Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
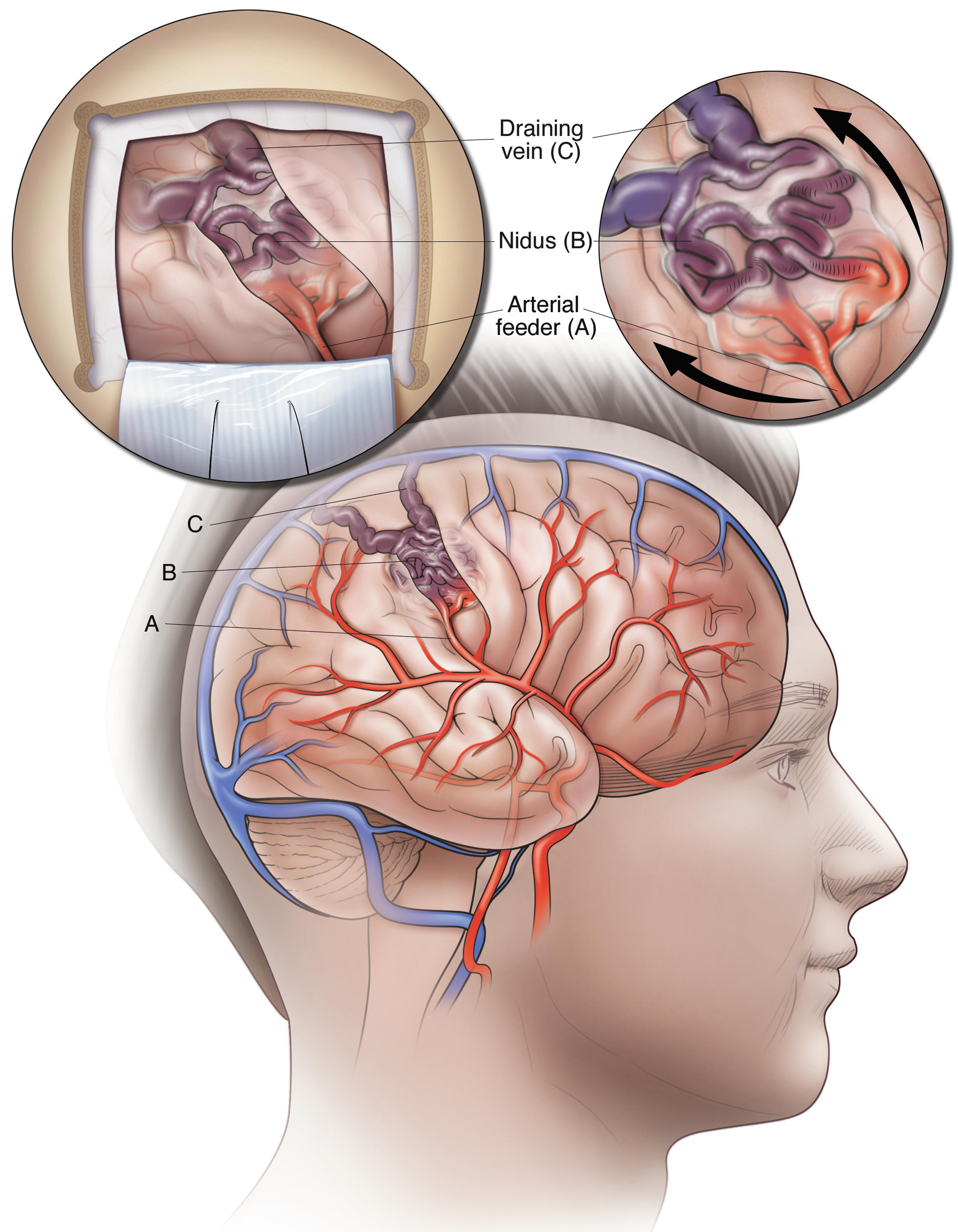
Intracranial AVMs are focal abnormal conglomerations of dilated arteries and veins that pulsate in the sulci and gyri of the brain and can cause stroke if they rupture.
The pathogenesis of iAVMs remains poorly understood, and traditional theories regarding the congenital etiology of these lesions are being challenged and replaced by more comprehensive pathophysiological hypotheses.
Features such as exclusive deep venous drainage, deep brain location, and associated aneurysms appear to be risk factors for hemorrhage.
The management of an iAVM in any given patient should be based on factors such as patient age and medical comorbidities as well as the anatomic and vascular features of the AVM.
Innovations in imaging technology, such as 3D imaging, functional imaging, and brain tract mapping, have the potential to improve surgical precision and safety in removing iAVMs and preserving surrounding vessels.
Intracranial arteriovenous malformations (iAVMs) are focal abnormal conglomerations of dilated arteries and veins that are typically encountered pulsating in the sulci and gyri of the brain ( Fig. 1.1 ) and can cause stroke when they rupture. The care of patients with iAVMs requires a thorough understanding of the pathological, anatomical, and clinical features that determine the natural history of the lesions, define the risk of treatment, and indicate the preferred method of treatment.
Normal brain function is entirely dependent upon adequate supply of oxygen and nutrients from the blood through a dense network of blood vessels. About 15% of one’s daily cardiac output is delivered to the brain. This is accomplished through two high-flow arterial systems: an anterior system, which is supplied by the internal carotid arteries; and a posterior system, which is supplied by the vertebral arteries. The vessels in both of these arterial systems all have extensive networks of small branches that extend out to perfuse brain tissue.
Blood supplying tissues travels through a system of capillaries, where oxygen-rich blood is delivered and oxygen-depleted blood is emptied into small veins ( Fig. 1.2 A ). In the brain, the small veins empty into larger veins that run within the dural covering of the brain, known as dural venous sinuses. The dural venous sinuses collect blood from veins around the brain and drain that blood into the left and right internal jugular veins.
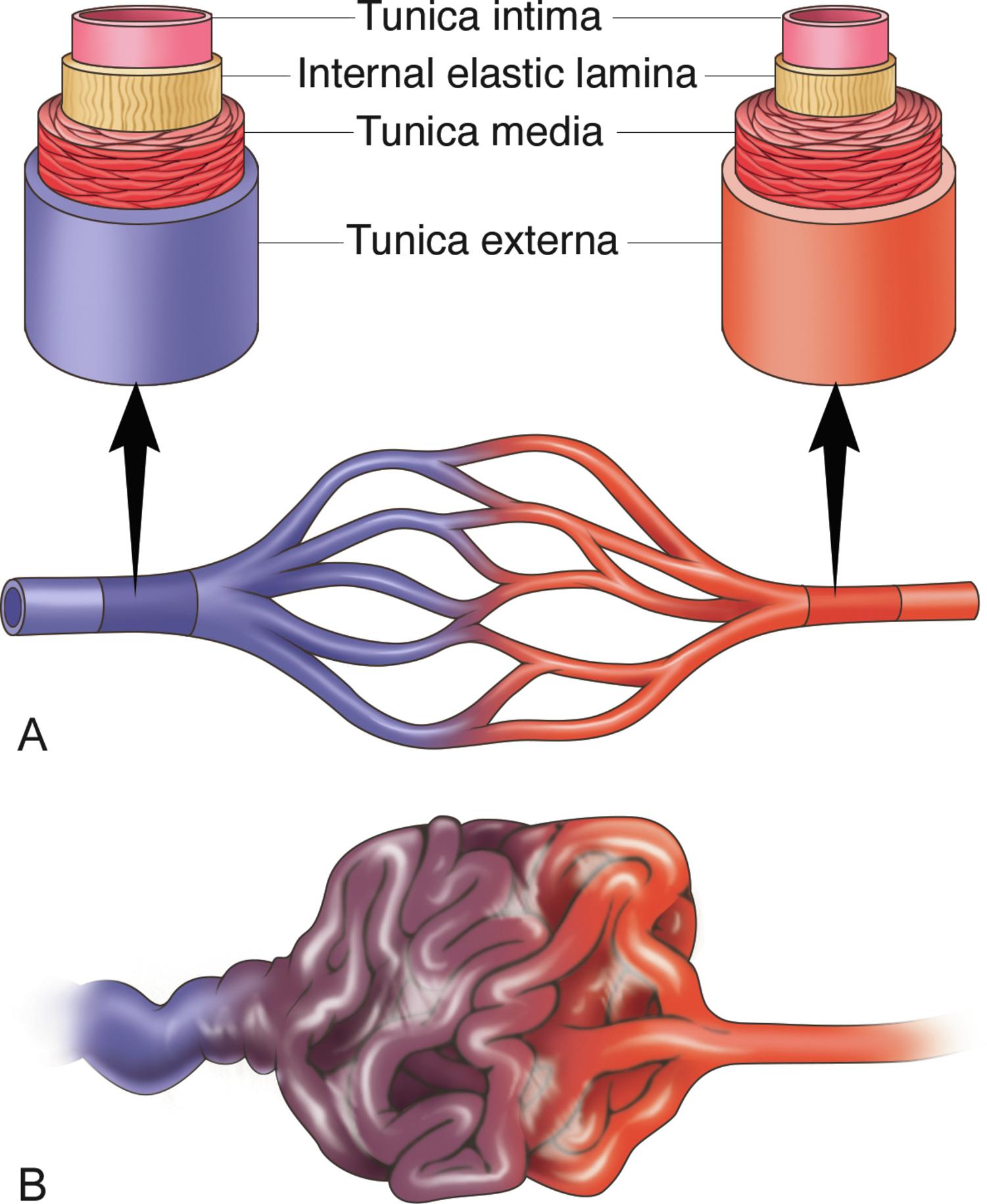
The human cortex is perfused by long arteries with many branches, resulting in a marked decrease in blood pressure between the large arteries at the base of the brain and the small arterioles perfusing the subcortical regions over the convexity. Blood pressure drops as flow is divided among branches, so at the distal end of the long arteries with many branches, blood pressure is much lower than in the parent artery. The veins of the brain are thin walled and valveless, and they would not be able to withstand the arterial pressure of the large-capacity intracerebral arterial vessels were it not for the successive branching and the fine network of capillaries interposed between the two systems, which reduce the arterial pressure before blood enters the venous bed ( Fig. 1.2 A).
Because iAVMs are composed of tangles of abnormally developed arteries and veins without intervening capillaries, there is an abnormal shunting of blood between arteries and veins (nonnutritive blood flow), resulting in high-pressure vascular channels that are at a risk of rupturing and bleeding out, often with catastrophic results. This abnormal tangle, known as a nidus, has a main arterial feeding vessel that is connected directly to the draining veins ( Fig. 1.2 B).
Arteriovenous malformations can be challenging for neurosurgeons, as the risks of surgical treatment may outweigh the benefits, and alternative management strategies (including the option of medical management or observation without interventional treatment) may be preferable in specific cases. Different grading systems have been widely used for determining surgery-related morbidity; however, high-risk factors such as history of hemorrhage, young age, deep venous drainage, and female sex may impact the choice and timing of surgical treatment in light of their effect on the patient’s lifetime risk of hemorrhage. There is also histopathological evidence of AVM features in association with identified risk factors. This chapter describes the anatomy of the cerebral vasculature and types of collateral circulation that are recruited by these lesions and the histopathological features of AVMs, which will create the basis for treatment considerations covered in the rest of this book.
The distribution of blood vessels in the brain was well studied in the last years of the 19th century in France and Germany, by Duret and Heubner, respectively. These two anatomists performed their research simultaneously in their own countries—each man working without the knowledge of the other’s efforts—and both contributed enormously to the understanding of cerebral vascularization.
Blood is supplied to the brain, face, and scalp via two major sets of vessels: the right and left common carotid arteries and the right and left vertebral arteries. The common carotid arteries bifurcate into the internal and external carotid arteries. The internal carotid arteries principally supply the cerebrum, whereas the external carotid arteries principally supply the face and neck. The two vertebral arteries join distally to form the basilar artery, and branches of the vertebral and basilar arteries supply blood for the cerebellum and brainstem. An anastomotic ring of arteries (the circle of Willis) located at the base of the brain connects the two major arterial systems via the posterior communicating arteries. (The circle of Willis is discussed in more detail in a subsequent section of this chapter.) Fig. 1.3 shows a simplified overview of some of the major arteries and the areas of the brain that they supply.
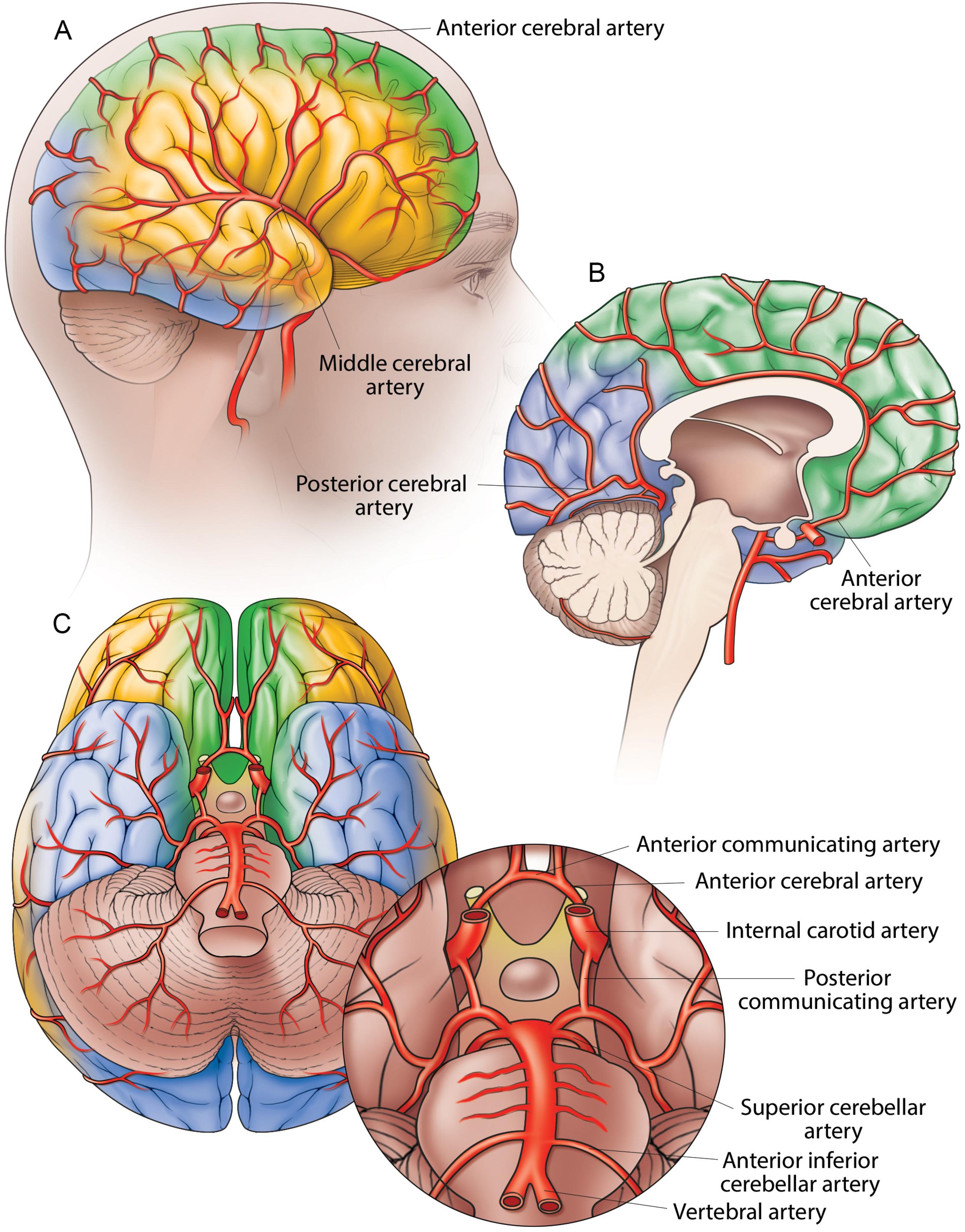
The two vertebral arteries, running toward each other, surround the medulla oblongata and join at the midline to form the basilar artery, a relatively large vessel that ascends along the ventral surface of the pons, in its basilar groove, within the pontine cistern. The basilar artery then bifurcates to form the paired posterior cerebral arteries. The basilar artery has different branches along its course, including the anterior inferior cerebellar artery, which arises from the proximal segment of the basilar artery and courses posterolaterally to supply the inferior aspect of the cerebellum. The anterior inferior cerebellar artery also anastomoses with the posterior inferior cerebellar artery, a branch of the vertebral artery. The superior cerebellar artery branches off in a lateral direction at the distal aspect of the basilar artery just prior to its bifurcation. It courses around the cerebral peduncles to supply the superior aspect of the cerebellum along with the tela choroidea of the third ventricle, the pineal gland, the pons, and the superior medullary velum. It also forms an anastomosis with derivatives of the inferior cerebellar arteries. The basilar artery gives off numerous pontine arteries from its lateral surface bilaterally as well as many perforators from the posteromedial aspect of the distal bifurcation. The basilar artery terminates as it divides, at the level of the anterior margin of the protuberance, into two terminal and divergent branches, the posterior cerebral arteries.
The posterior cerebral artery arises from the bifurcation of the basilar artery at the superior border of the pons, posterior to the dorsum sellae. Soon after its origin, the posterior cerebral artery continues laterally along the superior border of the pons, surrounds the inferior aspect of the cerebral peduncles, and runs posteriorly along the lateral parts of the transverse fissure (of Bichat) to reach the medial surface of the cerebral hemisphere, where it supplies the temporal and occipital lobes. The posterior cerebral artery is divided into four segments:
The precommunicating segment (P 1 ) extends from the termination of the basilar artery to the posterior communicating artery, within the interpeduncular cistern.
The postcommunicating segment (P 2 ) extends from the posterior communicating artery around the midbrain and terminates as it enters the quadrigeminal cistern.
The quadrigeminal segment (P 3 ) courses posteromedially through the quadrigeminal cistern and terminates as it enters sulci of the occipital lobe.
The cortical segment (P 4 ) runs within the sulci of the occipital lobe (calcarine artery, within the calcarine fissure).
The posterior cerebral artery gives off branches that are categorized as either central or cortical. The central branches supply the subcortical structures and include the thalamoperforating, thalamogeniculate, and posterior choroidal arteries. The cortical branches are distributed to different parts of the cortex, are named accordingly, and involve the temporal, occipital, parietooccipital, and calcarine arteries. The posterior cerebral artery curls around the cerebral peduncle and passes above the tentorium to supply the posteromedial surface of the temporal lobe and the occipital lobe. The visual cortex responsible for the contralateral field of vision lies in its territory. The macular part of the visual cortex often receives blood supply from both the posterior cerebral artery and the middle cerebral artery.
The internal carotid artery is responsible for supplying a large portion of the anterior and middle parts of the brain. A new classification system divides the internal carotid artery into four parts: cervical in the neck, petrous at the base of the skull, cavernous within the cavernous sinus, and intracranial above the cavernous sinus. The two internal carotid arteries, in their last or intracranial segment, after supplying the ophthalmic artery, each resolve into a tuft of four divergent branches: the anterior cerebral artery, the middle cerebral artery, the anterior choroidal artery, and the posterior communicating artery.
The anterior cerebral artery is a much smaller branch of the internal carotid artery (compared to the middle cerebral artery). It begins at the terminal segment of the internal carotid artery (after the ophthalmic branch is given off) on the medial part of the sylvian fissure. It runs anteriorly and medially toward the interhemispheric fissure, providing in this initial course a few small branches to the orbitofrontal cortex. Shortly after its origin, the anterior cerebral artery connects with its contralateral side via a 1- to 3-mm-long transverse anastomosis, the anterior communicating artery. The paired arteries then travel through the longitudinal cerebral fissure in a posterior direction along the genu of the corpus callosum, where they divide into its two major branches: the pericallosal and callosomarginal arteries. The anterior cerebral artery is also subdivided for clinical purposes into five segments:
The horizontal or precommunicating segment (A 1 ) extends from the terminal bifurcation of the internal carotid artery to the anterior communicating artery.
The vertical, postcommunicating, or infracallosal segment (A 2 ) originates at the anterior communicating artery, extends anteriorly to the lamina terminalis and along the rostrum of the corpus callosum, and terminates either at the genu of the corpus callosum or at the origin of the callosomarginal artery.
The precallosal segment (A 3 ) extends around the genu of the corpus callosum or distal to the origin of the callosomarginal artery and terminates where the artery turns directly posterior above the corpus callosum.
The supracallosal segment (A 4 ) courses above the body of the corpus callosum anterior to the plane of the coronal suture.
The postcallosal segment (A 5 ) courses above the body of the corpus callosum posterior to the plane of the coronal suture.
The anterior cerebral artery also gives off central and cortical branches. The cortical branches, which are named for the regions they supply, are responsible for the areas of somatosensory and motor cortex related to the lower limbs. Parietal branches perfuse the precuneus while the orbital branches supply the frontal lobe, olfactory cortex, medial orbital gyrus, and gyrus rectus. The frontal arteries supply the paracentral lobule, medial frontal and cingulate gyri, and the corpus callosum. The central branches are given off proximally (A 1 , anterior communicating artery, and proximal A 2 ) and supply the anterior perforated substance, the lamina terminalis, the rostrum of the corpus callosum, the septum pellucidum, the anterior part of the putamen, the head of the caudate nucleus, and the anteromedial part of the anterior limb of the internal capsule.
The anterior communicating artery has several anteromedial central arteries, which are responsible for supplying the cingulate gyrus, the anterior columns of the fornix, the hypothalamus lamina terminalis, the optic chiasm, and the paraolfactory regions.
The middle cerebral artery is the largest terminal branch of the internal carotid artery. It travels in the sylvian fissure and after a short course reaches the insula, where it bifurcates into the superior and inferior trunks. The trunks travel together through the sylvian fissure in a posterosuperior direction and reach its posterior end toward the lateral surface of the brain. The middle cerebral artery can be subdivided into four parts:
The sphenoid segment (M 1 ) extends from the termination of the internal carotid artery to the bifurcation or sometimes trifurcation of the middle cerebral artery.
The insular segment (M 2 ) travels posterosuperiorly in the insular cleft and terminates at the circular sulcus in the sylvian fissure.
The opercular segment (M 3 ) courses laterally along the frontoparietal operculum and terminates at the external/superior surface of the sylvian fissure.
The cortical segment (M 4 ) emerges through the lateral fissure to reach the surface of the brain.
The middle cerebral artery also gives off central and cortical branches. They supply most of the lateral surface of the hemisphere, with the exceptions being the superior portion of the parietal lobe (which is supplied by the anterior cerebral artery) and the occipital lobe and inferior portion of the temporal lobe (which are supplied by the posterior cerebral artery). In addition, the central and cortical branches of the middle cerebral artery supply part of the internal capsule and basal ganglia. The numerous central branches are also called striate or lateral lenticulostriate arteries and arise from the M 1 and M 2 segments within the sylvian fissure. Their main function is to supply the deep structures of the brain. They supply the basal ganglia (i.e., the striatum), much of the head and body of the caudate nucleus, and large portions of the lenticular nucleus and of the external and internal capsules.
The cortical branches of the middle cerebral artery arise from all of its segments. They supply most of the lateral surface of the brain (i.e., the orbital, frontal, parietal, and temporal parts of the cerebral cortex) and are named according to the region of the brain that they supply. The anterior temporal arteries vascularize the temporal pole of the brain, which is the most anterior aspect of the temporal lobe. The lateral frontobasal artery supplies the lateral part of the orbital surface of the frontal lobe as well as the inferior frontal gyrus. The artery of the prefrontal sulcus supplies the anterior aspects of the inferior and middle frontal gyri. The artery of the precentral sulcus supplies the posterior aspect of the inferior and middle frontal gyri, Broca’s area, and the precentral gyrus. The artery of the central sulcus travels within the central sulcus and contributes to the blood supply of the pre- and postcentral gyri. The artery of the postcentral sulcus supplies the anterior aspect of parietal lobe and the postcentral gyrus, which contains the primary somatosensory cortex for the head, upper limbs, and trunk. The angular artery supplies the angular and supramarginal gyri of the parietal lobe, the posterior part of the superior temporal gyrus, and the superior part of the lateral surface of the occipital lobe. The middle temporal branches supply the middle aspect of the superior and middle temporal gyri, as well as the primary auditory cortex and Wernicke’s area.
The anterior choroidal artery is divided into two segments: cisternal and intraventricular. It originates from the posterior wall of the internal carotid artery between the origin of the posterior communicating artery and the internal carotid termination. After reaching the lateral geniculate body, it traverses in the posterolateral direction above the uncus to enter the choroidal fissure, at the so-called plexal point, where it becomes intraventricular (intraventricular segment). It then continues superiorly around the thalamus, until the interventricular foramen (foramen of Monro). The anterior choroidal artery finishes its course at this point by anastomosing with the medial posterior choroidal branch of the posterior cerebral artery. It supplies several subcortical structures (limbic system, basal ganglia, diencephalon), the midbrain, the temporal lobe, and the visual pathway. Therefore these structures will be the main ones affected by an anterior choroidal artery stroke.
The posterior communicating artery originates from the posterior aspect of the communicating segment of the internal carotid artery and extends posteromedially to anastomose with the ipsilateral posterior cerebral artery and form part of the circle of Willis connecting the anterior or carotid system and the posterior or vertebral system to each other. The posterior communicating artery gives off many fine, scarcely visible, perforating branches. The largest perforating branch is called the premammillary (or anterior thalamoperforating) artery. Perforators from the posterior communicating artery supply the posterior part of the optic chiasm and optic tract, the posterior part of the hypothalamus and mammillary bodies, and part of the thalamus.
The middle cerebral arteries, the anterior cerebral arteries, and the anterior communicating artery form the anterior cerebral circulation. The vertebral arteries, basilar artery, and posterior cerebral arteries, together with the posterior communicating artery, form the posterior cerebral circulation. Through various anastomoses, the anterior and posterior arterial circuits of the brain unite in a completely closed arterial system at the base of the brain. This area is known as the circle of Willis, although it is actually a heptagon because it has seven sides. It is formed anteriorly by the two anterior cerebral arteries, connected to each other by the anterior communicating artery; posteriorly by the two posterior cerebral arteries; and on the sides by the two posterior or lateral communicating arteries (see Fig. 1.3 C). The main function of the circle of Willis is to provide collateral blood flow between the anterior and posterior arterial systems of the brain. Additionally, it offers alternative blood flow pathways between the right and left cerebral hemispheres. This system ensures optimal vascularization of the brain. A single trunk would have been sufficient in the absence of pathology, but instead, there are four trunks connected to each other by short—and generally very wide—anastomoses. The anastomoses provide alternative routes for blood flow in the event of vascular occlusion. Because the carotid and vertebrobasilar arteries form a circle, if one of the main arteries is occluded, the distal smaller arteries that it supplies can receive blood from the other arteries (collateral circulation).
It is thus understandable how one of these trunks can become occluded by an embolus or be suppressed by a ligature without always necessarily resulting in neurological dysfunction. Additionally, the circle is believed to function as a pressure relief system to accommodate increased blood flow in instances of raised intracranial pressure. Because of the combination of the pattern of collateral circulation that accompanies iAVMs and the compensatory circulation of the circle of Willis, some patients may have complete occlusion of certain segments of the circle of Willis and adjacent major branches without suffering distal infarction in the cerebral hemisphere or in the critical areas occupied by the AVM itself.
In many patients with complex, deep AVMs in the lenticulostriate territory, both anterior cerebral arteries fill from the contralateral internal carotid artery, as seen angiographically, and the shunting of blood into the deeper portions of the hemisphere is accompanied by extensive development of cortical anastomotic arteries joining the anterior, middle, and posterior cerebral arterial territories. These vascular features create the possibility of occluding the major arterial trunks at certain segments of the circle of Willis without significantly reducing the distal hemispheric circulation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here