Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Amniotic fluid (AF) is necessary for normal human fetal growth and development. The fluid volume cushions the fetus, protecting it from mechanical trauma, and its bacteriostatic properties may help to maintain a sterile intrauterine environment. The space created by the AF allows fetal movement and aids in the normal development of both the lungs and the limbs. Finally, AF offers convenient access to fetal cells and metabolic byproducts, and it has been used for fetal diagnosis more often than any other gestational tissue.
The existence of AF has been appreciated since ancient times. Leonardo da Vinci drew the fetus floating in the fluid, and William Harvey hypothesized that AF provided nourishment to the fetus. However, it was only in the late 19th century that AF became available for study other than at delivery, and fluid sampling by amniocentesis was rarely performed until the second half of the 20th century. Genetic amniocentesis for fetal diagnosis (i.e., for sex determination) was first performed in 1956. Research on the characteristics of AF is therefore a relatively recent phenomenon. This chapter reviews the current state of knowledge regarding the volume, composition, production, resorption, and volume regulation of AF.
In the first trimester of pregnancy the amnion does not contact the placenta or decidua, and the amniotic cavity is surrounded by the fluid-filled exocoelomic cavity. The exocoelomic fluid participates in the exchange of molecules between mother and fetus; the function of the AF at this early gestational period is uncertain. Importantly, coelomic fluid may contain antioxidants and proinflammatory cytokines and cytokine receptors that may influence placental function and fetal and placental development. , By the end of the first trimester of human gestation the exocoelomic cavity progressively obliterates, and the amniotic cavity is the only significant deposit of extrafetal fluid.
AF volumes have been directly measured in the first half of pregnancy, during which time the volume increases logarithmically. AF volumes were first estimated in the latter two-thirds of human pregnancies through the use of dye dilution techniques. , These original quantitative findings have been supported by semiquantitative measurements of AF volume performed with ultrasound ( Fig. 4.1 ).
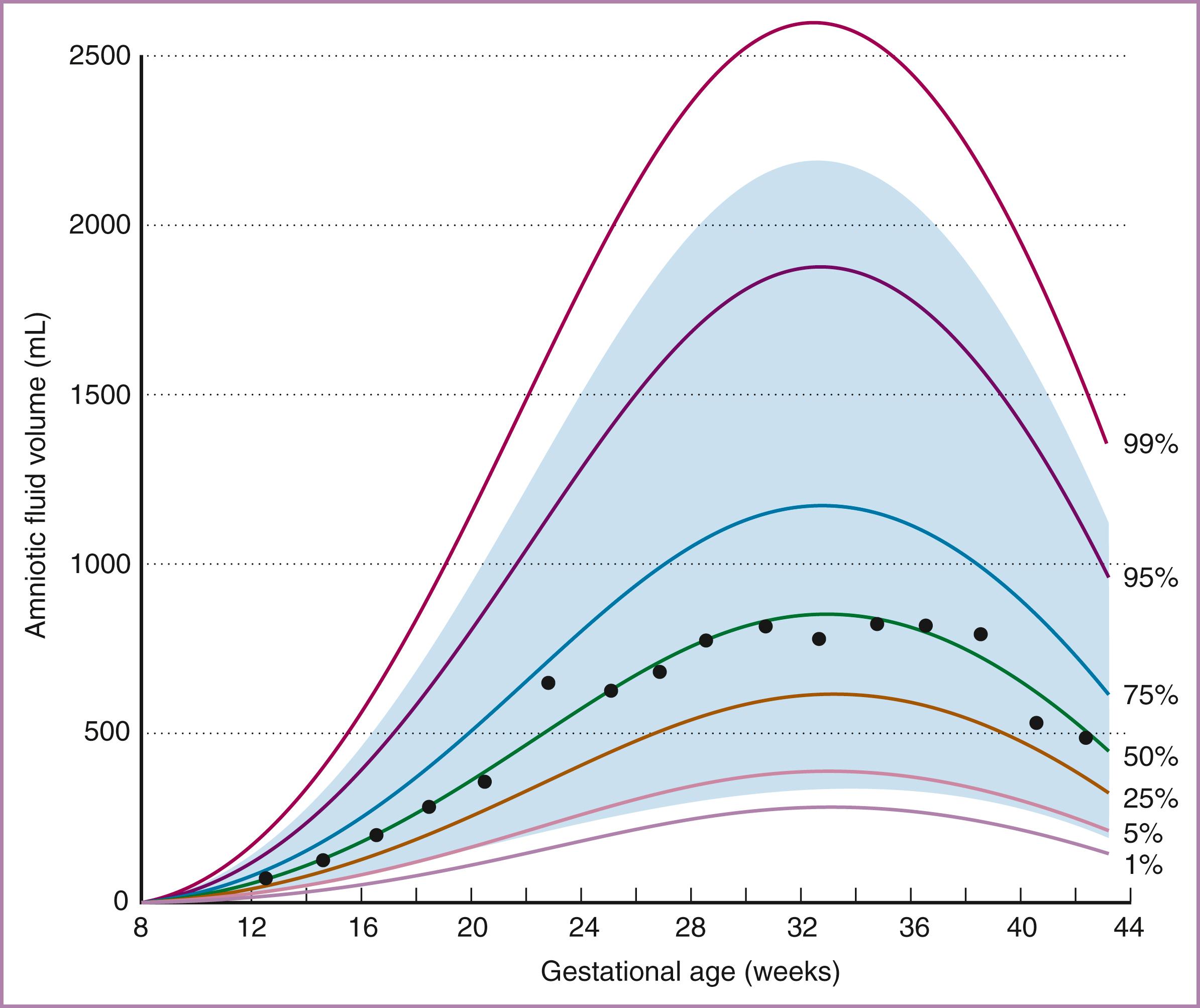
Each of these methods demonstrates that AF volume increases progressively between 10 and 30 weeks of gestation. Typically, volume increases from less than 10 mL at 8 weeks to 630 mL at 22 weeks and 770 mL at 28 weeks of gestation. After 30 weeks the increase slows, and AF volume may remain unchanged until 36 to 38 weeks of gestation, when it tends to decrease. As a pregnancy proceeds past the due date, AF volume decreases sharply, averaging 515 mL at 41 weeks. Subsequently, there is a 33% decline in AF volume per week, consistent with the increased incidence of oligohydramnios in postterm gestations.
The rate of change of AF volume depends on the gestational age. Total AF volume increases at a rate of 10 mL/wk at the beginning of the fetal period, increasing to 50 to 60 mL/wk at 19 to 25 weeks’ gestation and then decreasing until the rate of change equals zero (i.e., volume is at maximum) at 34 weeks. Thereafter total AF volume falls, with the decrease averaging 60 to 70 mL/wk at 40 weeks’ gestation. Although the complete mechanisms that produce these alterations in AF volume throughout gestation are unclear, it is important to note that, when expressed as a percentage, the rate of change decreases consistently throughout the fetal period. Therefore the decrease in AF volume near term represents a natural progression rather than an aberration.
The volume of AF may be dramatically altered in pathologic states. Excessive AF volume (polyhydramnios) may total many liters, and the volume of AF in conditions of reduced fluid (oligohydramnios) may be near zero. Fetal anatomic abnormalities such as renal agenesis or esophageal atresia may affect the normal processes for production and resorption of AF, respectively, leading to abnormal AF volumes. In addition, transient changes such as maternal dehydration or fetal anemia may alter AF flow and therefore AF volume. AF volume abnormalities may also occur without apparent cause. Abnormalities of AF volume in general have been associated with poorer perinatal outcomes. Specific issues are discussed elsewhere in the text.
The AF in the first trimester of pregnancy has rarely been the subject of study. It appears that human AF in the first trimester is isotonic with maternal or fetal plasma but contains minimal protein components. First-trimester AF also demonstrates an extremely low oxygen tension and an increased concentration of sugar alcohols, the product of anaerobic metabolism. In the second half of pregnancy the human fetus produces dilute urine, which is a major component of AF, causing the AF composition to diverge from that of serum. In particular, human AF osmolality decreases by 20 to 30 mOsm/kg with advancing gestation to levels approximately 85% to 90% of maternal serum osmolality. In the same period, AF urea, creatinine, and uric acid increase, resulting in AF concentrations of urinary byproducts two to three times higher than in fetal plasma.
It is thought that early AF arises as a transudate of plasma, either from the fetus through nonkeratinized fetal skin or from the mother across the uterine decidua or the placenta surface or both; however, the actual mechanism is unknown. Production and resorption of AF have been extensively studied in the latter half of pregnancy, most commonly in the sheep model, though recent ultrasound studies have provided insights into human fetal urine production and swallowed volumes. Evidence suggests that the entire volume of AF turns over on a daily basis, making this a highly dynamic system. The volume of AF is influenced by a complex interplay of productive and absorptive mechanisms ( Fig. 4.2 ). These mechanisms act to maintain AF volume, and there is some evidence that they may be regulated to normalize AF volume in pathologic conditions.
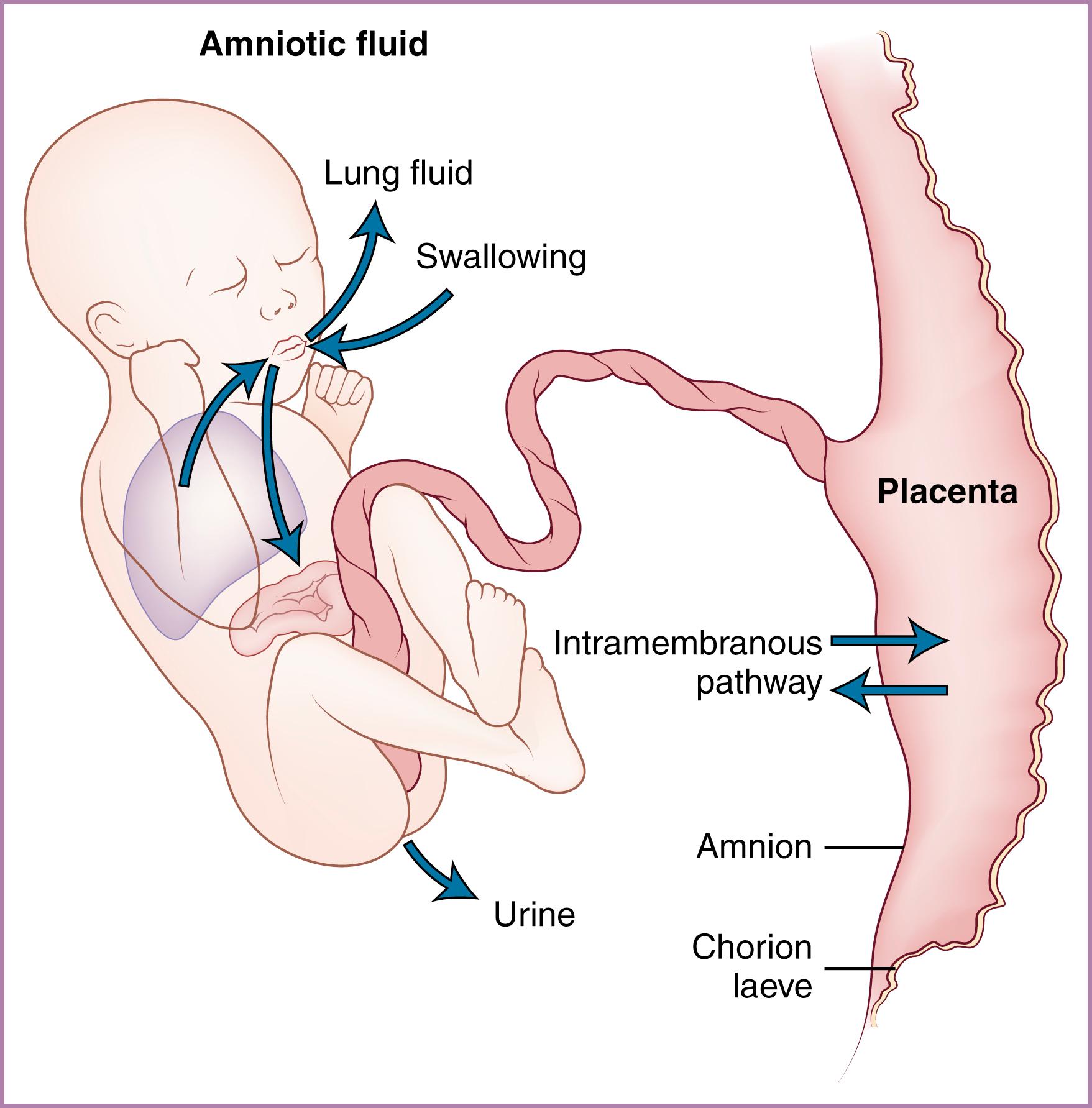
The major contributors to AF volume in the latter portion of pregnancy are fetal urine and fluid produced by the fetal lung. Minor contributors are transudation across the umbilical cord and skin and water produced as a result of fetal metabolism. Although some data on these processes in the human fetus are available, the bulk of the information about fetal AF circulation derives from animal models, primarily the sheep.
Although the mesonephros can produce urine by 5 weeks of gestation, the metanephros (the adult kidney) develops later, with nephrons formed at 9 to 11 weeks, at which time fetal urine is excreted into the AF. The amount of urine produced increases progressively with advancing gestation, and it constitutes a significant proportion of the AF in the second half of pregnancy. The amount of urine produced by the human fetus has been estimated by the use of ultrasound assessment of fetal bladder volume. Although there is uncertainty regarding the accuracy of noninvasive measurements, human fetal urine output appears to increase from 110 mL/kg/24 h at 25 weeks to almost 200 mL/kg/24 h at term, , in the range of 25% of body weight per day or almost 1000 mL/day near term. , In near-term fetal sheep, with direct methods used for measuring urine production rates, similar high values have been found. There may be a tendency for the urine flow rate to decrease after 40 weeks’ gestation, particularly if oligohydramnios is present.
Reduction or absence of fetal urine flow is commonly associated with oligohydramnios, indicating that urine flow is probably necessary to maintain normal AF volume. The mature fetus can also respond to changes in internal fluid status by modulating urine flow via endocrine regulation of renal blood flow or tubular resorption. In sheep, increased fetal blood pressure stimulates fetal secretion of atrial natriuretic factor and an accompanying diuresis, whereas increased plasma osmolality stimulates fetal vasopressin secretion and an antidiuretic response. , These findings indicate that AF volume could be regulated through the mechanism of altered fetal urine flow. However, in sheep, fetal hypoxia increases urine flow, but AF volume is maintained. These data suggest that regulation of AF volume is mediated by other mechanisms in addition to changes in urine production.
Three-dimensional ultrasound studies have provided assessments of hourly human fetal urine production rates (HFUPR). Among patients with oligohydramnios at term, HFUPR was decreased in those with intrauterine growth restriction (IUGR) when compared to normal controls at term but was not decreased in patients with isolated oligohydramnios. Similarly, HFUPR is significantly increased in cases with increased amniotic fluid index (AFI) without fetal anomalies. These findings emphasize the critical role of urine flow in abnormalities of AF volume and emphasize the potential value of ultrasound assessments to differentiate causes of altered AF volume.
It appears that all mammalian fetuses secrete fluid from their lungs. The AF phospholipids (lecithin, sphingomyelin, and phosphatidylglycerol) used to predict human fetal lung maturity are evidence that human fetuses are not exceptions to this statement. The rate of fluid production by the human fetal lungs has not been measured, and available data are derived from the ovine fetus. During the last third of gestation, the fetal lamb secretes an average of 100 mL/day per kilogram of fetal weight from the lungs. Under physiologic conditions, half of the fluid exiting the lungs enters the AF and half is swallowed ; therefore although total lung fluid production approximates one-third that of urine production, the net AF contribution made by lung fluid is only one-sixth that of urine. Fetal lung fluid flow is mediated by active transport of chloride ions across the lung epithelium and is isotonic to plasma, in contrast to the increasingly hypotonic urine. Lung fluid production is affected by diverse fetal endocrine factors. Increased arginine vasopressin, catecholamines, and cortisol decrease lung fluid production, effects that may help to explain the enhanced clearance of lung fluid in fetuses delivered after labor compared with elective cesarean delivery. , Almost all active stimuli have been demonstrated to reduce production of fetal lung liquid, indicating that lung liquid production functions at maximal capacity. Ovine fetuses with tracheal occlusion (used as a treatment for severe diaphragmatic hernia) demonstrate only a minor reduction in AF volume. Modulation of lung fluid production is therefore unlikely to be a significant regulator of AF volume. In addition, experiments in instrumented ovine fetuses failed to demonstrate any effect of lung liquid on the regulation of AF volume. , Current opinion is that fetal lung fluid secretion is likely most important in providing for pulmonary expansion, which promotes airway and alveolar development.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here