Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiac and vascular gross and microscopic anatomy has been appreciated for many years. Knowledge of ultrastructural anatomy and physiology followed. Disease was noted long before pathogenesis was clarified and it is humbling to realize that this is still so. We continue to learn about both normal and abnormal heart function.
Cardiac specimens were originally postmortem in nature. Surgical procedures on the heart are relatively new in the history of medicine. The pathologist (and surgeon, radiologist, and interventionalist) must understand normal cardiac anatomy before they can appreciate pathology. One is also faced with a rapidly changing specialty of our peers. New procedures are developed, structures are repaired rather than replaced, and there is a plethora of interventions and devices used for treatment. Molecular biology and personalized genomics are rapidly evolving, changing the landscape of both cardiology and medicine. Imaging has also evolved to the point where some even postulate it may replace, or at least complement, traditional pathology examination. Specimens are smaller and expectations are higher. Faced with this growing body of knowledge, the pathologist has become the data integrator, interpreting the clinical information, imaging, and pathological findings to make a clinically relevant diagnosis.
This chapter attempts to give some prerequisite anatomic information useful for diagnosis. Appreciation of gross and microscopic anatomy allows the pathologist to understand the range of normal findings. Herein, we outline recommendations for gross heart examination, provide some general suggestions for surgical specimen triage and examination, and give an in-depth overview of normal light and electron microscopic features of the heart and blood vessels.
The pathologist must dissect the heart so that it best exhibits alterations of cardiac structure brought on by disease. Numerous preparation and dissection methods are available and the prosector must choose the best method for a particular heart ( Table 2.1 ). Understanding of the clinical picture is requisite for planning the approach to dissection and evaluation. Regardless of the method chosen, a systematic approach to the examination of cardiac specimens is necessary. In addition to the gross examination, a comprehensive diagnosis is usually formulated in conjunction with well-chosen histologic, ultrastructural, and histochemical/immunohistochemical studies. Moreover, samples may also be procured for microbiologic, serologic, biochemical, or molecular and genetic studies. Even if there has been no indication of suspected heart disease, forethought on the part of the pathologist and his or her team may prompt appropriate sampling of tissues that can be used for future diagnostic or research studies.
| Preparation technique | Indication |
|---|---|
| Perfusion fixation | Photographic documentation, creation of teaching specimens, general cardiac evaluation |
| Radiography |
|
| Angiography | Localization and quantitation of coronary artery disease |
| Decalcification | Evaluation of calcified tissue |
| Stent dissolution | Evaluation of stented segment of vessel |
| Dissection technique | Indication |
| Short-axis with inflow–outflow | Routine evaluation |
| Tomographic planes | |
| Left ventricular long-axis | Assessment of the left ventricle outflow tract (hypertrophic cardiomyopathy, chronic aortic stenosis) |
| 4-Chamber |
|
| Base of heart |
|
| Right ventricular long-axis |
|
| Inflow-outflow | Congenital heart disease |
Ultimately, every member of the cardiac team studies the cardiac cell and its adaptation, injury, or death. The pathologist is the team member best acquainted with cellular morphology and best trained to interpret the cause and mechanism of disease at the cellular and molecular level. In the heart, more than most other organs, cellular adaptations are attended by highly predictable gross and histologic alterations. This allows the pathologist to add substantially to the overall understanding of the disease and its course.
The heart and great vessels are situated in the midthorax within the mediastinum. The mediastinum is divided into four specific regions: anterior, middle, posterior, and superior. The heart is located within the middle mediastinum, the aortic arch within the superior mediastinum and the descending thoracic aorta in the posterior mediastinum. Anteriorly, it is overlaid on either side by the lungs and pleura, which also cover the lateral borders of the heart. The cardiac apex is normally directed leftward, anteriorly, and inferiorly.
Both the location and orientation of the heart within the chest can be affected by underlying cardiac malformations and/or abnormalities of adjacent structures. Terms such as levocardia, dextrocardia, and mesocardia are commonly employed to describe position, but are not specific to which point they are addressing. Therefore with respect to position in the chest, heart location and apical direction should be described independently to avoid any ambiguity. Description terminology such as left-sided (normal), right-sided, or midline can be employed for cardiac location. Likewise, apical direction can be described as leftward (normal), rightward, or midline.
Beginning its embryologic development as bilateral structures that fuse to form a single midline structure, the cardiovascular system later acquires asymmetry and is thereby characterized by sidedness (situs or handedness) . Sidedness is genetically determined and may be normal, mirror-image, isomeric, or indeterminate. While abnormalities of sidedness may affect the entire body (as in total mirror-image sidedness or situs inversus totalis), they can also involve individual organ systems. Therefore sidedness of individual organ systems should be independently described.
Cardiac sidedness is determined by the position of the morphologic right atrium. It is not determined by the apical direction, cardiac position, or sidedness of the noncardiac viscera. Therefore accurate distinction between left and right atrial morphology is vital (see below). The right atrium is normally right-sided (situs solitus), but is left-sided in mirror-image heart (situs inversus). Bilateral right atria define right cardiac isomerism and bilateral left atria connote left cardiac isomerism (both considered forms of situs ambiguous). In circumstances where atrial morphology cannot be determined, the term indeterminate sidedness may be employed.
The pericardium is a roughly conical structure that both covers and surrounds the heart as the serous visceral and fibrous parietal pericardia, respectively ( Fig. 2.1A ). Between these two layers, in a space called the pericardial sac, is contained 15–50 mL of straw-colored, serous fluid .
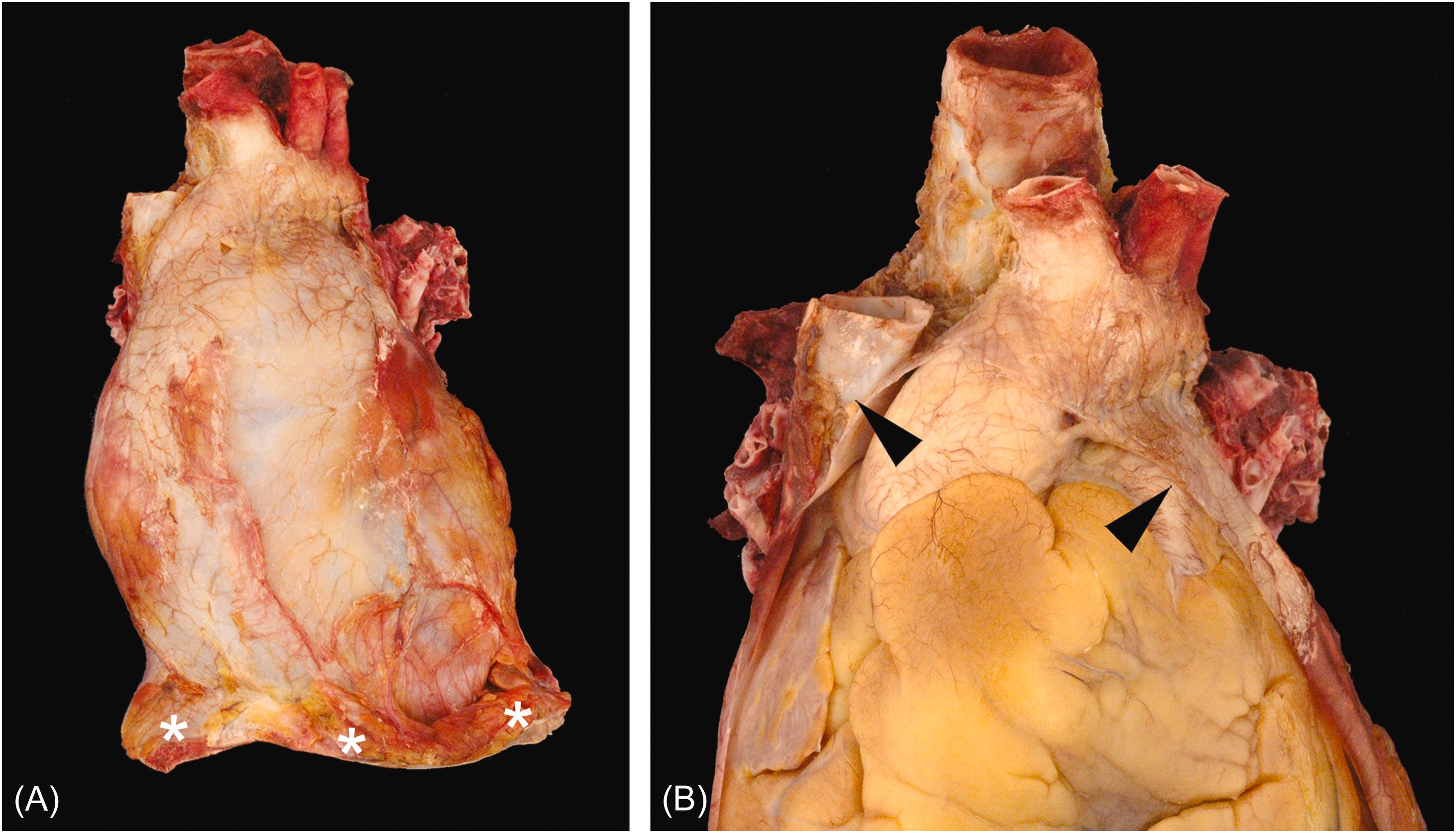
The parietal pericardium attaches along the great vessels such that the ascending aorta, main pulmonary artery, terminal superior vena cava, and terminal pulmonary veins are intrapericardial ( Fig. 2.1B ). The pericardium is attached to the central tendon of the diaphragm and the adjacent diaphragmatic muscle at its base. The parietal pericardium consists of an outer fibrous layer with an inner layer of mesothelial cells. Its outer surface also contains variable amounts of adipose tissue that may contribute to the cardiac silhouette radiologically. The dense collagen limits the acute distensibility of the parietal pericardium such that as little as 200 mL of rapidly accumulated fluid can compromise diastolic filling (cardiac tamponade) (see Chapter 15 : The Pericardium and its diseases).
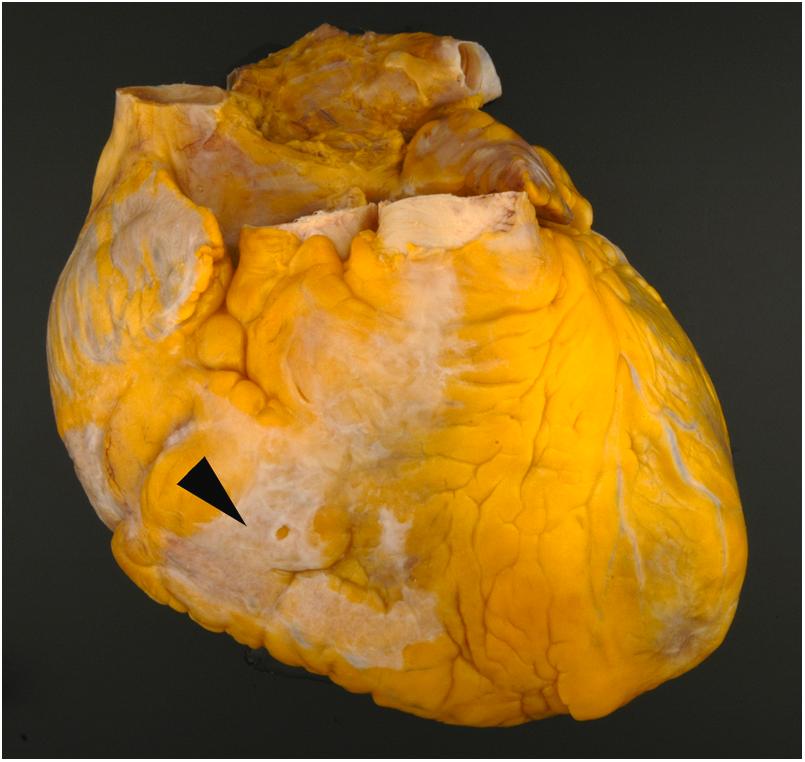
The visceral pericardium (also called the epicardium) consists of a lining of mesothelial cells with variable deposits of subjacent adipose tissue. Typically, the visceral pericardium is transparent with the subjacent structures readily visible; however, focal pale white thickening can be encountered in many structurally normal specimens. Often referred to as “soldier’s plaques,” the incidence of these epicardial collagen plaques rises with age and may represent healed pericarditis ( Fig. 2.2 ) . The fatty deposits are most pronounced in the region of the atrioventricular, interventricular, and interatrial grooves. Prominent fat tags also cover the origins of the coronary arteries between the aorta and atrial appendages.
Embryologically, rapid bulboventricular growth pushes the developing heart into the pericardial space analogous to a fluid-filled balloon with a fist being pressed into it. The parietal and visceral pericardia meet along the great vessels at the pericardial reflection. Likewise, the pericardium reflects around the region of the great veins, forming the oblique pericardial sinus immediately posterior to the left atrium. A tunnel-shaped reflection is also formed by the great arteries and the atrial walls, called the transverse pericardial sinus. The appreciation of these sinuses is increasingly important with catheter-based intrapericardial procedures being developed.
If there is no reason to suspect a major cardiac malformation after careful external assessment in situ (see below), the heart is best examined after its removal from the chest or from the thoracic organ block. The dissection method below describes removal from the thoracic organ block after it has been removed from the chest cavity. If indicated, and before proceeding, a pericardial fluid sample can be obtained by needle puncture through an area of the pericardium that has been seared for sterilization. The anterior parietal pericardium is then incised in situ and the pericardial contents are noted; 15–50 mL of clear, straw-colored fluid is a normal finding .
The parietal and visceral pericardial serosal membranes are inspected; their surface is normally smooth and shiny. Papillary fibrous tags, which are of no significance, may be observed on the visceral pericardium covering the atrial appendages . The vascular connections of the heart are defined in situ, including the ligamentum arteriosum that joins the origin of the left main pulmonary artery to the aortic arch, in case there is a patent ductus arteriosus. A persistent left superior vena cava, seen externally to join the left atrium, is sometimes found in an otherwise normal cardiovascular system; it drains into the coronary sinus, which, as a result, has a prominent and enlarged ostium in the right atrium. Otherwise, its remnant, the ligament of Marshall, can be identified at the left edge of the transverse pericardial sinus. The azygos and hemiazygos veins are best viewed in situ while they are still filled with blood.
The main pulmonary artery is then incised and opened, and its lumen and those of its right and left branches are explored by digital probing for thrombus, embolus, or other pathology. Similarly, the superior vena cava is transected approximately 1 cm above its connection to the right atrium and similarly explored. Turning to the posterior surface of the thoracic block, the posterior parietal pericardium is incised at the midline, inferior to superior, to the level of the carina. A note can be made of the situs and appearance of the bronchi. Reflecting the left and right halves of the posterior parietal pericardium to their respective sides will reveal the pulmonary veins, usually two on each side (an upper and a lower). These can be traced out and transected approximately 1 cm from their connection to the left atrium. The aorta and the pulmonary artery are then transected 0.5–1 cm distal to their valve commissures. At this point, the heart will be held to the thoracic block by the dorsal mesocardium, which can be cut along the inferior edge of the pulmonary arteries.
The amount of epicardial fat should be gauged at this stage. Normally, it fills the atrioventricular and interventricular grooves, encasing the epicardial coronary arteries. When fat is increased, it may blanket the epicardial surface of all chambers, especially along the course of coronary blood vessels; in extreme cases, more than half of the heart weight may be composed of fat . Epicardial fat does tend to positively correlate with visceral adiposity, in general . Fat also extends anterior to the lung hila and spreads into the myocardium between the myocytes and along the intramyocardial vessels, particularly in the right ventricle and atrial septum . Grossly, in a “fatty heart” (also called pathologic adiposity), typically only the outer half or two-thirds of the myocardium contains adipocytes. This contrast with the pattern is seen in arrhythmogenic cardiomyopathy (see Chapter. 10, Chapter. 11 ).
The right ventricle forms the anterior border of the heart, being located behind the sternum ( Fig. 2.3 ). The right atrium forms the right lateral border of the heart, receiving both the superior and inferior vena cavae. The left ventricle forms the posterior, inferior, and left lateral surfaces of the heart, with the left atrium occupying a midline-posterior position. Most commonly, four pulmonary veins will drain back to connect to the left atrium (two from the left lung and two from the right lung).
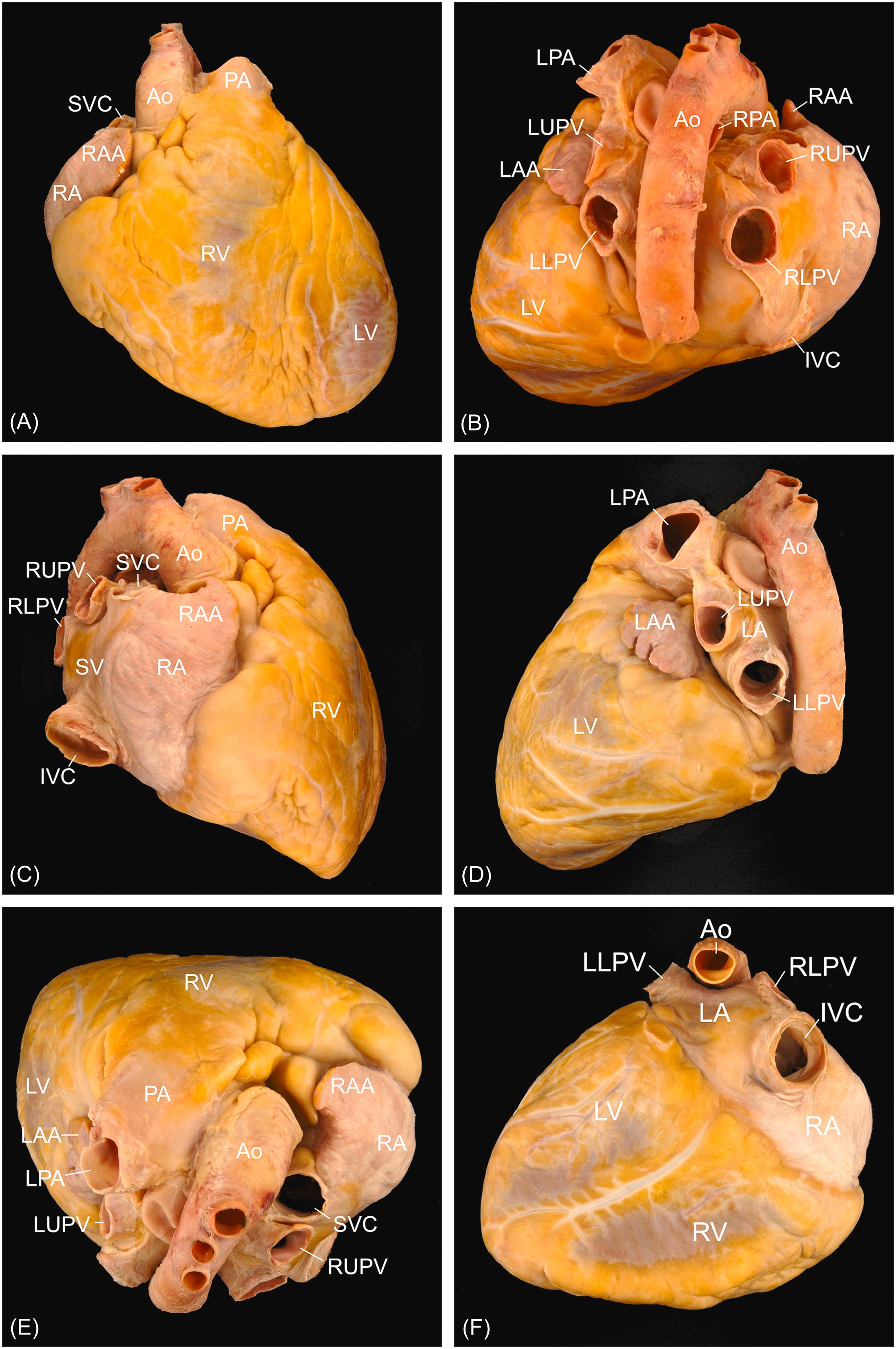
The relationship between the great vessels and the investing pericardium is best understood when their roots are viewed after removal of the heart ( Fig. 2.4 ). An investment of serous pericardium encloses the great arteries, and a second contains all the veins. Thus the pericardium encloses the distal half of the superior vena cava, most of the ascending aorta, and pulmonary trunk. Compromise of any portion of the vascular wall, within the encased area, may result in hemopericardium.
The main pulmonary trunk/artery arises from the right ventricle and travels to the left of the ascending aorta, directed toward the left shoulder. At its bifurcation, the left pulmonary artery continues over the left bronchus, whereas the right pulmonary artery courses beneath the aortic arch and posterior to the superior vena cava.
The aorta arises at the level of the aortic valve annulus and terminates at its bifurcation into the common iliac arteries. It can be divided into four distinct regions: the ascending aorta (beginning at the aortic annulus and extending to the origin of the brachiocephalic artery), aortic arch (extending to the ligamentum arteriosum), descending thoracic aorta (extending to the diaphragm), and the abdominal aorta (extending to the iliac bifurcation). The ascending aorta includes both a bulbous sinus portion at its proximal end and a more tubular portion extending toward the arch. The regions of the aorta are differentiated not only by their location but also by their embryologic derivation. The aortic root is derived from the second heart field (lateral plate mesoderm), the ascending aorta and arch from the neural crest, and the descending aorta from the paraxial mesoderm .
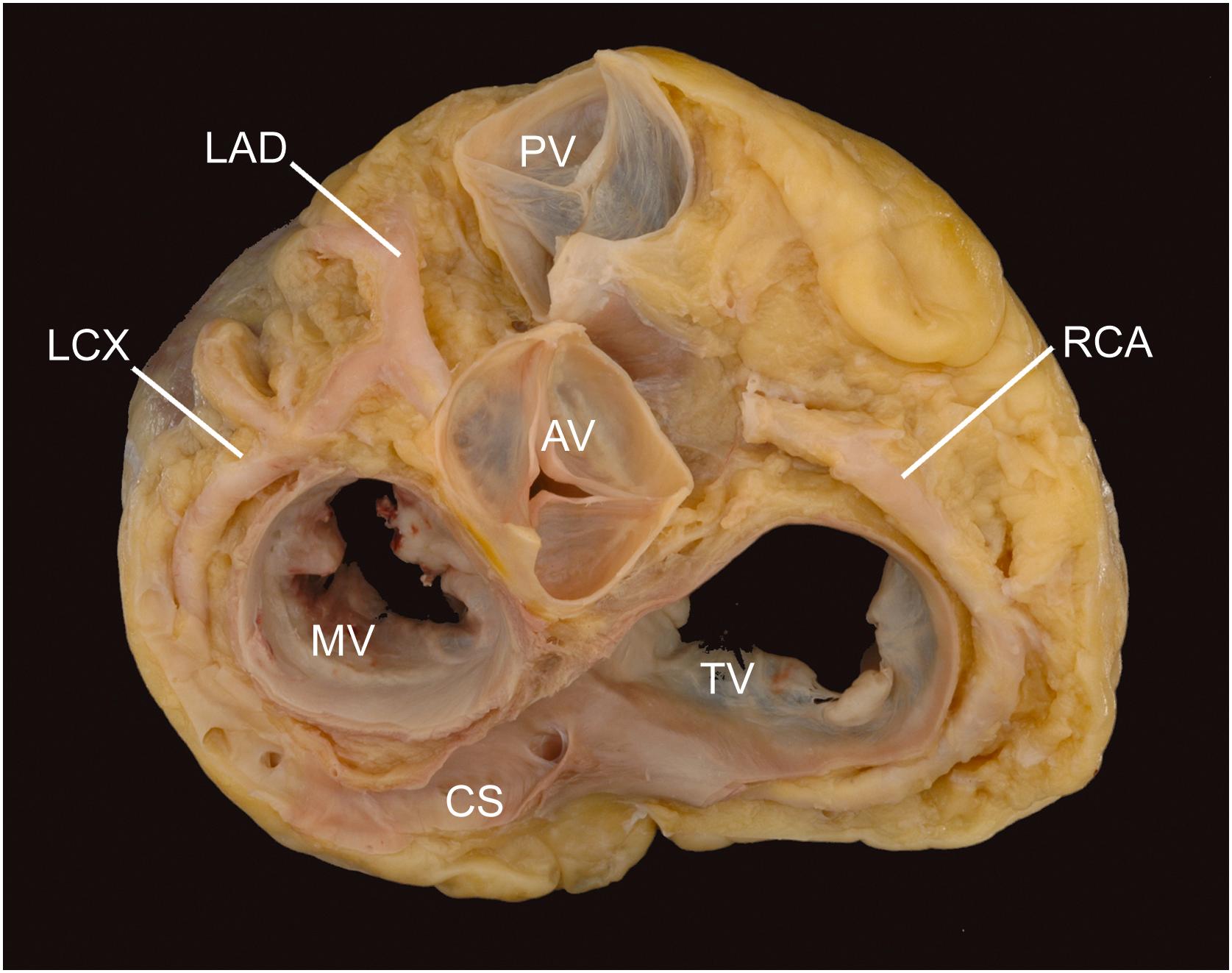
The epicardial coronary arteries are examined before incising the heart chambers. The right and left coronary arteries arise from their respective aortic sinuses (of Valsalva) ( Fig. 2.4 ). Their ostia arise midway between the aortic valve commissures, just below the sinotubular junction (the ridge within the ascending aorta formed by the virtual ring connecting the tips of the aortic valve commissures). The right coronary artery (RCA) usually arises perpendicular to the aortic sinus, whereas the left main coronary artery will often arise at an acute angle and travel downward, before branching to become the left anterior descending (LAD) and circumflex (LCX) coronary arteries. The LCX and RCA travel in their respective atrioventricular grooves, defining the plane of the cardiac base. The anterior and posterior descending arteries travel in their respective interventricular grooves and will indicate the plane of the ventricular septum. The RCA will supply the posterior descending coronary artery in 70% of individuals, so-called right-sided coronary dominance . The LCX will supply the posterior descending coronary artery in 10% of individuals (left-sided coronary dominance), with the remaining individuals (20%) having shared coronary dominance (codominance).
Epicardial branches include diagonals arising from the LAD and marginals arising from the RCA and LCX (acute and obtuse, respectively). In 60% of individuals, the first branch of the RCA is the conus coronary artery, which supplies the right ventricular outflow tract (conus or infundibulum); in the remaining 40%, the artery arises independently from the right aortic sinus, when a separate ostium will be noted . The sinoatrial nodal artery arises from the RCA in approximately 60% of hearts and from the LCX in the balance. The aortic valve (AV) nodal artery, in contrast, will arise from the dominant coronary artery, with the RCA supplying it in approximately 90% of hearts.
If the prosector has ready access to postmortem radiography, dissection can be complemented by postmortem angiography, which can indicate the presence of calcific coronary artery disease as well as assess the disease burden. Otherwise, palpation of the coronary arteries should be performed to evaluate for calcification. If identified, the entire epicardial arterial tree must be dissected from the heart and decalcified ( Table 2.1 ). Each artery should be removed and placed in a separate container. Decalcification prevents artifactual damage caused by cutting through a calcified arterial wall, crushing the lumen and plaque disruption. Performing decalcification is the best way to prepare calcified coronary arterial tissue for histologic examination. In the absence of any suspicion of coronary artery disease, especially if the patient is young and vessels are apparently noncalcified, they may be opened in situ, by cross sectioning at 0.2–0.5 cm intervals. Opening the vessels longitudinally is not optimal as it becomes difficult to assess disease severity and luminal narrowing. Diseased areas should be submitted for histology (4–6 cross sections per cassette), to include both hematoxylin-eosin staining and an elastic stain (e.g., Verhoeff-van Gieson, Movat pentachrome, etc.). Dividing each of the coronary arteries into thirds (proximal, middle, and distal) and designating as such can facilitate interpretation and reporting.
The presence of coronary artery stents presents a unique challenge in the evaluation of coronary artery disease. Methods that involve cutting the stented vessel with a diamond microtome, electrolytic stent dissolution, and physical stent removal have all been described . Regardless of the method chosen, effort should be made to evaluate these regions of the vessel to evaluate for disease progression as well as device failure.
Coronary veins follow, in an antiparallel fashion, the course of the arteries. They are more superficial in the epicardial fat and eventually join the coronary sinus, which passes from left to right in the posterior atrioventricular groove to open into the posterior wall of the right atrium just anterior to the inferior vena cava. Some small (accessory) coronary veins drain the free wall of the right ventricle and open directly into the anterior wall of the right atrium. The smallest veins, Thebesian or luminal veins, form within the myocardium and drain directly into each of the heart chambers . They are remnants of sinusoidal intramyocardial blood vessels present in the embryonic heart, at the stage of the noncompacted (spongy) myocardium.
The coronary sinus ( Fig. 2.4 ) is the most easily evaluated cardiac vein and is guarded by the valve of the coronary sinus (Thebesian valve) at its opening into the right atrium. It may be larger than normal if it drains anomalous vessels (e.g., a persistent left superior vena cava via an oblique vein of Marshall or vessels from the lung—anomalous pulmonary venous drainage) or in the presence of severe right heart failure. Alternatively, the coronary sinus opening may be smaller than normal or even atretic if coronary sinus blood is passing from the heart through a persistent left superior vena cava to the innominate vein and, hence, via a right superior vena cava to the right atrium. The coronary sinus may be absent, as in those conditions in which a left superior vena cava connects directly to the left atrium (an unroofed sinus). Such variations in the size of the coronary sinus may be isolated and incidental, but they often accompany serious cardiac malformations.
A cardiac lymphatic system has been well described . Lymphatic plexi within the subendocardium, myocardium, subepicardium, and pericardium have all been demonstrated. When engorged, these can grossly manifest as white streaking in the heart.
Following external examination, the heart should be opened to evaluate the internal cardiac anatomy. A variety of dissection methods are available and the prosector should be guided by the clinical circumstances and the gross findings of the external cardiac examination when choosing which will be employed ( Table 2.1 ). The method described below is an excellent general dissection technique that can be used for both normal and abnormal cardiac specimens after external examination and evaluation of the epicardial coronary arteries. Additionally, it leaves the venous connections intact and does not disrupt the major conduction system components (sinoatrial node, atrioventricular node, bundle of His, and proximal bundle branches).
A long blade is used to cut along the short axis at 1 cm intervals from the apex to the midventricular level, leaving the tops of the papillary muscles still attached to the tendinous cords. The sections are consecutively laid out (apical surfaces up) and the myocardium is evaluated for mural thickness and general uniformity. Any lesion should be documented, both in size and location. With respect to location, the chamber (left ventricle, right ventricle, or ventricular septum), level (apical or midventricular), and anatomic direction (anterior, inferior, or lateral) should be noted.
Attention can then be turned to the cardiac base. Starting at the right atrium, using scissors, a cut can be made approximately 1 cm anterior to the orifice of the inferior vena cava, just proximal to the tricuspid valve annulus. This cut is then extended superiorly to the tip of the right atrial appendage. The right atrial free wall can then be reflected back, and the chamber can be inspected. Assessment for a patent foramen ovale can be done at this time as well. Incising between the superior and inferior vena cava, while advocated by some, is discouraged primarily because it may disrupt the sinoatrial node as well as a Chiari network (if present).
Moving downward to the right ventricle, a cut can be made through the inflow portion of the ventricle approximately 1 cm away from the ventricular septum, through the posterior tricuspid valve leaflet. The outflow tract of the right ventricle can be opened by cutting along the anterior portion of the ventricular septum, through the infundibulum and out the pulmonary valve. Generally, the moderator band (trabeculum septomarginalis) will be cut at this point. Staying along the ventricular septum will usually direct the cut to the commissure between the anterior and left pulmonary valve cusps.
The left atrium is best approached from the posterior aspect. A vertical cut, using scissors, is made between the left- and right-sided pulmonary veins. The cut is extended superiorly to the top of the atrial dome and then inferior toward the mitral valve annulus. At the level of the mitral annulus, the incision is extended leftward around the left atrium, parallel to the left atrioventricular groove, to the tip of the left atrial appendage. Evaluation for mural thrombus is important, as the left atrial appendage is a common site to find such .
The inflow portion of the left ventricle is incised by positioning a long blade down through the mitral valve (sharp end toward the free wall) in the middle of the anterior leaflet. The free wall is then cut (at the midportion of the P2 segment of the posterior mitral valve leaflet). Cutting the lateral left ventricular wall between the papillary muscles may also be done. Opening the left ventricular outflow tract is best done, using either scissors or a long blade, from the ventricle out to the aorta. Staying within 1 cm of the ventricular septum will help ensure that the anterior mitral valve leaflet is not inadvertently damaged in the process. This final cut will usually cut through the left aortic valve cusp.
The right atrium serves as the receiving chamber for blood returning from the systemic veins and lies posterior to the right ventricle and anterior to the left atrium in a right lateral position ( Fig. 2.5 ) . Its basic anatomic components are that of the free wall and the septum. It has variable thickness, measuring 2–6 mm in some regions and <1 mm in others.
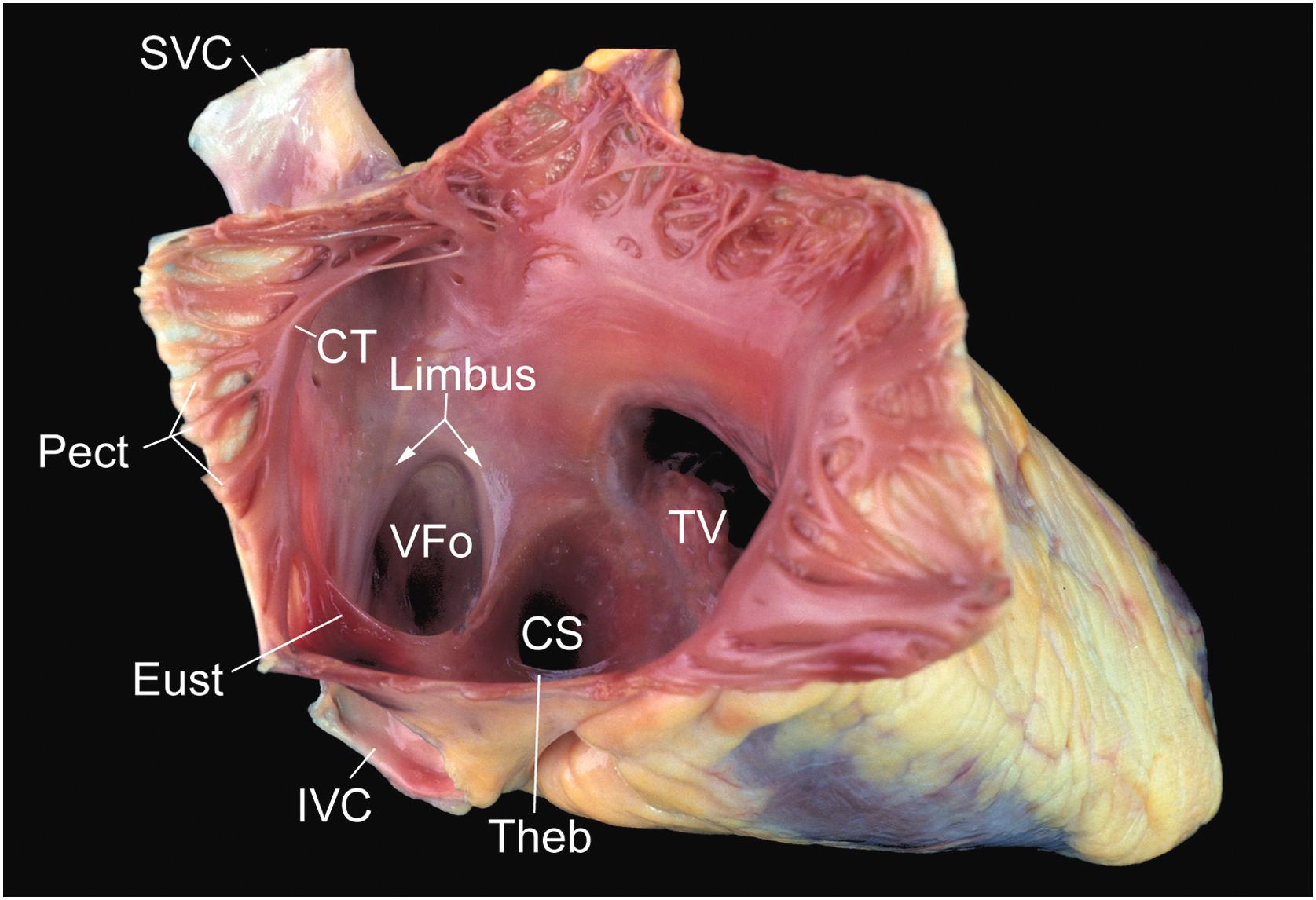
The free wall, internally, has a smooth-walled posterior portion, derived from the embryological sinus venosus that receives blood from the vena cavae and the coronary sinus. The remaining free wall is derived from the primitive atrium and contains prominent small muscular ridges arranged in parallel, called pectinate muscles. The crista terminalis, corresponding internally to the external sulcus terminalis, serves as the dividing line between these two portions.
Pectinate muscles arise from the crista terminalis, which is a prominent C-shaped ridge of muscle that separates the smooth-walled portion of the atrium from the trabeculated portion. The inferior aspect of the right atrium, between the ostium of the coronary sinus, posteriorly, and the tricuspid annulus, anteriorly, is a region referred to as the cavotricuspid isthmus. It is in this region of the heart where atrial flutter is frequently thought to arise . The midportion of the isthmus containing a shallow pouch with trabeculated lining is called the sub-Eustachian sinus of Keith .
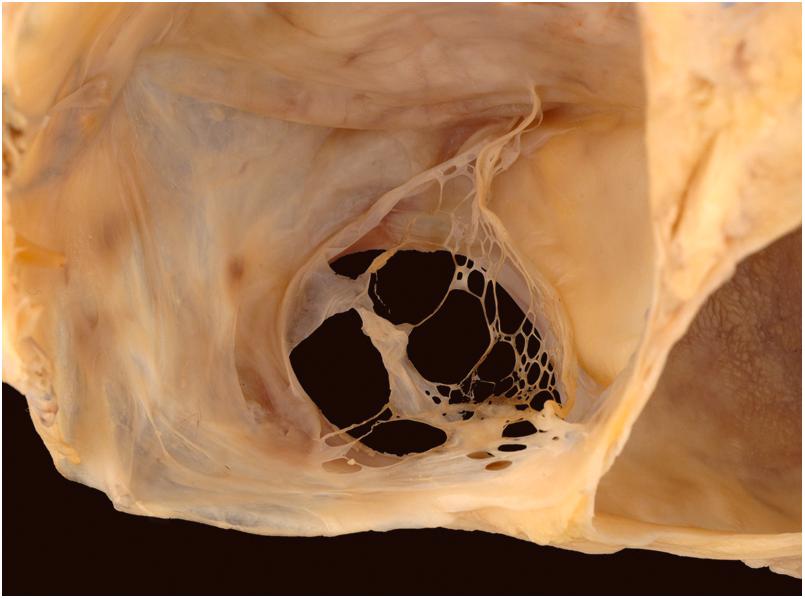
Three distinct valves can be visualized inside the right atrium: the valve of the inferior vena cava (Eustachian), the valve of the coronary sinus (Thebesian), and the valve of the fossa ovalis. The first two valves, remnants of the right venous valve, vary greatly in size and may be fenestrated or absent, but the Eustachian valve is well developed in approximately 90% of normally formed hearts . The Thebesian valve is generally less well developed, covering the ostium of the coronary sinus in approximately 40% of subjects with normal heart weight . A Chiari network, usually a web or lace-like sheet of tissue, may span the atrial cavity from the region of the Thebesian or the Eustachian valve to insert into the crista terminalis ( Fig. 2.6 ) . The left venous valve is usually absorbed into the atrial septum, but remnants may be found as bands of tissue bridging the posteroinferior margin of the fossa ovalis.
The most prominent feature of the septal portion of the right atrium is that of the fossa ovalis ( Fig. 2.5 ). This structure can be as large as 3.5 cm in diameter and is usually well defined superiorly and anteriorly by the raised limbus of the fossa ovalis . The horseshoe-shaped limbus surrounds a relatively thin membrane, the valve of the fossa ovalis. This valve is thin and translucent early in life, but progressively thickens throughout life . Probing under the limbus anteriorly shows whether the foramen ovale is obliterated or patent; the latter may be seen in a third of people beyond the first year of life .
An aneurysmal bulge of the septum primum may occur through the fossa ovalis into either atrium . The association in adults of aneurysms of the fossa ovalis with increased frequency of embolic stroke has been attributed to its significant association with mitral valve prolapse, dilated atria, intracardiac thrombi, and patent foramen ovale; the latter condition is present in 70% of such subjects . An aneurysm of the fossa ovalis bulging to the left atrium is also seen during fetal life and is associated with fetal arrhythmia and other causes of fetal heart failure .
By transillumination, part of the membranous interventricular septum is seen in the right atrium just proximal to the tricuspid annulus where it joins the base of the septal leaflet of the tricuspid valve. A defect in this area permits communication between the left ventricle and the right atrium, whereas a defect distal to the septal leaflet is a membranous ventricular septal defect at its most common location. Usually, the anterior and septal leaflets of the tricuspid valve cross the membrane to meet at their commissure. Rarely, they insert at the edge of the membrane without forming a commissure. This does not produce valvular regurgitation. The right aortic sinus is also closely related to the right atrium and may cause a smooth protuberance of the right side of the anterosuperior atrial septum, the torus aorticus . The interventionalist should be mindful of this anatomic relationship during procedures such as transseptal puncture or placement of a septal occluder device.
The tendon of Todaro is a ligament attached to the fibrous skeleton of the heart. It may represent the commissure of the Eustachian and Thebesian valves. This tendon probably functions during fetal life to strengthen the free edge of the Eustachian valve and helps to direct venous return from the inferior vena cava across the foramen ovale . The tendon of Todaro is sometimes seen in adult hearts, but it is more obvious in children. The tendon is a white cord deep to the right atrial endocardium, passing from the sinus septum, which separates the ostia of the inferior vena cava and the coronary sinus, toward the membranous septum. When this tendon is visible, it is a useful landmark for the atrioventricular node, which lies within an anatomic triangle (the triangle of Koch, described below).
The tricuspid valve is a three-leaflet valve with a saddle-shaped annulus and its valvular plane facing toward the right ventricular apex . Its normal annular circumference is 10–11.1 cm in women and 11.2–11.8 cm in men . The three leaflets hang from the valve ring like a veil, with the septal and posterior leaflets against the septal and inferior walls of the right ventricle, respectively ( Fig. 2.7 ). The anterior leaflet forms a diagonal curtain separating the inflow and outflow tracts of the right ventricle. A papillary muscle is located directly beneath each commissure, sending tendinous cords up to the adjacent leaflets (roughly half to each) . Each leaflet has a distal rough zone and a proximal basal zone into which the cords insert, as well as a clear zone free of cord insertion that lies between the rough and the basal zones.
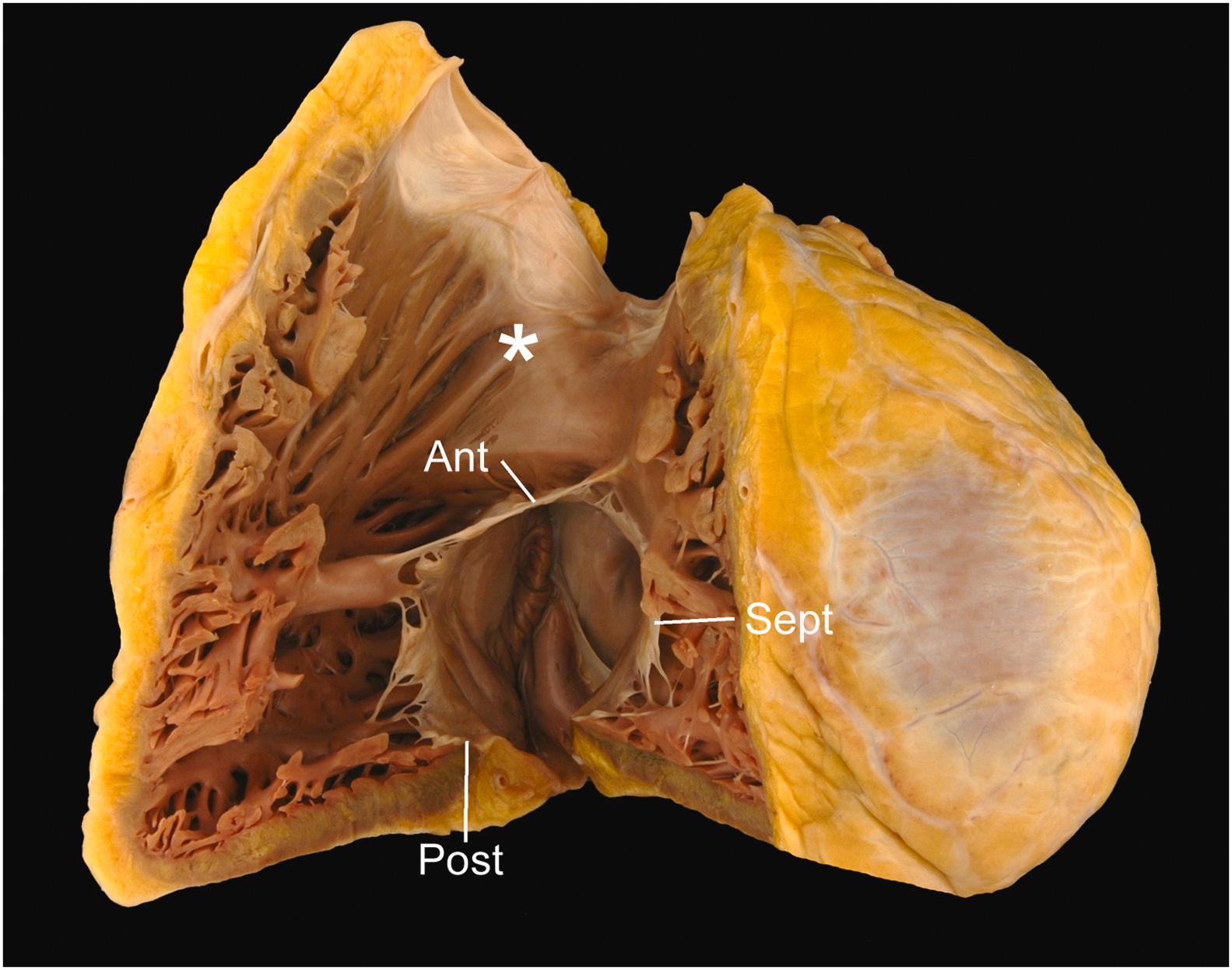
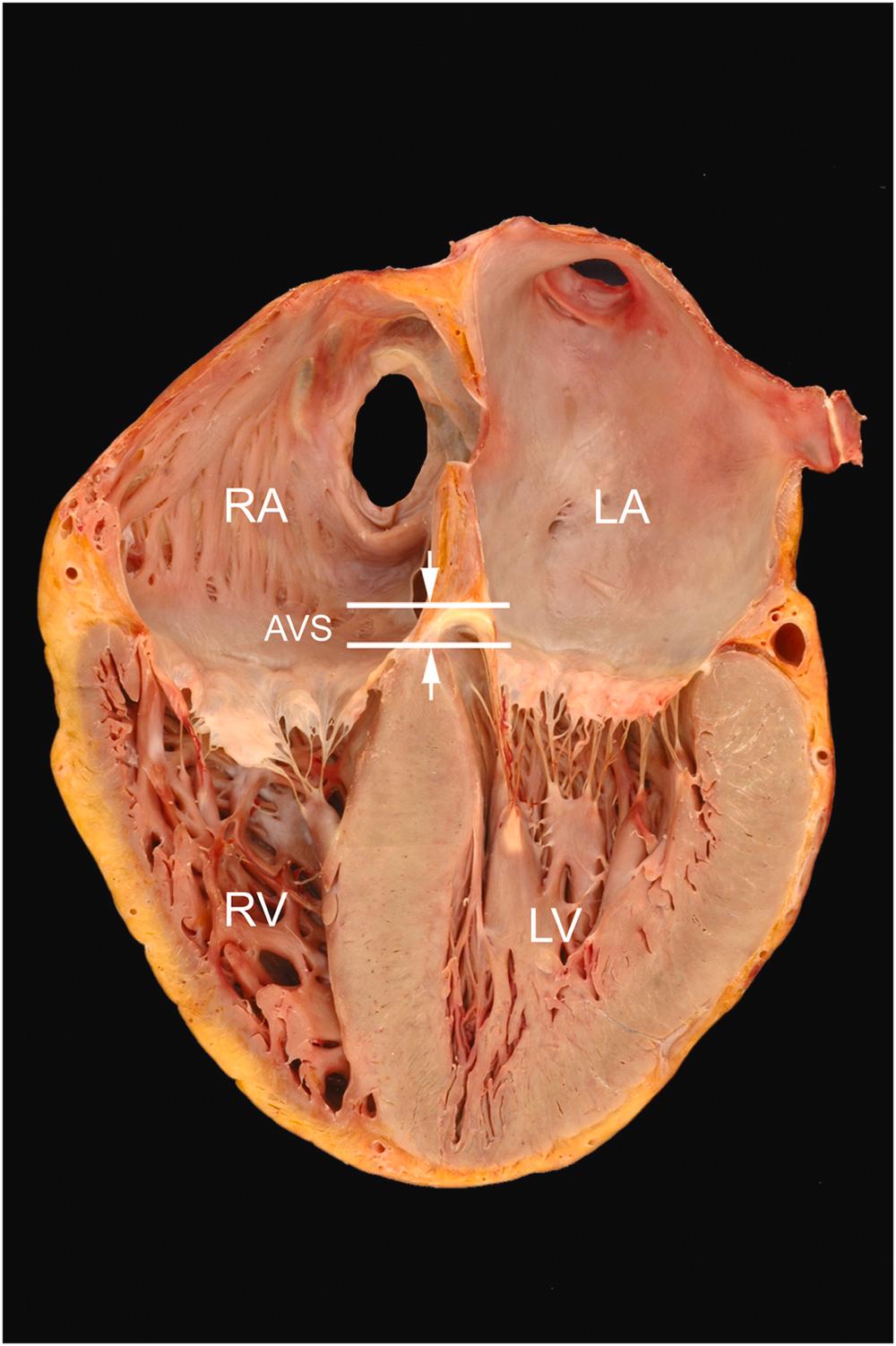
The annulus of the tricuspid valve is more apically positioned than that of the mitral valve annulus ( Fig. 2.8 ). This nonplanar arrangement of the atrioventricular valves allows for three distinct regions to be defined in the septum that divide the right and left heart: the interatrial component (atrial septum), separating the right and left atria; the atrioventricular component (atrioventricular septum), separating the right atrium from the left ventricle; and the interventricular component (ventricular septum), separating right and left ventricles.
The anterior leaflet is usually the longest (average 2.2 cm). It is semicircular or quadrangular and is 3.7 cm in average width at its base. Rarely, it has a distinct scallop in its medial side near the anteroseptal commissure. The septal leaflet is semioval and is 1.6 cm long by 3.6 cm wide on average. It shows a characteristic fold on its atrial surface in the angle where its base passes from the posterior ventricular free wall to the membranous area. The posterior leaflet, which averages 2.0 cm long by 3.6 cm wide at its base, has a variable number of scallops, usually two or three, produced by indentations in its free edge. The cords are of varying length (0.3–2.2 cm) and thickness (0.05–0.15 cm) .
Of the three papillary muscles, the anterior is the largest and most well formed. It may be single or bifid and provides cordal insertions to the anterior and posterior leaflets. The posterior papillary muscle arises from the inferior wall sending cords to the septal and posterior leaflets. The medial papillary muscle (also called the papillary muscle of the conus or the muscle of Lancisi) arises along the superior aspect of the septal band, at the level of the membranous septum and has cordal attachments to both the septal and anterior leaflets. It is important to note that in addition to cords to the papillary muscles, the tricuspid valve characteristically has cords to trabeculations of the septum. These septal cord attachments serve to help differentiate the tricuspid from the mitral valve in the setting of congenital heart disease.
The right ventricle lies anterior to the other heart chambers . Posteriorly and to the left, it is related to the left ventricle from which it is separated by the interventricular septum, which is vertically oriented and lies at a 45° angle to the median plane of the body. The right ventricle is crescentic in shape in short-axis (transverse) section ( Fig. 2.9 ), with its free wall forming an acute angle often referred to as the acute margin of the heart.
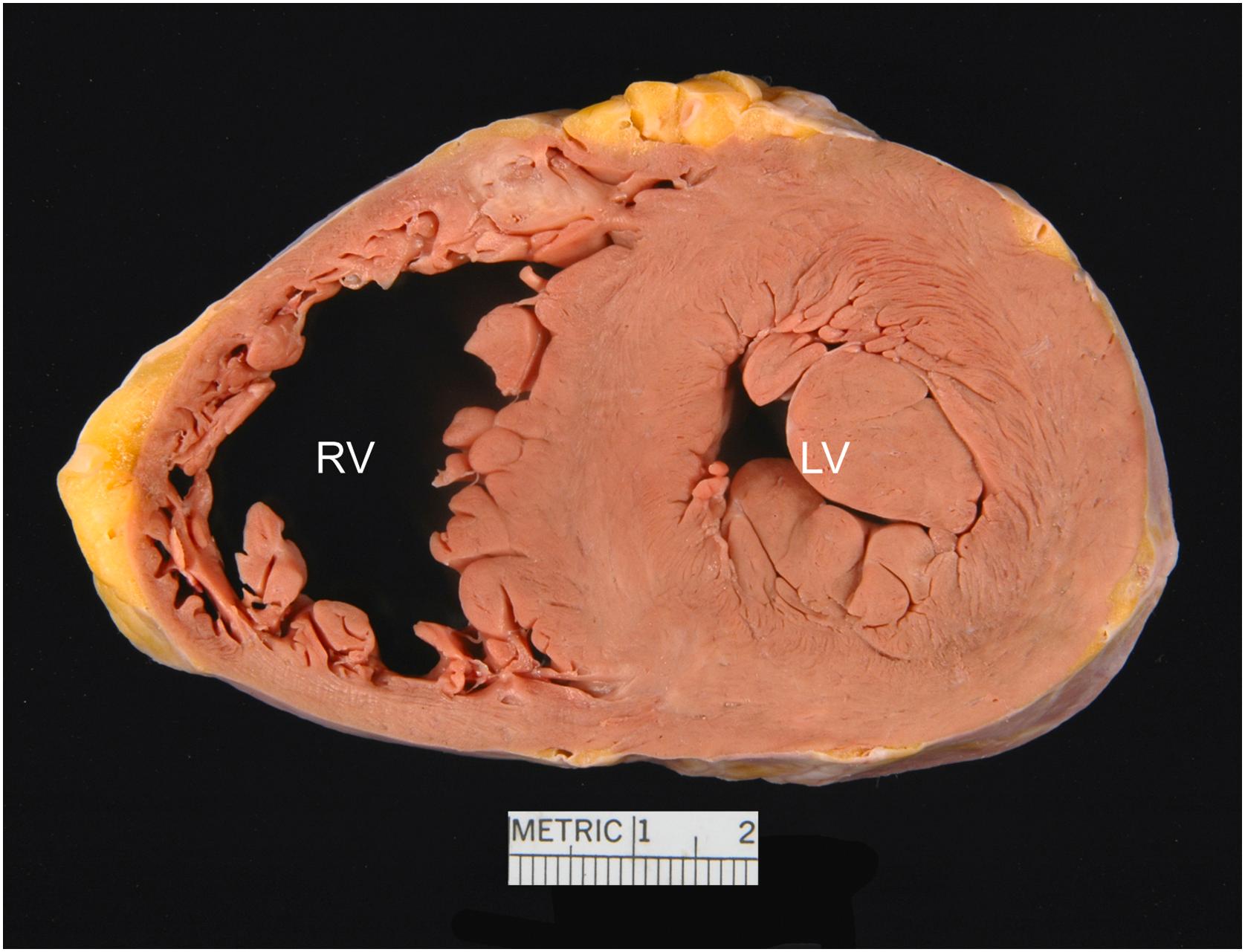
The anatomic right ventricle has an inflow portion, an apical trabeculated portion and an outflow portion, the latter of which is often referenced as the infundibulum or conus. The infundibulum is separated anatomically from the sinus by a muscular crest or arch, the crista supraventricularis. This crest, which separates tricuspid and pulmonary valves, merges medially where it meets the septum, with muscular ridges of variable prominence called the septal and parietal bands that course in those walls of the right ventricle. The site of insertion of the crest between these trabeculations may be smooth or marked by either a vertical ridge or a groove. The papillary muscle of the conus arises from the septal wall just proximal to the crista; it is an important surgical landmark for the conduction system .
The junction of the inflow tract with the infundibulum is defined anteriorly by the upper edge of another smooth arch of muscle formed by the junction of the septal band, the moderator band, and the anterior papillary muscle. The apex of the papillary muscle often points toward the commissure between the anterior and posterior tricuspid valve leaflets. The moderator band is not always as distinct in human hearts as in those of animals. The entire luminal surface of the right ventricle, with the exception of the septal and parietal bands and the posterior wall of the infundibulum above the crest (conus muscle), is coarsely trabeculated and, on section, the trabeculations form the inner two-thirds or three-fourths of the ventricular wall. Aberrant ventricular bands, usually muscular rather than fibrotic, are common in the distal part of the right ventricle and may be congenital or acquired . The conus muscle separates the pulmonary valve ring from the aortic valve ring and is responsible for the higher position by approximately 1.5 cm of the former structure ( Fig. 2.7 ). Within this muscle is a variable fibrous attachment between the rings of the two semilunar valves; the conus ligament.
Ventricular septal defects or the sites of patch repair may be seen proximal to, or, rarely, distal to the crest. If there are defects between the inflow portions of the ventricles, they will open among the trabeculations on the interventricular septum . Such defects may be multiple, small, and tortuous. Small ones often close spontaneously, being obliterated either by adherent tricuspid leaflet tissue or by endocardial proliferation about their edges and sometimes small aneurysms result.
The pulmonary valve lies between the infundibulum and the pulmonary artery and is attached to the pulmonary annulus, which points posteriorly, superiorly, and to the left (pointing toward the left shoulder) . Its ring circumference is 5.7–7.4 cm in women and 6.0–7.5 cm in men . The pulmonary infundibulum winds across the anterior aspect of the aortic root, with the annulus approximately 1.5 cm higher than the aortic annulus. The three cusps are named the anterior, the right (closest to the right aortic cusp), and the left (closest to the left aortic cusp).
Both the aortic and pulmonary semilunar valves consist of an annulus, cusps, and commissures. Semilunar valves lack tendinous cords and papillary muscles; hence their opening and closing are a passive function of hemodynamics. The annuli of the semilunar valves are shaped like a triradiate crown, with the three points representing the three commissures where adjacent cusps meet.
The three pulmonary cusps are half-moon (semilunar) shaped structures made of fibroelastic tissue. The distal edge is referred to as the free edge, beneath which lies a biscalloped ridge, the closing edge or margin, along the ventricular aspect of the cusp. A fibrous nodule, named for both Arantius and Morgagni, is located centrally on the free edge of each cusp. Extending away from the nodule, on either side, is a crescent-shaped area, referred to as the lunula, which represent the closing surfaces of each cusp.
The left atrium, with a wall 0.3 cm in average thickness, lies in a midline-posterior position immediately anterior to the lower esophagus . The descending thoracic aorta lies immediately to the left and the bifurcated pulmonary artery and left bronchus, superiorly.
As with the right atrium, the left atrium can be divided into its septal and free walls. The left side of the atrial septum contains the valve of the fossa ovalis, the remnant of septum primum. Most of the free wall consists of a dome-shaped, smooth-walled portion that receives the four pulmonary veins. Like the sinus venosus of the right atrium, this smooth-walled portion of the left atrium is of venous origin (arising from the common pulmonary vein, embryologically). Anteriorly is the base of the left atrial appendage.
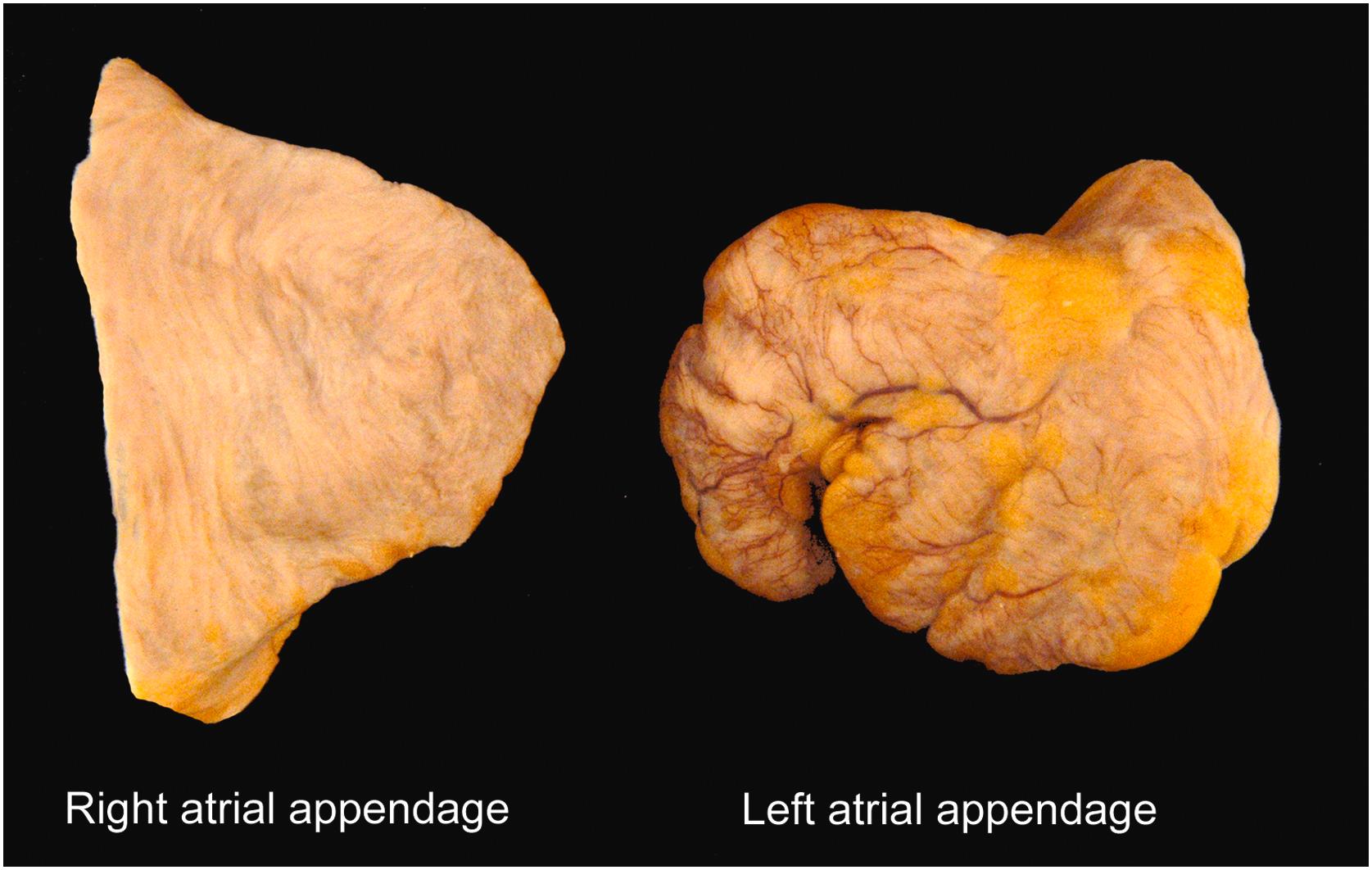
Unlike the pyramidal right atrial appendage ( Fig. 2.10 ), the left atrial appendage has a more variable morphology that has been typed into four major categories: cauliflower, cactus, windsock, and chicken wing. Of note, the latter is thought to be somewhat protective of thromboembolic events . The atrial appendage is lined by pectinate muscles like those in the right atrial appendage, but unlike the right atrium, those in the left are fine, radially arranged, and do not exist outside of the appendage. There may be tiny ostia of Thebesian veins in the left atrium as there are in the right; the largest, the ostia of Lannelongue, are central in the wall. The endocardial lining of the left atrium is thicker and more opaque than that of the right atrium, in keeping with the higher pressure within the left side of the heart.
The mitral valve is a two-leaflet valve with a saddle-shaped annulus and its valvular plane facing anteriorly, inferiorly, and to the left ( Fig. 2.11 ) . The valve’s normal annular circumference is between 8.2 and 9.1 cm in women and 9.2 and 9.9 cm in men . The two leaflets, anterior and posterior, are attached around the annulus, meeting at the commissures.
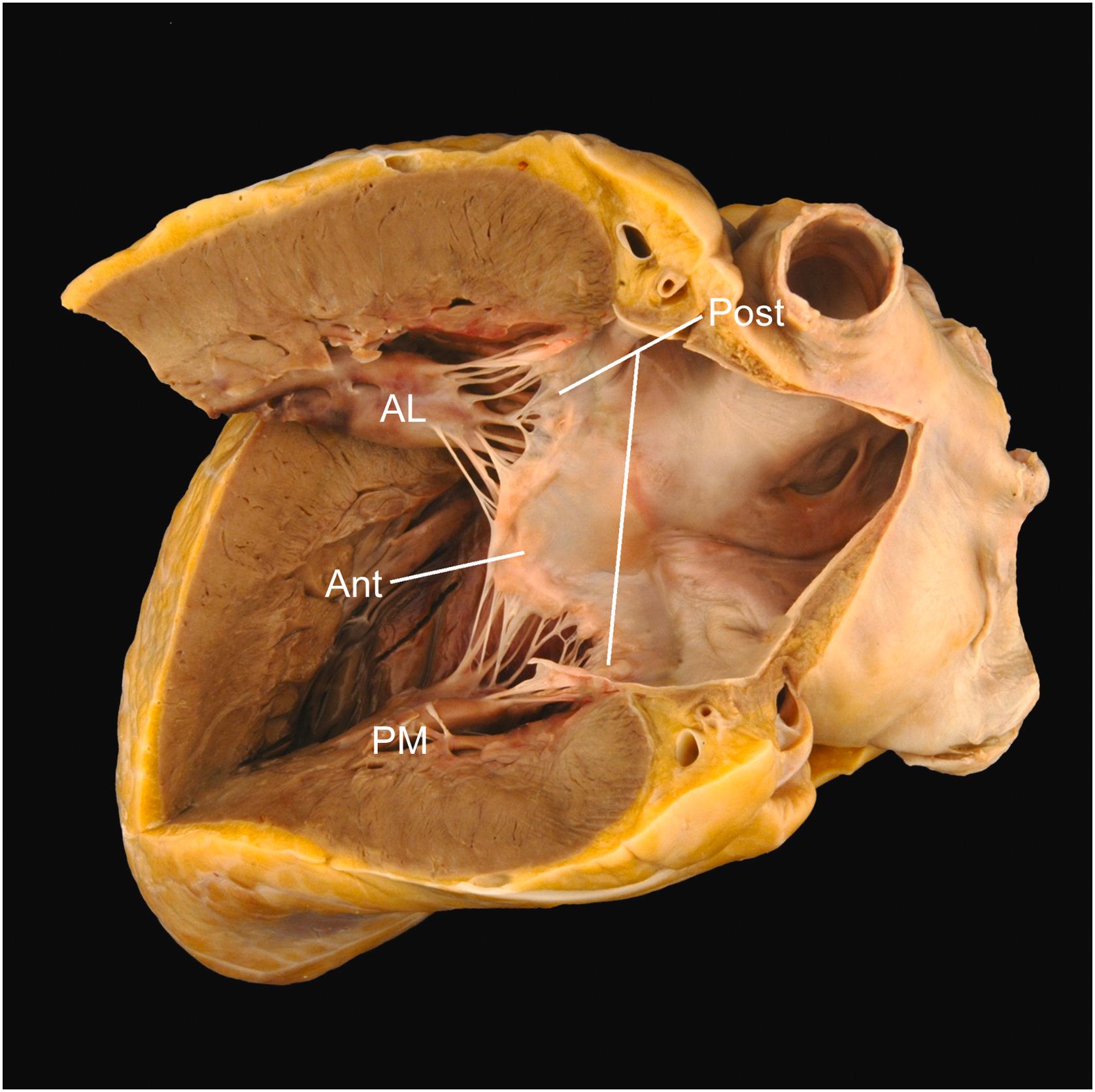
The anterior mitral leaflet is in fibrous continuity with the aortic valve proximal to its posterior (noncoronary) and left aortic cusps ( Fig. 2.12 ) . Although the entire annular circumference connects to the atrium because of this fibrous continuity, only a C-shaped portion attaches to the underlying left ventricular free wall. The anterior mitral leaflet forms a curtain that, like the anterior tricuspid leaflet, separates the inflow and outflow portions of its respective ventricle. The anterior leaflet is long (average 3 cm) and triangular, or almost sail-like, having an average width of 3.3 cm at its base. A ridge on its atrial aspect, 0.8–1.0 cm from the free edge, defines the line of valve closure. The rough zone of cordal attachment on the ventricular surface lies between the line of closure and the free edge.
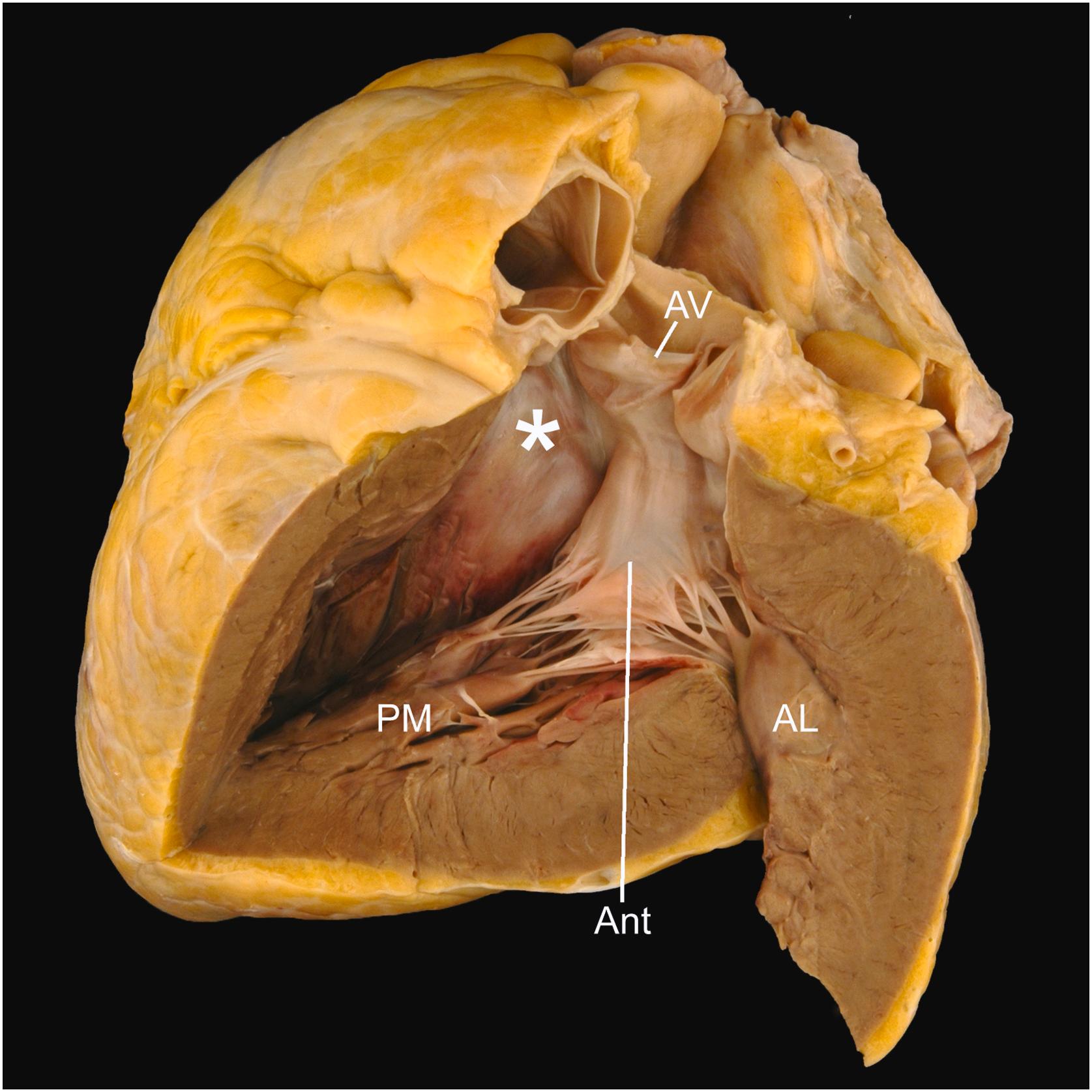
The posterior mitral leaflet attaches to the parietal part of the mitral annulus. Although more shallow than the anterior leaflet, it occupies approximately two-thirds of the mitral annular circumference . It is divided into scallops, usually three: P1, P2, and P3. The posterior leaflet has an average width of 4.8 cm, and the longest scallop, usually the middle one, has an average length of 1.3 cm. Unlike the anterior leaflet, the posterior leaflet has a basal zone of cord attachment that is separated from the rough zone near the free edge by a narrow clear zone. The line of valve closure, located on the atrial surface of the leaflet, lies approximately 0.2 cm from the free edge.
The mitral valve cords vary in number, length, and thickness . So-called primary cords attach to the free edge of the valve and are responsible for leaflet coaptation, while secondary cords attach to the ventricular surface and serve to help maintain ventricular geometry . A large, thick cord can usually be seen arising from each papillary muscle and inserting on the anterior mitral leaflet. These specialized secondary cords are often referred to as strut cords. Commissural cords are also recognized.
The two papillary muscles of the left ventricle are the anterolateral and posteromedial. They are of approximately equal size and are often double throughout or bifid at their tips, the posterior muscle showing subdivision into two or more columns more often than the anterior muscle . The papillary muscles have broad bases in the apical trabecular muscle, and their tips give attachment to cords that tether the adjacent halves of each mitral leaflet. The cords from the anterior papillary muscle attach to the anterior halves of each leaflet.
Left ventricular pseudotendons (also termed false cords) arise from a papillary muscle and insert onto either the septum or the opposite papillary muscle. These ventricular bands may be seen in upwards of 50% of subjects . Their lack of attachment to valves helps to distinguish them from congenital bands (like that seen in straddling valves associated with atrioventricular canal defects) .
The left ventricle forms the posterior and left lateral surfaces of the heart . It is circular in short-axis cross section, but somewhat triangular when viewed along the long axis. Its shape approximates that of a truncated ellipsoid. Trabeculations are present in the left ventricular myocardium, but are finer and more numerous than in the right ventricle. They make up less than the inner one-fourth of the wall; the outer three-fourths are composed of compact muscle, spirally arranged, from apex to base.
Like the right ventricle, the left ventricle has three distinct regions: an inflow portion, apical portion, and an outflow portion. Blood flowing through the left ventricle changes direction by nearly 180° in passing the free edge of the anterior mitral leaflet. The inflow portion contains two papillary muscles, as described previously; in a contracted heart, they abut on each other and the interventricular septum to virtually obliterate the ventricular cavity. The apical portion contains trabeculations that are both finer and more numerous than the apical trabeculations of the right ventricle. The outflow portion has a smooth septal wall formed by the upper third of the interventricular septum anteriorly and to the right, and by the ventricular surface of the anterior mitral leaflet posteriorly and to the left ( Fig. 2.12 ). The smooth muscular interventricular septum bulges somewhat into the left ventricular outflow tract and may become more prominent with increasing age, producing a “sigmoid” septum (see Chapter 3 ) .
The triangular membranous septum lies proximal to and between the posterior and right aortic valve cusps and can be transilluminated. A ventricular septal defect may occur in the membranous septum, but commonly involves the muscular septum immediately proximal and anterior to this region. Small muscular ventricular septal defects often heal spontaneously and may be marked by gray–white endocardial dimples or small septal aneurysms.
The aortic valve lies between the left ventricular outflow tract and the aorta . Its normal annular circumference is 5.7–7.9 cm in women and 6.0–8.5 cm in men . The valve annulus is a midline structure and occupies a central position in the cardiac base. The plane of the valve points posteriorly, superiorly, and to the right (directed toward the right shoulder) ( Fig. 2.4 ). The annulus and cusps are very similar to those of the pulmonary valve, as previously described. The higher, left-sided pressures typically make the closing edge and midline cusp nodule (nodule of Arantius) more evident than in that of the pulmonary valve ( Fig. 2.13 ). Lambl excrescences are frequently found on the nodules and along the line of valve closure ( Fig. 2.14 ) (see Chapter 3 ).
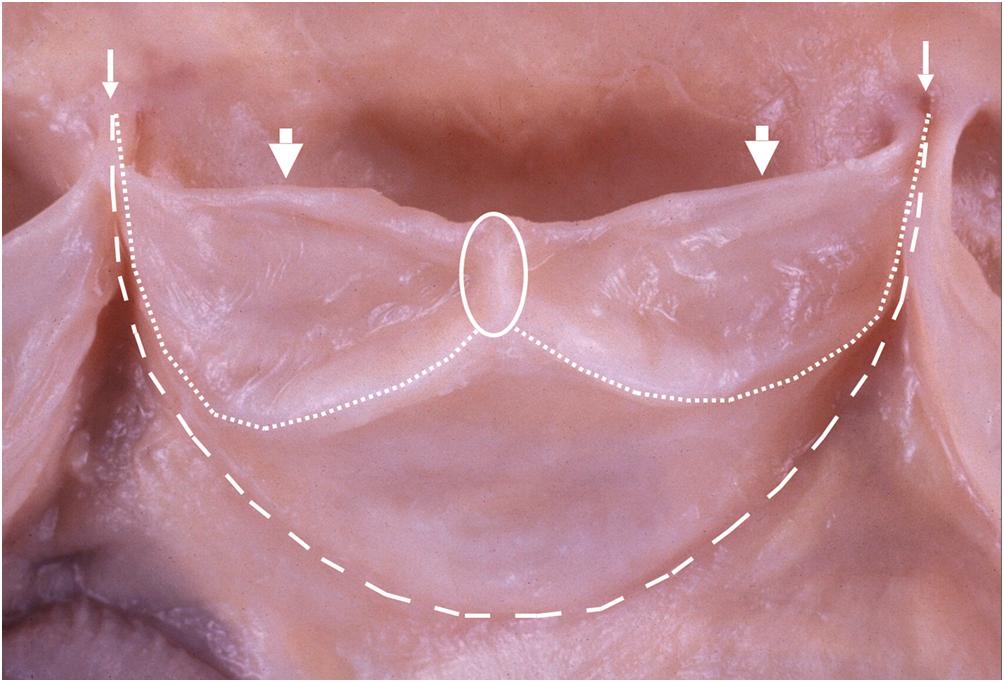
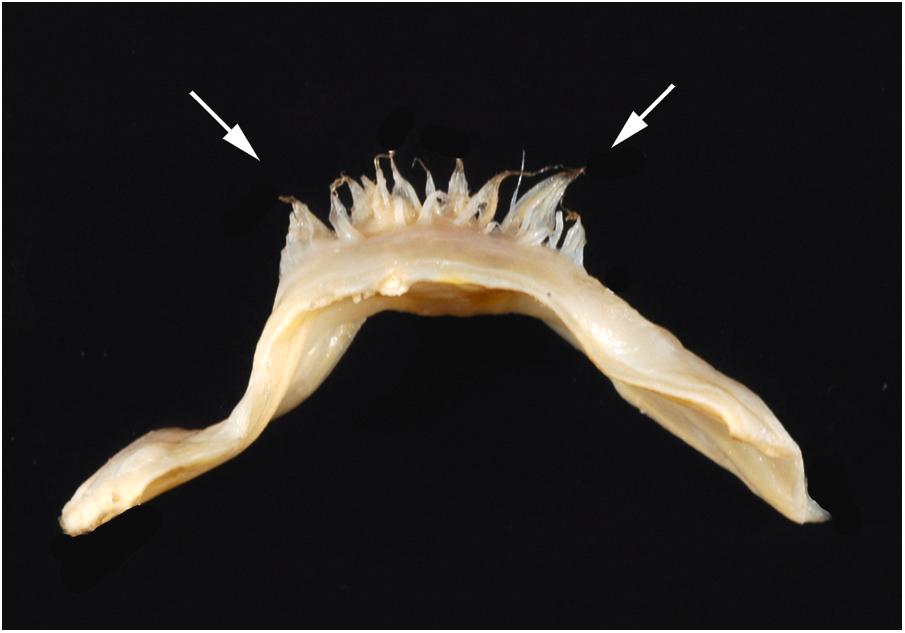
The posterior noncoronary cusp is posterior in position, and the right and left coronary cusps are anterior ( Fig. 2.4 ). The valve cusps are similar in size, though are usually not equal. In some instances, this may hasten calcification . The aortic and mitral valve rings are fused (intervalvular fibrosa) over the length of attachment of the anterior mitral leaflet, which has a variable degree of fibrous continuity with the adjacent halves of the posterior and left aortic valve cusps ( Fig. 2.15 ) .
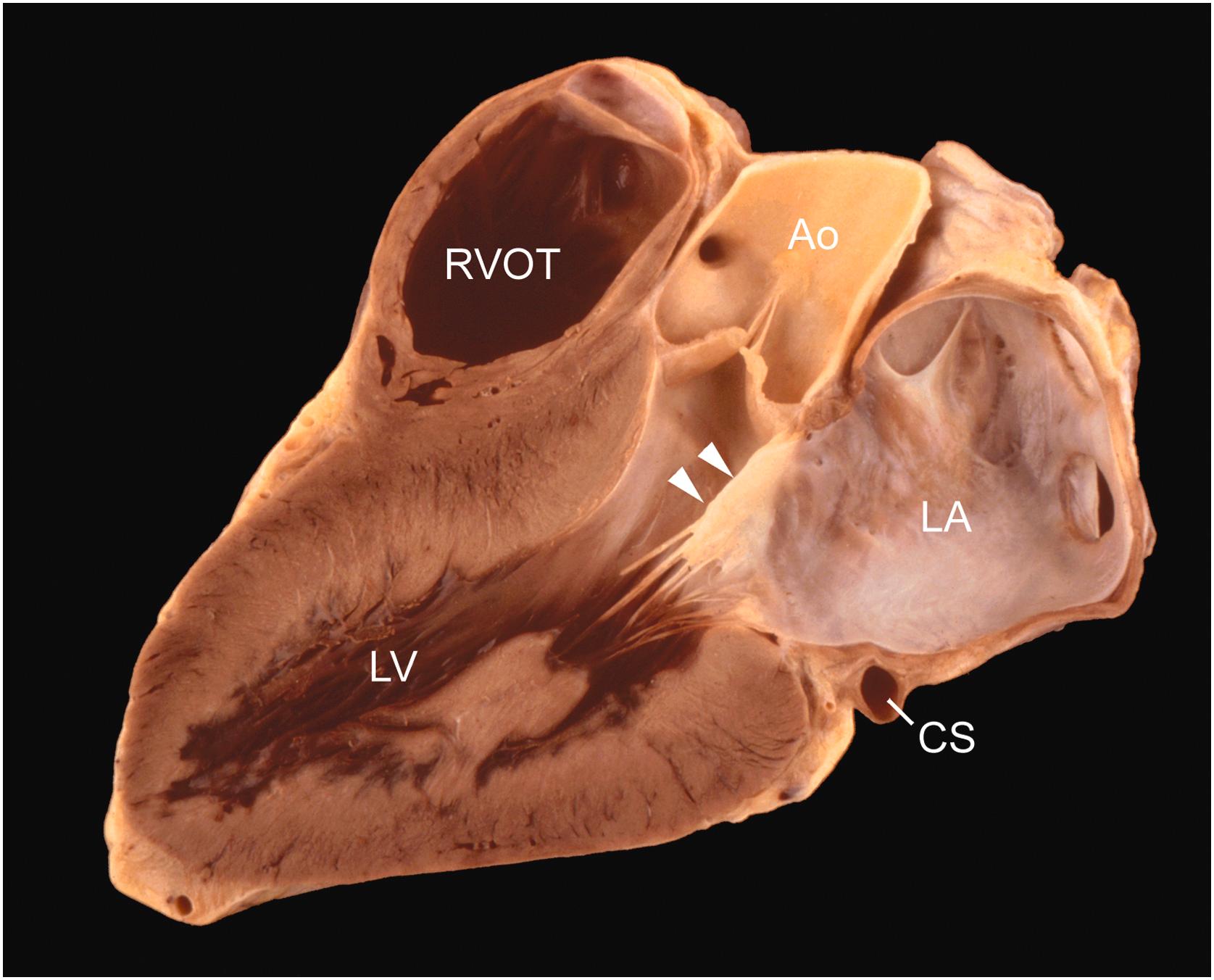
The sinus (of Valsalva) of each aortic cusp is the pocket enclosed by the cusp attachment proximally and a ridge encircling the aortic lining at the level of the commissures. An imaginary line connecting the tips of the commissures (prongs of the triradiate annulus) forms the sinotubular junction where the aortic sinus becomes the tubular portion of the ascending aorta. The sinus is 1.5 times wider than the proximal tubular ascending aorta, but this is better appreciated in angiograms taken during life than in gross specimens.
The coronary arterial ostia may be variable in number, position, and size. The mean diameter of the right vessel is 0.32 cm, and of the left, 0.40 cm. They are usually single, centrally located within the wall of the aortic sinus, and approximately equal in size. If unequal, the left is more often larger, but the right is often double, with one ostium representing the conus artery ostium.
Normal variations of coronary ostia are distinguished from anomalies (e.g., both the LAD artery and the LCX coronary artery may arise from the same coronary sinus) . Nevertheless, a solitary coronary ostium, stenosis of an ostium, acute angulation or arterial takeoff, or an intussusception of an ostium may be associated with sudden death (see Chapter 10: Myocardial Ischemia and Its Complications ) .
The cardiac conduction system consists of the sinus node, internodal pathways, and atrioventricular conduction tissues. Its function is influenced by the innervation of the heart (described below). The sinus and atrioventricular nodes are both right atrial structures. It is important to note that all structures of the conduction system are specialized cardiac myocytes (not nerves) whose primary function is impulse propagation rather than contraction and relaxation.
The sinus node is located subepicardially, along the superior aspect of the sulcus terminalis, near the confluence of the superior vena cava, sinus venosus, and muscular right atrial free wall. These latter two structures are the reason the sinus node is sometimes referred to as the sinoatrial node. It is elliptical in shape with the sinoatrial nodal artery, which is often grossly identifiable, running centrally through the nodal tissue.
The internodal pathways serve as preferential tracts by which impulse propagation is transmitted from the sinus node to the atrioventricular node. They have been described in the atrial septum, right atrial free wall, and the crista terminalis. While electrophysiologically identifiable, these structures have not been definitively teased out morphologically, except for the Bachman bundle between the atria .
The atrioventricular node is located subendocardially (rather than subepicardially, like the sinus node) within the triangle of Koch. The triangle of Koch is the anatomic region bordered by the tricuspid annulus (of the septal tricuspid leaflet), the ostium of the coronary sinus, and the tendon of Todaro (described above). The apex of the triangle is adjacent to the central fibrous body. The atrioventricular nodal artery does not necessarily travel intranodally, like that of the sinus nodal artery.
The atrioventricular bundle (bundle of His) extends from the node, traveling through the central fibrous body, to the basal ventricular septum, adjacent to the membranous septum. It then splits into both its right and left bundle branches. The right bundle branch is a cord-like structure that travels subendocardially to the moderator band and then out to the free wall of the right ventricle. The broaderleft bundle branch also travels subendocardially toward the left ventricular apex, while fanning out into fascicles of Purkinje cells.
The nerve supply to the heart is autonomic, including both sympathetic and parasympathetic innervation via both efferent and afferent fibers . It is conveyed to the cardiac plexus in branches from the vagus and phrenic nerves and also from the cervical and thoracic cardiac nerves. The cardiac plexus is a group of nerve ganglia located between the arch of the aorta and the bifurcation of the trachea. The largest ganglion, the ganglion of Wrisberg, lies below the arch of the aorta. Nerve ganglia are seen frequently in microscopic sections of the subendocardial muscle of the right atrium, particularly in the interatrial septum and in the epicardium near the roots of the great vessels. Both sympathetic and parasympathetic nerves innervate the nodes, myocardium, and coronary arteries.
The opened heart, emptied of postmortem clot and vessels trimmed to within 1 cm of the heart, should be weighed, measurements of valve rings and ventricular wall thicknesses should be made, and the results compared to accepted normograms . In individual cases, normality of heart weight should be judged by consideration of body size, weight, and sex. Generically, the following useful guidelines for fresh heart weight may be used:
Adult male heart weight : 0.45% of body weight, average 300 g, range 250–350 g.
Adult female heart weight : 0.40% of body weight, average 250 g, range 200–300 g.
Heart weight tends to be related to body weight . Table 2.2 predicts a normal ( formalin-fixed ) heart weight from the body weight of adult men and women; it is based on a large study group . Both increases and decreases (of approximately 6%) have been noted following formalin fixation . Heart weight is also related to body length, but less so than to body weight . Although heart weight is said to increase with age, this is more likely true in women than in men, and it decreases in the elderly of both sexes . Adult heart weight is typically reached between the ages of 17 and 20 years. Heart weight in children is related to age and body size . Table 2.3 shows normal ( formalin-fixed ) heart weights predicted from body weights of male and female subjects up to 19 years of age .
| Body weight | Women | Men | |||||
|---|---|---|---|---|---|---|---|
| kg | lb | L95 | P | U95 | L95 | P | U95 |
| 30 | 66 | 133 | 196 | 287 | 162 | 213 | 282 |
| 32 | 71 | 137 | 201 | 295 | 167 | 220 | 291 |
| 34 | 75 | 141 | 206 | 302 | 172 | 227 | 300 |
| 36 | 79 | 144 | 211 | 310 | 177 | 234 | 309 |
| 38 | 84 | 148 | 216 | 317 | 182 | 240 | 317 |
| 40 | 88 | 151 | 221 | 324 | 187 | 247 | 325 |
| 42 | 93 | 154 | 226 | 331 | 191 | 253 | 334 |
| 44 | 97 | 157 | 230 | 337 | 196 | 259 | 341 |
| 46 | 101 | 160 | 234 | 344 | 200 | 265 | 349 |
| 48 | 106 | 163 | 239 | 350 | 205 | 270 | 357 |
| 50 | 110 | 166 | 243 | 356 | 209 | 276 | 364 |
| 52 | 115 | 169 | 247 | 362 | 213 | 281 | 371 |
| 54 | 119 | 171 | 251 | 368 | 217 | 287 | 379 |
| 56 | 123 | 174 | 255 | 374 | 221 | 292 | 386 |
| 58 | 128 | 177 | 259 | 379 | 225 | 297 | 392 |
| 60 | 132 | 179 | 262 | 385 | 229 | 302 | 399 |
| 62 | 137 | 182 | 266 | 390 | 233 | 307 | 406 |
| 64 | 141 | 184 | 270 | 395 | 237 | 312 | 412 |
| 66 | 146 | 187 | 273 | 401 | 240 | 317 | 419 |
| 68 | 150 | 189 | 277 | 406 | 244 | 322 | 425 |
| 70 | 154 | 191 | 280 | 411 | 248 | 327 | 431 |
| 72 | 159 | 194 | 284 | 416 | 251 | 331 | 437 |
| 74 | 163 | 196 | 287 | 420 | 255 | 336 | 444 |
| 76 | 168 | 198 | 290 | 425 | 258 | 341 | 450 |
| 78 | 172 | 200 | 293 | 430 | 261 | 345 | 455 |
| 80 | 176 | 202 | 297 | 435 | 265 | 349 | 461 |
| 82 | 181 | 205 | 300 | 439 | 268 | 354 | 467 |
| 84 | 185 | 207 | 303 | 444 | 271 | 358 | 473 |
| 86 | 190 | 209 | 306 | 448 | 275 | 362 | 478 |
| 88 | 194 | 211 | 309 | 453 | 278 | 367 | 484 |
| 90 | 198 | 213 | 312 | 457 | 281 | 371 | 489 |
| 92 | 203 | 215 | 315 | 461 | 284 | 375 | 495 |
| 94 | 207 | 217 | 318 | 465 | 287 | 379 | 500 |
| 96 | 212 | 219 | 320 | 470 | 290 | 383 | 506 |
| 98 | 216 | 221 | 323 | 474 | 293 | 387 | 511 |
| 100 | 220 | 222 | 326 | 478 | 296 | 391 | 516 |
| 102 | 225 | 224 | 329 | 482 | 299 | 395 | 521 |
| 104 | 229 | 226 | 331 | 486 | 302 | 399 | 526 |
| 106 | 234 | 228 | 334 | 490 | 305 | 403 | 531 |
| 108 | 238 | 230 | 337 | 494 | 308 | 406 | 536 |
| 110 | 243 | 232 | 339 | 497 | 311 | 410 | 541 |
| 112 | 247 | 233 | 342 | 501 | 314 | 414 | 546 |
| 114 | 251 | 235 | 345 | 505 | 316 | 418 | 551 |
| 116 | 256 | 237 | 347 | 509 | 319 | 421 | 556 |
| 118 | 260 | 239 | 350 | 513 | 322 | 425 | 561 |
| 120 | 265 | 240 | 352 | 516 | 325 | 429 | 566 |
| 122 | 269 | 242 | 355 | 520 | 327 | 432 | 570 |
| 124 | 273 | 244 | 357 | 523 | 330 | 436 | 575 |
| 126 | 278 | 245 | 360 | 527 | 333 | 439 | 580 |
| 128 | 282 | 247 | 362 | 531 | 335 | 443 | 584 |
| 130 | 287 | 249 | 364 | 534 | 338 | 446 | 589 |
| 132 | 291 | 250 | 367 | 537 | 341 | 450 | 593 |
| 134 | 295 | 252 | 369 | 541 | 343 | 453 | 598 |
| 136 | 300 | 253 | 371 | 544 | 346 | 456 | 602 |
| 138 | 304 | 255 | 374 | 548 | 348 | 460 | 607 |
| 140 | 309 | 257 | 376 | 551 | 351 | 463 | 611 |
| 142 | 313 | 258 | 378 | 554 | 353 | 466 | 616 |
| 144 | 317 | 260 | 381 | 558 | 356 | 470 | 620 |
| 146 | 322 | 261 | 383 | 561 | 358 | 473 | 624 |
| 148 | 326 | 263 | 385 | 564 | 361 | 476 | 629 |
| 150 | 331 | 264 | 387 | 567 | 363 | 479 | 633 |
| Body weight | Females | Males | |||||
|---|---|---|---|---|---|---|---|
| kg | lb | L95 | P | U95 | L95 | P | U95 |
| 3 | 7 | 13 | 19 | 29 | 11 | 16 | 24 |
| 4 | 9 | 16 | 24 | 37 | 14 | 21 | 31 |
| 5 | 11 | 19 | 29 | 44 | 18 | 26 | 38 |
| 6 | 13 | 22 | 33 | 51 | 21 | 30 | 45 |
| 7 | 15 | 25 | 38 | 58 | 24 | 35 | 51 |
| 8 | 18 | 28 | 42 | 64 | 27 | 39 | 58 |
| 9 | 20 | 30 | 46 | 71 | 30 | 44 | 64 |
| 10 | 22 | 33 | 50 | 77 | 33 | 48 | 71 |
| 12 | 26 | 38 | 58 | 89 | 39 | 57 | 83 |
| 14 | 31 | 43 | 66 | 101 | 45 | 65 | 96 |
| 16 | 35 | 48 | 74 | 113 | 50 | 74 | 108 |
| 18 | 40 | 53 | 81 | 124 | 56 | 82 | 120 |
| 20 | 44 | 58 | 88 | 135 | 61 | 90 | 132 |
| 22 | 49 | 62 | 95 | 146 | 67 | 98 | 143 |
| 24 | 53 | 67 | 102 | 156 | 72 | 106 | 155 |
| 26 | 57 | 71 | 109 | 166 | 78 | 111 | 167 |
| 28 | 62 | 76 | 116 | 177 | 83 | 122 | 178 |
| 30 | 66 | 80 | 122 | 187 | 89 | 130 | 190 |
| 32 | 71 | 84 | 129 | 197 | 94 | 137 | 201 |
| 34 | 75 | 88 | 135 | 207 | 99 | 145 | 212 |
| 36 | 79 | 93 | 142 | 216 | 104 | 153 | 223 |
| 38 | 84 | 97 | 148 | 226 | 110 | 160 | 235 |
| 40 | 88 | 101 | 154 | 236 | 115 | 168 | 246 |
| 42 | 93 | 105 | 160 | 245 | 120 | 175 | 257 |
| 44 | 97 | 109 | 166 | 254 | 125 | 183 | 268 |
| 46 | 101 | 113 | 172 | 264 | 130 | 190 | 279 |
| 48 | 106 | 117 | 179 | 273 | 135 | 198 | 289 |
| 50 | 110 | 121 | 184 | 282 | 140 | 205 | 300 |
| 55 | 121 | 130 | 199 | 304 | 153 | 224 | 327 |
| 60 | 132 | 140 | 214 | 326 | 165 | 242 | 354 |
| 65 | 143 | 149 | 228 | 348 | 178 | 260 | 380 |
| 70 | 154 | 158 | 242 | 370 | 190 | 278 | 406 |
| 75 | 165 | 167 | 256 | 391 | 202 | 295 | 432 |
| 80 | 176 | 176 | 269 | 412 | 214 | 313 | 458 |
| 85 | 187 | 185 | 283 | 432 | 226 | 331 | 484 |
| 90 | 198 | 194 | 296 | 453 | 238 | 348 | 509 |
| 95 | 209 | 202 | 309 | 473 | 250 | 365 | 535 |
| 100 | 220 | 211 | 322 | 493 | 262 | 383 | 560 |
Although total heart weight has a wide normal range, it is the most practical and reproducible guide to myocardial hypertrophy when combined with a visual assessment of heart chamber musculature. It should be remembered that total heart weight best reflects left rather than right ventricular hypertrophy. Visual assessment is particularly needed to judge the presence of right ventricular hypertrophy. Hypertrophied muscle produces brawny and prominent trabeculations. In some situations (e.g., pulmonary arterial hypertension), it may be useful to weigh the ventricles independently for a more detailed assessment of the hypertrophy . This partitioning method involves stripping the epicardial fat and coronary vasculature from the specimen, removing the atria and great arteries, and separation of the right ventricular free wall from the heart (leaving the septum with the left ventricular free wall). When the total weight of the ventricles is >250 g, the ratio of left ventricle to right ventricle is <2.1 in cases of right ventricular hypertrophy and >3.3 in cases of left ventricular hypertrophy . Because it can be difficult to maintain orientation after the specimen has been partitioned, it has been recommended that partitioning of the ventricles occur after dissection and description using the short-axis method described above .
In assessment of cardiac hypertrophy, an increase (above the mean for age and body size) between 26% and 50% can be considered mild, 51% and 100% as moderate, and >100% as marked. The presence of cardiomegaly typically correlates with either moderate or marked hypertrophy. It may be particularly difficult to gauge degree of hypertrophy in surgical explants (where the atria have been retained in the patient) or hearts with extensive pericardial adhesions or installed hardware. In these cases, the prosector should use their judgment to best adjust the expected weight accordingly.
Documentation of thicknesses of the compact myocardium (wall thickness) is also requisite. These measurements are typically taken at the midventricular (papillary muscle) level and should include the left ventricular free wall, ventricular septum, and right ventricle. Care should be taken not to include trabeculations or the papillary muscles in the measurements. Generally, the left ventricle in adult hearts is 0.8–1.2 cm in thickness and the right ventricle is 0.3–0.5 cm in thickness. The ratio of the thickness of the ventricular septum to that of the left ventricular free wall (VS:LV) should be <1.3. A ratio of greater than 1.3 is considered asymmetric septal hypertrophy and may raise concern for hypertrophic cardiomyopathy.
Chamber sizes should also be measured and documented at the midventricular level. When viewed in short axis, the left ventricle is circular, and the chamber size is generally <3.0 cm (excluding trabeculations and papillary muscles). The crescent-shaped right ventricle is generally <3.5 cm in the anterior-posterior dimension and <5 cm in the lateral dimension. The ventricular walls may artifactually thicken (emulating systole) in the postmortem state, owing to the effect of rigor (which occurs in a time- and environment-dependent manner) . Similarly, decomposition can cause artifactual chamber dilatation (with concomitant thinning of the walls). The variability nature of these postmortem phenomena reemphasizes the importance of heart weight in the determination of cardiac hypertrophy.
Finally, valve circumferences should be measured ( Table 2.4 ). Atrioventricular valves can be measured along their annuli. Semilunar valve circumference should be measured at the sinotubular junction (the ring that connects the commissures).
| Valve | Circumference (cm) | |
|---|---|---|
| Women | Men | |
| Tricuspid | 10–11.1 | 11.2–11.8 |
| Pulmonary | 5.7–7.4 | 6.1–7.5 |
| Mitral | 8.2–9.1 | 9.2–9.9 |
| Aortic | 5.7–7.9 | 6.0–8.5 |
The nature of surgical pathology specimens taken from cardiovascular cases has evolved over the years. More aggressive treatment, the greater use of noninvasive or structure-preserving measures, and the increasing usage of endomyocardial biopsy (for both primary diagnosis and transplant surveillance) have changed and continue to change the landscape . The processing of these specimens in surgical pathology is considered under a series of headings and then the handling of particular specimens is discussed.
When handling specimens, universal safety precautions should always be employed. Appropriate protective clothing and personal protective items should be worn. No tissue, even fixed material, should ever be handled with bare hands. It must be kept in mind that noxious agents are present in the laboratory. They must be treated with respect and appropriate precautions. Formaldehyde solution soon irritates nasal mucosa, making its presence known, but long exposure can lead to debilitating corneal ulceration, respiratory problems, dermatitis, or other pathology. Other agents are flammable and still others toxic. Potential untoward actions must be recognized and prevented. Knowledge of safety regulations is essential, and the location of safety features in the laboratory should be known by all. It is especially important to be cognizant of the procedures to be followed by individuals and staff if a toxic spill occurs or a fire starts.
Specimens, irrespective of their nature, are received with a consultation request or requisition bearing demographic details about the patient, as well as clinical data. All this information is vital and is incorporated into generating the final pathology report. Surgeons and/or clinicians should realize that a final pathology report may be delayed unless this information is provided. The pathologist must ensure that specimen containers are correctly labeled and must not accept tissue from more than one anatomic site in a container. Errors at this stage are best dealt with by promptly contacting the clinical team. The tissue may be in a saline fixative solution, or fresh, and is given a laboratory accession number; correct identification must be maintained at all subsequent stages of the process. However, in some cases, before collection is made, and in many others, before dissection and blocking for histology are performed, the pathologist must consider the need for any other action. For example, should an individual specimen be collected fresh or should it be placed in a special fixative? Communication between the pathologist and the clinician may be invaluable in choice of specimen collection and triage.
In many instances, photography can add depth to the gross report and may help when reviewing histology or in subsequent conferences. Edwards provides guidance for this type of activity . Radiography can assist in determining the degree of calcification of the valves and coronary arteries. Also, radiography can locate stents or other prostheses ( Fig. 2.16 ). Microbiologic cultures or use of electron microscopy may be indicated, and frozen tissue is required for some examinations.
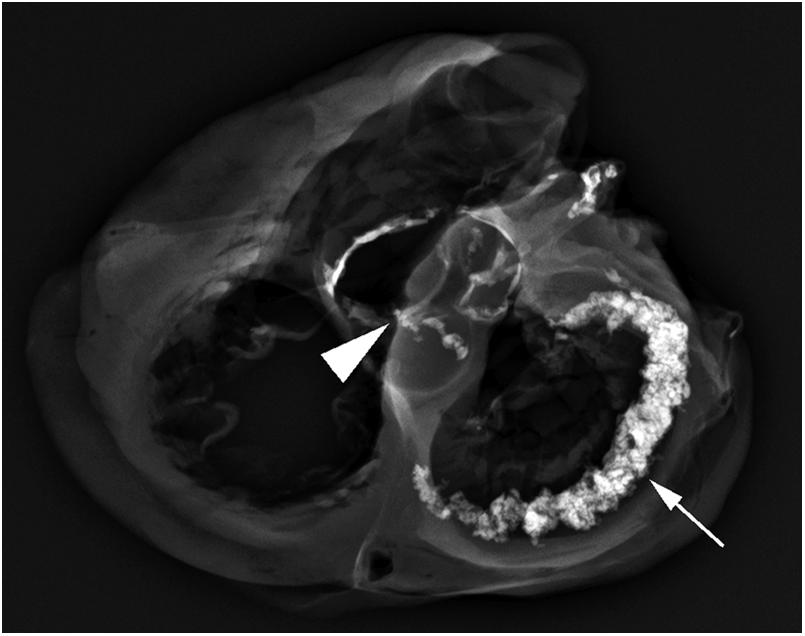
Economically responsible practice is good practice. Therefore consideration must be given as to whether to perform histologic sections on all specimens. For medicolegal purposes, pathologists must practice according to the standards established in their communities. In other words, if all cardiovascular pathologists in a city or region submit every specimen for histology, it would be prudent to do so. Useful recommendations have come from the Society for Cardiovascular Pathology and the Association of European Cardiovascular Pathology . For example, some groups do not perform routine histologic examinations of heart valves, incidentally removed right atrial appendages, varicose veins, and aortic buttons removed during bypass grafting. However, one must have a low threshold to perform histologic analysis should a suspicious gross finding be encountered. If gross examination alone is performed, the report should reflect such. Retaining the gross specimen for a period of time after case sign-out is strongly encouraged, particularly if only gross analysis was done. If additional information comes to light that would prompt histological evaluation, it will then be available for such.
Cleanliness and organization at the workbench are essential to prevent contamination of the area and to prevent the spread of tissue contamination from one case to another. Copious use of water and handling each specimen on a clean surface helps to maintain a clean workspace. No more than one specimen container should be open at a time.
In examination, the description must be exact and detailed. The description must include information about the source of the specimen, the number and size of fragments included in the container, measurements, and (if appropriate) the specimen weight. The specimen should also be described in terms of shape, surface, edge, color, consistency, and relationships. With the widespread use of digital photography, a relatively low threshold should be maintained for deciding whether or not to photograph gross specimens.
Histologic sampling must be representative. In some instances, all of a specimen, especially if it is small, is embedded, with that fact noted in the report. Very small specimens may be wrapped in tissue paper and stained grossly with 10% eosin to help in identification when the paper is unwrapped. Tissue blocks must be no thicker than 3 mm and should not overfill the cassette in which they are placed. Histopathology cannot be expected to deliver good sections if the material supplied to them is less than ideal. Tissue should always be decalcified if the blade grates during sectioning; rapid decalcification does not delay processing. Only rarely, when calcification is very extensive (e.g., calcification that affects the posterior mitral annulus), need processing be prolonged and done before sections are cut. Metal staples should be removed. Also, if blocks must include suture, cloth, or other unusual material, the histopathology laboratory should be advised of their presence. Processing may be completed overnight or by more rapid methods.
Of the routine stains available, hematoxylin and eosin (H&E) and Verhoeff-van Gieson stains are the most commonly employed (an elastic stain being essential for assessment of vascular specimens). In examining cardiovascular lesions, a pathologist employs the full barrage of other histochemical, immunohistochemical, and molecular diagnostic techniques available and may employ morphometry or other techniques in examination.
For some specimens, protocol examinations and templates may be appropriate. Protocols help to teach, and ensure consistency, whereas templates speed report preparation and help consistency. Again, time can be saved by predetermining the type of special stains that are required for certain gross findings and by ordering stains when blocks are submitted.
Whether the pathologist supplies both gross and microscopic descriptions with diagnosis in a report or a gross description with a diagnosis, depends on local practice. In either instance, an explanatory comment relating clinical and pathologic findings is often useful to the clinical team.
When the pathologist dictates reports, it is appropriate to establish both the patient’s name and surgical accession number. Use of short sentences is best, with avoidance of traditional yet nonsensical duplications, such as “yellow in color .” Ultimately, the pathologist is responsible for the report.
While it is not possible to account for each and every cardiovascular specimen that may be encountered in clinical practice, we will attempt to address the most commonly encountered types and present general approaches to the handling and processing of such. No mention is made in this chapter of certain devices used to treat or ameliorate cardiovascular disease (such as ventricular assist devices or artificial hearts). Additionally, the handling of native or donor hearts from patients who have received a transplant or of endomyocardial biopsies is also not discussed. That information is presented in Chapter 8, Chapter 14, Chapter 17 , respectively.
Vascular specimens include a wide variety of sample types, including (but not limited to) aortic resections, temporal artery biopsies, endarterectomy specimens, and varicose veins. The one commonality among these specimens is the necessity for the pathologist to evaluate the specimens with a standard H&E stain as well as some type of elastic stain (e.g., Verhoeff-van Gieson, Movat pentachrome, Elastic-van Gieson). The latter is essential for proper examination of the vessel wall. In general, vascular specimens are typically evaluated in cross section. One exception to this rule is when the clinical concern is fibromuscular dysplasia, where longitudinal evaluation can help to see the segmental nature of the disease over the course of the vessel .
Aortic specimens are generally procured during the course of surgical repair of aortic aneurysms, dissections, or congenital forms of aortic disease (e.g., coarctation, vascular rings). The type of resection should be noted as either segmental or partial, with the former typically consisting of a complete circumference of aorta, the latter representing an opened specimen. Overall (aggregate) size, thickness, and any gross pathology (atherosclerosis, dissection, fibrosis) should be documented. Generally, examination of between six and eight full-thickness sections of aortic wall sampled from various areas of the specimen is sufficient for evaluation.
Temporal arteries, biopsied to evaluate for vasculitis, should be serially cross-sectioned following documentation of the size and character of the sample. Sectioning is best done after processing at the time of embedding. It is imperative to evaluate complete cross sections of the vessel, so proper orientation is paramount. In general, each section should be evaluated at multiple levels (20–40). Several levels may be put on a single slide. Findings may be focal and examination of levels may be evaluated for features of healed arteritis or prior traumatic injury.
Endarterectomy specimens are procured during the course of the therapeutic removal of a luminal obstruction, usually by atherosclerosis or intimal fibroplasia. Not all institutions routinely process these specimens for gross or histologic examination. However, vascular pathology can be identified by the pathologist when the vessel wall is examined. The specimens should be measured and the remaining description should include degree of luminal obstruction (stenosis).
Both atrioventricular (mitral and tricuspid) and semilunar (aortic and pulmonary) valves may be resected for stenosis or regurgitation. Of these, aortic and mitral valves are the most frequently encountered, with the former typically being resected for stenosis and the latter typically being resected for regurgitation. Regardless of which valve is being evaluated, knowing the reason for resection as well as whether there is a clinical suspicion of endocarditis is essential at the time of grossing. Occasionally, the entire valve will have been resected, but part of the tissue will have been sent to the microbiology laboratory for culture. Correlating with these microbiologic reports and incorporating the culture results creates a more complete pathology report .
Atrioventricular valves should be measured, both in terms of the size of resected leaflet(s) and the length of the attached cords and whether papillary muscle is included. The presence and location of calcification should be noted as well as the thickness of the leaflet and cord tissue. A good rule of thumb is if the valve is translucent and color can be seen through the tissue, it is probably not appreciably thickened. Myxomatous degeneration (which often causes prolapse and regurgitation) will often impart a thickened and rubbery appearance to the valve, which should be spelled out in the gross description. Both the cords and the commissures (if present) should be evaluated for fusion. The former is usually only evident with complete valvular resection. The leaflets should be evaluated for the presence of perforation (which may indicate active or healed endocarditis) and the entire specimen should be evaluated for thrombus or vegetation.
Valves are best examined, histologically, with an H&E stain and an elastic stain. Stains for microorganisms (Gram, silver stains, Giemsa, periodic acid Schiff with diastase digestion) should be ordered if vegetation is found or if there is clinical concern for endocarditis . If papillary muscle is received, it too should be evaluated histologically, usually by sectioning it longitudinally.
In the case of semilunar valves, the cusps should be quantified and measured. Lining the cusps up and measuring the total annular circumference and then the average cusp width is often adequate. If one cusp is remarkably larger or smaller than the others, it also should be indicated. The presence and size of Lambl excrescences (arising on the closing surface of the valve) should also be noted. As in the case of the atrioventricular valves, the specimen should be examined for calcification, vegetation, commissural fusion, and perforation. In terms of commissural fusion, some attempt should be made to decipher whether the fusion appears to be congenital or acquired.
Congenital fusion typically produces an obtuse angle between adjacent cusps, and the ventricular aspect of the conjoined cusps appears relatively smooth. Acquired fusion typically results in an acute angle between the fused cusps and a small cleavage plane between them that can be seen on their ventricular aspects. Congenitally bicuspid valves will also often have a raphe at the point of fusion on the arterial aspect of the valve. The raphe may be either fenestrated (atypical bicuspid variant) or nonfenestrated.
Routine histologic evaluation of semilunar valves, particularly those resected for degenerative stenosis or congenitally bicuspid valvular stenosis, may not be seen as necessary in all institutions. However, if any vegetation or suspicious lesion is present on the cusp tissue, microscopic evaluation is mandatory. As with atrioventricular valves, H&E and an elastic stain is sufficient to evaluate the valve structure and special stains for microorganisms can be added at the dissection of the pathologist.
Prosthetic heart valves can be either of the mechanical or bioprosthetic variety. Bioprosthetic valves can be further subdivided into bovine pericardial, porcine bioprosthetic, and homograft valves. New generation transcatheter valves (deployed percutaneously) may also be encountered by the pathologist . Like native valves, close correlation with the hemodynamic profile that prompted removal is paramount. Importantly, prosthetic valves removed in the setting of periprosthetic leak may have no gross pathology whatsoever. Radiography is useful for valve type identification, assessment of integrity of the prosthesis, and assessment of calcifications.
The mechanical or bioprosthetic leaflets/cusps should move freely at the time of grossing. The valves should be examined for the presence of thrombus, obstructive fibrous ingrowth (pannus), and vegetation. Careful evaluation of the sewing ring for vegetation is also necessary. Histologic evaluation of vegetation and thrombus is mandatory.
Myocardial specimens are typically produced during septal myomectomy (for either hypertrophic cardiomyopathy or with aortic stenosis), ventricular assist device implantation, atrial appendectomy, and endomyocardial biopsy. The amount of tissue received varies based not only by the type of procedure, but can be highly variable within each procedure. The gross report should include the size and number of specimens received. It should also document the character and consistency of the samples. Sample weight is also a useful indicator of size (particularly for septal myomectomies). Samples should be cut to include all layers of the sample wall (from endocardium to epicardium). The presence of mural thrombus should always be documented, both grossly (if apparent) and histologically.
In addition to H&E staining, special stains for amyloid (Congo red, sulfated Alcian blue) may be helpful. Generally, if the clinical history is suspicious for amyloidosis or the patient is >65 years old, amyloid stains ordered up front can be helpful. Other special histochemical and immunohistochemical stains can be added as needed.
Pericardial tissue, procured most commonly during pericardiectomy for recurrent effusion or pericardial constriction, is usually sectioned easily but may be heavily calcified. After describing the specimen, 4–6 full-thickness samples should be evaluated histologically. Sampling of the heavily calcified areas is not often informative. In trying to determine the cause of pericarditis, it is better to see H&E sections before ordering special stains that may define pathogenesis.
Cardiac masses are the most likely cardiovascular specimen to prompt a surgeon to ask for an intraoperative diagnosis, necessitating frozen section analysis by the pathologist. Cardiac masses are typically handled like masses in other locations. They are described in terms of size and character (color, consistency, etc.). Additionally, whether or not they appear infiltrative or well circumscribed should be noted as well as whether or not they are sessile or polypoid. Ink may be useful to help keep the sample oriented and in the evaluation of resection margins. In general, the adage of one section of tumor examined histologically for each centimeter of greatest tumor size serves as a good starting point for evaluation if the diagnosis is not well defined at the time of grossing. Communication with the surgeon is essential as the specimen location and correlation with preoperative imaging may be helpful.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here