Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The neurodegenerative disease spinal muscular atrophy (SMA) is the leading genetic cause of infant mortality worldwide. Incidence and prevalence estimates vary widely across populations sampled; studies have estimated the incidence of SMA at 7.8 in 100,000 births (Northeast Italy), 9.80 in 100,000 births (West-Thuringen, Germany), and 3.53 per 100,000 births (Cuba). The study in Cuba further analyzed incidence by self-reported race, and noted a stark contrast between the incidence of SMA in whites (8 per 100,000 births) and blacks (0.89 per 100,000 births). A summary of incidence studies is provided in Table 5.1 .
| Authors | Year | Location | Years | Method | Subtypes | Reported annual incidence | Incidence per 100,000 live births |
|---|---|---|---|---|---|---|---|
| Pearn | 1978 | Northeast England | 1956–72 | Census and hospital registry data | Type II, III | 1/24,100 | 4.15 |
| Spiegler et al. | 1990 | Warsaw | 1976–85 | Hospital and genetic center survey | Type I, II, III | 1/19,474 | 5.14 |
| Burd et al. | 1991 | North Dakota | 1980–87 | Review of death certificates | Type I | 1/6,720 | 14.88 |
| Mostacciuolo et al. | 1992 | Veneto, Italy | 1960–83 | Hospital survey | Type I, II, III | 7.8/100,000 | 7.80 |
| Thieme et al. | 1993 | West-Thüringen, Germany | 1974–87 | Hospital survey | Type I | 1/10,202 | 9.80 |
| Zalvidar et al. | 2005 | Cuba | 1996–2002 | National database review | Type I | 3.53/100,000 | 3.53 |
Overall prevalence of SMA is estimated at 1 per 10,000 people. Twice as many boys as girls are affected, and female cases tend to be less severe, with female incidence of SMA decreasing with age. Approximately 60% of cases are classified as Type I (inability to sit, onset <6 months, early respiratory failure), 27% of cases are Type II (onset 6–18 months, ability to sit but not stand), 12% are Type III (onset >18 months, ability to stand), and 1% are Type IV (adult-onset, proximal limb weakness but no major muscular impairment).
SMA is caused by mutation or deletion of the survival motor neuron (SMN) gene. Since the autosomal recessive inheritance pattern of the condition was discovered, many studies have taken advantage of large sets of screening data to examine rates of carrier frequency across populations. Screening tests identify carriers as individuals with one functional copy of the SMN1 gene. Carrier frequencies vary substantially based on race. A 2012 study of 72,453 specimens at the Genzyme Genetics Molecular Diagnostic Laboratory in Westborough, MA, USA, found the following carrier frequencies across six racial categories: Caucasian, 1 in 47; Ashkenazi Jew, 1 in 67; Asian, 1 in 59; African American, 1 in 72; Hispanic, 1 in 68. The overall carrier frequency was calculated to be 1 in 54. A population-based cohort study in Taiwan found a carrier frequency of 1 in 48 based on screening of 107,611 pregnant women.
The first documentation of SMA appeared in the literature in the 1890s. As many additional cases were described over the next century, the disease was gradually organized into subtypes based on severity. By the middle of the 20th century, three subtypes were established, based on onset and clinical progression criteria. In 1995, the discovery of the genetic underpinnings of SMA revolutionized our understanding of this disease and offered the promise of new therapeutics.
Written documentation of the natural history of SMA dates to 1891, when Guido Werdnig, an Austrian neurologist at the University of Graz, wrote case studies on 3 year old Wilhelm Bauer and his 1 year old brother. Wilhelm developed weakness in his proximal limbs at about 1 year of age, and over the subsequent 2 years progressively lost muscle tone, voluntary movements, and the ability to swallow and hold up his head. Just before Wilhelm’s 3rd birthday, Werdnig noted that the boy exhibited weakened muscles, large fat deposits, flexed legs, tremor in hands and arms, and very limited, labored movement.
Less than a month after this clinical examination, Wilhelm developed rales and exhibited retraction of intercostal muscles. Two days later, he developed dyspnea and fever; several days after the onset of dyspnea, he passed away. His younger brother lived until 6 years of age and died of similar respiratory complications. Werdnig completed a microscopic examination of the older brother’s spinal cord cross sections and gastrocnemius muscle. Within the second and third cervical nerves, he noted several abnormal findings: regions of the lateral tracts lacking in myelinated fibers, degenerated anterior funiculi, indistinct processes in the ganglion cells of the anterior horn of the spinal cord, and many empty cell-beds. Posterior horns and roots were observed to be normal ( Fig. 5.1 ). These findings were consistent across many of the cervical, thoracic, and lumbar sections.
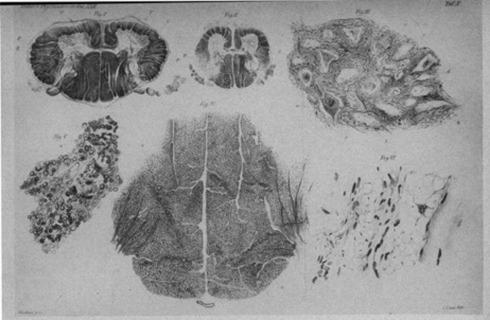
Upon examination of the gastrocnemius muscle, he noted groups of muscle fibers separated from one another by large masses of fatty tissue. These fibers included single fibers with disrupted cross-striations and degraded contractile tissue that resembled flattened tubes with nuclei ( Fig. 5.1 ).
Based on the clinical history and autopsy, Werdnig concluded that Wilhelm, along with his younger brother who similarly lost muscle function in his legs yet maintained normal sensation, had an infantile, familial muscular atrophy. This illness, Werdnig posited, resembled a neurological illness due to its swift course of atrophy. Thus, it was distinct from muscular dystrophy, or a slow degradation of muscle mass due to a lack of functional protein in the muscle.
Over the next decade, German neurologist Johann Hoffmann published five papers in accord with Werdnig’s conclusions. He documented a total of seven cases across four families, all with onset around 1 year of age. Age of death varied between 14 months and 5 years. Hoffmann’s autopsies echoed two of Werdnig’s key observations: atrophy of muscle in the extremities (with proximal onset) and damaged cells in the anterior horn of the spinal cord. Hoffmann agreed with the majority of Werdnig’s evidence, disputing only his finding that affected children exhibited hand tremor. Through these case studies, Hoffmann reinforced evidence for a familial, early-onset motor neuron disease, which he called spinale muskelatrophie , or “spinal muscular atrophy.”
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here