hepatocytes and the intrahepatic bile ducts. Hepatocyte nuclear factor (HNF)4α expression drives further hepatocyte differentiation and epithelial transformation into the characteristic sinusoidal architecture.
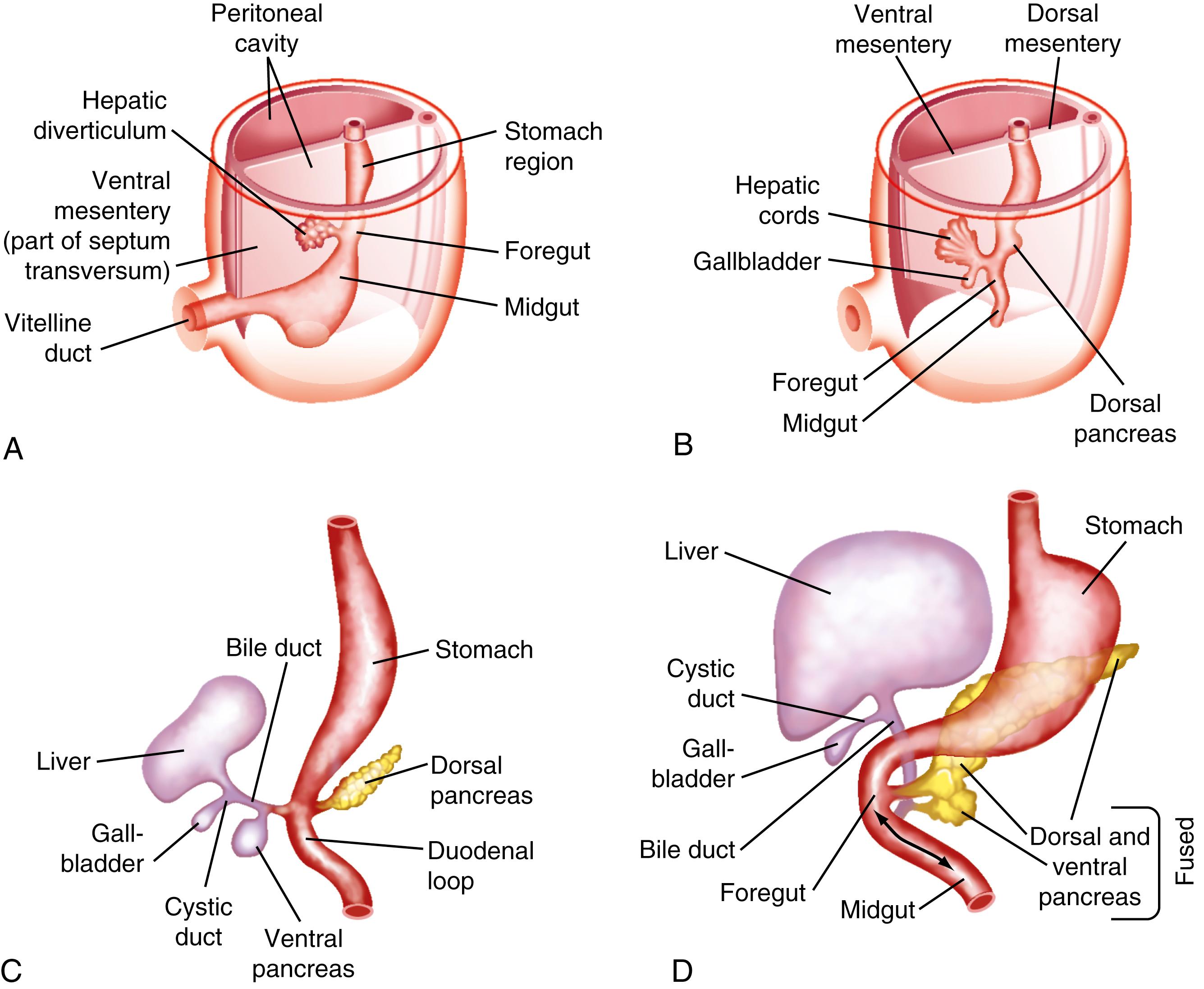
Bipotential hepatoblasts express alpha fetoprotein and markers for hepatocytes such as albumin and for keratin and cholangiocytes (cytokeratin 19, CK19). The transcription factor sex-determining region Y- box 9 (SOX9) is the earliest specific biliary marker detected in endodermal cells that line the hepatic diverticulum. Its expression disappears as soon as hepatoblasts invade the septum transversum but reappears in cells of the biliary lineage throughout the development. This early change occurs on the eighteenth day of gestation and corresponds to the 2.5-mm stage of the embryo. The homeobox gene Hhex is essential for proper hepatoblast differentiation and bile duct morphogenesis. Members of the transforming growth factor (TGF)-β, Wnt, FGF, Hippo, GATA, FOXA, ONECUT2, and HNF3/forkhead transcription factor families and HNF6 are also required for formation and differentiation of gut endoderm tissues. The septum transversum consists of mesenchymal cells and a capillary plexus formed by the branches of the 2 vitelline veins. At the 3- to 4-mm stage, between the third and fourth weeks of gestation, the growing diverticulum projects as an epithelial plug into the septum transversum.
The intrahepatic bile ducts develop from primitive hepatocytes around branches of the portal vein. The process is initiated near the hilum of the liver and progressively asymmetrically toward the periphery through a series of remodeling stages. Cholangiocytes are associated with the basement membrane throughout bile duct development, suggesting that cholangiocytes receive morphogenic signals from components of the extracellular matrix, including laminin and type IV collagen. A ring of hepatocytes in proximity to the portal vein branches first transform into bile duct-type cells. A second layer of primitive hepatocytes is similarly transformed and produces a circular cleft around the portal vein that is lined on both sides by bile duct epithelial cells. This double-walled cylinder with a slit-like lumen, the ductal plate, can be detected at 9 weeks of gestation. Thus the entire network of interlobular and intralobular bile ductules develops from the limiting plate. The periportal mesenchyme secretes TGF-β and produces a portal-to-parenchymal gradient of TGF-β signaling, with the highest activity near the mesenchyme. The mesenchyme also produces Jagged1, which via cell-cell contacts induces Notch2-mediated signaling in the differentiating biliary cells. TGF-β and Notch signaling, in cooperation with other signaling pathways, stimulate sequential differentiation of the 2 layers of biliary cells (ductal plate) to induce asymmetrical tubulogenesis. Secreted factors of the Wnt family also regulate differentiation of hepatoblasts to biliary cells. Bile ducts develop according to 2 axes: ducts mature along their radial axis, and they also grow in length according to an axis that extends from the hilum of the liver to the periphery of the liver lobes.
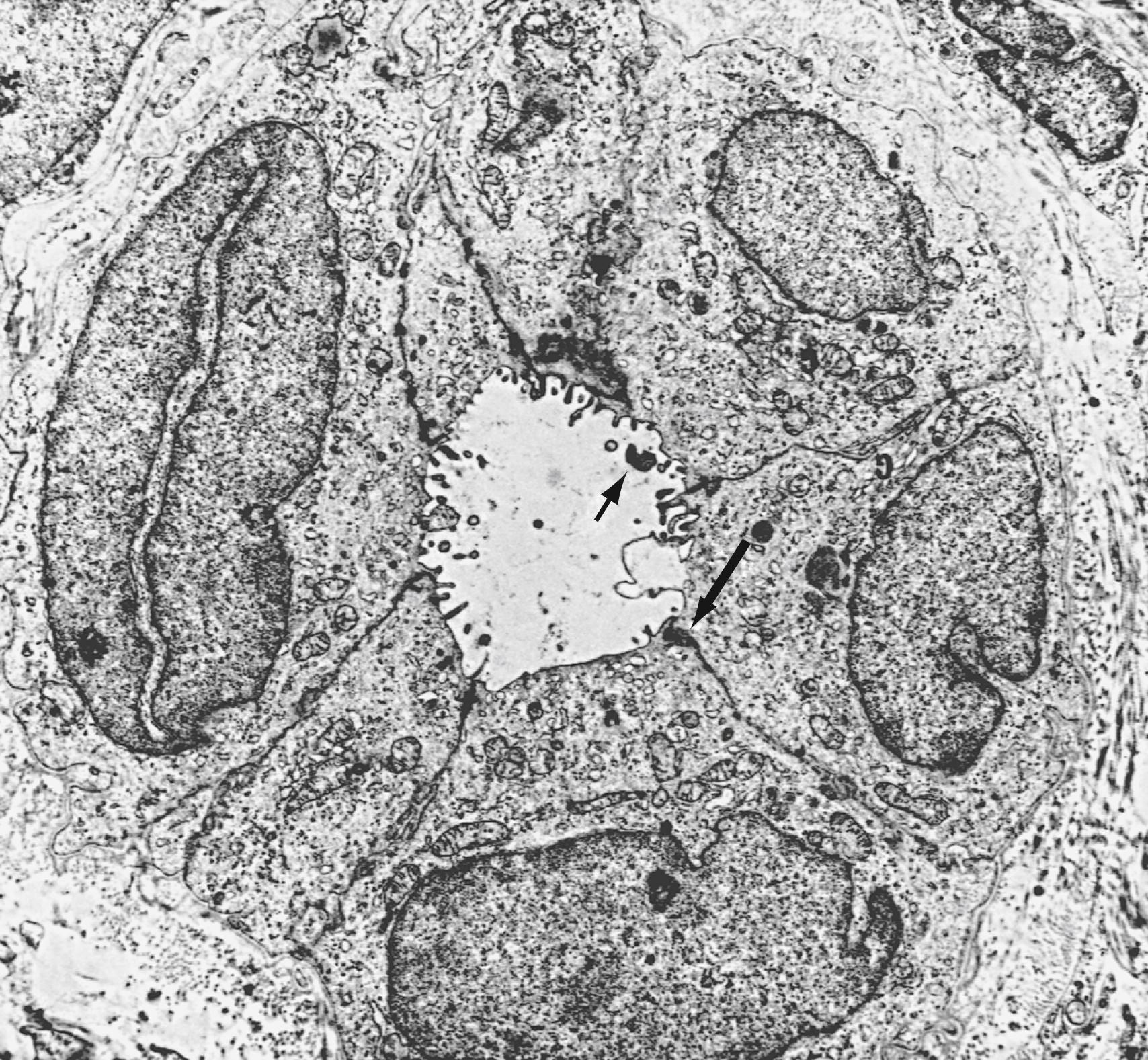
In sections of the 10-mm embryo, many of the liver cords are traversed by double-walled canals that branch and are morphologically indistinguishable from bile capillaries of the adult. These structures differ from those of the adult in that they are bounded by 6 or more liver cells instead of 2. The process of differentiation of bile ductular epithelial cells (cholangiocytes) from primitive hepatocytes has been documented in humans using immunohistochemical staining with several anticytokeratin antibodies. During the phenotypic shift toward bile duct–type cells, hepatocytes first display increased reactivity for CKs 8 and 18 and express CK19 at 20 to 25 weeks’ gestation. Cholangiocyte-mesenchymal cell interaction is important for the formation of bile ducts. During the transition from ductal plates to bile ducts, portal myofibroblasts expand significantly and surround newly formed bile ducts. Periportal connective tissue, corticosteroid hormones, and basal laminar components may play important roles in the differentiation of bile ducts. The ductal plate structure requires extensive remodeling through a process of reabsorption, possibly through apoptosis, to yield the characteristic anastomosing system of biliary channels that surround the portal vein. Proteins that appear to have a role in the promotion of apoptosis, specifically Fas antigen and c-Myc, are consistently detected in primitive intrahepatic ductal cells. Lewis antigen, which is expressed in damaged and apoptotic cells, is also present. Bcl-2 protein, an inhibitor of apoptosis, is not found in early stages of intrahepatic bile duct cell development but becomes detectable later. Computed three-dimensional (3D) reconstruction of the developing ductal plate has shown that the ductal plate remodeling process starts at the porta hepatis at approximately 11 weeks’ gestation and progresses toward the periphery of the liver. The process is in large part completed at term, but even at 40 weeks’ gestation, some of the smallest portal vein branches may not be accompanied by an individual bile duct and may still be surrounded by a (discontinuous) ductal plate. Ductal plate malformation is a common feature of pediatric biliary disorders and may be initiated at distinct stages of bile duct morphogenesis, owing to defects in ductal plate remodeling, abnormal differentiation of hepatoblasts into ductal plate cells, or abnormal maturation of primitive ductal structures.
The extrahepatic bile ducts, bile duct (common bile duct), cystic duct, and gallbladder arise from a region of ventral foregut proximate to the liver and ventral pancreas and share a common origin with the ventral pancreas but not the liver. Development of the extrahepatic biliary system precedes that of intrahepatic bile ducts. The extrahepatic system (but not intrahepatic bile ducts) are derived from a progenitor cell expressing the transcription factor pancreatic-duodenal homeobox 1 (Pdx1). The Sox17 transcription factor is an important determinant of how cells within the Pdx1 domain are assigned to the 2 different fates of pancreas or extrahepatic biliary system. Appropriate segregation of extrahepatic biliary system and ventral pancreatic lineages is also regulated by hairy and enhancer of split-1 (Hes1), a transcriptional effector of Notch signaling.
The primitive extrahepatic bile duct maintains continuity with the ductal plate, from which intrahepatic bile ducts are eventually formed. Contrary to long-held concepts of biliary development, no “solid stage” of endodermal occlusion of the bile duct lumen is found at any time during gestation. At 16 mm, the cystic duct and proximal gallbladder are hollow, but the fundus of the gallbladder is still partially obstructed by remnants of the epithelial plug. The gallbladder is patent by the third month of gestation. Further development until birth consists primarily of continued growth. The characteristic folds of the gallbladder are formed toward the end of gestation and are moderately developed in the neonate. Bile secretion starts at the beginning of the fourth month of gestation; thereafter, the biliary system continuously contains bile, which is secreted into the gut and imparts a dark green color to the intestinal contents (meconium).
The adult human liver has more than 2 km of bile ductules and ducts. Quantitative computer-aided 3D imaging has estimated the volume of the entire macroscopic duct system of human liver to be a mean of 20.4 cm. In these studies, the mean internal surface of 398 cm 2 is magnified approximately 5.5-fold by the presence of microvilli and cilia at the apical surfaces of cholangiocytes that play an important role in the regulation of cholangiocyte functions. These structures are far from being inert channels; they are capable of modifying biliary flow and composition significantly in response to hormones such as secretin. A general feature of bile ductules is their anatomic intimacy with portal blood and lymph vessels, which potentially allows selective exchange of materials between compartments. No major ultrastructural differences exist between cholangiocytes lining small and large bile ducts, but the functional properties of cholangiocytes are heterogeneous. For example, large, but not small, intrahepatic bile ducts are involved in secretin-regulated bile ductal secretion. Correspondingly, the secretin receptor and chloride-bicarbonate exchanger messenger RNAs have been detected in large, but not small, intrahepatic bile duct units.
Bile secretion begins at the level of the bile canaliculus, the smallest branch of the biliary tract. Its boundaries are formed by a specialized membrane of adjacent apical poles of liver cells. The canaliculi form a meshwork of polygonal channels between hepatocytes with many anastomotic interconnections. Bile then enters the small terminal channels (the canals of Hering), which have a basement membrane and are lined partly by hepatocytes and partly by cholangiocytes. The canals of Hering provide a conduit through which bile may traverse the limiting plate of hepatocytes to enter the larger perilobular or intralobular ducts. These smallest of biliary radicles are less than 15 to 20 μm in diameter, with lumens surrounded by cuboidal epithelial cells. At the most proximal level, one or more fusiform-shaped ductular cells may share a canalicular lumen with a hepatocyte; gradually, the ductules become lined by 2 to 4 cuboidal epithelial cells as they approach the portal canal. Bile flows from the central lobular cells toward portal triads (from zone 3 to zone 1 of the liver acinus) (see Chapter 71 ). The terminal bile ductules are thought to proliferate as a result of chronic extrahepatic bile duct obstruction.
The interlobular bile ducts form a richly anastomosing network that closely surrounds the branches of the portal vein. These bile ducts ( Fig. 62.2 ) are initially 30 to 40 μm in diameter and are lined by a layer of cuboidal or columnar epithelium that displays a microvillar architecture on its luminal surface. The cells have a prominent Golgi apparatus and numerous vesicles that likely participate in exchange of substances among cytoplasm, bile, and plasma through the processes of exocytosis and endocytosis. These ducts increase in caliber and possess smooth muscle fibers within their walls as they approach the hilum of the liver. The muscular component may provide the morphologic basis for the narrowing of the ducts at this level, as observed on cholangiography. Because the ducts become progressively larger, the epithelium becomes thicker, and the surrounding layer of connective tissue grows thicker and contains many elastic fibers. These ducts anastomose further to form the large hilar intrahepatic ducts, which are 1 to 1.5 mm in diameter and give rise to the main hepatic ducts.
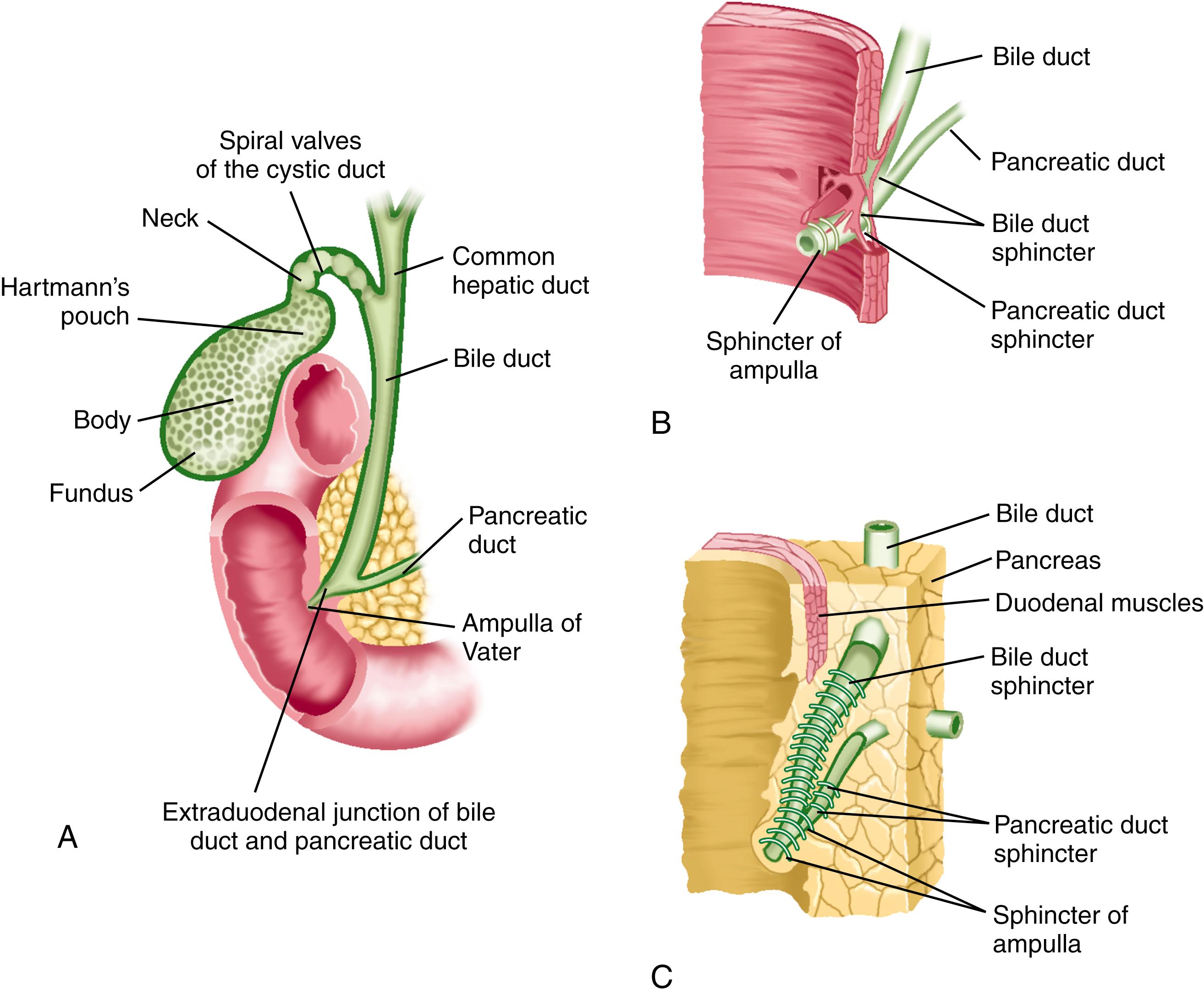
The common hepatic duct emerges from the porta hepatis after the union of the right and left hepatic ducts, each of which is 0.5 to 2.5 cm long ( Fig. 62.3 ). The confluence of the right and left hepatic ducts is outside the liver in some 95% of cases; uncommonly, the ducts merge inside the liver, or the right and left hepatic ducts do not join until the cystic duct joins the right hepatic duct. Because the hepatic ducts leave the porta hepatis, they lie within the 2 serous layers of the hepatoduodenal ligament. This sheath of fibrous tissue binds the hepatic ducts to the adjacent blood vessels. In the adult, the common hepatic duct is about 3 cm long and is joined by the cystic duct, usually at its right side, to form the common bile duct (or simply bile duct). The length and angle of junction of the cystic duct with the common hepatic duct are variable. The cystic duct enters the common hepatic duct directly in 70% of patients; alternatively, the cystic duct may run anterior or posterior to the bile duct and spiral around it before joining the bile duct on its medial side. The cystic duct may also course parallel to the common hepatic duct for 5 to 6 cm and enter it after running posterior to the first portion of the duodenum.
In humans, the large intrahepatic bile ducts at the hilum (1- to 1.5-mm diameter) have many irregular side branches and pouches (150- to 270-μm diameter) that are oriented in one plane, corresponding anatomically to the transverse fissure. Smaller pouches of the side branches are also found. Many side branches end as blind pouches, but others, particularly at the hilum, communicate with each other. At the bifurcation, side branches from several main bile ducts connect to form a plexus. The functional significance of these structures is unknown. The blind pouches may serve to store or modify bile, whereas the biliary plexus provides anastomoses that may allow exchange of material between the large bile ducts.
The anatomy of the hepatic hilum is particularly important to the surgeon (see also Chapter 71 ). A plate of fibrous connective tissue in the hepatic hilum includes the umbilical plate that envelops the umbilical portion of the portal vein, the cystic plate in the gallbladder bed, and the Arantian plate that covers the ligamentum venosum. Histologic examination of the sagittal section of the hilar plate reveals abundant connective tissue, including neural fibers, lymphatic vessels, small capillaries, and small bile ducts. The bile ducts in the plate system correspond to the extrahepatic bile ducts, and their lengths are variable in every segment.
Similar to the intestine, the cystic, common hepatic, and bile ducts possess mucosa, submucosa, and muscularis. The ducts are lined by a single layer of columnar epithelium. Mucus-secreting tubular glands can be found at regular intervals in the submucosa, with openings to the surface of the mucosa. The common bile duct is 6.0 to 8.0 cm long, runs between layers of the lesser omentum, and lies anterior to the portal vein and to the right of the hepatic artery. The bile duct is normally 0.5 to 1.5 cm in diameter. The wall of the extrahepatic bile duct is supported by a layer of connective tissue with an admixture of occasional smooth muscle fibers. The smooth muscle component is conspicuous only at the neck of the gallbladder and at the lower end of the bile duct. The bile duct passes retroperitoneally behind the first portion of the duodenum in a notch on the back of the head of the pancreas and enters the second part of the duodenum. The duct then passes obliquely through the posterior medial aspect of the duodenal wall and joins the main pancreatic duct to form the ampulla of Vater (see Fig. 62.3 ). The mucous membrane bulge produced by the ampulla forms an eminence, the duodenal papilla. In 10% to 15% of patients, the bile and pancreatic ducts open separately into the duodenum. The bile duct tapers to a diameter of 0.6 cm or less before its union with the pancreatic duct.
As they course through the duodenal wall, the bile and pancreatic ducts are invested by a thickening of both the longitudinal and circular layers of smooth muscle (see Fig. 62.3 ) of the sphincter of Oddi (see also Chapter 63 ). There is considerable variation in this structure, but it is usually composed of several parts: (1) the sphincter choledochus—circular muscle fibers that surround the intramural portion of the bile duct immediately before its junction with the pancreatic duct; (2) the sphincter pancreaticus, which is present in approximately one third of persons and surrounds the intraduodenal portion of the pancreatic duct before its juncture with the ampulla; (3) the fasciculi longitudinales—longitudinal muscle bundles that span intervals between the bile and pancreatic ducts; and (4) the sphincter ampullae—longitudinal muscle fibers that surround a sparse layer of circular fibers around the ampulla of Vater. The sphincter choledochus constricts the lumen of the bile duct and, thus, prevents bile flow. Contraction of the fasciculi longitudinales shortens the length of the bile duct and, thus, promotes the flow of bile into the duodenum. The contraction of the sphincter ampullae shortens the ampulla and approximates the ampullary folds to prevent reflux of intestinal contents into the bile and pancreatic ducts. When both ducts end in the ampulla, however, contraction of the sphincter may cause reflux of bile into the pancreatic duct.
The intrahepatic and extrahepatic bile ducts are highly dependent on arterial blood supply for oxygenation. An abundant anastomotic network of blood vessels from branches of the hepatic and gastroduodenal arteries supplies the bile duct. The supraduodenal portion of the duct is supplied by vessels running along its wall inferiorly from the retroduodenal artery and superiorly from the right hepatic artery. Injury to these blood vessels can result in bile duct ischemia and stricturing.
The surface of the intrahepatic and extrahepatic bile ducts is drained by fine venous plexuses that communicate with each other. A fine reticular epicholedochal venous plexus lies on the surface of the bile ducts, and a paracholedochal venous plexus lies outside the bile ducts and courses parallel to the ducts.
An extraordinarily rich plexus of capillaries surrounds bile ducts as they pass through the portal tracts. Blood flowing through this peribiliary plexus empties into the hepatic sinusoids via the interlobular branches of the portal vein. The peribiliary plexus may modify biliary secretions through the bidirectional exchange of proteins, inorganic ions, and bile acids between blood and bile. Because blood flows in the direction (from the large toward the small ducts) opposite to that of bile flow, the peribiliary plexus presents a countercurrent stream of biliary-reabsorbed substances to hepatocytes.
The intrahepatic arteries, veins, bile ducts, and hepatocytes are innervated by adrenergic and cholinergic nerves. In the autonomic nervous system, there are a number of regulatory peptides, such as neuropeptide tyrosine, calcitonin gene-related peptide, somatostatin, vasoactive intestinal polypeptide, enkephalin, and bombesin. Neuropeptide tyrosine-positive nerves present in extrahepatic bile ducts may serve to regulate bile flow by autocrine or paracrine mechanisms.
The lymphatic vessels of the hepatic, cystic, and proximal portions of the bile duct empty into glands at the hilum of the liver. Lymphatics draining from the lower portion of the bile duct drain into glands near the head of the pancreas.
The gallbladder (see Fig. 62.3 ) is a storage reservoir that allows bile acids to be delivered in a high concentration and in a controlled manner to the duodenum for the solubilization of dietary lipid (see Chapter 64 ). It lies in a fossa on the undersurface of the right lobe of the liver. This distensible pear-shaped structure is 3 cm wide and 7 cm long in the adult and has a capacity of 30 to 50 mL. The gallbladder has a thin muscular layer with the smooth muscle cells largely oriented around the circumference of the gallbladder. The absorptive surface of the gallbladder is enhanced by numerous prominent folds. The gallbladder is covered anteriorly by an adventitia that is fused with the capsule of the liver. On its posterior aspect and at the apex, it is covered by the visceral peritoneum. The portions of the gallbladder are the fundus, body, infundibulum, and neck. The anterior portion of the fundus is located at the level of the right lateral border of the musculus rectus abdominis and the ninth costal cartilage. The posterior aspects of the fundus and body lie close to the transverse colon and duodenum, respectively; with perforation of the gallbladder, gallstones can readily penetrate these structures. The infundibulum is an area of tapering between the gallbladder body and neck. Hartmann pouch is a bulging of the inferior surface of the infundibulum that lies close to the neck of the gallbladder. Gallstones can become impacted in Hartmann pouch, thereby obstructing the cystic duct and producing cholecystitis. Extensive inflammation in Hartmann pouch can lead to obstruction of the adjacent common hepatic duct (Mirizzi syndrome) (see Chapter 65 ).
The gallbladder is connected at its neck to the cystic duct, which empties into the bile duct (see Fig. 62.3 ). The cystic duct is about 4 cm long and maintains continuity with the surface columnar epithelium, lamina propria, muscularis, and serosa of the gallbladder. The mucous membrane of the gallbladder neck forms the spiral valve of Heister, which is involved in regulating flow into and out of the gallbladder.
The gallbladder is supplied by the cystic artery, which usually arises from the right hepatic artery. The artery divides into 2 branches near the neck of the gallbladder: a superficial branch that supplies the serosal surface and a deep branch that supplies the interior layers of the gallbladder wall. Variations in the origin and course of the cystic artery are common. Because the cystic artery is an end artery, the gallbladder is particularly susceptible to ischemic injury and necrosis that result from inflammation or interruption of hepatic arterial flow.
The cystic vein provides venous drainage from the gallbladder and cystic ducts and commonly empties into the portal vein and occasionally directly into the hepatic sinusoids. The lymph vessels of the gallbladder are connected with the lymph vessels of Glisson capsule. Subserosal and submucosal lymphatics empty into a lymph gland near the neck of the gallbladder. Sympathetic innervation of the gallbladder originates from the celiac axis and travels with branches of the hepatic artery and portal vein. Visceral pain is conducted through sympathetic fibers and is frequently referred to the right subcostal, epigastric, and right scapular regions. Branches of both vagus nerves provide parasympathetic innervation that likely contributes to the regulation of gallbladder motility.
The gallbladder is lined by a mucosa that manifests multiple ridges and folds and is composed of a layer of columnar epithelial cells. The gallbladder wall consists of a mucosa, lamina propria, tunica muscularis, and serosa. The tunica muscularis is thick and invested with an interlocking array of longitudinal and spiral smooth muscle fibers. Tubuloalveolar glands are found in the region of the neck of the gallbladder and are involved in mucus production. The Rokitansky-Aschoff sinuses are invaginations of the surface epithelium that may extend through the muscularis. These structures can be a source of inflammation, most likely as a result of bacterial stasis and proliferation within the invaginations. The ducts of Luschka may be observed along the hepatic surface of the gallbladder and open directly into the intrahepatic bile ducts rather than into the gallbladder cavity. These structures are thought to represent a developmental anomaly, and when they are present in the gallbladder bed may be a source of a bile leak after cholecystectomy.
Accessory bile ducts are aberrant ducts that drain individual segments of the liver; they may drain directly into the gallbladder, cystic duct, right and left hepatic ducts, or bile duct. In rare cases, the right hepatic duct may connect to the gallbladder or cystic duct. These anomalies must be recognized on cholangiography in order to prevent inadvertent transection or ligation of bile ducts during surgery.
Complete duplication of the bile duct occurs rarely. In most cases, separate ducts drain the right and left hepatic lobes and open into the duodenum.
Variation in the drainage and course of the cystic duct is common. Duplication of the cystic duct may also be encountered. The cystic duct is absent in most cases of agenesis of the gallbladder (see later); rarely, the duct alone may be absent, and the gallbladder empties directly into the common hepatic duct.
Most structural anomalies of the gallbladder are of no clinical importance, but occasionally the abnormal gallbladder may be a predisposing factor for bile stasis, inflammation, and formation of gallstones. Gallbladder disease in an anomalous or malpositioned gallbladder may cause diagnostic confusion.
Agenesis of the gallbladder may be an isolated anomaly or occur in association with other congenital malformations. The abnormality has a frequency at autopsy of 0.04% to 0.13% and likely reflects a lack of development of the gallbladder bud or failure of the normal process of vacuolization. Incomplete vacuolization of the solid endodermal cord during development can result in congenital strictures of the gallbladder or cystic duct. Ectopic tissues of foregut endodermal origin, including gastric, hepatic, adrenal, pancreatic, and thyroid tissues, may be found within the gallbladder wall.
A double gallbladder is another rare malformation that occurs in 1 to 5 per 10,000 persons in the general population. The 2 gallbladders may share a single cystic duct, forming a Y-shaped channel, or each may have a distinct cystic duct that enters the bile duct separately. Vesica fellea triplex, or triplication of the gallbladder, is another rare congenital anomaly. Multiple gallbladders are usually discovered because of cholelithiasis, sludge, cholecystitis, or neoplasia. Bilobed gallbladders and gallbladder diverticula are other rare anomalies. A single gallbladder may be divided by longitudinal septa into multiple chambers, probably secondary to incomplete vacuolization of the solid gallbladder bud during morphogenesis. Diverticula and septations of the gallbladder may promote bile stasis and gallstone formation.
Various malpositions of the gallbladder have been described. Rarely, the gallbladder lies under the left lobe of the liver, to the left of the falciform ligament. This defect likely results from migration of the embryonic bud from the hepatic diverticula to the left rather than to the right. Some researchers have proposed that a second gallbladder may develop independently from the left hepatic duct, with regression of the normal structure on the right. In other cases, a caudal bud that advances farther than the cranial bud may become buried within the cranial structure, creating an intrahepatic gallbladder. It is thought that if the caudal bud lags behind the movement of the cranial bud, a floating gallbladder results. In this setting, the gallbladder is covered completely with peritoneum and suspended from the undersurface of the liver by mesentery to the gallbladder or cystic duct; the gallbladder is abnormally mobile and prone to torsion. Rarely, gallbladders have been found in the abdominal wall, falciform ligament, and retroperitoneum.
Several forms of “folded” gallbladders have been described. In one variant, the fundus appears to be bent, giving the appearance of a “Phrygian cap.” The gallbladder is usually located in a retroserosal position, and the anomaly is thought to result from aberrant folding of the gallbladder within the embryonic fossa. Aberrant folding of the fossa during the early stages of development can result in kinking between the body and the infundibulum of the gallbladder. Kinked gallbladders probably do not lead to clinical symptoms but may be a source of confusion in the interpretation of imaging studies.
Cholestatic liver disease results from processes that interfere with either bile formation by hepatocytes or bile flow through the intrahepatic and extrahepatic biliary tract. A number of these disorders are due to defective ontogenesis or a failure of postnatal adaptation to the extrauterine environment. Box 62.1 provides a list of disorders that affect the biliary tract and occur in both infants and older children; they are discussed later in this chapter. There is a particular emphasis on neonatal cholangiopathies and the unique aspects of biliary disease in the older child. The general features of the many cholestatic liver diseases of the neonate are similar, and a central problem of pediatric hepatology is differentiating intrahepatic from extrahepatic cholestasis ( Table 62.1 ). The treatment of metabolic or infective liver diseases and the surgical management of biliary anomalies require early diagnosis. Even when effective treatment is not possible, infants and children with progressive liver disease benefit from optimal nutritional support and medical management of chronic liver disease before they are referred for LT.
Allograft rejection
Bile duct obstruction due to pancreatic disease (inflammatory or neoplastic)
Bile plug syndrome
Biliary helminthiasis
Caroli disease
Choledochal cysts
CF
Extrahepatic biliary atresia
Graft-versus-host disease
Idiopathic bile duct stricture (possibly congenital)
Paucity of intrahepatic bile ducts (syndromic and nonsyndromic)
Post-traumatic bile duct stricture
Sclerosing cholangitis (IBD-associated, immunodeficiency-related, neonatal)
Spontaneous perforation of bile duct
Tumors intrinsic and extrinsic to the bile duct
Acalculous cholecystitis
Acute cholecystitis
Acute hydrops of the gallbladder
Anomalies
Cholelithiasis
Chronic cholecystitis
Tumors
| Disorder | Frequency (%) |
|---|---|
| Idiopathic neonatal hepatitis | 30-35 |
| Extrahepatic biliary atresia | 30 |
| α 1 -Antitrypsin deficiency | 7-10 |
| Intrahepatic cholestatic syndromes (Alagille syndrome, progressive familial intrahepatic cholestasis, others) | 5-6 |
| Hepatitis (CMV, rubella, HSV, others) | 3-5 |
| Choledochal cyst | 2-4 |
| Bacterial sepsis | 2 |
| Endocrinopathy (hypothyroidism, panhypopituitarism) | ≈1 |
| Galactosemia | ≈1 |
| Inborn errors of bile acid metabolism | ≈1 |
| Other metabolic disorders | ≈1 |
Because of the immaturity of hepatobiliary function, the number of distinct disorders that exhibit cholestatic jaundice may be greater during the neonatal period than at any other time of life (see Box 62.1 ). Genomic sequencing is identifying new defects that were previously labeled as idiopathic neonatal hepatitis. Liver dysfunction in the infant, regardless of the cause, is commonly associated with bile secretory failure and cholestatic jaundice. Although cholestasis may be traced to the level of the hepatocyte or the biliary apparatus, in practice there may be considerable overlap among disorders with regard to the initial and subsequent sites of injury. For example, damage to the biliary epithelium is often a prominent feature of neonatal hepatitis due to CMV infection. Mechanical obstruction of the biliary tract invariably produces liver dysfunction and in the neonate may be associated with abnormalities of the liver parenchyma, such as giant cell transformation of hepatocytes. Whether giant cells—a frequent nonspecific manifestation of neonatal liver injury—reflect the noxious effects of biliary obstruction or whether the hepatocytes and the biliary epithelium are damaged by a common agent during ontogenesis, such as a virus with tropism for both types of cells, is unknown. Another common histologic variable that often accompanies neonatal cholestasis is bile ductular paucity or a diminution in the number of interlobular bile ducts. This finding may be of primary importance in patients with Alagille syndrome and may also occur as an occasional feature of many other disorders, including idiopathic neonatal hepatitis, congenital CMV infection, and α 1 -antitrypsin deficiency. Serial liver biopsies usually show a progressive decrease in the number of bile ductules per portal tract, with a variable amount of associated inflammation and fibrosis.
In most infants with cholestatic liver disease, the condition appears during the first few weeks of life. Differentiating conjugated hyperbilirubinemia from the common unconjugated physiologic hyperbilirubinemia of the neonate or the prolonged jaundice occasionally associated with breast-feeding is essential. The possibility of liver or biliary tract disease must be considered in any neonate older than 14 days with jaundice (see also Chapter 77 ). The stools of a patient with well-established biliary atresia are acholic, but early in the course of incomplete or evolving biliary obstruction, the stools may appear normal or only intermittently pigmented. Life-threatening but treatable disorders such as bacterial infection and a number of inborn errors of metabolism must be excluded. Success of surgical procedures in relieving the biliary obstruction of biliary atresia or a choledochal cyst depends on early diagnosis and surgery.
The approach to evaluation of an infant with cholestatic liver disease is outlined in Box 62.2 . The initial assessment should promptly establish whether cholestatic jaundice is present and assess the severity of liver dysfunction. A more detailed investigation may be required and should be guided by the clinical features of the case. All relevant diagnostic tests need not be performed in every patient. US may promptly establish a diagnosis of a choledochal cyst in a neonate with jaundice and, thus, obviate the need to exclude infectious and metabolic causes of liver disease. Numerous routine and specialized biochemical tests and imaging procedures have been proposed to distinguish intrahepatic from extrahepatic cholestasis in infants and thereby avoid unnecessary surgical exploration. Standard liver biochemical tests usually show variable elevations in serum direct bilirubin, aminotransferase, alkaline phosphatase, and lipid levels. Unfortunately, no single test has proved to have satisfactory discriminatory value, because at least 10% of infants with intrahepatic cholestasis have bile secretory failure sufficient to lead to an overlap in diagnostic test results with those suggestive of biliary atresia. The presence of bile pigment in stools is sometimes cited as evidence against biliary atresia, but coloration of feces with secretions and epithelial cells that have been shed by the cholestatic patient may be misleading.
Details of family history, pregnancy, presence of extrahepatic anomalies, and stool color
Fractionated serum bilirubin analysis
Liver biochemical tests (AST, ALT, alkaline phosphatase, 5′-nucleotidase, GGTP)
Tests of liver function (prothrombin time, partial thromboplastin time, coagulation factors, serum albumin level, serum ammonia level, serum cholesterol level, blood glucose)
CBC
Bacterial cultures of blood, urine, and other sites if indicated
Viral cultures
Serologic tests (HBsAg, TORCH, EBV, parvovirus B19, HIV, others)
Paracentesis if ascites
α 1 -Antitrypsin level and phenotype if level is reduced
Metabolic screen (urine and serum amino acids, urine organic acids)
Urine for reducing substances
Red blood cell galactose-1-phosphate uridyl transferase activity
Serum iron and ferritin levels
Sweat chloride analysis
Thyroid hormone, thyroid-stimulating hormone (evaluation of hypopituitarism as indicated)
Urine and serum analysis of bile acids and bile acid precursors
Genetic studies for Alagille syndrome and progressive familial intrahepatic cholestasis
US of liver and biliary tract (first)
Hepatobiliary scintigraphy
MRCP
Radiography of long bones and skull for congenital infection and of chest for lung and cardiac disease
Percutaneous or endoscopic cholangiography (rarely indicated)
Bone marrow examination and skin fibroblast culture for suspected storage disease
Duodenal intubation to assess fluid for bile pigment
Percutaneous liver biopsy (for light and electron microscopic examination, enzymologic evaluation)
Exploratory laparotomy and intraoperative cholangiography
US can be used to assess the size and echogenicity of the liver. Even in neonates, high-frequency real-time US can usually define the presence and size of the gallbladder, detect stones and sludge in the bile ducts and gallbladder, and demonstrate cystic or obstructive dilatation of the biliary system. Extrahepatic anomalies can also be identified. A triangular cord or band-like periportal echogenicity (≥3 mm in thickness), which represents a cone-shaped fibrotic mass cranial to the portal vein, appears to be a specific ultrasonographic finding in the early diagnosis of biliary atresia. The gallbladder “ghost” triad , defined as gallbladder length less than 1.9 cm, lack of smooth or complete echogenic mucosal lining with an indistinct wall, and irregular or lobular contour, has been proposed as an additional criterion for biliary atresia.
CT is less suitable in children younger than 2 years of age because of exposure to radiation and the paucity of intra-abdominal fat that is necessary for contrast.
MRCP, performed with T2-weighted turbo-spin echo sequences, is widely used to assess the biliary tract in all age groups. In a 1999 study, MRCP reliably demonstrated the bile duct and gallbladder in normal neonates. In some patients with biliary atresia, nonvisualization of the bile duct and demonstration of a small gallbladder have been characteristic MRCP findings. Another study found that MRCP is 82% accurate, 90% sensitive, and 77% specific for depicting extrahepatic biliary atresia. Contrary to previous reports, false-positive and false-negative findings occur with MRCP. Differentiation of severe intrahepatic cholestasis from biliary atresia may be difficult because the ability of MRCP to delineate the extrahepatic biliary tract depends on bile flow. MRCP can accurately define choledochal cysts and has emerged as the procedure of choice in the diagnosis of PSC.
The use of hepatobiliary scintigraphic imaging agents such as 99m Tc iminodiacetic acid derivatives may be helpful in differentiating extrahepatic biliary atresia from other causes of neonatal jaundice. Unfortunately, a 1997 study showed that in 50% of patients who had a paucity of interlobular bile ducts but no extrahepatic obstruction, biliary excretion of radionuclide was absent. In patients who had idiopathic neonatal hepatitis, 25% also demonstrated no biliary excretion. Nevertheless, the modality remains useful for assessing cystic duct patency in patients with a hydropic gallbladder or cholelithiasis.
ERCP may be useful in evaluating children with extrahepatic biliary obstruction and has been performed successfully in cholestatic neonates. Considerable technical expertise is required of the operator to complete this procedure in infants. Most neonates require general anesthesia for a satisfactory examination. Percutaneous transhepatic cholangiopancreatography may be of value in visualizing the biliary tract in selected patients, but the technique is more difficult to perform in infants than in adults because the intrahepatic bile ducts are small and because most disorders that occur in infants do not result in dilatation of the biliary tract. Interventional ERCP is commonly used to dilate biliary strictures and to remove common bile duct stones in children.
Percutaneous liver biopsy is particularly valuable in evaluating cholestatic patients and can be undertaken in even the smallest infants with only sedation and local anesthesia. For example, a diagnosis of extrahepatic biliary atresia can be made based on clinical and histologic criteria in 90% to 95% of patients. When doubt about the diagnosis persists, the patency of the biliary tract can be examined directly by a minilaparotomy and operative cholangiography. In a meta-analysis of various diagnostic methods used in 11 studies involving 646 patients, the accuracy rate of percutaneous liver biopsy was better than that of all noninvasive methods, with a pooled sensitivity of 98% (95% CI 96% to 99%), specificity of 93% (95% CI 89% to 95%), a positive predictive value of 93.0%, and a negative predictive value of 97.7%. Liver biopsy is also valuable in demonstrating bile duct paucity or biliary damage from drugs or viruses.
Biliary atresia is characterized by complete obstruction of bile flow as a result of the destruction of all or a portion of the extrahepatic bile ducts. As part of the underlying disease process or as a result of biliary obstruction, concomitant injury and fibrosis of the intrahepatic bile ducts also occurs to a variable extent.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here