Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The field of plastic surgery originated with the first early attempts to reconstruct the face, especially the nose.
The face tells the world who we are and materially influences what we can become.
The restoration of a normal appearance and an open airway to allow comfortable breathing remains the goal.
Treatment choices will depend on an understanding of the deformity and wound healing, missing anatomic layers, available donor tissues, surgical planning, the surgeon's ability to modify them into “like” tissue, and the advantages, disadvantages, and limitations of the technique, and its ability to achieve the desired outcome.
![]()
![]() Access video and video lecture content for this chapter online at Elsevier eBooks+
Access video and video lecture content for this chapter online at Elsevier eBooks+
The primary functions of the nose are to look normal and allow easy nasal breathing.
The reconstructive approach depends upon the site, size, and depth of the defect, donor availability and, most importantly, the patient’s goal and surgeon's choices in material, method, and approach. This requires an understanding of the deformity, both anatomically and aesthetically, wound healing, and tissue transfer. The advantages, disadvantages, and limitations of each material, technique, and stage must be understood. First, make a diagnosis and then determine what is missing and replace it – external skin which matches adjacent facial skin in color and texture, a midlayer support of soft tissue, bone and cartilage, and internal lining. Covering skin must be thin, conforming, and vascular. Lining must be thin, supple, and vascular, neither occluding the airway nor distorting external nasal shape due to excessive bulk or stiffness. A rigid midlayer must support, shape, and brace the soft tissues against gravity, tension, and scar contraction to prevent collapse and create contour. The surgeon must replace tissues similar in kind to those which are missing, if available. However, although some donor tissues may have some similar characteristics, most are not. All tissues must be modified – thinned and shaped – to become “like” tissue. Transferred skin, ear or rib cartilage, and traditional lining replacements have modest similarity to the “normal” nose in quality, shape, or dimension.
Nasal deformity may follow congenital malformation, trauma (including burns), skin cancer after excision or radiation, infection, or immune disease. In general, reconstruction can be routinely delayed for weeks to years to allow wound stabilization, and maturation, and disease control.
Repair is initiated when both the patient and defect are ready. Associated medical illness may limit the extent, methods, or timing of repair. Since the nose continues to grow until early teen years, it is often best to delay repair in young children to avoid further injury to the nasal growth centers and avoid the need to perform major secondary surgeries when the child’s nose has completed growth.
Precancerous and very superficial cancers (actinic keratosis, basal and squamous skin cancer) may be treated with curettage, cryosurgery, and modern topical medications. Radiation therapy is occasionally employed, especially in elderly patients, those with non-associated medical problems precluding extensive or multiple operations, or patient choice. However, most small, and large skin cancers are excised surgically. Due to their aggressive character and high risk of metatastic spread, melanomas are always treated by surgical excision. In all instances, complete cancer control or excision is required. The margins of the excision must be “clear”.
The dimension and outline of the required skin excision (the cancer and adjacent clinically normal skin which may contain less visible tumor extensions) can be estimated using several methods. The visual extent of the tumor can be estimated and an additional margin of normal tissue excised, based on clinical judgment, or published “rules of thumb”. The border of the specimen can be examined by either permanent histologic section after surgery or by intraoperative frozen sections prior to wound closure to rule out tumor extension along the cut specimen margin. However, large tissue excisions with multiple margins are burdensome for the hospital pathologist, being time-consuming and disruptive to their other duties, and can result in incomplete margin examination.
To better evaluate the entire peripheral and deep margin, the “Margin Check Technique” can be applied. An en bloc excision is performed, based on physical examination, histology, and clinical judgment. Additional 1–2 mm slivers of the entire lateral and deep margins are then excised, oriented for the hospital pathologist, and the wound dressed. Between 24 and 72 hours later, pending verification of complete tumor excision, the defect is repaired in a delayed primary fashion.
Another option is Mohs histographic surgery, which is normally performed in an outpatient setting and utilizes a unique serial horizontal section and mapping technique to maximize cure rate. It is especially useful for difficult tumors – skin cancers greater than 2 cm in size, recurrent, those with poorly defined visible borders, morphea or sclerotic basal cell cancers, and those in locations that are especially difficult to repair, such as nose, ear, or eye, where maximum tissue preservation is desirable.
Although immediate repair of an open defect is attractive, a staged excision with delayed repair is especially advantageous when repairing more extensive cancers which requires more complex reconstruction. Ideally, the patient is seen prior to tumor excision. The diagnosis is verified, the likely extent of excision and reconstruction are discussed, and the treatment options outlined. Preoperative medical clearance is obtained, if necessary, and operative time is scheduled for the future. Excision is performed, and a follow-up appointment is made to evaluate the defect after tumor clearance is assured. During the post-excisional consultation, the true extent of the defect is confirmed, and anatomic and aesthetic losses are defined. Reconstruction follows within 48–72 h. Because the extent of the defect is identified prior to repair, the patient understands the requirements of reconstruction and is an informed participant in their care.
A coordinated excision and repair provide an opportunity to think, plan, and discuss options with the patient in a leisurely manner prior to entering the operating room. A surgical plan is developed preoperatively – decreasing patient and surgeon anxiety and ensuring the best result. Because tumor clearance is confirmed prior to repair, the length of anesthesia and operative times are shortened. Most importantly, disruption of the operative schedule and intraoperative decision-making are minimized.
The preoperative consultation should clarify the diagnosis, define anatomic and aesthetic defects, ensure a healthy wound and patient, provide patient education, instill confidence and the patient's participation, formulate a surgical plan, and identify donors, methods, and staging. Medical history and physical examination are evaluated with special emphasis on the etiology of the nasal injury, disease remission, cancer clearance, and general health. Facial photographs, combined with calibrated photographs and normative facial measurements, identify anatomic and aesthetic injury, old scars, landmark malposition, injury to available donor sites, and provide measurements which are useful intraoperatively. In complex three-dimensional (3D) injuries, a facial moulage can be obtained preoperatively and a clay model of the desired result designed. This allows the surgeon to intellectually visualize the dimension and contours which require replacement. Prior pathologic exams and old operative reports are examined. Facial X-rays, computed tomography (CT) scans, or magnetic resonance imaging (MRI) are occasionally employed to clarify bony and soft-tissue injury to the midface when rebuilding composite defects of the nose and cheek.
All defects are different. They may be superficial, deep or full-thickness, small or large, lie within flat or highly contoured regions, be limited to the nose, or extend onto the lip and cheek, or present within a previously injured or repaired nose. So, the surgeon has many questions: What is my goal? Healed, “filled”, a normal appearance? How do I describe what is “normal”? Is it proportion, mathematics, rules of thumb, artistic vision? How do I determine what is missing? Anatomically, aesthetically, with a measuring ruler, guesstimate? What should be replaced? Cover, lining, support? Should the wound be altered – made smaller, larger, changed in shape or depth? How do I determine the size, border outline, and position of tissue replacements? What are the best materials to restore the missing skin cover, lining, and support? We are told to use tissue with qualities similar to what is missing, but most donor materials are not similar to the nose. They are too thick or thin, of incorrect dimension or of poor quality. How do I restore three-dimensional shape – hard tissue, soft tissue, timing, modification? How do I avoid unsightly scars? How do I prevent unnecessary donor injury? How many operations will it take? What should I do first, second, third etc. and in what order?
Younger surgeons whose training and experience are limited may have additional questions. Do I just “make it up” as I go, or can I employ guiding principles applicable to all facial reconstruction that can guide my decision- making? Should I and the patient just “give up” or can I realistically expect to restore a normal life to most patients?
Traditionally, the defect determined the repair. Surgeons simply “filled the hole” to obtain a healed wound. The design and dimension of a skin graft or flap were determined by the apparent, but often distorted, defect. The emphasis was on tissue transfer (skin graft or flap), blood supply, or the replacement of anatomic layers (cover, lining, support). Avoiding scars and donor injury were overriding concerns. A comprehensive plan to restore multiple, exact, independent, 3D facial features was rarely envisioned. This traditional approach failed to appreciate the strong motivation of patients to look as they did before. It followed a “less is more” cautious approach, employing principles with little expectation of restoring the normal.
The history of facial and nasal reconstruction is the history of plastic surgery.
Early surgeons focused on the replacement of external skin. Although cartilage, bone, and mucous membrane were missing in larger defects, skin was the most obvious deficiency.
The origins of forehead rhinoplasty (the Indian method) are obscure. Nasal repair was described in the Hindu Book of Revelation, the Sushruta Samhita, and probably was performed well before the first century BCE. Italian surgeons Branca and Tagliocozzi reconstructed the nose with skin from the upper arm in the late 1400 s. .
The first written English account of an Indian midline forehead rhinoplasty appeared in the Madras Gazette in 1793 and was republished in the Gentleman's Magazine of London one year later. Carpue, an English surgeon, published his account of two successful operations in 1816. This classic, vertically oriented median forehead flap design was popularized in the United States by Kazanjian in 1946. The base of the flap, which harvested from midline forehead tissues and twisted 180°, was supplied by paired supratrochlear vessels. Its pedicle base was located above the eyebrows.
Early repairs were unlined. So, the external shape of the nose and its airways became distorted by the contracting scar on the underlying raw surface of the external skin flap. The importance of lining replacement did not become clear until the period between 1840 and World War I. Residual intranasal mucosa seemed inadequate. Petralli folded the distal end of the flap onto itself for lining around 1842. However, because of the high pivot point of the classic median flap above the brows, its length was inadequate to create a long columella, satisfactory projection, or to permit infolding for lining without transferring hair-bearing skin on the distal end of the flap.
To increase flap length distally, Auvert, in 1850, slanted the flap's design obliquely across the forehead. German surgeons designed forehead flaps horizontally, supplied by the supraorbital vessels on one side. In 1935, Gillies described an up-and-down flap which was centered over one supraorbital pedicle, passed into the hair-bearing scalp, and then descended back into the forehead. In 1942, Converse modified the up-and-down flap by creating a long pedicle based on the lateral blood supply of the scalp to camouflage scars within the hair-bearing skin. Other designs included New's sickle flap, which transferred skin from the temple recess based on the ipsilateral superficial temporal vessels, or Washio's flap, which transferred skin from behind the ear, based on the anastomosis of the post-auricular and superficial temporal vessels.
The Converse scalping flap, lined by folding its distal end onto itself or by prelamination, became the most commonly used method during the late twentieth century. Unfortunately, these modifications created a forehead defect that was harder to close.
To avoid the limitations created by the high pivot point of the median forehead flap, other surgeons lengthened its design by modifying incisions at the pedicle's base. Lisfranc, in 1829, extended one incision lower than the other at the base of the pedicle. Dieffenbach lengthened one incision until it reached the defect. Labat curved his incisions proximally, centering the flap over the medial brow and canthus on one side, creating a unilaterally based vertical flap.
These innovations reduced the twist of the pedicle base and brought the flap closer to the recipient site by lowering its point of rotation. This vertical paramedian flap transferred forehead tissue on a unilateral pedicle blood supply, located near the medial canthus. The anatomic studies of McCarthy and Reece demonstrate that the forehead is perfused by an arcade of vessels supplied by the supraorbital, supratrochlear, infraorbital, dorsal nasal, and angular branches of the facial artery and on branches of the superficial temporal artery. The rich plexus of vessels, centered on the medial canthus, reliably perfuse a unilateral flap.
Refinements developed to minimize donor deformity. Velpech, in 1828, designed his flap as a reversed Ace of Spades, with its stem forming the columella and its tapering tip as the pedicle. Labat, in 1834, designed a similar tripod-shaped flap, with limbs extending obliquely across the forehead. Millard, in the 1960s, used a seagull-shaped flap with a central vertical component, which covered the dorsum, tip and columella, and horizontal lateral wings, which extended horizontally to resurface the ala. Undermining of adjacent wound edges allowed partial closure and a relatively inconspicuous midline T-shaped scar, even in large defects.
Today, the vertical paramedian forehead flap, with a unilateral supratrochlear pedicle, is the first choice for nasal repair because of its vascularity, size, reach, reliability, and relatively minimal morbidity. Importantly, because a unilateral vertical flap does not encroach on the opposite forehead, a second vertical flap can be harvested with relative ease.
In modern times, forehead expansion has been used to increase the available surface area of the forehead, ease closure, and minimize donor deformity when forehead dimension is limited in height or width due to a low hairline or prior forehead scarring.
As Harold Gillies stated in 1920, “One is struck chiefly with the lack of appreciation of the need for lining membrane for all mucus lined cavities. In actual fact, except that the forehead skin most closely resembles nose skin, the origin of cover is the least important.” .
For at least 2000 years, surgeons placed a flap over a full-thickness defect but left its raw undersurface to heal secondarily. Unfortunately, the external shape of the nose and the airways became distorted by contracting scar. In the mid-eighteenth century, Petralli folded the distal end of the flap on itself to form, in a manner of speaking, a tip, ala, and columella. Volkman, in 1873, turned down tissue lying adjacent to the defect as hinge-over flaps. Thiersch transferred flaps from other facial areas in 1879. Millard, in the twentieth century, rolled over bilateral nasolabial flaps to line both the alae and columella.
In 1898, Loosen applied a skin graft to the underlying raw surface of a covering flap during flap transfer. However, the “take” was inconsistent and late contracture was common. Others placed a split- or full-thickness skin graft on the deep surface of the forehead flap during a preliminary operation. Weeks later, once the viability of the graft was assured, the skin grafted flap was transferred to supply both cover and lining. Gillies, in 1943, popularized prelaminating the forehead flap with composite chondrocutaneous grafts. In 1956, Converse recommended septal mucoperichondrial cartilage grafts. However, these methods of prelamination (formerly referred to as prefabrication), delayed formal repair and created a relatively unsupported and shapeless nose.
Gillies developed the skin graft inlay method for the saddle nose deformity of syphilis and leprosy. If lining and support were lost but the overlying nasal skin remained intact, he released scar on the undersurface of the external skin and applied a skin graft to the underlying raw surface. A permanent internal prosthesis was worn to splint the graft and maintain nasal shape and airway patency.
Burget, realizing that the forehead is composed of skin, subcutaneous fat, and underlying frontalis muscle, tunneled a cartilage graft within a vascularized pocket within the subcutaneous fat of a full-thickness forehead flap. The deep undersurface of the frontalis muscle was skin-grafted for lining. The buried cartilage graft “stented” the skin graft lining, much like Gillies' external splint.
Burget and Menick popularized the use of residual intranasal lining flaps, based on axial vessels, for lateral, hemi-nasal, and near-total nasal defects. Because such intranasal lining flaps were thin and relatively reliable, primary cartilage grafts could be employed simultaneously to build a delicate hard-tissue layer to support and shape the nose during the initial stages of reconstruction.
Menick modified the traditional folded flap and lining skin graft approaches. Because the distal end of a folded forehead flap or a lining skin graft heal to the adjacent residual nasal lining within 3–4 weeks, the new reconstructed lining is not dependent on the flap's pedicle for survival. The distal end of a folded flap (cut free from the proximal flap) or a skin graft (which was initially revascularized from the forehead flap's deep surface) survive, even when the covering flap is completely re-elevated. This permits the placement of a delayed primary support framework over the newly vascularized lining, prior to pedicle division.
Over the last decade, distant tissue has been transferred for lining, as a free flap. Until recently, the technical elegance of microsurgery has not matched the artistry required to make a normal nose. However, recent advancements by Burget and Walton, employing multiple individual forearm skin paddles and a two-stage forehead flap with an intermediate to debulk the midvault and Menick and Salibian's folded single paddle radial forearm flap, combined with a three-stage forehead flap, have produced good results.
Historically, lining necrosis, infection, or excessive bulk precluded the early placement of cartilage grafts. Prelaminated composite chondrocutaneous grafts for the nostril margins or large cantilever bone grafts, fixed to the nasal bones, provided incomplete support initially. Without primary support placement, unchecked forces of healing led to contracted scars, pin cushioning, and airway collapse. Additional support grafts were sometimes placed secondarily but the results were limited due to the difficulty of molding soft tissues after collapse and scar contraction.
Realizing that a complete cartilage and bone framework must be placed early to support, shape, and brace the repair, Burget and Menick combined primary cartilage grafts with thin, vascular intranasal flaps. Menick recommended a three-stage full-thickness forehead flap approach, combining primary and delayed primary cartilage grafts and staged soft-tissue debulking during an intermediate operation, prior to pedicle division.
Traditionally, skin grafts or flaps were designed to replace the existing defect. Unfortunately, the defect does not reflect what is missing and what needs to be replaced. Fresh wounds are enlarged by edema, local anesthesia, tissue tension, and gravity. Old wounds may be contracted by secondary intention healing or distorted by prior injury or repair, but to restore the normal, the “true” tissue loss must be identified, and the “true” defect replaced. This requires that the remaining normal tissues adjacent to the defect be returned to their normal position and defect filled with tissue replacements which replace what is missing in exact dimension, shape, and quality.
Transferring extra tissue for “good measure” only complicates the reconstruction. If too much tissue is transferred, the wound is “stuffed”, and the borders of the defect and adjacent landmarks are pushed outward. Further surgery will be needed to excise the extra bulk and reposition the facial landmarks.
Sharing tissue from an area of excess to one of deficiency is a basic surgical tool. Although it is tempting to transfer a flap smaller that the true defect to preserve the donor site, the impulse must be avoided. Central features, such as the nose or lip, have an exact border outline and position. If the flap is too small, the borders of the defect are dragged inward, distorting the size, shape, and position of the nose and adjacent structures. Missing tissues should be replaced exactly, in most instances.
Surgeons are taught to preserve tissue. However, when skin is transferred to resurface only part of a facial feature, it may appear as a distracting patch, outlined by scars. It is often helpful to alter the defect and discard extra skin prior to repair, even if this makes the defect larger. Discardable tissues may be used for other purposes – hinge-over lining, subcutaneous bulk, or shifted to resurface an adjacent injury.
A poor facial repair is identified by incorrect dimension, volume, position, and contour, not by the presence of scars. Scars can be effectively camouflaged by positioning them in the joins between subunits.
Traditionally, cover and lining were replaced without support to avoid the risk of extrusion and infection. Occasionally during a preliminary operation, flimsy cartilage strips were placed over a skin graft within a prefabricated flap. More often, bone and cartilage grafts were placed, months later, as crude cantilevered grafts to lift the tip and dorsum.
Unfortunately, unsupported soft tissues shrink and are rapidly distorted by gravity and tension and fixed by scar. Late re-expansion with secondary cartilage grafts and soft tissue “thinning” may not be successful.
When a defect extends within multiple facial units, it is difficult to reproduce the delicate 3D character of face with a single flap. The shortest distance between two points is a straight line and a single flap often fails to supply enough skin to resurface a 3D defect. Myofibroblasts within the bed of scar under the flap contracts, drawing the single flap into a dome-like pin-cushioned mass, obliterating normal nasal and facial contours (the alar crease, the nasolabial fold etc.). It is often preferable to repair individual facial units with separate grafts and flaps.
A healed wound, tissue survival, or the replacement of anatomic layers are necessary, but not sufficient, to restore normal facial appearance and function. Aesthetic results depend on the surgeon's and patient's choices. The modern approach relies on the visualization of the “normal” to determine what is missing, both anatomically and aesthetically. This is a regional unit approach based on the judicious choice and modification of recipient and donor tissues to provide for the exact replacement of tissues and restoration of facial units. The principles of facial reconstruction have switched from a wound perspective (how big, how deep, anatomy, and flap blood supply) to a visual one. The mature surgeon, with training and experience, “sees the future”. They conceptualize the available options, while visualizing the desired result and then decide which one will work. A plan is formulated, principles outlined, and techniques and methods chosen.
Observation is the basis of diagnosis.
Diagnose before you treat.
Make a pattern of the defect and a plan.
Replace the normal in the normal position and retain it there.
Do something positive.
Treat the primary defect first. Do not let the secondary defect endanger the final result.
Borrow from Peter to pay Paul, if Peter can afford it.
Losses must be replaced in kind.
Never throw anything away, unless you are sure you don’t want it.
Never let routine methods become your master.
Never do today what you can honorably put off until tomorrow. When in doubt, don’t.
Fortunately, although each defect is different, all repairs are simplified because the “normal” is unchanging. The “normal” nose is visually defined by its dimension, volume, position, projection, platform, symmetry, expected skin quality, border outline, and 3D contour. Major facial landmarks are identified as regional units – adjacent topographic areas of characteristic quality, outline, and 3D contour. Often, the contralateral normal remains as a visual standard for comparison. If not, the ideal is the guide. This regional unit approach helps the surgeon conceptualize the goal, define the requirements of repair and priorities, choose materials and methods, determine staging, and measure the success of the result with the ideal normal in mind ( Fig. 3.1 ).
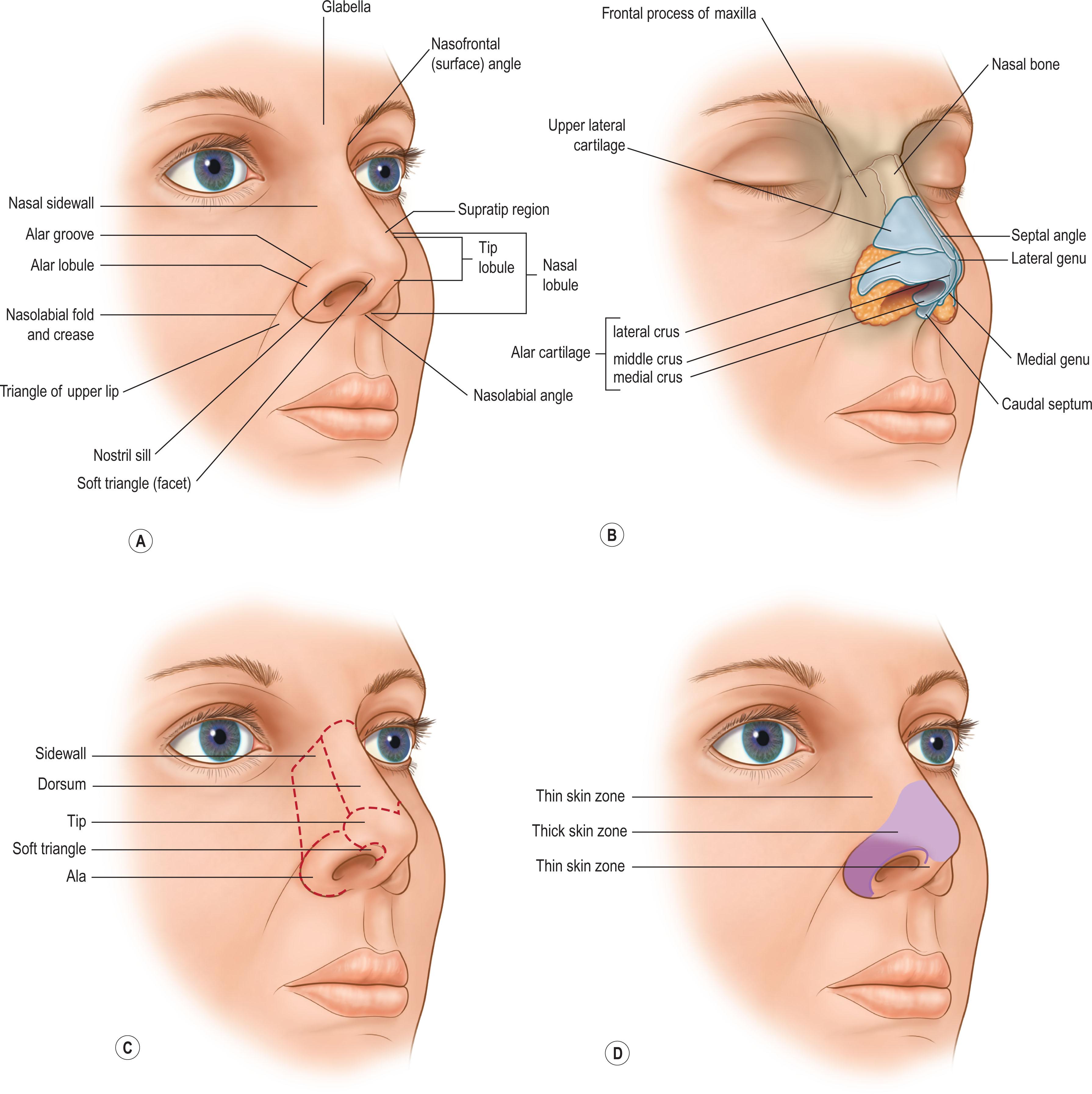
In the latter half of the twentieth century, Gonzalez-Ulloa divided the face into regions, based on skin thickness. Millard envisioned major facial landmarks as “units” and recommended replacing them in their entirety with “like” tissue of similar color and texture to avoid a patch-like repair. Burget and Menick divided the nose and face into “subunits”, based on skin quality, border outline, and 3D contour.
The face can be divided into peripheral and central units’ areas based on their skin quality, border outline, and 3D contour. This regional unit approach to repair directs clinical observation, planning, and treatment recommendations. .
The forehead and cheek are peripheral facial units. Like a “picture frame”, they lie at the periphery of the face and are of a secondary visual importance. Their surfaces are largely flat and expansive and their border outlines are variable due to changing hairline and eyebrow position. Because the entire unit is not visible in in the frontal view, symmetry and outline of the peripheral units cannot be compared to the contralateral normal side. Because the peripheral units are variable, inexact, and peripheral, their repair is less demanding and of secondary importance. The accurate restoration of skin quality determines success, not outline or 3D contour.
A small-moderate forehead defect can heal secondarily. The resulting shiny, flat scar, supported by the underlying rigid bony platform of the skull, blends into the normal shiny, tight surface of the forehead without significant distortion or malposition of adjacent landmarks. In contrast, small skin grafts usually appear as a depressed “patch” within the remaining forehead unit. Rarely, a large skin graft is useful if used to replace the entire forehead unit or a lateral forehead subunit, after residual skin is discarded within a large defect. The uniform, shiny, flat quality of a skin graft simulates the expected quality and contour of the entire forehead unit or subunit and its peripheral scars lie along the hairline, brow, or contour lines between the lateral and central forehead subunits. For similar reasons, skin grafts are rarely used to resurface the cheek. Most often, the lax skin within the adjacent cheek is shared with the defect by advancing matching skin adjacent to the defect as a rotation flap. Enlarging the wound so that the entire forehead or cheek is resurfaced with one flap is impractical due to the paucity of available donor excess and the unreliability of flap blood.
The central midfacial units are the nose, lip, and eyelids. They require a different reconstructive approach than for peripheral units. The concept of regional unit reconstruction applies primarily to the repair of central facial defects, not to the peripheral units. The central units are seen in primary gaze and demand the highest priority of repair.
The surface subunits of the nose have a fixed border outline, a 3D shape, and skin quality. The tip, nostril margin and alar base positions, nasal landmarks, such as the alar crease, and nasolabial folds etc., are all visible on frontal view. An asymmetry, incorrect landmark or unit dimension, shape, border outline, and position are easily compared from right to left by the observer. If the reconstructed part of the nose does not match the contralateral normal or the ideal, it will look abnormal. Although skin quality is important, subunit border outline, contour, size, shape, and symmetry are of greater importance.
Guiding principles of a regional unit repair are listed in Box 3.2 . Following these, residual tissue within the unit or subunit may be discarded to enlarge the wound to a unit shape. Or the defect may be decreased in size by local advancement rotation flaps or changed in border outline by a combination of excision and tissue rearrangement, rather than just patching the defect. Practically speaking, smaller nasal defects are often closed with non-subunit grafts or local flaps, especially in patients who want a simple repair and do not demand an aesthetic result. However, when the ideal is the goal, it is often useful to alter the defect to a unit or subunit shape and resurface the wound with a regional transposition flap of exact subunit dimension, thereby creating a unit defect and filling it with a unit-shaped flap.
Human beings wish to look normal.
The normal is defined by 3D contour, border outline, and skin quality which describe regional units. Units are not determined by wrinkle or resting skin tension lines.
The nasal unit consists of the tip, dorsum, columella, and paired alae, sidewalls, and soft subunits.
Restore units, do not fill defects.
If a defect, within part of a central unit, is filled without regard to unit outline, the tissue replacement may appear as a distracting patch within the subunit. The goal is to restore the character of the unit, rather than simply fill the “hole”.
If an aesthetic outcome is desired, large or deep defects of the nose are altered in site, size, outline, and depth – to improve the result.
Subunit resurfacing positions scars so that they are camouflaged within the joins between subunits. More importantly, myofibroblasts lie in the recipient bed under a transferred flap and contract, causing the transposed skin flap to rise above the level of adjacent skin. When an entire convex subunit is resurfaced, the pin-cushioned flap shrink-wraps around the underlying cartilage framework, augmenting, rather than distorting, the contour of a convex subunit.
If a defect of a central convex subunit, such as the tip or ala, is greater than 50% of the subunit, discard adjacent normal tissue within the subunit and resurface the entire subunit, rather than merely “patching the hole”. This rule applies to the convex tip and alar subunits but has limited application to the sidewall and dorsum. The lateral border of the sidewall or superior border of the dorsum at the radix are indistinct. Although resurfacing the dorsum to the lateral border of its subunit is effective, subunit excision of the sidewall or complete excision of the dorsal skin to the radix is not recommended.
The defect may be enlarged, and the donor requirement increased. A larger defect may preclude closure with local tissue and necessitate a regional flap repair.
The number of stages and the complexity of the repair may be increased.
The amount of cartilage material required to support the soft tissues may be increased.
Patient morbidity may increase if regional flaps or cartilage grafts are needed.
However, when appropriately applied, the result may be significantly improved because the contour is correct, and the scars are less apparent. It should be emphasized, however, that a good result does not depend on any one surgical maneuver. It reflects a series of choices, methods, and tissue manipulations that transfers a thin flap which blends into neighboring tissues, establishes 3D contour, and replaces missing tissues in exact dimension and outline. Resurfacing a facial defect as a unit can be helpful, but it is only a single tool.
If a flap is larger than the defect, its size and bulk pushes adjacent landmarks outward, creating malposition and asymmetry. If the flap is smaller than the true defect, neighboring structures are pulled inward. Tension may crush and collapse underlying cartilage grafts. So, the missing tissue must be replaced in exact dimension and border outline. Exact 3D patterns are designed which fit the needs of the “true” tissue defect, rather than a wound distorted by edema, tension, scar, or prior repair.
The apparent defect does not reflect what is missing. Due to edema, tension, gravity, scar, or past repair, the wound may be larger, smaller, altered in shape, or shifted in position. Because the deformity does not reflect the actual tissue loss, the surgeon cannot rely on observation or measurements of a fresh open wound, a contracted healed wound or an inadequate repair to determine what is missing and the dimension, shape, thickness or position of the materials that need to be replaced. Instead the surgeon can use the contralateral normal – the opposite ala, hemi-tip or hemi-nose, or hemi-lip subunit to design a foil template that reflects the size and shape of the missing subunit. If the contralateral normal is absent, a template can be designed from an ideal clay model, based on a moulage of the patient's face, or from a universal pattern.
Surgeons should replace missing tissues with material with similar qualities, if possible. Millard's admonition to use “like for like” applies. Practically speaking, this applies primarily to matching skin quality of local and regional skin – local flaps for a small nasal defect, cheek for cheek, and a forehead or nasolabial flap for major nasal resurfacing. The use of full-thickness Abbe and Estlander lip flaps, containing matching skin, muscle, and mucosa, is an exception. Distant tissues do not match the face in skin quality and are best employed for lining, to fill dead space, create a facial platform, or vascularize an ischemic, contaminated, or radiated wound. Although distant skin can be placed for temporary cover, it should be discarded later and replaced with matching local or regional skin to obtain a more normal result.
Traditionally, the method of tissue transfer was chosen based on the vascularity and depth of the defect. Skin grafts were employed to resurface well-vascularized superficial defects when skin and a small amount of subcutaneous tissue were missing. Skin flaps were used to supply bulk to a deep defect or cover a poorly vascularized recipient site, a wound with exposed vital structures, or exposed or restored cartilage and bone.
Skin grafts and flaps should instead be used based on their expected final appearance and qualities. Healed skin grafts are typically shiny, atrophic, and hypopigmented or hyperpigmented. Even though a skin graft donor may match the color and texture of the recipient site, the transient ischemia associated with skin graft “take” leads to unpredictable skin color and texture changes. Full-thickness skin grafts shrink modestly but are less likely to create a trapdoor deformity. In contrast, a skin flap maintains its own perfusion and retains the skin quality of its donor site. However, the scar between the flap and the recipient bed contracts, which leads to pin cushioning. This effect of flap healing will typically result in a convex surface shape.
Considering these final qualities, skin grafts are best employed to resurface flat or concave recipient sites, such as the nasal side wall, while skin flaps are used to resurface convex surfaces, such as the tip or ala subunits. When an entire convex subunit is resurfaced with a flap as a unit or subunit, wound healing and tissue transfer are harnessed, and pin cushioning contributes to the desired contour.
Nasal defects often extend from the nose onto the adjacent cheek and lip, creating a composite defect. Normally, the nose sits on the lip and cheek platform in specific position and projection. Unfortunately, edema, gravity, tension, and postoperative scar contraction may shift a repaired platform postoperatively. The larger and deeper the defect, the greater is the risk. If the lip/cheek platform is unstable and the nose is reconstructed during the same operative procedure, the nose may be dragged inferiorly and laterally over time.
Although a small superficial defect of the nose, cheek, and lip can often be repaired during a single stage, large deep defects of the cheek and lip are more reliably reconstructed during a preliminary operation to re-establish a stable platform. Then the nose is positioned in the ideal position during a later stage.
A nose looks normal because it has a nasal shape which is dependent on both cartilage and soft-tissue contours. Primary and delayed primary cartilage grafts restore support and shape, bracing the cover and lining against collapse and contraction. Although the alar lobule and soft triangle normally contain no cartilage, cartilage must be placed to support the nostril margin to brace the alar rim and prevent constriction inward, contraction outward, and airway collapse. Precise soft-tissue sculpting also adds 3D shape. Secondary placement of cartilage grafts and soft-tissue excisions are less effective after pedicle division due to scarring and more limited blood supply, but remain useful.
Surgeons and patients wish to minimize incisions because they fear unsightly scars. In actuality, incisions usually heal with fine scars. A reconstructed nose which looks normal is “worth” a fine forehead scar if a forehead flap is the best option. Poor results, in most facial repairs, are due to poor contour, malposition and asymmetry and the failure to restore a normal appearance, not unsightly scars. It is frequently preferable to disregard old scars and add additional incisions.
Rather than attempting to thin bulky flaps secondarily by elevating the peripheral edges of the flap through its border scar during a late revision, it is frequently preferable to disregard old scars and place new incisions within the surface of a transferred flap to permit direct exposure and exact soft-tissue contour.
Using accurate templates, based on the contralateral normal or ideal, the position of the alar crease or nasolabial fold are marked with ink and incised. The wound edges on either side of the “new” incision are elevated. Under direct vision, the underlying soft tissue is sculpted to create a flat sidewall, a round ala, a full medial cheek, etc. The overlying skin is then reapproximated to the newly contoured subcutaneous bed with quilting sutures. The wound is closed. Although a new incisional scar is created, it lies in the border outline of the newly contoured subunits within the contour depression between units.
Surgical staging is most often employed to transfer and divide a pedicled flap. Equally important, surgical staging is also an opportunity to recreate the defect, return normal to normal, ensure viability, prepare excess tissue for other uses (hinge-over lining flaps, soft-tissue bulk, etc.), surgically delay, prefabricate, transfer, and modify tissues by debulking or shaping, add or alter support grafts, improve imperfections, and treat complications. Following the old general surgery adage “A chance to cut is a chance to cure”, take advantage of all opportunities to improve the final result.
A preliminary operation prior to formal nasal repair is often indicated. The diagnosis may need clarification, the wound or the donor may need preparation, and the problem may need to be analyzed to prepare a plan. The extent of true defect is often obscured after secondary healing, prior skin grafts, or flaps. Although past history, physical examination, old operative reports, or radiographs may provide information, the extent of the true defect may only become apparent after recreating the defect. A preliminary operation excises scars and releases remaining adjacent tissues to their normal position to restore the dimension, depth, and position of injury and identify the required tissue needs. An occluded airway can be opened by excision of scar and soft-tissue bulk. Defects of the lip and cheek may be repaired, establishing a stable platform on which to place the nose in the future. Donor materials can be surgically delayed to maximize blood supply, especially when scar lies within its territory or injury to the flap's pedicle is suspected or a flap can be prefabricated. Ischemic or chronically infected tissue may be debrided or the wound biopsied to ensure complete clearance of tumor or immune disease remission.
The nose is covered by skin and an underlying layer of subcutaneous fat and nasalis muscle which lay over a rigid bony framework of cartilage and fibrofatty tissue. However, the quality of the skin is not uniform, unless atrophic due to old age, sun injury, or radiation injury. In the normal nose, it can be divided into areas of thin smooth skin and thickly pitted skin. Note that the zones of skin quality do not correspond to the nasal units, which are defined by contour, not by skin quality.
In the superior half of the nose, dorsal and sidewall skin is thin, smooth, pliable, and mobile. A modest excess of skin permits repair with primary closure or a local flap without distortion of adjacent mobile landmarks. A modest amount of skin can be recruited from the cheek to resurface a small sidewall or ala defect. A simultaneous rhinoplasty to decrease the size of the nasal skeleton and increase the available skin is rarely helpful to ease the closure of small defects.
The inferior half of the nose is covered by tight skin, pitted with sebaceous glands, and adherent to the underlying deep structures. No excess is present. The thick skin zone begins in the alar groove, crosses 5–10 mm above the supratip region, and extends inferiorly towards the caudal borders of the tip and alar rims. About 2–3 mm above the alar margins and a few millimeters below the inferior point of the tip and onto the columella, the skin thins and loses its sebaceous quality. The lower half of the infratip lobule, including the soft triangle and columella, is covered by thin, but adherent skin fixed to the underlying structures. There is no excess within the inferior nose and the tip and alar margins are mobile and easily distorted by contracting scar or inaccurate tissue replacement.
Nasal defects are classified into small, superficial, large, deep, or composite defects. The difficulty of repair is determined by the site, size, and depth of injury.
Small defect : If the defect is larger than 1.5 cm, local flaps are precluded because there is not enough residual skin to “share” over the entire nasal surface without excessive closure tension and landmark distortion. A skin graft can be employed to resurface larger defects.
Superficial defects : The defect includes only skin and a small amount of underlying subcutaneous fat and nasalis muscle. Vascularized soft tissue remains in the depth of the wound. So, the defect can be resurfaced with a skin graft or flap. A small defect can heal secondarily if periosteum or perichondrium is intact.
Adversely located defects: If the defect is closer than 0.5 to 1 cm of the nostril margin, local flaps may distort the tip and nostril rim. Local flaps cannot reach the infratip lobule or columella. Adversely located defects require a regional flap, even though the wound may not be not large.
Large defects : If the defect is greater than 1.5 cm in size, a skin graft or regional flap from the cheek or forehead are required.
Deep defects : If a cartilage graft is needed or lining is missing, a skin graft or local flap cannot be employed. A skin graft will not “take” over bare cartilage grafts. Cartilage grafts collapse under the wound tension associated with local flaps. So, although a small rim defect can be closed with a composite skin graft, significant full-thickness defects require a vascularized regional flap for cover. Even in the ala which contains no cartilage, if significant alar skin and underlying compact fibro-fatty middle layer are absent, cartilage support must be placed to prevent collapse.
Composite defects : A nasal defect may extend onto the adjacent cheek and upper lip to include multiple units differing visually, anatomically, and functionally and in unit quality, outline, and contour. The tissue loss and required cover, lining, and support will vary from unit to unit. The simplest solution is to “fill the hole” with a single flap even though it cannot reproduce the 3D character of a composite defect. Geometrically, the shortest distance between two points is a straight line and a single flap often takes a “surgical shortcut” and fails to provide enough skin to restore 3D contours. Scar contraction of a single flap also draws a single flap into a dome-like mass, outlined by patch-like peripheral scars. So, it is often preferable to use separate grafts or flaps for each facial unit to position scars in the joins between landmarks and control pin cushioning.
Although “simple” at first glance, small and superficial defects are difficult to repair. Surgeons and patients fail to appreciate the complexity of nasal contour, the paucity of excess tissue, the difficulty of matching the skin in color and texture, and the risk of distorting the residual mobile tip and nostril margins. The time, trouble, morbidity, donor and recipient scars, number of stages, time to wound maturity, and the cost must be balanced against the likelihood of secondary deformity. Many treatment options are available.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here