Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Microscopically, the pancreas is composed of epithelium (acini, islets, and ducts), connective tissue, and lymphoid tissue. These components may be variably present within any individual pancreatic biopsy, depending on the location and adequacy of the tissue sample. The parenchyma is divided into lobules separated by loose connective tissue septa. Because the pancreas functions as both an exocrine (secretory) and endocrine organ, its parenchyma includes both exocrine and endocrine elements intimately mixed. The exocrine glands (acini) and their associated duct system comprise the majority of the lobular parenchyma and up to 85% of the total volume of the adult pancreas. The exocrine glands are composed of a single layer of triangular-shaped cells radially arranged to form spherical or tubular acini that each surround a central lumen. These acinar cells have round, basally-located nuclei with evenly distributed chromatin and small nucleoli. The cells appear granular because of their cytoplasmic secretory granules, including the basophilic basally located granules and the eosinophilic granules concentrated toward the lumen.
The duct system transports the luminal acinar secretions to the duodenum. This system begins with the intercalated ducts that drain each acinus. These ducts then fuse to form the intralobular ducts. The intralobular ducts converge to form the interlobular ducts and ultimately the large main pancreatic ducts. All of these ducts demonstrate a smooth and regular contour without angulation and vary in their epithelial lining. The intercalated ducts are lined by a single layer of cuboidal epithelium lacking mucin that transitions to columnar, mucinous epithelium within the larger pancreatic duct. Accessory mucous glands surround larger pancreatic ducts. The duct epithelial nuclei are round, basally located, and have evenly distributed chromatin.
The endocrine component is present primarily within the islets of Langerhans cells, which are randomly distributed throughout the pancreas. The islets are usually found as single, compact clusters of cells associated with a rich capillary network. They can also be seen as diffuse islets with ill-defined borders in the inferior portion of the pancreatic head. The cells within the islets are a mixed population of four major types, including beta, alpha, delta, and pancreatic polypeptide (PP) cells, each of which secrete a different hormone. These endocrine cells typically contain nuclei with coarsely clumped chromatin and abundant cytoplasm.
Variable amounts of connective tissue, including collagen and adipose tissue, are present within and outside the pancreas. In fact, the pancreatic parenchyma is divided into lobules by loose connective tissue septa supporting ducts, vessels, and nerves. The interlobular and main pancreatic ducts are surrounded by a thicker layer of collagen than the smaller ducts. Adipocytes can be scattered within the pancreas and are usually most abundant in the surrounding peripancreatic tissue. The distribution of collagen and fat in the pancreas is important to keep in mind when considering the location of a particular biopsy and whether it represents intraparenchymal or extraparenchymal tissue in the biopsy sample.
Lymphoid tissue may be present within the pancreas as intrapancreatic lymph nodes, lymphoid follicles, or small aggregates of lymphocytes. The lymphoid follicles include mucosa-associated lymphoid tissue (MALT). The follicles can be unstimulated primary follicles or secondary follicles with a germinal center. Small and mature lymphocytes, specifically T-lymphocytes, are the predominant cell population within the lymphoid tissue.
Plasma cells may be present within the lymphoid tissue as well. Normal, mature plasma cells show abundant deep blue cytoplasm and one to two nuclei with a coarse “clock face” chromatin pattern. A perinuclear clearing, or “hof,” representing the prominent Golgi apparatus may be visible as well. Occasionally, intracellular or extracellular immunoglobulin in the form of a variably sized and well-circumscribed round pink structure may be present. The coarse nuclear chromatin pattern and abundant cytoplasm of the plasma cell may appear similar to the endocrine cell histology described earlier. Anecdotally, endocrine cells and plasma cells can be difficult to distinguish from each other, particularly during an intraoperative frozen-section consultation. In scenarios in which neoplastic plasma cells are suspected and cannot be confirmed immediately, fresh tissue may be processed or held for immediate or future flow-cytometry studies, respectively.
Inflammatory cells found outside of normal lymphoid tissue are considered abnormal, especially in the setting of active epithelial injury (such as in acute pancreatitis) and/or the chronic reactive changes of stromal fibrosis (chronic pancreatitis). In acute pancreatitis, destruction of acinar parenchyma is accompanied by varying amounts of necrosis and acute inflammation including neutrophils and/or eosinophils. Chronic pancreatitis is believed to occur secondary to multiple episodes of acinar cell destruction followed by an associated inflammatory response. This response stimulates repeated tissue reactions, leading to increased stromal fibrosis and changes to the residual ductal epithelium. These ducts retain a lobular configuration despite the atrophic or absent parenchyma in chronic pancreatitis. They are arranged in lobules such that a larger branched duct is surrounded by smaller round ductules. The stroma between lobules is denser than within the lobules. These residual ducts demonstrate smooth contours without sharp angles and a complete epithelial lining. The lining is made of relatively uniform cells without significant variations in their nuclear sizes. Histologically, the inflammation present in a biopsy of chronic pancreatitis is typically composed of a lymphocytic or lymphoplasmacytic population of cells. If clinically significant inflammation is present in a benign biopsy, the predominant inflammatory cell type and assessment of the type and degree of connective tissue often helps pathologists subclassify the type of pancreatitis. Accurate subclassification of an inflammatory process is clinically important because some diseases, such as autoimmune pancreatitis (AIP), are steroid responsive.
Pancreatic biopsies are most often performed for evaluation of a tumor, mass, or ill-defined lesion on imaging. Thus the main primary differential diagnosis of a pancreatic lesion typically includes neoplasia versus an inflammatory process that may, both clinically and radiologically, mimic a neoplasm. As discussed previously, chronic pancreatitis–associated lesions are believed to develop as a result of multiple repeated episodes of acinar cell destruction and subsequent inflammatory response within the parenchyma. Inflammation then stimulates repeated tissue reactions that lead to increased levels of stromal fibrosis. This stromal fibrosis can appear as a lesion radiologically, which ultimately leads to tissue sampling.
Biopsies of the pancreas can be obtained via endoscopic retrograde cholangiopancreatography or endoscopic ultrasound. Endoscopic ultrasound evaluates the pancreatic head and uncinate process through the duodenum. The pancreatic body and tail are imaged via the stomach. If clinically indicated, these areas of the pancreas can be sampled along with the proximal lymph nodes. As noted earlier, these biopsies can vary in the amount of pancreatic epithelium, connective tissue, and lymphoid tissue present.
An artifact to consider related to the tissue acquisition procedure and subsequent laboratory processing is poor fixation caused by pancreatic autolytic enzymes. Reasons for additional variations of normal pancreatic histology include patient age and the precise anatomical location of the biopsy. For example, the endocrine component is relatively increased in neonates compared with adults and can compose up to 20% of the organ. Also, diffuse islets of Langerhans can mimic a neoplastic process and are concentrated in the inferior portion of the pancreatic head.
In this chapter, we suggest an approach to pancreatic biopsies based broadly on the type and degree of the cellular and inflammatory components of the sample ( Fig. 33.1 ). With this approach, one can often place a biopsy into one of several major histological patterns of injury from which a more narrow differential diagnosis can be generated, often with the help of additional clinical or serological studies. When evaluating a biopsy, major patterns of disease include the presence of (1) normal or near-normal–appearing tissue, (2) acute pancreatitis–like changes, (3) granulomatous inflammation, and (4) chronic pancreatitis–like changes. These patterns of disease are discussed more thoroughly and individually in the remainder of this chapter.
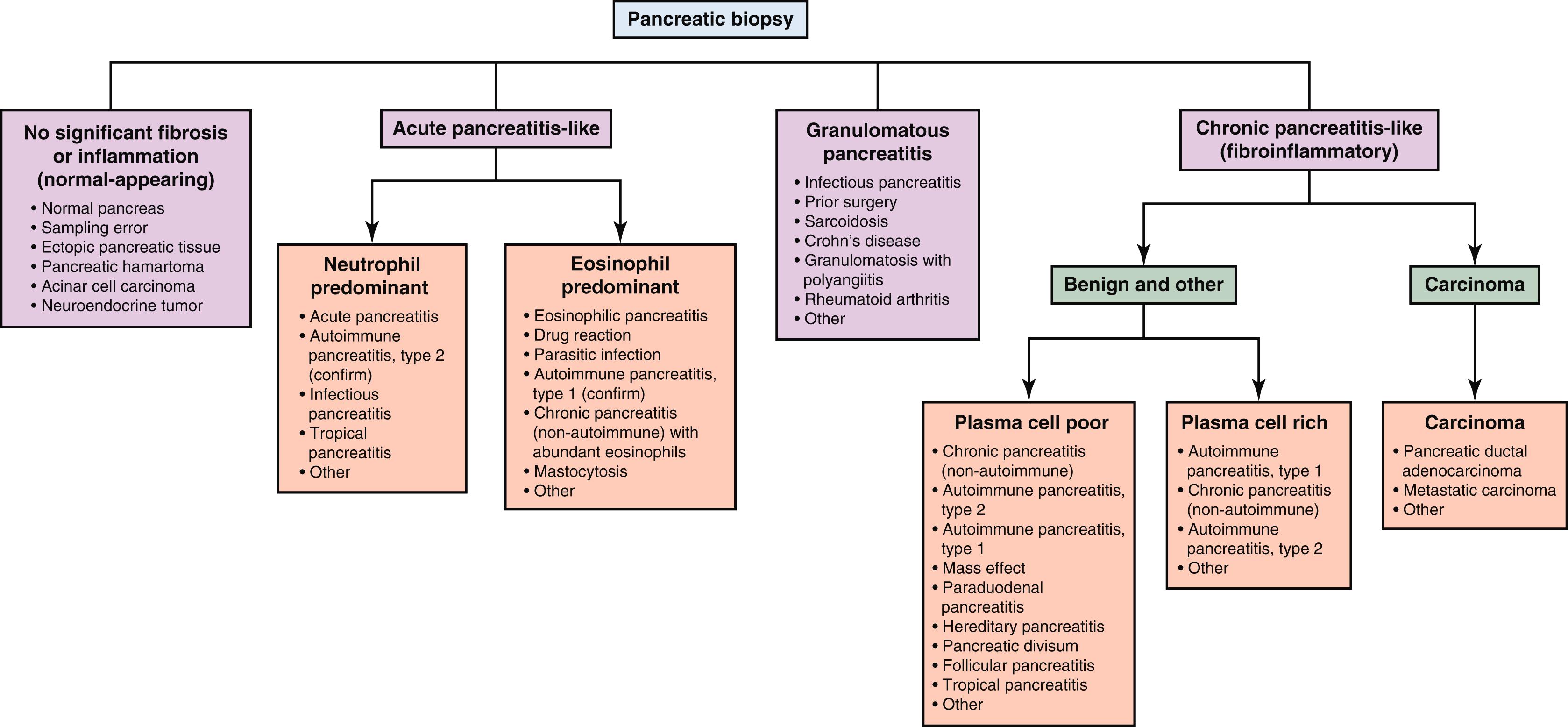
Because most pancreatic biopsies are performed for evaluation of a targeted lesion, the first step of a pathologist is to determine whether a biopsy is simply benign (inflammatory) or malignant. This can be challenging when a tumor, specifically carcinoma, is desmoplastic and/or the inflammatory process is quite fibrotic, as in some forms of chronic pancreatitis, particularly the autoimmune types. Relative to the normal highly cellular acinar/endocrine parenchyma, the normal pancreas contains only a minimal amount of stromal connective tissue. This connective tissue includes varying amounts of collagen organized around pancreatic ducts, between lobules, and within the peripancreatic tissue. Thus it is histologically “striking” when one sees an increase in paucicellular connective tissue relative to atrophic or absent parenchyma on a biopsy, and this should always raise concern for a malignant tumor.
Increases in connective tissue can be present in both epithelial malignancies and chronic pancreatitis. The most common epithelial malignancy of the pancreas, pancreatic ductal adenocarcinoma, typically displays desmoplastic stroma. Chronic inflammatory, or “chronic pancreatitis-like,” diseases can demonstrate a “fibroinflammatory” histological pattern, which includes significant stromal fibrosis accompanied by variable amounts of inflammation. Thus an initial assessment of connective tissue may be the first step in assessing a biopsy as benign or malignant.
After an epithelial malignancy, such as pancreatic ductal adenocarcinoma or metastatic carcinoma, is excluded, the presence, type, and amount of inflammation relative to fibrosis, if present, will help further subclassify the biopsy. Inflammation in the pancreas may include neutrophils, eosinophils, histiocytes (and granulomas), lymphocytes, and plasma cells. Active inflammation without granulomas or increased stromal fibrosis suggests acute pancreatitis or an “acute pancreatitis–like pattern” of injury. The inflammatory cells in an acute pancreatitis–like pattern are typically neutrophils and/or eosinophils. A fibroinflammatory pattern, as described later in this chapter, consists of increased fibrosis relative to inflammation. The chronic inflammatory cells are typically lymphocytes with variable amounts of plasma cells. Biopsies without significant inflammation, granulomas, or stromal fibrosis are considered normal or near-normal.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here