Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
With the increasing use of cross-sectional imaging, adrenal lesions are frequently identified in routine practice and are seen in up to 5% of abdominal computed tomograph (CTs). Recent lung-screening data report 2% of screening low-dose CTs demonstrate the presence of an incidental adrenal lesion. Pathognomonic imaging features have been established for many of these lesions, including myelolipomas, adenomas, haematomas and cysts. Most of these adrenal lesions are benign. However, as the adrenal gland is also a frequent site for metastatic disease and malignant adrenocortical carcinomas (ACCs) and malignant phaeochromocytomas are becoming more frequent, distinguishing between benign and malignant masses on imaging in patients with primary cancers elsewhere is essential. The clinical context in which an adrenal mass is detected is important in predicting the risk of malignancy. Although several imaging techniques can be applied, CT has a pivotal role in both detection and characterisation of adrenal lesions. In functioning adrenal disease, clinical and biochemical findings should direct the radiologist to the correct interpretation and diagnosis. This chapter looks at adrenal anatomy, physiology, the characteristic imaging features of common adrenal lesions and application of modern imaging techniques in evaluating an incidental adrenal mass and functional adrenal disease.
The adrenal gland is a composite gland made up of two neuroendocrine organs, the adrenal cortex of mesodermal origin and the adrenal medulla of neuroectodermal origin. Modern imaging techniques cannot distinguish between cortex and medulla as separate zones.
Each gland is found in the suprarenal fossa: the left gland is crescentic and the right gland is pyramidal in adults. Although variable, the approximate size of each gland is 5 × 3 × 1 cm and each weighs between 4 and 6 g. Prolonged stress, however, can induce hypertrophy and hyperplasia, increasing both size and weight. Classically, the adrenal gland is described as having arterial supply from three arteries—the superior, middle and inferior adrenal arteries arising from the inferior phrenic, aorta and renal arteries, respectively—although there is variation. The short right adrenal vein drains directly to the inferior vena cava (IVC), whilst the longer left adrenal vein usually drains to the left renal vein (although there are several rare variations—e.g. directly to the IVC). Lymphatic channels are only present in the capsule and not within the glands.
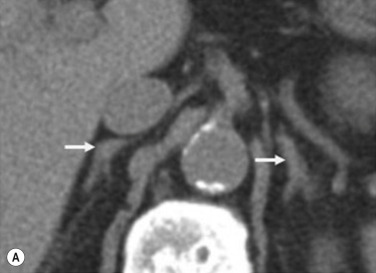
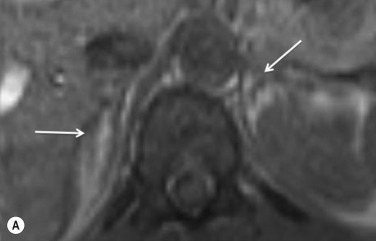
The recommended imaging parameters for the adrenal glands on CT and magnetic resonance imaging (MRI) are summarised in Tables 37.1 and 37.2 , respectively. On cross-sectional imaging, both adrenal glands can be divided into a body and medial and lateral limbs. The right adrenal body should not exceed 8 mm and the left adrenal body should not exceed 10 mm in maximum dimension. The maximum normal adrenal limb thickness is 5 mm. The normal adrenal demonstrates uniform contrast enhancement on the arterial and venous phase CT and the cortex cannot be distinguished from the medulla ( Fig. 37.1 ). On MRI, both glands have a uniform intermediate T 1 and a low to intermediate T 2 signal intensity (SI) and are better demonstrated on T 1 fat-saturated images as nulling the signal from surrounding retroperitoneal fat augments the adrenal signal ( Fig. 37.2 ).
| Slice Thickness | Plane | |
|---|---|---|
| Unenhanced | 3–5 mm | Axial |
| 60 s postcontrast | 1–3 mm | Axial and coronal |
| 15 min postcontrast | 1–3 mm | Axial and coronal |
| Weighting | Plane | Slice Thickness/Gap | |
|---|---|---|---|
| Standard sequences | T1 | Axial | 3–5 mm/3 mm |
| T2 | Axial and coronal | 3–5 mm/3 mm | |
| CSI (in- and out-phase) | Axial | 3–5 mm | |
| T1 fat sat | Axial | 5–7 mm/3 mm | |
| Optional sequences | T1 fat sat with gadolinium | Axial and coronal | 5–7 mm/3 mm |
| DWI ( b = 0, 250, 500, 1000, 1200) | Axial | 3–5 mm |
The adrenal cortex, consisting of three layers, synthesises and secretes corticosteroids, which include mineralocorticoids, glucocorticoids and sex hormones, all derived from cholesterol ( Fig. 37.3 ).
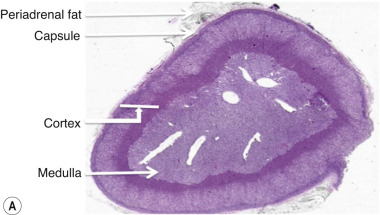
The outer zona glomerulosa layer produces aldosterone and other mineralocorticoids and is chiefly under the control of angiotensin II. The zona fasciculata and zona reticularis are influenced primarily by adrenocorticotrophic hormone (ACTH) and produce glucocorticoids and some androgens and oestrogens, respectively. The main glucocorticoid is cortisol, and it has an important role in carbohydrate, fat and protein metabolism.
The central adrenal medulla contains the chromaffin cells, producing catecholamines (noradrenaline and adrenaline), and is the major source of noradrenaline (in addition to the organ of Zuckerkandl located at the aortic bifurcation). The medulla synthesises and secretes the catecholamines in response to signals from preganglionic nerve fibres in the sympathetic nervous system.
Incidentally detected adrenal masses in patients with no known malignancy occur in 5% of all abdominal CT examinations. The indications for the CT are variable, with abdominal pain, trauma, cancer staging and non-specific symptoms as the most frequent indications.
Most often, these masses are benign, but when detected they often present a diagnostic dilemma and require clinical evaluation to exclude endocrine disease, primary adrenal cancer and metastases from other malignancies. The important questions to answer before a suitable management plan can be made is to determine if the lesion is functioning or non-functioning and if it is benign or malignant. Function is clinically and biochemically established, whilst benign adrenal masses such as lipid-rich adenomas, myelolipomas, adrenal cysts and adrenal haemorrhage have pathognomonic cross-sectional imaging appearances, as discussed later.
Adrenal adenomas are more common in some inherited diseases, including multiple endocrine neoplasia type I, Beckwith–Weidmann syndrome and the Carney complex. The likelihood of developing an adenoma increases with age. Based on pathological studies, about 6% of the population over 60 years of age harbour an adrenal adenoma. Of these, 70% to 80% are benign non-functioning adenomas. The prevalence of adrenal masses increases to 9% to 13% in patients with a known underlying malignancy. The adrenal gland is a relatively frequent site for metastatic disease, but even in patients with a known carcinoma, only 26% to 36% of adrenal masses are metastatic. This incidence of metastatic adrenal lesions increases to 71% if the adrenal mass is larger than 4 cm and demonstrates an increase in size on follow-up imaging within 1 year.
The nature of incidentally detected adrenal masses can be determined with a high degree of accuracy using CT or MRI alone. [ 18 F]-Fluorodeoxyglucose positron-emission tomography (FDG PET) is also increasingly used in clinical practice in patients being staged for cancer and can assist in characterising adrenal lesions.
A few adrenal masses elude precise characterisation on cross-sectional imaging and remain indeterminate. These are some lipid-poor adenomas, adrenal metastases, adrenal carcinomas and phaeochromocytomas which have an overlap in their imaging appearances. It is clearly important to make the distinction between these lesions in patients undergoing staging for a known carcinoma, as the presence of metastases may contraindicate radical curative surgery or radiotherapy. CT and MRI techniques are optimised to maximise specificity for benign adrenal adenomas whilst still maintaining an acceptable sensitivity. Conversely, PET techniques are optimised for detection of malignant disease.
A variety of combinations of imaging techniques are used for characterisation of adrenal masses, and these are discussed in the following section.
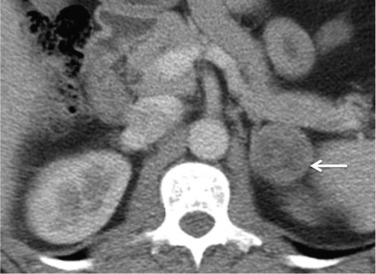
On unenhanced CT, features that suggest a higher likelihood of malignancy include a large lesion size, irregular contour, heterogeneous appearance and a temporal increase in size. Lesions greater than 4 cm in diameter have a higher likelihood to be either metastases or primary adrenal carcinomas. However, size alone is a poor discriminator between adenomas and non-adenomas. In a study by Lee et al., using 3.0 cm as the maximum size cut-off, the sensitivity and specificity for adenomas was only 79% and 84%, respectively ( Figs 37.4 and 37.5 ). Although it has been suggested that adenomas have a smooth contour, whilst malignant lesions have an irregular shape, this, as a single feature, is insufficient in discriminating.
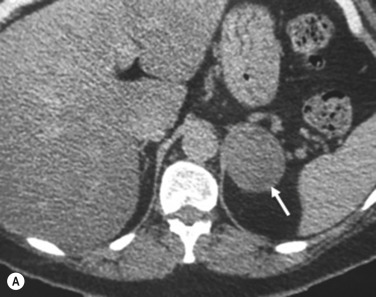
Rapid change in size does raise the suspicion of malignancy, as adenomas are slow-growing lesions.
Guidelines published by the American College of Radiology suggest that for lesions greater than 4 cm in size, adrenal resection without any other additional imaging work-up should be considered once biochemical evaluation to exclude phaeochromocytomas has been performed if typical imaging features such as those seen in benign lesions such as myelolipomas, adenomas, cysts are not present. The same guidelines also controversially suggest that in patients with no history of previous malignancy who present with a less than 4 cm adrenal mass with benign imaging features on postcontrast imaging such as smooth external contour and homogeneous appearance, a follow-up in 6 to 12 months is adequate and no additional imaging with contrast enhancement or chemical-shift imaging (CSI) MRI is required. In the authors’ experience, other confirmative features of a benign lesion are needed before this guideline can be safely applied.
A number of recommendations have been made recently for the imaging of adrenal incidentalomas in the recent European Society of Endocrinology Clinical Practice Guideline. These include
Unenhanced CT should be performed to confirm a lipid-rich adenoma (HU < 10).
If unenhanced CT is indeterminate and there is no clinically significant hormone excess, then three options can be considered:
Immediate imaging with another technique (e.g. contrast-enhanced adrenal protocol CT, MRI with CSI or FDG PET). In the setting of known extra-adrenal malignancy, FDG PET/CT can be performed in the place of other imaging techniques
Interval imaging in 6 to 12 months to assess lesion growth
Surgical resection.
MRI is recommended as the primary imaging technique in children and adults below 40 years old and in pregnant women.
In the setting of known extra-adrenal malignancy, if CT/MRI imaging is conclusive of a benign lesion then no further imaging work-up is necessary.
Biopsy is only recommended for an adrenal lesion in the setting of a known extra-adrenal primary malignancy when the following criteria are met:
Lesion is hormonally inactive
Lesion is indeterminate on imaging
Management will be altered by knowing the histology of the lesion.
Most (>70%) adenomas have a high intracellular lipid content, which lowers their unenhanced CT density and hence their attenuation value. If an adrenal mass measures 0 HU or less, the specificity of the mass being a benign lipid-rich adenoma is 100%, but the sensitivity is only an unacceptable 47%. Boland et al. performed a meta-analysis of 10 studies demonstrating that if a threshold attenuation value of 10 HU was adopted, specificity was 98% and sensitivity increased to a clinically acceptable 71%. Therefore, 10 HU is the most widely used threshold value for the diagnosis of a lipid-rich adrenal adenoma. In another study by Song et al., in 973 consecutive patients with 1049 incidental adrenal masses, adenomas accounted for 75% of incidental masses, of which 78% were lipid-rich adenomas, with an unenhanced CT attenuation value of less than 10 HU. By using a threshold of 10 HU, the sensitivity and specificity for the detection of an adenoma at non-enhanced CT is 89% and 100%, respectively. Therefore, most lesions can be fully characterised on unenhanced CT alone and require no further confirmatory imaging ( Fig. 37.6 ). Lesions with an unenhanced CT attenuation greater than 10 HU require further evaluation with either contrast washout CT, MRI or scintigraphy.
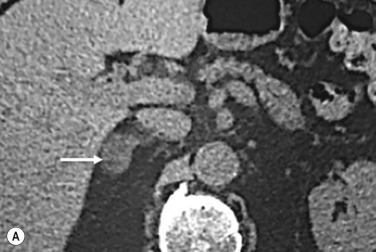
On non-contrast-enhanced CT, up to 12% to 30% of benign adenomas have an attenuation value of greater than 10 HU and are considered lipid poor. Malignant lesions and phaeochromocytomas are also lipid poor. Characterisation of adrenal masses using contrast-enhanced CT (CECT) relies on the unique physiological perfusion patterns of adenomas. Adenomas enhance rapidly after contrast administration and also demonstrate a rapid washout of contrast medium—a phenomenon termed ‘contrast medium washout’. Malignant lesions and phaeochromocytomas enhance rapidly but demonstrate a slower washout of contrast medium.
The difference in contrast enhancement washout characteristics between adenomas and malignant lesions has been shown to be a consistent and reliable technique. The percentage of contrast enhancement washout between enhanced images acquired 60 seconds after contrast administration and the delayed images acquired 15 minutes after contrast administration can be used to differentiate adenomas from malignant lesions. Measurement of the attenuation values of the mass before injection of contrast medium, at 60 seconds following injection of contrast medium, and then again at 15 minutes, are made using an electronic cursor that encompasses at least two-thirds of the mass. These contrast medium enhancement washout values are only applicable to relatively homogeneous masses without large areas of necrosis or haemorrhage. Both lipid-rich and lipid-poor adenomas behave similarly, as this property of adenomas is independent of their lipid content (see Fig. 37.6 ). The percentage of absolute contrast enhancement washout (ACEW) can be calculated as follows:
The CECT value is the attenuation value of the mass, in Hounsfield units, 60 seconds after commencement of intravenous contrast administration. The delayed attenuation value is the attenuation value of the mass, in Hounsfield units 15 minutes after commencement of contrast administration. Unenhanced CT attenuation is the attenuation value of the mass before administration of contrast medium.
If a 15-minute delayed protocol is used, an ACEW of 60% or higher has a sensitivity of 86% to 88% and a specificity of 92% to 96% for the diagnosis of an adenoma.
However, the measurement of this absolute contrast medium enhancement washout requires an unenhanced image. Frequently, in clinical practice, only postcontrast CT is available ( Fig. 37.7 ). In these patients, by performing a delayed 15-minute scan the percentage relative contrast enhancement washout can be calculated as follows:
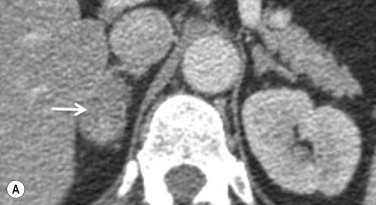
The enhanced and delayed attenuation values are measured as described previously. After 15 minutes, if a relative enhancement washout of 40% or higher is achieved, this has a sensitivity of 96% and a specificity of 100% for the diagnosis of an adenoma.
Phaeochromocytomas may cause confusion as they may be of sufficiently low attenuation (1.8 to 42 HU) on non-contrast-enhanced CT to be mistaken for adenomas and show contrast washout profiles similar to adenomas (absolute washout 40% to 89% and relative washout 16% to 83%). Phaeochromocytomas, although rare, may present as incidental masses and on CT and mimic both benign adenomas and malignant masses. Therefore, if there is doubt, a phaeochromocytoma should be excluded by clinical and biochemical evaluation.
Although contrast enhancement washout criteria have a high sensitivity and specificity for adenomas, the technique is somewhat cumbersome as it requires contrast administration and delayed images up to 15 minutes after contrast enhancement. Unenhanced CT alone, when using a threshold of 10 HU or less, has a 100% specificity but a low sensitivity for adenomas. CT histogram analysis is a technique that has been applied to both non-contrast-enhanced and contrast-enhanced images. A region of interest (ROI) cursor is drawn covering at least two-thirds of the adrenal mass, excluding areas of necrosis. The individual attenuation values of all the pixels in the ROI are plotted against their frequency and the amount of lipid in the mass is proportional to the number of negative pixels (<0 HU). The original studies demonstrated 97% of adenomas contain negative pixels. Eighty-five per cent have more than 5% negative pixels, and 83% have more than 10% negative pixels. No metastases had negative pixels. The performance of histogram analysis is variable in the literature and subsequent studies have reported negative pixels in non-adenomas, including metastases, phaeochromocytomas, and carcinomas.
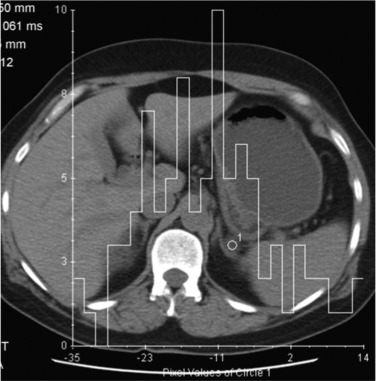
CT histogram analysis on unenhanced images may be a useful adjunct to the CT attenuation values, as the combination of CT attenuation value less than 10 HU or greater than 10% negative pixel content, would correctly identify 91% of adenomas compared with 66% using CT attenuation values alone ( Fig. 37.8 ). On enhanced CT, its low sensitivity precludes clinical usefulness.
Dual-energy CT has received much interest recently and applications in many different clinical setting have been researched. A recent retrospective study of 47 adrenal lesions by Mileto et al. suggests that dual-energy contrast-enhanced CT with material density analysis has excellent ability to discriminate between adrenal adenomas (lipid rich and lipid poor) and non-adenomas (sensitivity 96% and specificity 100%). Connolly et al. performed a meta-analysis of five studies that showed equivalent sensitivity and specificity for dual-energy CT-derived virtual unenhanced CT compared to actual unenhanced CT to diagnose adrenal adenomas using an attenuation threshold of 10 HU. Further prospective studies will be required to confirm these findings before dual-energy CT can be applied in clinical practice in this setting.
On conventional T 1 and T 2 images, considerable overlap exists between the signal intensities of adenomas and malignant lesions and up to a third of lesions remain indeterminate.
The accuracy of MRI in differentiating benign from malignant masses can be improved by using intravenous gadolinium injection and T 1 weighted sequences. After gadolinium enhancement, 90% of adenomas demonstrate homogeneous or ring enhancement while 60% of malignant masses have heterogeneous enhancement. Again, the early peak enhancement the time to reach peak enhancement is the strongest discriminator between adenomas and malignant adrenal masses. The value of peak enhancement has no statistical difference between adenomas and metastases. However, as with signal characteristics alone, there is considerable overlap in enhancement characteristics of benign and malignant masses, limiting its sole application in distinguishing adenomas from malignant masses.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here