Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Whatever the cause, airway narrowing leads to expiratory flow limitation, gas trapping and hyperinflation of the lung, which manifests itself as breathlessness.
Asthma involves intermittent, reversible airway obstruction caused by airway inflammation and bronchial smooth muscle contraction, both as a result of mediators released from mast cells and eosinophils.
Chronic obstructive pulmonary disease is progressive, with a poorly reversible airway narrowing caused by airway inflammation and loss of lung tissue elasticity, mostly as a result of smoking-induced activation of airway neutrophils.
Cystic fibrosis is an inherited disease in which abnormal chloride transport in the airway impairs the normal pulmonary defence mechanisms, leading to chronic and destructive pulmonary infections.
This chapter considers the physiological changes seen in the three most common diseases of the pulmonary airways: asthma, chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF). The first two of these have many clinical and physiological features in common, and together constitute the vast majority of respiratory disease seen in clinical practice.
It is estimated that 4.3% of people have asthma worldwide, with a prevalence that has increased by approximately 50% per decade. In industrially developed countries the increasing prevalence is believed to have now levelled off at 10% to 15% of the population, and may be decreasing in some countries. Meanwhile asthma prevalence is increasing rapidly in developing countries as lifestyles change. The prevalence of asthma amongst children in the developed world increased two- to threefold in the last 50 years. Although the worldwide prevalence is no longer increasing, hospital admissions for asthma continue to rise. Fortunately, improved treatments mean that deaths attributable to asthma have been falling consistently since the 1980s, but the years of life lost because of asthma remains considerably higher in low sociodemographic index countries.
Asthma causes recurrent episodes of chest ‘tightness’, wheezing, breathlessness and coughing as a result of airway narrowing from a combination of inflammation of the small airways and contraction of bronchial smooth muscle in the lower airway. In an acute episode of asthma there are three closely related phases.
Bronchoconstriction occurs early in an asthma ‘attack’. This is particularly prominent in allergic asthma, when, within minutes of exposure to an allergen, wheezing develops. Narrowing of small airways occurs because of contraction of airway smooth muscle (ASM) in response to the cellular mechanisms described in the next section. Bronchoconstriction in different regions of the lung may not be uniform, and two different scanning techniques have shown that, in many patients with asthma, ventilation becomes heterogeneous, with clusters of poorly ventilated lung regions ( Fig. 28.1 ). , This might be expected to cause maldistribution of ventilation and perfusion, but a further study showed that blood flow to the poorly ventilated areas is also reduced, presumably illustrating the efficiency of hypoxic pulmonary vasoconstriction (page 80). It is not clear why only some asthmatic patients develop ventilation defects; one possible explanation is asymmetric airway branching patterns in susceptible subjects. What is more clear is that heterogeneity of bronchoconstriction is associated with more severe clinical disease and has significant implications for inhaled therapy, as these studies suggest that most of an inhaled drug will be deposited in the better-ventilated regions rather than where it is most needed.
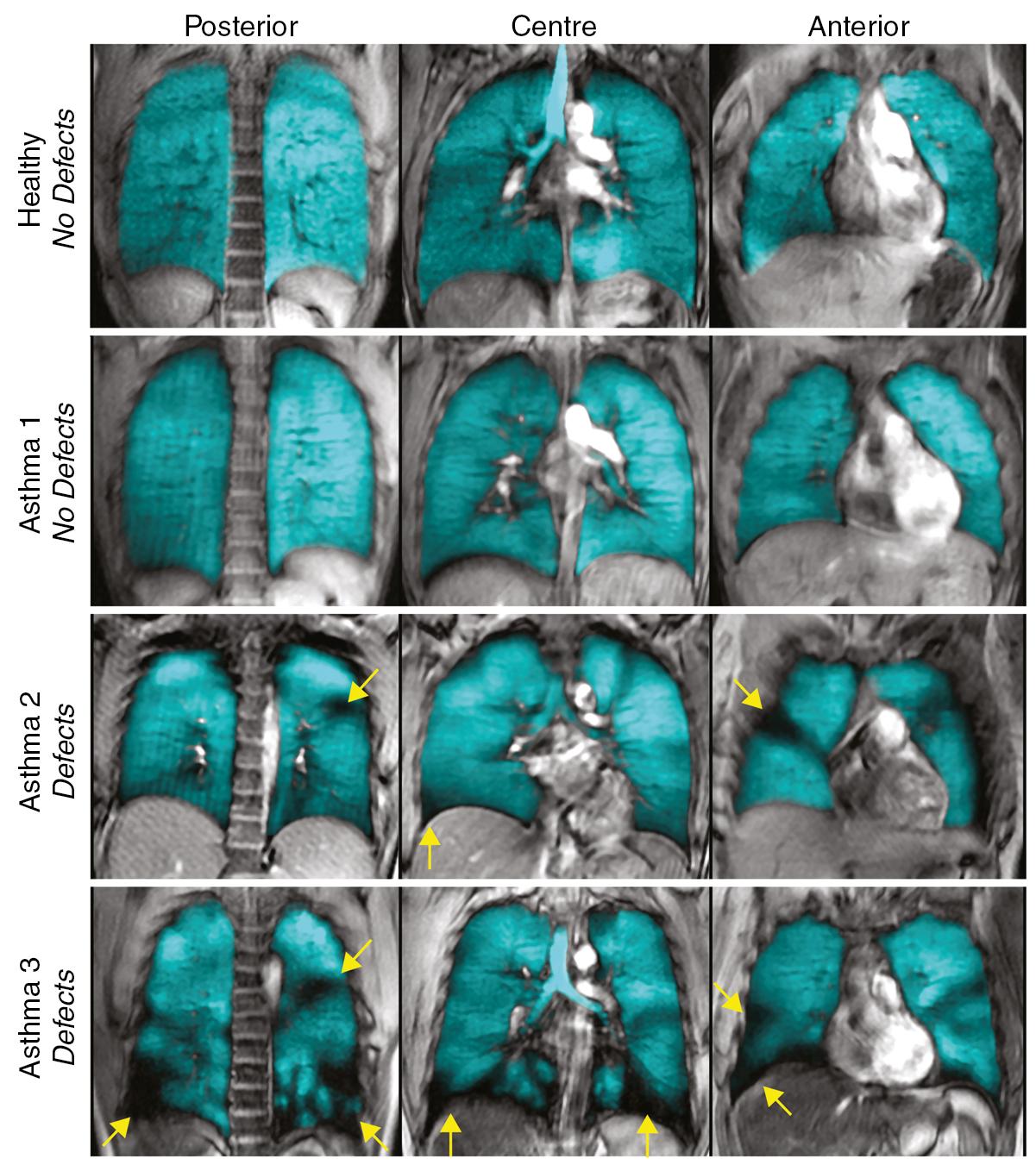
With more severe bronchoconstriction airway closure begins to occur during expiration, gas trapping occurs, and the lungs become hyperinflated. Eventually the patient is attempting to breath in when the lungs are almost at total lung capacity, and a sensation of inspiratory dyspnoea results, even though the defect is with expiration. Physiological effects of hyperinflation are described on page 332.
Bronchoconstriction may quickly subside, either spontaneously or with treatment, but more commonly progresses to a late-phase reaction.
Late-phase reactions are characterized by inflammation of the airway, and develop a few hours after the acute bronchoconstriction. Airway obstruction continues, and cough with sputum production develops. Asthma precipitated by respiratory tract infection may ‘bypass’ the acute bronchoconstriction phase, and the onset of symptoms is then more gradual.
Airway hyperresponsiveness (AHR) describes the observation that asthmatic subjects become wheezy in response to a whole range of stimuli that have little effect on normal individuals. Stimuli include such things as cold air, exercise, pollution (page 241) or inhaled drugs, and occur via the neural pathways present in normal lungs (page 34). Methacholine or histamine can be used to measure AHR accurately by determining the inhaled concentration that gives rise to a 20% reduction in forced expiratory volume in 1 second (FEV 1 ).
The term ‘asthma’ includes a wide spectrum of illnesses, varying from a wheezy 6-month-old baby with a viral infection to a young adult with repeated episodes of wheezing or an older patient with chronic lung disease. Asthma is therefore not a single disease, but more a heterogeneous group of underlying disease processes with a variety of presentations, physiological characteristics and patient outcomes. As a result, the concept of asthma phenotyping has emerged, which involves integrating biological and clinical features with the ultimate goal of providing more personalized therapy. Commonly used clinical phenotypes include:
Allergic asthma. Onset usually in childhood, associated with a history of atopy, with sputum examination typically showing eosinophilic inflammation.
Nonallergic asthma. Adult onset, not associated with allergic triggers of bronchospasm; sputum examination may reveal neutrophilia, eosinophilia or few inflammatory cells.
Late-onset asthma. Presents in adult life, more commonly in women; manifests as nonallergic wheezing.
Asthma with fixed airflow limitation. Patients with longstanding asthma develop fixed airflow limitation, thought to be secondary to airway remodelling.
Asthma with obesity. This is described on page 202.
Many cell types are involved in the pathophysiology of asthma. A summary of the interactions between these cells in allergic asthma is shown in Figure 28.2 , which also shows the principal cytokines that facilitate communication between the cells.
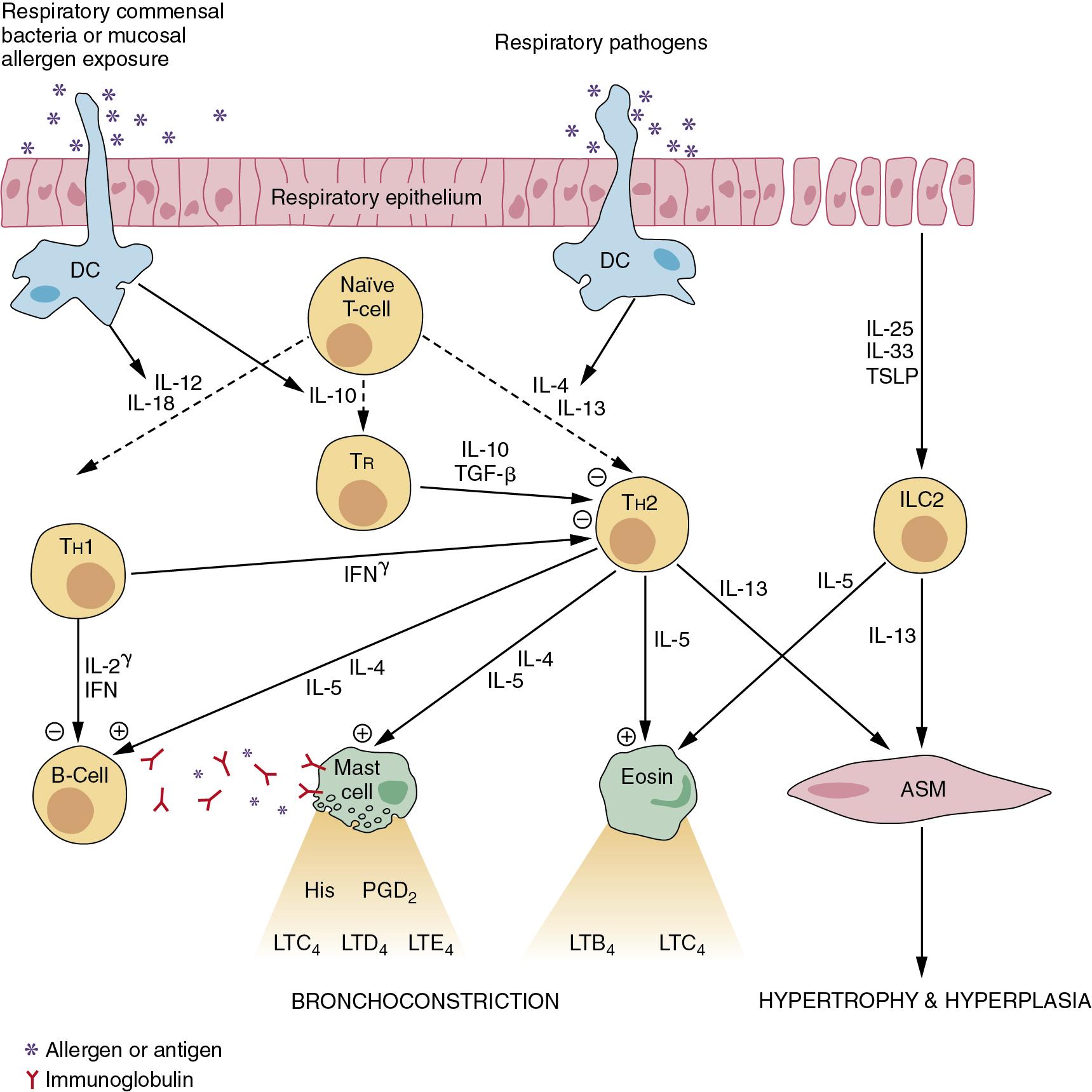
Mast cells are plentiful in the walls of airways and alveoli, and also lie free in the lumen of the airways. where they may be recovered by bronchial lavage. Mast cell activation is the main cause of the immediate bronchospasm seen in allergic asthma. The surface of the mast cell contains a large number of binding sites for the immunoglobulin IgE. Activation of the cell results from antigen bridging of only a small number of these receptors, and may also be initiated by complement fractions C3a, C4a and C5a and substance P, physical stimulation and many drugs and other organic molecules.
The triggering mechanism of the mast cell is thus extremely sensitive, and is mediated by an increase in inositol triphosphate and intracellular calcium ions. Within 30 seconds of activation, there is degranulation, with discharge of a range of preformed mediators listed in Table 28.1 . Histamine acts directly on H 1 receptors in the bronchial smooth muscle fibres to cause contraction, on other H 1 receptors to increase vascular permeability and on H 2 receptors to increase mucous secretion. The granules also contain proteases, mainly tryptase, which can detach epithelial cells from the basement membrane, resulting in desquamation and possibly activating neuronal reflexes causing further bronchospasm.
| Preformed Mediators | Newly Generated Mediators | Cytokines |
|
|
|
The second major event after mast cell activation is the initiation of synthesis of arachidonic acid derivatives (see Fig. 11.3 ). The most important derivative of the cyclooxygenase pathway is the prostaglandin PGD 2 , which is a bronchoconstrictor, although its clinical significance is still not clear. The lipoxygenase pathway results in the formation of leukotriene (LT) C 4 , from which two further leukotrienes, LTD 4 and LTE 4 , are formed (see Fig. 3.10 ).
Finally, mast cells also release a variety of cytokines. Interleukin-5 (IL-5) and granulocyte/macrophage colony-stimulating factor (GM-CSF) are chemotactic for eosinophils, and IL-4 stimulates IgE production by B-lymphocytes, amplifying the activation of mast cells.
Eosinophils are freely distributed alongside mast cells in the submucosa, and are believed to be the principal cell involved in the late-phase reaction of asthma. In particular, they release LTB 4 and LTC 4 , which are potent bronchoconstrictors with a prolonged action. They are attracted to the area by GM-CSF, which is released by many inflammatory cells, before being activated by IL-5 originating from mast cells and lymphocytes.
Lymphocytes have an important role in the control of mast cell and eosinophil activation. Activated B-lymphocytes are responsible for production of the antigen specific IgE needed to cause mast cell degranulation. B-cells are in turn controlled by various subsets of T-’helper’ lymphocytes.
Th2 cells are important proinflammatory cells in asthma, promoting both bronchospasm and inflammation by stimulation of mast cells, eosinophils and B-lymphocytes. The Th2 cell is nonspecific in its response, and relies on stimulation by IL-4 and IL-13 from dendritic (antigen-presenting) cells (DCs) both for its generation from naive T-cells and its subsequent activation to produce its own proinflammatory cytokines. The airway epithelium contains many DCs which have toll-like receptors, as do the epithelial cells themselves (page 167). Once activated by their specific antigen, the DCs migrate to lymphoid tissue in the lungs to control the division of naive lymphocytes into their various subtypes.
Th1 cells are also generated from naive T-cells in lymphoid tissue in response to cytokines released by activated DCs. Th1 cells normally act as antiinflammatory cells by producing interferon and IL-2, which inhibit the activity of Th2 and B-cells.
The relative activity of the opposing effects of Th1 and Th2 lymphocytes was, until recently, believed to play an important role in the development and severity of asthma. However, this convenient explanation, based mainly on studies in animals, is now thought to be an oversimplification of the situation in humans, particularly with respect to the generation of Th1 cells. The third subtype of T-lymphocyte involved in immune regulation of the lung are regulatory T-cells (Tregs), which are again generated from naive T-cells, this time in response to IL-10 released by activated DCs. Activation of the DCs to produce the antiinflammatory cytokines IL-10, IL-12 and IL-18 is believed to occur in response to antigens from respiratory tract commensal bacteria or from exposure to high levels of allergens. Tregs exert an antiinflammatory effect by secretion of IL-10 and transforming growth factor β, which modify the activities of both Th1 and Th2 cells. Finally, a recently described fourth regulatory T-cell (group 2 innate lymphoid cell) has been described which is generated in response to IL-25 and IL-33 released by damaged epithelial cells. These cells reside within the epithelium and produce large amounts of IL-5, which further activates eosinophils and, more importantly, IL-13, which leads to AHR and airway remodelling, including goblet cell hyperplasia. ,
Neutrophils are important contributors to the nonallergic phenotypes of asthma, and when activated lead to airway narrowing and inflammation. A different series of cytokines to those already described are involved, with predominantly IL-6 activating Th1 and Th17 lymphocytes, which in turn release a variety of forms of IL-17 to activate neutrophils. ,
Stimulation of bronchial smooth muscle by the substances shown in Figure 28.2 and Table 28.1 explains some of the airway narrowing seen in asthma, particularly during the acute and early stages. During deliberately induced bronchoconstriction ASM cells in asthmatic subjects also respond differently to stretching (by taking a deep inspiration) compared with in nonasthmatic subjects. In the normal lung deep inspiration causes ASM relaxation which ameliorates the bronchoconstriction, whereas in asthmatic subjects the ASM fails to respond or even contracts, exacerbating the bronchoconstriction.
Airway narrowing during the late-phase response, or in severe asthma, results from inflammation of the airway. Many cytokines released during asthma have effects on blood vessel permeability, causing oedema of the epithelium and basement membrane. This airway narrowing because of oedema may be exacerbated by rostral fluid shifts when supine, for example, at night. Protease enzymes break down normal epithelial architecture, generating defects in the epithelial barrier, leading to further inflammation and eventually detachment of the epithelium from the basement membrane. Finally, hypersecretion of mucus and impaired mucociliary clearance are both recognized features of asthma. These changes in the thickness of the airway lining translate into a significant reduction in airway cross-sectional area, and thus a large increase in resistance. Mucus, inflammatory cells and epithelial debris cause obstruction of small airways, compound flow limitation and prevent an effective cough.
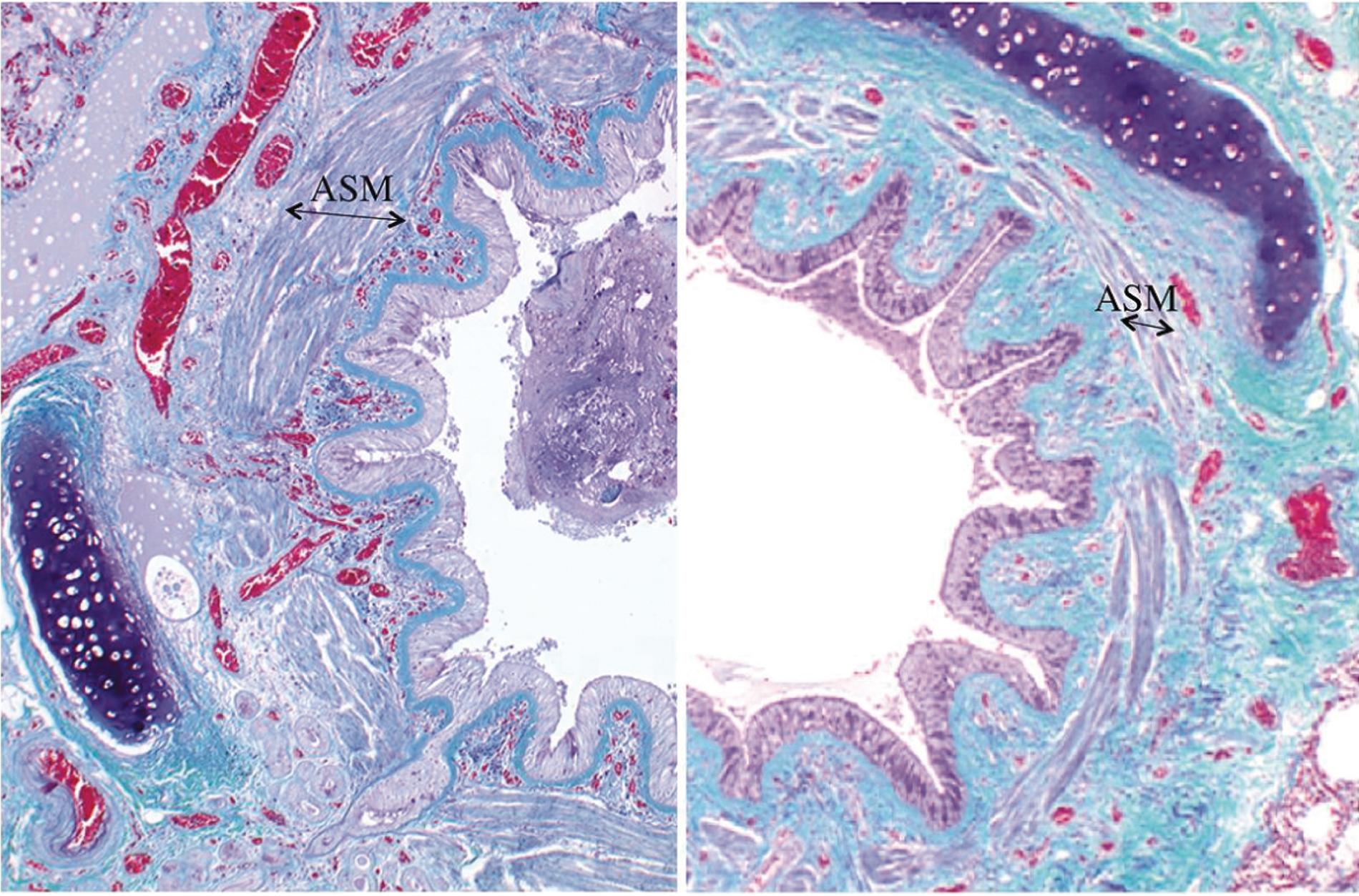
Repeated activation of inflammatory pathways inevitably leads to attempts by the body to repair the tissue concerned. In the lung, this results in morphological changes to both the ASM and the respiratory epithelium. Hypertrophy and hyperplasia of ASM cells cause thickening of the airway wall, even when the muscle is relaxed ( Fig. 28.3 ), and exacerbates the airway narrowing that occurs with muscle contraction because a lesser degree of muscle shortening now causes a greater reduction in the airway lumen. Epigenetic changes occur in ASM cells in asthma, most commonly by acetylation or methylation of histone proteins, which consequently modifies the expression of the genes within ASM cells. Goblet cell hyperplasia also occurs, worsening the hypersecretion of mucus seen with airway inflammation. Finally, in asthmatic patients, there is thickening of the lamina reticularis of the epithelial basement membrane and changes to the extracellular matrix, ultimately resulting in collagen deposition and long-term loss of lung function. Most of these structural changes are stimulated by Th2 cytokines, particularly IL-13 ( Fig. 28.2 ). The clinical significance of airway remodelling in asthma is unknown, but remodelling is believed to be responsible for the long-term decline in lung function seen in some asthma patients. Widespread ASM hyperplasia is more common in fatal cases of asthma, and measures of ASM mass may in future be useful to predict which patients will develop severe asthma later in life. Airway remodelling may begin before asthma becomes severe or is even diagnosed at all, but unfortunately, effective drugs to reverse the structural changes are as yet to be discovered.
Asthma, along with other allergic diseases, has a substantial genetic component, with several genomic regions known to be linked with developing the disease or its progeression. Environmental factors invariably contribute to the development of clinical disease, but genetic susceptibility to asthma is strong. Two reasons explain this observation. First, the genes for most of the cytokines involved in asthma are found close together on chromosome 5, and asthmatic patients may have increased expression of these. Second, human lymphocyte antigens (HLAs), which are involved in sensitization of DCs to specific antigens, are part of the major histocompatibility complex allowing immunological ‘self-recognition’, and so are inherited. It is possible that some HLA types are particularly active in the processing of common allergens, and thus the stimulation of Th2 cells or the suppression of Treg cells.
Genome-wide scans of patients have identified several genetic loci linked to different phenotypes of asthma. These genes facilitate asthma development by many different mechanisms, for example, by affecting the barrier function of the airway mucosa and so changing the interaction between allergens or pathogens and the immune cells in the airway. The most widely studied example of a gene affecting asthma is ADAM33 , (A Disentegrin And Metalloprotease protein), which encodes a large family of proteins with diverse functions, including the control of cell–cell and cell–matrix interactions. In lung tissue, the ADAM33 protein is found in smooth muscle and fibroblasts, but not epithelial cells, indicating its possible role in airway remodelling in asthma.
Changes in living conditions are believed to have contributed to the increase in asthma prevalence. In the developing world, population shifts from rural to urban environments have reduced exposure to parasitic infections and increased exposure to other allergens, and it seems likely that the extensive immunoglobin (Ig)E and mast cell systems that formally inactivated parasites now respond to urban allergens. In the developed world, changes in living conditions have resulted in a dramatic increase in allergen exposure, in particular house dust mite ((HDM); Dermatophagoides pteronyssinus ), domestic animals and fungi. Asthma is more common in affluent families, and correlates with exposure to HDM, which thrives in warm, humid houses with extensive carpeting and bedding. These conditions are ideal for the HDM and its food supply of shed skin flakes. Simply inhaling allergens is only part of the explanation of how allergen exposure may cause asthma, and maternal allergen exposure during pregnancy may also play a role. Despite decades of research across the world, the contribution of HDM allergy to causing asthma remains controversial.
Viral respiratory tract infections cause wheezing in many patients with asthma, and account for over half of the acute exacerbations of asthma. The most likely pathogen in adults is the ‘common cold’ rhinovirus. In infants, respiratory syncytial or some subtypes of rhinovirus are associated with developing asthma in later life, but causation has not yet been established. Viral infection gives rise to an immune response involving many cells and cytokines, but T-lymphocytes are particularly important and undergo both virus-specific and generalized activation. Inevitably, Th2 activity is increased giving rise to wheeze and airway inflammation by the mechanisms described earlier ( Fig. 28.2 ). In addition, stimulation of allergic mechanisms in susceptible individuals continues for some time after the viral symptoms have subsided. For example, after a simple rhinovirus infection, allergen-induced histamine production and eosinophil-induced late-phase reactions remained increased for 4 to 6 weeks.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here