Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter addresses endometrial carcinomas, including those with mesenchymal differentiation (carcinosarcomas). Primary endometrial carcinomas constitute a range of morphologies, including carcinomas closely resembling preexisting endometrial epithelium (endometrioid), clear cell carcinomas, uterine serous carcinomas (USCs; also referred to as uterine papillary serous carcinomas), carcinomas sharing one or more of these features, undifferentiated carcinomas, and tumors with mixed epithelial-mesenchymal differentiation (carcinosarcomas). They can also be divided according to clinical features, including the following: (1) excess estrogen effect via obesity, hormonal replacement, or abnormal estrogen production by ovarian lesions; (2) the absence of hyperestrogenism; (3) tamoxifen therapy; and (4) prior radiation therapy.
The issues most critical to proper diagnosis and management are as follows:
Identifying the patient at risk for these neoplasms
Determining appropriate methods for identifying or excluding endometrial adenocarcinoma
Appreciating the different forms of endometrial carcinomas that have prognostic significance
Assessing variables that affect outcome and influence therapy
Recently, at least four distinct molecular subgroups have also been described for endometrial carcinomas (although limited to endometrioid and serous histologies): (1) POLE -mutated carcinomas; (2) microsatellite instability (MSI)–high carcinomas; (3) copy number low carcinomas; and (4) copy number high (serous-like) carcinomas. They will be discussed in greater detail later in the chapter.
Endometrial carcinoma is the most common invasive cancer of the female genital tract in the United States, estimated by the American Cancer Society to have reached 40,000 new cases/year, with approximately 7500 deaths in 2008. It accounted for 7% of all invasive cancers in women, excluding skin cancer. Previously, it was far less common than cancer of the cervix, but earlier detection and effective treatment of cervical intraepithelial neoplasia (CIN) and an increase in endometrial carcinomas in younger age groups have reversed this ratio. In the United States, there are now 34,000 new endometrial cancers/year, compared with fewer than 10,000 new cervical cancers. Endometrial cancers usually arise in postmenopausal women, and more than 80% present with abnormal (postmenopausal) bleeding, which often permits detection and cure at an early stage. From 2% to 14% of endometrial carcinomas occur in women younger than 40 years. The peak incidence is in the 55- to 65-year-old age group, with a median age of 63 years.
Two general patient groups have emerged from demographic and histopathologic studies ( Table 19.1 ). The first is endometrioid adenocarcinomas and their variants, which are often termed type I endometrial carcinomas . As a group, these account for about 90% of endometrial cancers and arise in a background of chronic estrogen stimulation. The source of the estrogen may be ovarian dysfunction, polycystic ovarian disease or cortical stromal hyperplasia (stromal thecosis), estrogen-secreting tumors (rare), and unopposed estrogen in the form of continuous estrogen therapy. Obesity, which encourages the conversion of androstenedione to estrone in adipose tissue via aromatase activity, is strongly associated with this subset of endometrial tumors. Nulliparity is another risk factor. Because ovarian dysfunction and obesity are associated with adult-onset diabetes mellitus, the latter is also a risk factor. However, the reader is cautioned that not every woman with a type I tumor will share these risk factors, and attention to these variables alone will not determine which women with postmenopausal bleeding merit diagnostic studies.
| Parameter | Type I | Type II |
|---|---|---|
| Age | 50s–60s | 60s–70s |
| Obesity | Common | Uncommon |
| Estrogenic stimuli | Common | Uncommon |
| Endometrium | Anovulatory | Atrophic |
| Precursor | Endometrial intraepithelial neoplasia | Presumed EGD |
| Transition | Slow | Unknown |
| Type | Endometrioid | Papillary serous or mixed |
| Molecular genetics | MSI, PTEN mutation; loss of PAX2 | p53 mutation, 1p deletions; loss of PAX2 |
| Familial | Hereditary nonpolyposis colonic cancer syndrome | |
| Spread | Lymph nodes | Peritoneum |
| Concurrent ovarian | Common | Uncommon |
| Prognosis | Good | Poor |
Endometrial carcinomas that are associated with unopposed estrogen tend to be well differentiated, mimic normal endometrial glands (hence, the term endometrioid ) in histologic appearance, and are associated with a favorable prognosis. The presumed pathway to endometrial cancer involves excess estrogen, development of anovulatory endometrium, subsequent endometrial intraepithelial neoplasia (atypical hyperplasia), and, ultimately, adenocarcinoma. Predictably, this subset of tumors affects women in their middle to late 50s but encompasses most adenocarcinomas seen in the younger age groups.
The second and less common group of endometrial carcinomas (type II) is more often seen in the seventh and eighth decades, does not arise in association with hyperestrogenism, obesity, or diabetes, and has a distinctly different morphologic growth pattern. In this group, tumors are generally more poorly differentiated and include clear cell carcinoma and serous carcinoma. These tumors overall have a poorer prognosis than endometrioid tumors, and the factors predisposing to their development are less well defined. A small proportion of these tumors arise in association with endometrioid carcinomas, indicating independent processes or, more likely, a single tumor that has evolved to develop heterogeneity through multiple pathogenetic pathways.
Although most endometrial carcinomas can be subdivided into these two categories, the reader is reminded (and may already believe) that not all endometrial carcinomas are so easily pigeonholed. A more modern division of endometrial carcinomas into four subgroups is based on molecular alterations ; however this is also limited in the ability to assign carcinomas to one particular category definitively. These molecular insights have highlighted some diagnostic issues that pathologists may encounter. First, high-grade adenocarcinomas, including high-grade endometrioid carcinomas, may be immunophenotypically and molecularly similar to serous carcinomas, creating a category with an ambiguous or mixed endometrioid and serous phenotype. Second, some tumors that are architecturally endometrioid may harbor TP53 mutations and stain aberrantly for p53. These conundrums will be addressed and placed in a workable system for laboratory management.
Another mechanism of carcinogenesis is radiation-related. In one study of 23 women who developed endometrial cancer following radiation therapy, the average duration from radiation exposure to cancer development was 14 years. Some 69% were in unfavorable histologic categories, including carcinosarcomas.
Studies have shown that endometrial carcinoma is less common in African American women, but this group nonetheless has a higher mortality. Black women have a cancer incidence of 13/100,000, compared with 23/100,000 for white women and 14/100,000 for Hispanic women. However, black women have a fourfold higher risk of dying from endometrial cancer. The excess mortality in this group has been attributed to one or more variables, including later stage at diagnosis, unfavorable tumor type or stage, sociodemographic factors, treatment, and comorbidities. Tumor histologic type probably accounts for the bulk of this effect because black women have significantly higher incidence rates of poor prognostic cell types of serous and clear cell carcinoma, carcinosarcoma, and sarcoma. Rare aggressive tumor types accounted for 53% of mortality among black women, compared with 36% among white women in one study. However, survival is poorer for black women than for white women, even when controlled for in histopathologic category, stage, grade, and age.
Continuous (unopposed) estrogen exposure is associated with an increased risk of endometrial adenocarcinoma, with a risk approximately sixfold higher than that of controls ( Table 19.2 ). Shorter intervals of estrogen followed by progesterone, such as encountered in the use of sequential contraceptives and sequential replacement therapy, have a normal or marginal increase in risk. Screening of the latter population with endometrial sampling has not proven to be cost-effective. In two studies combined, only one malignancy was discovered in 1398 patients on replacement therapy who were screened by biopsy.
| Factor | Relative Risk |
|---|---|
| Overweight (lb) | |
|
3.0 |
|
10.0 |
| Nulliparous | |
|
2.0 |
|
5.0 |
| Late menopause (>52 vs. 49 yr) | 2.4 |
| Diabetes mellitus | 2.7 |
| Unopposed estrogen therapy | 6.0 |
| Tamoxifen therapy | 2.0 |
| Sequential oral contraceptives | 7.0 |
| Combination oral contraceptives | 0.5 |
| Cowden syndrome ( PTEN mutation) | Three- to fivefold increased risk |
| Hereditary nonpolyposis colonic cancer syndrome | 40%–60% lifetime risk |
| Family member with endometrial cancer | 3.4 |
Tamoxifen is a nonsteroidal antiestrogen used chiefly for prophylaxis and as a maintenance drug for breast carcinoma. The dominant histologic pattern seen with tamoxifen use is an atrophic or inactive endometrium. However, in the mid-1990s, studies emerged linking more prolonged use with endometrial polyps and a range of endometrial neoplasms, including endometrioid, serous, and mixed epithelial and mesenchymal tumors. The risk of these conditions, which includes an increased endometrial stripe on ultrasound, increases as a function of dose and duration of therapy. A total of 18% of 317 treated patients developed some form of pathology in one study, and 5 (1.6%) developed malignancy after 3 years of exposure. The malignancy rate was 0.5% (5 patients) in another group of 1010 women followed an average of 51 months. The computed standard incidence ratio was 4.0 to 4.8.
Some authors have linked tamoxifen to more aggressive subtypes of endometrial cancers ; others have not demonstrated an increased risk of poor outcome. Some of the observed differences may be due to selection bias of consultation material-based studies compared with more inclusive studies of exposed populations.
Endometrial cancer is overwhelmingly a sporadic disease (>98% of occurrences), but it occasionally may be a manifestation of hereditary cancer syndromes that also include ovarian, colonic, and other neoplasms. What is known can be summarized by the following.
Endometrial carcinoma is the most common gynecologic cancer associated with Lynch syndrome, formerly known as hereditary nonpolyposis colonic cancer syndrome (HNPCC). Tumors from patients with Lynch syndrome display the microsatellite instability (MSI) phenotype attributed to defects in mismatch repair genes ( MSH2 , MSH6 , MLH1 , or PMS2 ) or, less commonly, EPCAM deletions resulting in epigenetic silencing of MSH2 . From 40% to 70% of women with Lynch syndrome develop endometrial cancer; the mean age is approximately 10 to 20 years younger than nonfamilial occurrences (46 years).
Risk can be assessed using several systems, as follows.
One is the modified Amsterdam criteria, which includes several factors:
Three relatives with any Lynch syndrome–associated tumor, including colorectal cancer. Other tumors in the Lynch syndrome link include those involving the endometrium, ovaries, stomach, small bowel, kidney, brain, and skin.
One affected family member is a first-degree relative of the other two.
Two successive generations of family members must be affected.
One family member is diagnosed with this cancer before the age of 50 years.
Familial adenomatosis coli must be excluded.
Another is the Bethesda criteria, which stipulates the following factors :
Colorectal cancer diagnosed in a patient who is younger than 50 years of age
Presence of synchronous, metachronous colorectal, or other Lynch syndrome–associated tumors, regardless of age
Colorectal cancer, with the histology diagnosed in a patient younger than 60 years
Colorectal cancer diagnosed in one or more first-degree relatives with a Lynch syndrome–related tumor, with one of the cancers diagnosed in a patient younger than 50 years
Colorectal cancer diagnosed in two or more first- or second-degree relatives with Lynch syndrome–related tumors, regardless of age
Approximately 2% of endometrial cancers are associated with Lynch syndrome (4% if <50 years) and, in more than 50% of women with Lynch syndrome, endometrial cancer will be their sentinel malignancy. However, it is estimated that nearly two-thirds of these could go unsuspected based on historical criteria because their pedigrees were small or the dominant familial tumor was endometrial.
Of the four mismatch repair (MMR) genes associated with Lynch syndromes ( MLH1 , MSH2 , MSH6 , and PMS2 ), MSH6 or MSH2 is the more commonly affected in endometrial cancer. Immunohistochemistry for loss of expression of one or more MMR proteins is sensitive but not specific because at least 25% of endometrial carcinomas have evidence of microsatellite instability and loss of expression of one or more of the mismatch repair genes (usually MLH1, PMS2), which will not discriminate between genetic and epigenetic silencing of MLH1 . Thus, one logical approach is to perform antibody screening followed by single-gene sequencing of the candidate revealed by loss of immunostaining.
At present, there are no formal guidelines for screening women with endometrial cancer for Lynch syndrome. One review has proposed that in addition to historical data, tumor morphology, such as tumor-infiltrating lymphocytes, tumor heterogeneity with dedifferentiated or undifferentiated features, lower uterine segment location, and synchronous ovarian clear cell carcinomas be used to help identify patients with endometrial carcinoma at risk for this syndrome. Although these features may be enriched for MSI-high endometrial carcinomas and patients with Lynch syndrome, they are neither sufficiently sensitive nor specific to use in clinical practice. Many groups are in favor of, and have already implemented, universal screening for Lynch syndrome in endometrial carcinomas using a combination of MMR immunohistochemistry, MLH1 promoter methylation studies, and referral to genetic counseling for consideration of germline testing. A flow chart of possible options for this process is presented in Fig. 19.1 , and examples of the reporting of the immunohistochemistry results are shown in Box 19.1 .
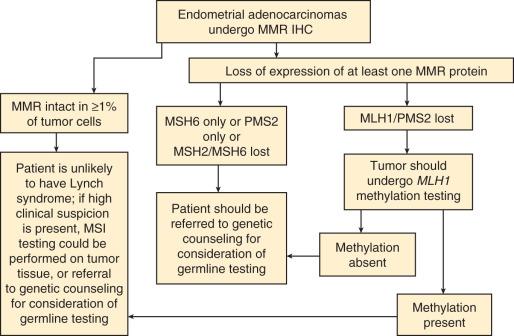
a At the Brigham and Women's Hospital. See also College of American Pathologists: Template for reporting results of biomarker testing of specimens from patients with carcinoma of the endometrium. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/endometrium-16biomarker-1100.pdf .
Intact MLH1, MSH2, MSH6, PMS2 expression
Immunohistochemistry for MLH1, MSH2, MSH6, and PMS2 shows retained expression. In a small subset of tumors, there is an underlying hereditary genetic defect despite intact nuclear expression in tumor cells. Further testing for microsatellite instability is available if the clinical suspicion for Lynch syndrome remains high, despite the intact mismatch repair protein expression.
Loss of MLH1 and PMS2 expression
Immunohistochemistry for MLH1, MSH2, MSH6, and PMS2 shows loss of MLH1 and PMS2 expression. Methylation of the MLH1 promoter is the most common cause of sporadic MLH1 protein loss in endometrial adenocarcinomas. Additional testing to assess the MLH1 promoter methylation status will be performed, and the results will be reported separately.
MLH1 promoter methylation testing was performed that did detect MLH1 promoter methylation. This result indicates a high likelihood of sporadic inactivation causing microsatellite instability, rather than a germline mutation.
MLH1 promoter methylation testing was performed, which did not detect MLH1 promoter methylation. Absence of MLH1 promoter methylation in tumors with MLH1 protein loss may predict a germline mutation in the MLH1 gene (Lynch syndrome–associated tumor).
Genetic counseling is recommended.
Loss of MSH2 and MSH 6 expression
Immunohistochemistry for MLH1, MSH2, MSH6, and PMS2 shows loss of MSH2 and MSH6 expression. In most tumors with loss of MSH2 and MSH6 staining, the defect is caused by a germline mutation in the MSH2 gene (or, rarely, in the MSH6 gene), usually in the setting of a family history of colorectal, endometrial, gastric, or other cancers (Lynch syndrome).
Genetic counseling is recommended.
Loss of MSH6 expression
Immunohistochemistry for MLH1, MSH2, MSH6, and PMS2 shows loss of MSH6 expression. In most tumors with loss of MSH6 staining, the defect is caused by a germline mutation in the MSH6 gene, usually in the setting of a family history of colorectal, endometrial, gastric, or other cancers (Lynch syndrome).
Genetic counseling is recommended.
Loss of PMS2 expression
Immunohistochemistry for MLH1, MSH2, MSH6, and PMS2 shows loss of PMS2 expression. In most tumors with loss of PMS2 staining, the defect is caused by a germline mutation in the PMS2 gene, usually in the setting of a family history of colorectal, endometrial, gastric, or other cancers (Lynch syndrome).
Genetic counseling is recommended.
The risk of synchronous or metachronous ovarian tumors is increased in women with endometrial cancer, ranging from 7% to 25%, with the lower figure applying to the general population. Like isolated endometrial adenocarcinoma, these usually occur in a sporadic nonfamilial setting. Most of these (>90%) are presumed to be concurrent and separate primary neoplasms, and reproductive tract endometrioid carcinomas are a strong endorsement of a field effect influencing the emergence of multiple neoplastic clones at different sites. The rate of bilaterality is highest in young women, with 25% reported in one study. This has implications for sparing the ovaries in these patients, with the risk appearing low but not zero in women with normal-appearing ovaries at surgery.
Some of the familial risk of endometrial cancer appears to be independent of the Lynch syndrome link. This strong relationship appears to be limited to endometrioid tumors only. Standard incidence (risk) ratios (SIRs) for synchronous or metachronous ovarian carcinomas in women with endometrioid carcinomas (or the reverse) may be as high as 140. The SIRs of endometrial cancer and endometrioid ovarian cancer in a daughter whose mother had endometrial cancer are similar (3.4 and 3.2). If a sister was affected by colorectal cancer, the SIR increased to 31.4 and the mean age predictably decreased. There is a mildly elevated endometrial cancer risk in familial kindreds with Cowden syndrome, caused by constitutive inactivation of the PTEN tumor suppressor gene. The low magnitude of increased risk (probably only three- to fivefold) may be due to lack of those nongenetic hormonal factors, which interact with mutations to define the lifetime cancer risk.
In summary, patients who present with familial endometrial cancer have a high frequency of genetic mutations in those mismatch repair genes inactivated in Lynch syndrome families. Usually, these are structural genomic defects in the DNA mismatch repair genes MLH1 , PMS2 , MSH2 , or MSH6 , and a genetic instability phenotype can be observed in histologically normal, as well as neoplastic, tissues. Sporadic nonfamilial endometrial cancers also have a high incidence (17%–23%) of loss of MLH1 and PMS2 expression caused by somatic epigenetic inactivation of the MLH1 gene. The accumulated data thus support a relationship between inactivation of mismatch repair genes and endometrioid tumors of the female genital tract. When the mismatch repair system is constitutively inactivated through a heritable mechanism, affected individuals tend to be younger at the time of cancer presentation and have a shared risk for other neoplasms, most notably colorectal cancer.
Serous and endometrioid endometrial cancer subtypes have a divergent pathogenesis. Alterations in the PTEN tumor suppressor gene and the MSI phenotype have long been known to be strongly associated with the endometrioid subgroup (see Table 19.1 ). Mutations in the PTEN tumor suppressor gene—active in cell cycle control and apoptosis—have been identified in as many as 80% of endometrioid carcinomas and are also commonly detected in isolated glands of histologically normal proliferative endometrium and endometrial intraepithelial neoplasia (EIN; see Chapter 17 ; Fig. 19.2 ). The pathogenesis of endometrioid carcinoma and its transition from normal to neoplastic also involve a wide range of other molecular disturbances, including KRAS mutation, CTNNB1 inactivation, and loss of cyclin-dependent kinase activity.
In contrast to the endometrioid carcinomas, serous tumors are not typically associated with PTEN inactivation or MSI. Instead, more than 80% of these tumors possess mutations in the TP53 tumor suppressor gene. Mutations in TP53 are found in the earliest identifiable manifestations of these tumors, producing intense diffuse nuclear staining or complete loss of p53 staining. Diffuse staining for p16 is also the rule ( Fig. 19.3 ).
Despite efforts to divide them into two distinct groups (types I and II), the pathogenesis of endometrial adenocarcinoma is complex. TP53 mutations can be associated with endometrioid carcinomas and a proportion of carcinosarcomas. Recently, studies of endometrioid and serous endometrial carcinomas using whole-exome sequencing have divided endometrial carcinomas into four distinct molecular subgroups, as follows.
This group of carcinomas comprises approximately 5% to 10% of all endometrial carcinomas, and is often high-grade (grade 3) endometrioid in histotype. Molecularly, they are characterized by mutations in POLE , which results in a high overall mutational rate (ultramutated). This molecular subgroup of endometrial carcinoma appears to have an improved prognosis, with better progression-free and overall survival. Importantly, a substantial percentage of this group is high-grade endometrioid (grade 3). POLE -mutated tumors may be misinterpreted as serous carcinomas, and about 35% have alterations in TP53, resulting in aberrant p53 expression patterns by immunohistochemistry.
This group of endometrial carcinomas has a defect in mismatch repair, either germline in nature (associated with Lynch syndrome) or somatic (usually MLH1 promoter methylation), as discussed above, resulting in hypermutation.
This group of endometrial carcinomas has both low numbers of mutations and low numbers of copy number alterations. Most tumors in this category are endometrioid in histotype and are grades 1 and 2. Only one gene is more frequently mutated in this group compared to MSI tumors, CTTNB1.
This group of carcinomas is characterized by genomic instability, as evidenced by high numbers of copy number variations (CNVs) and smaller numbers of mutations, but virtually all tumors harbor TP53 mutations. Although tumors in this subgroup have serous histology, a significant proportion of high-grade endometrioid carcinomas (grade 3) may also fall into this molecular group.
Preinvasive endometrial glandular neoplasia was discussed in detail in Chapter 17 . It is generally assumed that the common (endometrioid) forms of endometrial adenocarcinoma have their beginnings as endometrioid intraepithelial neoplasia (EIN). This is supported by temporal, genetic, and morphologic continuity between EIN and endometrioid endometrial adenocarcinoma, as well as the increased risk of endometrial cancer clinical outcomes associated with EIN.
The lag period between premalignant lesions and the development of endometrial cancers, and the precise percentage of endometrial cancers that transit a diagnosable premalignant phase, is unclear, because of previous imprecision in precancer diagnosis under the old hyperplasia system and difficulties in obtaining longitudinal tissue samples on individual patients. Koss screened asymptomatic women and found a prevalence rate of endometrial cancer of 6.96/1000 women-years, with an incidence of 1.71/1000 women-years. In spite of an active search for endometrial hyperplasias, the rate of these lesions was nearly identical to the prevalence and incidence rates for carcinoma—a striking contrast to the disparity in prevalence rates for invasive and preinvasive squamous neoplasms of the cervix. A systematic search for residual EIN in women having hysterectomies for endometrial adenocarcinoma has indicated that approximately 40% of women with the endometrioid types of carcinoma retain persistent premalignant lesions at the time of definitive therapy. Those cancers that transit to a premalignant phase are most likely to show inactivation of the PTEN tumor suppressor gene and downregulation of PAX2, changes noted in two-thirds of EIN lesions. This is strong evidence that not only is the endometrioid subtype of carcinoma most strongly associated with premalignant EIN lesions, but also that this progression scenario is functionally defined by accumulation of genetic defects in affected tissues over a period of years. The tempo of progression was first estimated by Hertig and Sommers, who found an average progression interval of 3 to 5 years from endometrial anaplasia to adenocarcinoma. This time frame was confirmed in a follow-up study of atypical endometrial hyperplasia, which showed a median progression interval to adenocarcinoma of 4.1 years. Studies have shown that lesions classified as EIN have as much as a 40% risk of an endometrial adenocarcinoma outcome. Uterine papillary serous carcinomas are associated with a less well-defined spectrum of precursor lesions and serous endometrial intraepithelial carcinoma (EIC), which is an early noninvasive form of the disease but is potentially lethal. The duration of this transition from normal to serous EIC or frank USC is likely quite variable; it could develop gradually from morphologically normal endometrial surface epithelium via a p53 signature or endometrial glandular dysplasia, making detection of an intervening premalignant phase difficult. Other carcinomas could emerge as a secondarily mutated subclone from a preexisting endometrioid adenocarcinoma.
Postmenopausal bleeding confers a 64-fold increase in risk of endometrial carcinoma, and the risk is maximized by recurrent bleeding. In one study, 91% of patients presented with postmenopausal or irregular bleeding. More ominous but less frequent symptoms were abdominal pain or other symptoms (9.4%). Important variables that influence the significance of bleeding include the following.
The risk of cancer is a function of the age when bleeding occurs, increasing from 9% in the sixth decade to 60% in the ninth decade. Overall, the risk in postmenopausal women with bleeding is approximately 8% to 11%. In most of them, atrophic (83%) and other benign nonatrophic (6%) patterns are present. However, any abnormal bleeding should be investigated, including intermenstrual bleeding and oligomenorrhea, particularly in the setting of ovarian dysfunction.
Hormone replacement therapy (HRT) is associated with a lower risk of carcinoma in the setting of bleeding. Elliott et al. have examined 299 postmenopausal women and 204 women on HRT. The incidence of endometrial carcinoma was significantly higher in older women and those who were not on HRT (relative risk [RR] > 10). Gemer and Segal observed a rate of only 0.08% for women younger than 50 years versus 3.7% for women older than 50 years.
Twu and Chen studied 77 patients with postmenopausal bleeding whose initial tissue diagnosis was benign. A total of 16 of them was subsequently diagnosed with carcinoma or hyperplasia, and the risk was particularly high for those older than 65 years (44.8%). The authors concluded that repeated bleeding in a postmenopausal patient may justify hysterectomy.
The recurrence rate of uterine bleeding was highest in carcinoma of the endometrium but was not exclusive to this group. However, another study of 40 hysterectomies for persistent postmenopausal bleeding, but with normal prior endometrial sampling, found no cancers and four (10%) hyperplasias, suggesting that persistent postmenopausal bleeding with nonmalignant pathology on endometrial sampling is not necessarily an indication for hysterectomy.
Transvaginal ultrasound (TVU) has increased in popularity, principally as a device to screen women on tamoxifen therapy and identify women at risk for endometrial cancer. The parameter of importance is endometrial thickness. The following factors are important variables when using TVU.
As a rule, the mean endometrial thickness increases as a function of the pathology. It averages 3.4, 9.7, and 18.2 mm for atrophic, hyperplastic, and malignant endometrium, respectively.
The cutoff level varies among studies, but generally ranges from 5 mm (for the general population) to 8 mm (for women on tamoxifen). Predictably, the lower the threshold, the higher the sensitivity and the lower the positive predictive value. In a screening population, nearly 100% of cancers can be excluded with the value set at 5 mm. However, the positive predictive value is poor, being 9% in one screening study. In a meta-analysis of four studies of women with postmenopausal bleeding, Gupta et al. noted a positive predictive value of 31.3% for a positive test (>5 mm); however, 2.5% of negatives were confirmed to have cancer on histologic examination. Other literature reviews and reports using a 5- or 6-mm cutoff have found similar results and, despite some showing a 100% sensitivity, the consensus is that symptomatic women should undergo endometrial sampling.
In many institutions, TVU is most popular in the follow-up of women on replacement therapy or tamoxifen. The cutoff value is usually 8 mm, but the test has been limited by lack of specificity. Endometrial thickness increases as a function of duration of treatment and will exceed 8 mm in half of patients after prolonged use. Moreover, there is little relationship between thickness and cancer. The cause of the endometrial thickness in most women on tamoxifen is subendometrial thickening.
It is generally accepted that hysteroscopy is a useful adjunct for evaluating the endometrium but is not sufficient without histologic examination. One meta-analysis, based on 208 articles, established a pretest probability of endometrial cancer at 3.9%; a positive and negative hysteroscopy yielded a cancer diagnosis in 71.8% and 0.6%, respectively. Others have concurred that although few cancers are identified in the setting of normal or atrophic findings, all patients must undergo biopsy.
One concern with hysteroscopy is that it will increase the risk of a positive peritoneal washing at the time of hysterectomy, effectively upstaging the tumor to an International Federation of Gynecology and Obstetrics (FIGO) stage IIIA. However, it does not appear to influence overall prognosis. In the setting of low-grade disease with no other risk factors, the significance of a positive peritoneal cytology appears to be minimal.
A potentially significant improvement in hysteroscopy is saline infusion sonohysteroscopy (SIS). In one study, SIS combined with targeted endometrial sampling helped make a diagnosis in 89% of cases versus 52% for blind endometrial biopsy. If this study is confirmed, this technique may prove superior to endometrial biopsy in diagnosing endometrial pathology in women with abnormal uterine bleeding.
The pathologist might be involved in four phases of endometrial carcinoma management, including the following: (1) detection of the tumor on a Papanicolaou (Pap) smear; (2) interpretation of a biopsy or curetting, which may be prompted by the cytology but much more frequently by abnormal bleeding; (3) intraoperative assessment of the uterus to facilitate staging; and (4) postoperative evaluation of the hysterectomy specimen to determine the risk of recurrence and guide therapy. Each of these components of laboratory management has its priorities, limitations, and diagnostic pitfalls and will be discussed subsequently. However, addressing each one first requires a comprehensive understanding of the histologic classification and grading of endometrial neoplasia. Before that, it is important to reemphasize the criteria for malignancy as opposed to precancers (EIN). These include confluent cribriform growth, rambling columnar epithelium that fails to round up into glands, villoglandular architecture, and conspicuous nuclear atypia ( Fig. 19.4 ). The latter may conform to normal gland architecture but often signifies a serous component.
Box 19.2 breaks down the different tumor types of endometrial adenocarcinoma, which include the three major categories—endometrioid, serous, and clear cell. In practice, some of these divisions are not as easy to make, primarily because some tumors with low-grade glandular architecture may have higher risk factors, such as TP53 mutations, and the separation of serous from endometrioid differentiation can be difficult. Thus, in essence, grading and tumor type cannot always be separated. However, the clinician will need to know whether the tumor is in a high-risk category to determine whether to perform a nodal dissection. To simplify this process, we will view endometrial carcinomas from the following perspective.
Precursors
Endometrial intraepithelial neoplasia (EIN)
Endometrial glandular dysplasia and p53 signatures
Common types
Endometrioid adenocarcinoma
With squamous differentiation
With mucinous differentiation
With ciliated (tubal) differentiation
With secretory differentiation
With squamotransitional differentiation
Villoglandular carcinoma
Uterine papillary serous carcinoma
Mixed endometrioid and serous carcinoma
Clear cell carcinoma
Carcinosarcoma undifferentiated/de-differentiated carcinoma
Rare variants
Condyloma
Squamous carcinoma (including verrucous carcinoma)
Small cell carcinoma (neuroendocrine carcinoma)
Giant cell carcinoma
Nongestational choriocarcinoma
Metastatic tumors
Cervix carcinoma
Breast carcinoma
Gastrointestinal carcinoma
Tumors easily classified as low grade include the following: (1) pure endometrioid adenocarcinomas, in the grade 1 architectural category; and (2) endometrioid carcinomas with squamous, secretory, tubal, and mucinous differentiation.
Intermediate- or indeterminate-grade carcinomas include the following: (1) endometrioid carcinomas in the architectural grade 2 category; and (2) endometrioid differentiation in the architectural grade 1 or 2 category, with higher nuclear grade or features worrisome for serous differentiation, including those with unusual glandular features, including villous or papillary morphology.
High-grade carcinomas include the following: (1) grade 3 endometrioid carcinomas; (2) serous carcinomas; (3) high-grade adenocarcinomas with ambiguous morphology, typically sharing endometrioid and serous features; (4) clear cell adenocarcinomas; (5) undifferentiated adenocarcinomas; and (6) neuroendocrine carcinomas.
When approaching these tumors, the pathologist should keep the following in mind. Adenocarcinomas of the endometrioid type, including those with other forms of differentiation described herein, are graded according to the FIGO system ( Table 19.3 ; Fig. 19.5A–C ). The distinction of grades 1, 2, and 3 is based on less than 5%, 5% to 50%, and greater than 50% solid growth, respectively.
| Grade | Features |
|---|---|
| 1 | Less than 5% solid growth (excludes squamous differentiation) |
| 2 | Solid growth involving 5%–50% |
| 3 | Solid growth involving over 50% |
a Tumor grade is increased by one step if conspicuously enlarged nuclei and prominent nucleoli are present. Lesser atypias do not justify an increase in grade.
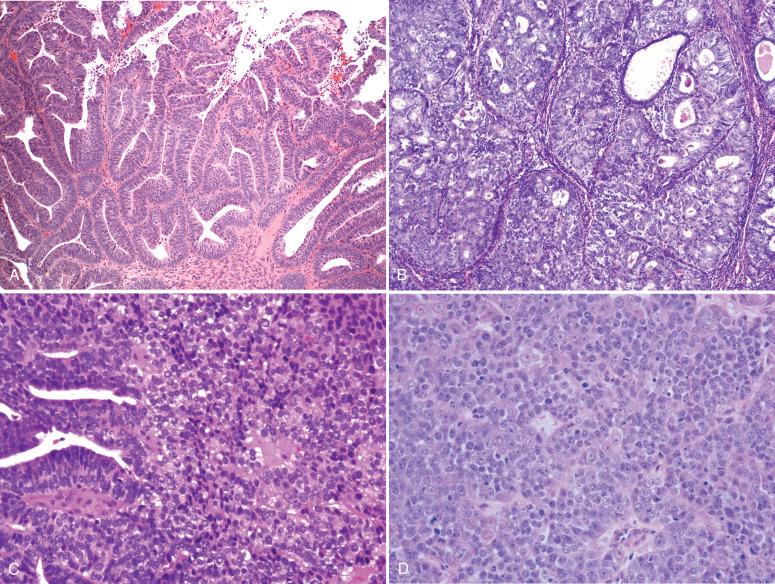
The pathologist is permitted to increase the grade by one step if conspicuous nuclear enlargement and atypia are present ( Fig. 19.6 ). Both the architectural and nuclear grading schemes are summarized in Table 19.3 .
The cutoff of 5% solid growth between grades 1 and 2 can be problematic because of the relatively small difference in prognosis between the two grades (92% vs. 88% 5-year survival) and a cutoff point of 5% solid growth. Other systems have been proposed. Taylor et al. offered a two-tiered system in which grade 2 was assigned to tumors with greater than 20% solid growth. They reported much higher interobserver reproducibility (κ = 0.97 vs. κ = 0.53 for a three-grade system) and improved agreement with outcome histology in the hysterectomy (90% vs. 63%). In another study, Lax et al. proposed a binary system in which grade 2 was assigned to tumors with two or more of the following: (1) more than 50% solid growth (squamous and nonsquamous were not distinguished); (2) diffusely infiltrative versus expansive myometrial invasion; and (3) tumor cell necrosis. For tumors that were confined to the endometrium, 50% solid growth or greater and tumor necrosis designated high grade. Interobserver agreement was also high in contrast to the traditional system (κ = 0.65 vs. κ = 0.22). In their analysis, grade 1, stage IA and IB had a 100% 5-year survival (FYS). Grade 1, stages IC and IV, and grade 2, stages IB and IC, had an FYS of 67% and 76%, respectively. However, because the pattern of myometrial infiltration was an important variable, this system is more appropriate to the hysterectomy specimen than the curetting specimen and will be revisited later in the chapter.
Tumors that are classified as serous carcinomas, clear cell carcinomas, and undifferentiated carcinomas are exempt from grading because they are by definition high-risk tumors and will require full surgical staging.
Grading is not a perfect science, for several reasons. Not all solid areas of tumor impose a higher grade; some may be subtle squamous differentiation. Assessment of nuclear grade, which can be used to upgrade endometrioid carcinomas, is subjective. Also, a small number of endometrioid tumors are strongly p53 immunopositive.
The following rules should be followed and will be repeated later in the chapter. Squamous differentiation does not factor as solid growth and does not upgrade the tumor. Cytologically bland grade 1 neoplasms with focally solid growth exceeding 5% do not merit upgrading if the solid areas resemble squamous metaplasia (so-called abortive squamous differentiation) and exhibit bland cytologic features ( Fig. 19.7A ). Conversely, solid areas of adenocarcinoma with clear to pale cytoplasm might mimic squamous differentiation (see Fig. 19.7B ). These areas of bland squamous epithelium pose a second problem because they may be misclassified as benign (see Fig. 19.7C and D ).
Some tumors are morphologically biphasic, consisting of juxtaposed grade 1 and grade 3 endometrioid tumors. If such cases contain less than 50% solid growth overall, the biologic potential may be more similar to a grade 3 tumor. In such cases the pathologist could call attention to the biphasic pattern and the fact that a discrete area of the tumor is essentially a grade 3 carcinoma ( Fig. 19.8 ).
Irrespective of the grading system, the presence of pronounced nuclear atypia warrants upgrading of the tumor. This is defined as anisokaryosis, macronuclei, nuclear pleomorphism, and prominent nucleoli (see Fig. 19.6B ).
Biomarkers such as p53 have a limited role but might be helpful in specific circumstances. The accumulation of p53 in a high percentage of tumor cell nuclei is usually but not always because of TP53 gene mutations ( Figs. 19.9 to 19.12 ). The precise cutoff level of staining that defines a TP53 mutation varies according to reports. In our practice, the p53 staining should be more or less diffuse (>90%) in its distribution and moderate to strong in intensity. Others use lower cutoff levels, between 30% and 70%. In practice, most will score less than 30% or more than 70%. The most common distribution patterns include the following: (1) diffuse intense nuclear staining (see Fig. 19.9A and B ); (2) no staining or cytoplasmic staining only (see Fig. 19.10A and B ); (3) wild-type staining, consisting of focal weak to intense staining of scattered neoplastic cells (<30%; see Fig. 19.11A and B ); and (4) complete absence of staining in all tumor cell nuclei, consistent with a deletion mutation in the gene (i.e., the null phenotype). A fifth pattern defines the juxtaposition of two distinct staining (and morphologic) patterns, including an abrupt transition from minimal to marked staining, befitting mixed histologic patterns (see Fig. 19.12 ). Reports differ on whether p53 immunostaining is an independent prognostic parameter, primarily because it correlates so closely with nuclear atypia and the serous phenotype. However, there has been increasing evidence that p53 staining is useful not only for sorting out tumors with problematic features, such as papillary patterns or tubular glandular arrangements, but also for conveying prognostic information.
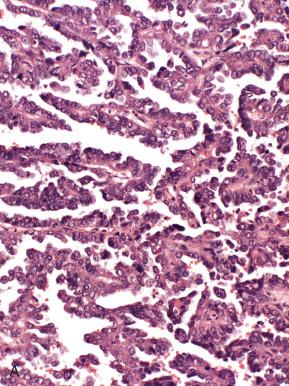
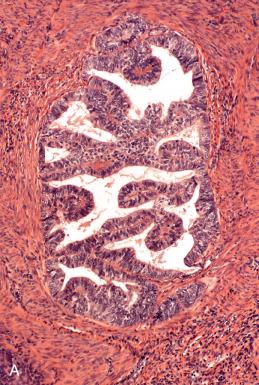
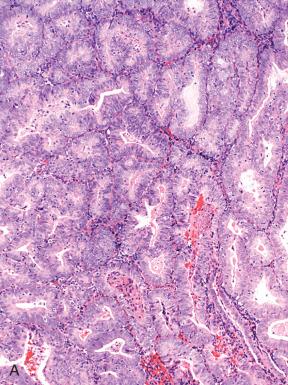
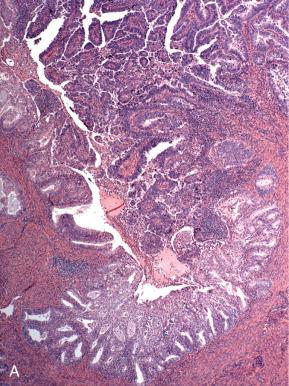
Endometrioid adenocarcinomas, including those with nonendometrioid forms of differentiation, account for nearly 90% of endometrial adenocarcinomas. Most endometrioid carcinomas are characterized histologically by well-defined glands lined by cytologically malignant columnar epithelial cells. However, they are subdivided into several morphologic subsets, each of which may vary in the degree of differentiation. Three important parameters to keep in mind when evaluating tumors in this category are as follows :
Glandular pattern, in terms of the shape of glands and degree of solid growth
Degree of nuclear atypia, with attention to the criteria for elevating the grade if present
Nonendometrioid cellular differentiation (or metaplasia), which can both confirm an endometrioid type and introduce some issues in grading
Patterns of endometrioid differentiation fall into the following categories: (1) conventional endometrioid carcinoma and other patterns accounting for a small or large percentage of the tumor; (2) squamous differentiation, which can range in degree of atypia; (3) mucinous differentiation, which can range in degree; (4) tubal differentiation, including cilia; (5) secretory differentiation, which must be distinguished from clear cell carcinomas and some primitive endometrioid patterns; (6) villoglandular, which can be misclassified as serous carcinoma; (7) subtle changes in the nuclear-to-cytoplasmic ratio or nuclear atypia within uniform endometrioid glands that might signal the presence of TP53 mutations and therefore merit classification as a higher grade tumor; and (8) other forms of differentiation. Many of the preceding patterns are often classified in other texts as nonendometrioid categories because they differ in appearance from normal endometrium. This is technically correct; however, the separation of endometrioid from these other patterns is superfluous, inasmuch as these variant patterns are characteristically associated with endometrioid carcinomas in contrast to serous or clear cell carcinomas and do not alter outcome. For this reason, they will be discussed with the endometrioid tumors.
Endometrioid adenocarcinomas are those in which the malignant glands resemble those of the normal proliferative phase endometrium. They are distinguished from normal endometrium and preinvasive disease (EIN) by features outlined in Fig. 19.4 and graded as illustrated in Fig. 19.5 . Most endometrioid carcinomas are grade 1 or 2. Because they resemble normal endometrium, endometrioid carcinomas usually display nuclear uniformity, with preservation of polarity (see Fig. 19.6A ). The neoplastic glands are larger and typically more irregular than those of the normal endometrium. When conspicuous atypia is present, it will influence grading (see Fig. 19.6B ). Rarely, some exhibit a uniform tubular pattern similar to Sertoli cell tumors of the ovary ( Fig. 19.13 ). Another characteristic feature of some endometrioid tumors is the presence of compact clusters of foamy histiocytes in the stroma adjacent to the neoplastic glands ( Fig. 19.14A ). In curettage specimens, foamy cells should be distinguished from smaller sheets of macrophages that are commonly seen in benign endometria (see Fig. 19.14B ). Foamy stromal histiocytes do not invariably signify cancer, but their presence in association with a uterine carcinoma signifies an endometrial rather than a cervical origin.
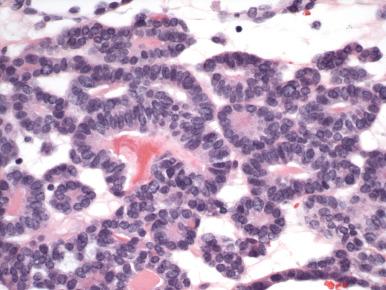
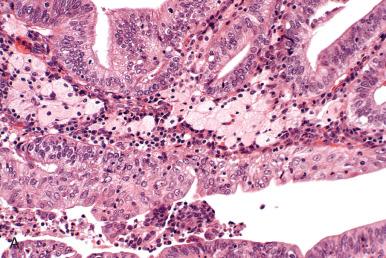
Squamous differentiation accompanies approximately 25% of endometrioid carcinomas, and the reader should be aware of the following. Squamous differentiation displays a range of atypia that parallels the degree of glandular atypia. This range includes the following:
Discrete nests (morules) or well-demarcated sheets of uniform, nonkeratinizing squamous cells, as commonly seen in EIN and metaplasias, typically accompany well-differentiated tumors, and lesions straddling the interface between EIN and well-differentiated adenocarcinoma ( Fig. 19.15A ).
In some cases, atypical squamous differentiation may be all that is present (see Fig. 19.15B ).
Conspicuous nuclear atypia or less well-defined squamous differentiation with focal keratinization and higher nuclear grade may be indistinguishable from squamous carcinoma; this pattern accompanies high-grade endometrioid adenocarcinomas (see Fig. 19.15C ).
In contrast, rare examples of exceedingly well-differentiated papillary squamous carcinomas are also seen (see Fig. 19.15D ).
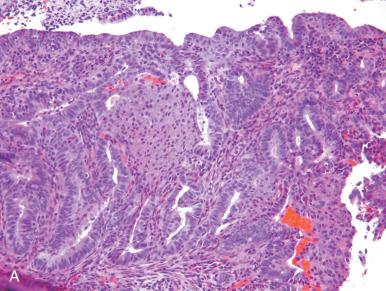
The clinical significance of squamous differentiation in endometrioid carcinomas has been debated in the past, with disagreement over whether squamous differentiation per se influences outcome. Traditionally, adenocarcinomas with squamous differentiation were assigned unique diagnostic categories, such as adenoacanthoma or adenosquamous carcinoma, on the assumption that they portended better and worse outcomes, respectively. Although this was generally correct, experts ultimately decided that the degree of glandular differentiation primarily governed the prognosis of these tumors. Consequently, nomenclature replaced the above terms with adenocarcinoma (of specific type and grade) with squamous differentiation . Because the range of squamous differentiation varies in endometrioid carcinomas and because the transitions between squamous and glandular components can be subtle, the pathologist may not always be able to determine whether the tumor in question is a poorly differentiated endometrioid carcinoma or a high-grade carcinoma with mixed differentiation. However, in high-grade tumors, this distinction is not critical because the tumor will be assigned a high-risk histology. In some tumors, the nonglandular component may appear transitional or squamotransitional (discussed later), reflecting further the plasticity of these tumors in terms of differentiation pathway ( Fig. 19.16A–C ).
In occasional cases, the tumor may be almost entirely squamous in appearance, and the degree of atypia may be so mild as to mimic squamous metaplasia of the cervix. The clinician must keep this possibility in mind when encountering unusual squamous epithelium. The mimics of and pitfalls associated with squamous differentiation include overgrading of the tumor (see Fig. 19.7A ), misclassifying eosinophilic sheetlike areas of glandular epithelium as squamous (see Fig. 19.7B ), and failing to recognize bland-appearing squamous differentiation—which can be in the endometrium and on the cervical surface—as a component of an adenocarcinoma (see Fig. 19.7C and D ). This latter circumstance could delay the diagnosis of cancer.
Mucinous differentiation is frequently present as a minor component in endometrioid adenocarcinomas and is defined by the presence of prominent intracellular mucin. As emphasized in Chapter 18 , bland-appearing mucinous glands, although they resemble endocervix, are much more commonly seen in endometrial than endocervical carcinomas, from which they must be distinguished.
A few endometrial carcinomas are purely mucinous and, in rare cases, marked mucin production will be visible on gross examination. In most cases, mucinous differentiation occurs in an otherwise well-differentiated endometrioid neoplasm, prompting the diagnosis of endometrioid adenocarcinoma (grade specified) with mucinous differentiation. If nearly pure, the term mucinous adenocarcinoma is permitted, but there is no clinical significance to this distinction. The range of atypia, however, may be significant in terms of influencing the diagnosis and prompt recognition of these tumors.
Histologically, tumors with mucinous differentiation range from banal to obviously malignant, and the reader should be aware of the following patterns:
The most subtle variants consist of loosely arranged columnar epithelia that exhibit mild complexity.
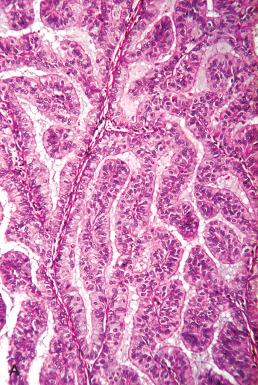
More conspicuous patterns exhibit cribriform growth lined by uniform cuboidal to low-stratified cells with round, slightly enlarged nuclei and mucin-containing cytoplasm ( Figs. 19.17 and 19.18 ). Those with microacinar architecture may closely resemble microglandular changes of the cervix.
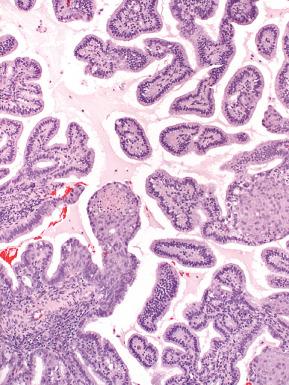
Other patterns include micropapillary or macropapillary architecture that must be distinguished from benign surface metaplasias.
These are discussed later, under “Differential Diagnosis,” and in Chapters 17 and 18 . Others contain more irregular architecture and conspicuous nuclear atypia and are unmistakably malignant ( Fig. 19.19 ).
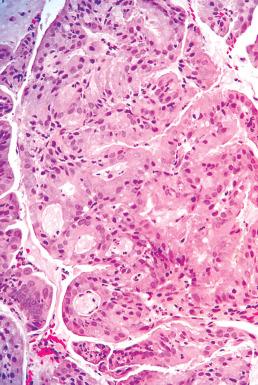
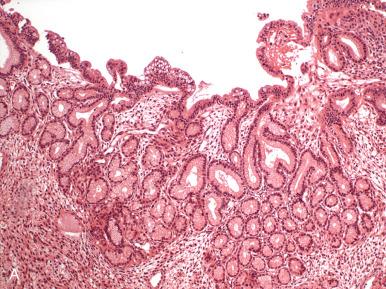
Rare variants of mucin-producing endometrial carcinomas include those with gastric or intestinal differentiation ( Fig. 19.20 ).
In general, lesions with mild atypia will be minimally invasive. However, there have been cases in which the mucinous component was superficial and focal, with a subjacent higher grade adenocarcinoma or, rarely, a carcinosarcoma.
There is a microglandular-like pattern of endometrioid carcinoma. Tumors with prominent microglandular architecture may be confused with benign microglandular changes of the cervix (see Chapter 14 ).
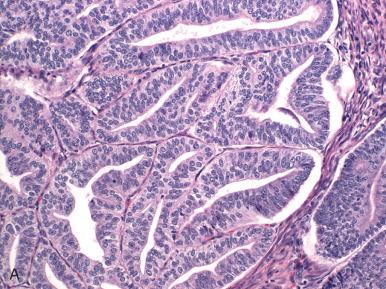
Tubal differentiation is discussed in Chapter 18 and is common in both benign and neoplastic endometrium. When the tumor has widespread ciliated cells, the term ciliated carcinoma has been used. By definition, these tumors are well differentiated ( Fig. 19.21 ). They have uniform but confluent glands composed of stratified cells with round nuclei, focal cytoplasmic clearing, prominent eosinophilic cytoplasm with terminal bars, and well-defined cilia projecting into the lumen above a portion of the cells. They portend a favorable outcome.
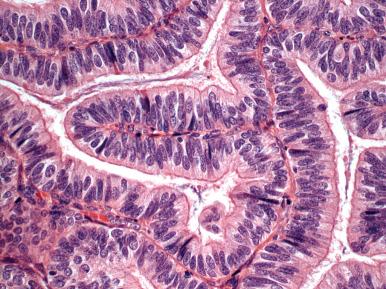
A small number of endometrioid adenocarcinomas are characterized by basal or supranuclear vacuoles akin to those seen in early secretory endometrium. The glands are similar in contour to early secretory endometrium but differ by their size (larger), back-to-back orientation, and prominent differential epithelial stratification with luminal scalloping. Although the pattern evokes a connection to progestins and may be associated with hormonal therapy or ovulatory endometrium in some premenopausal women, most patients are postmenopausal ( Fig. 19.22A ) and have no known source of progesterone. The most important exclusions are secretory endometrium with artifactual crowding (see Chapter 16 ) and EIN with secretory change (see Chapter 17 ). The second is more subtle and consists of glands with a higher nuclear-to-cytoplasmic ratio and small discrete vacuoles in the glandular epithelium or sheets of cells with cytoplasmic vacuolization lacking gland architecture. These features characterize more poorly differentiated adenocarcinomas, including the epithelial components of carcinosarcomas (discussed later; see Fig. 19.22B ). A related entity that can be particularly difficult to interpret is adenocarcinoma that has been treated with progestins (see Fig. 19.22C ).
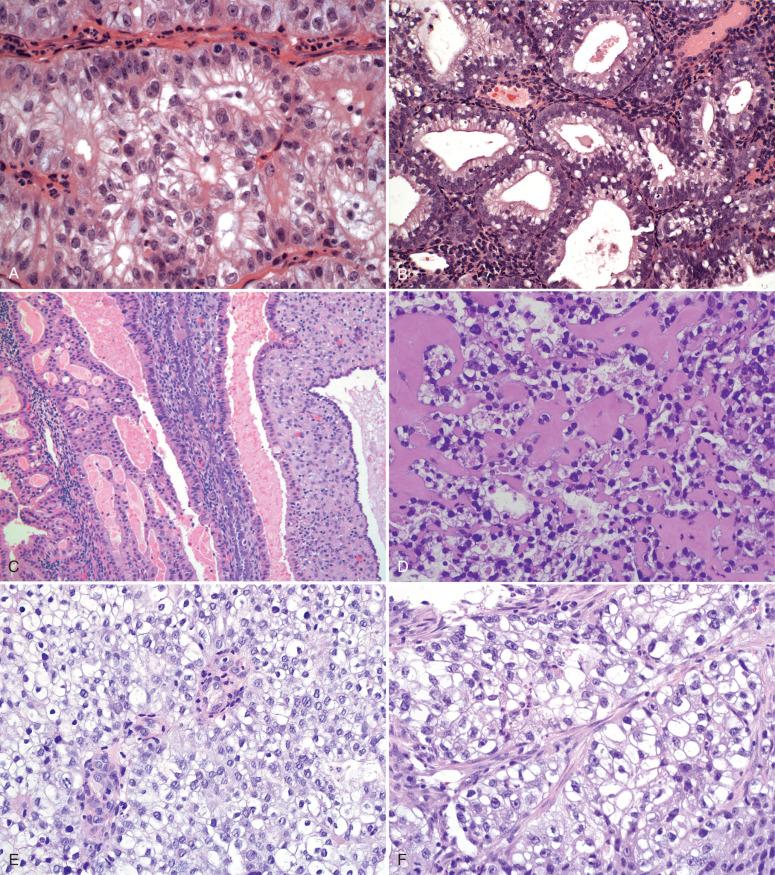
Clear cell changes are not uncommon and range from the uncommon clear cell carcinoma (see Fig. 19.22D ) to the more common endometrioid adenocarcinomas, with areas of clear cell differentiation (see Fig. 19.22E and F ).
Villoglandular endometrioid adenocarcinomas exhibit a predominantly treelike branching architecture with discrete fibrovascular cores lined by defined, pseudostratified, endometrioid-type epithelium ( Fig. 19.23A ). Included in this pattern is intraglandular papillary architecture (see Fig. 19.23B ). Occasionally, foci of degenerative changes with exfoliation might mimic serous carcinoma (see Fig. 19.23C ). Some degree of villoglandular architecture has been described in as many as 30% of endometrioid adenocarcinomas ; however, this pattern is seen as the predominant pattern in fewer than 5%. The prognostic importance of this pattern varies, depending on the study. One associated the villoglandular pattern with other aggressive histologic features. A larger study did not detect a difference in outcome between villoglandular carcinomas and conventional endometrioid adenocarcinomas.
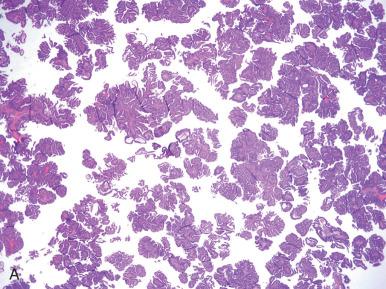
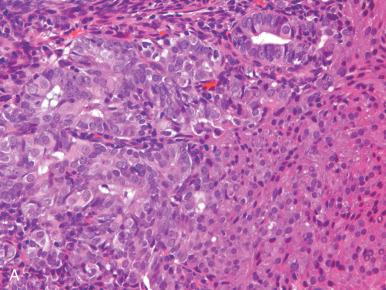
Corded and hyalinized endometrioid carcinoma is an uncommon form of endometrioid carcinoma that may be confused with carcinosarcoma due to its seemingly biphasic appearance. These endometrioid carcinomas may have strikingly hyalinized stroma, spindled cell morphology, and sex cord–like areas, without significant cytologic atypia. The distinction from carcinosarcoma is important because corded and hyalinized carcinomas tend to present at a low stage and are associated with a good prognosis, in contrast to the aggressive endometrial carcinosarcomas. Notably, the corded and hyalinized carcinomas demonstrate a transition from well-formed glands to the sex cord–like elements. Endometrioid carcinomas with a spindled component may also be misinterpreted as a carcinosarcoma; however, both components should be low grade, with merging between the gland-forming and spindled areas.
A variety of other endometrial lesions, some benign (e.g., papillary metaplasia) and others malignant (e.g., uterine serous, clear cell, mucinous, and transitional carcinomas), may exhibit villous architecture with fibrovascular cores. These will be discussed later, under “Differential Diagnosis.”
Diagnostic pitfalls with lower grade carcinomas include the following:
Confusing secretory differentiation with clear cell carcinoma
Confusing endometrioid villoglandular architecture with uterine serous carcinoma
Confusing micropapillary changes on the surface with serous carcinoma
Confusing the microglandular pattern of endometrioid carcinoma with microglandular hyperplasia of the cervix (see Chapter 14 )
Sheet-forming squamous epithelium misclassified as solid endometrioid growth
Misinterpreting bland-appearing but neoplastic squamous differentiation as benign
Misclassifying higher-grade (nuclear criteria) tumors with bland architecture as low-grade endometrioid carcinomas (see next section)
Architectural grade 2 endometrioid adenocarcinomas are defined as endometrioid adenocarcinomas that contain solid growth encompassing between 5% and 50% of the tumor. The cytologic grade should be low or at most moderate and not conspicuous (with markedly coarse chromasia and three- to fourfold differences in nuclear size). This is, in our experience, one of the three problematic areas of classification, with others including rather small differences in overall prognosis between grades 1 and 2 endometrioid carcinomas, potential confusion in separating solid glandular from squamous patterns (which do not merit upgrading), and the overall subjectivity of separating grade 1 from grade 2 based on solid growth. Moreover, the pathologist will encounter biphasic endometrioid carcinomas that juxtapose low grade and high grade endometrioid carcinoma. We do not classify these tumors based on percentage of the high-grade component. Rather, we classify these as the coexistence of two tumor types and assume that outcome is influenced by the high-grade component. We generally reserve grade 2 endometrioid designations for those tumors in which the transition from glandular to solid growth is more gradual and not accompanied by a marked change in nuclear grade.
Most endometrioid carcinomas can be readily separated into a serous or endometrioid category, but a minority will pose problems in classification, typically reflected in greater disagreement between observers. The indeterminate category is distinguished from the intermediate group by the difficulty in determining not whether it is grade 1 or grade 2 endometrioid, but whether it belongs in the endometrioid group. As we will discuss subsequently in the discussion of high-grade endometrioid carcinomas, the absolute distinction between a serous and endometrioid carcinoma is not always simple, although the distinction carries less importance in tumors that are indisputably high grade. The term indeterminate grade is used in this chapter to designate tumors that have a glandular or papillary architecture but also problematic features. The latter include the following: (1) a pseudostratified population, with greater nuclear hyperchromasia and nuclear pleomorphism; (2) loss of stratification, with a more cuboidal appearance to the cells; and (3) architectural patterns with papillary or tubular epithelial architecture. When we encounter tumors of this type, we perform p53 immunostaining ( Figs. 19.24 and 19.25 ). If positive (or globally negative), we classify these tumors as high-grade endometrial carcinomas of uncertain type, and note that this decision is based on the unusual pattern combined with the p53 immunophenotype.
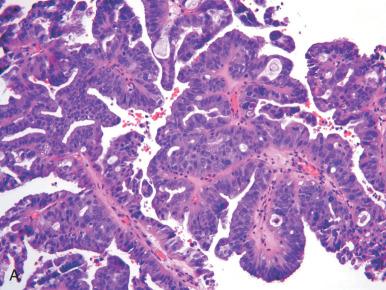
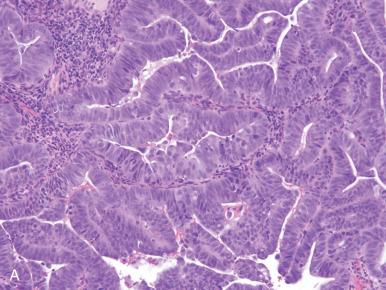
There are several entities in this group. All are approached as high-risk malignancies and, as a group, confer a higher risk of relapse and death than their lower grade counterparts ; however, there are differences in behavior, as summarized in Table 19.4 . High-grade endometrioid and serous carcinomas have a similar outcome albeit via different patterns of spread. Undifferentiated and neuroendocrine carcinomas have a worse prognosis than grade 3 endometrioid carcinomas, justifying their distinction, if not absolute separation. Clear cell carcinomas have a high rate of relapse but are largely extrapelvic, distinguishing them from serous carcinoma.
| Reference No. | Type | Stage I (<2 mm) (%) | Stage I (All) (%) | All Cases (%) |
|---|---|---|---|---|
| a | EM | 98 | 91 | 91 |
| VG | — | — | 94 b | |
| ASCA | — | 64 | 65 | |
| CCA | 90 | 43–59 | 39–42 | |
| UND | 100 | 79 | 58 | |
| USC | 60–93 c | 75 | 27–38 |
a Combines recurrences and deaths.
b Stages I and II combined 3-year survival.
c Survival in this group increases as a function of thorough staging to exclude extrauterine disease.
Grade 3 endometrioid carcinomas should exhibit clear evidence of endometrioid differentiation but with minimal architectural definition (see Figs. 19.5D and 19.7B ). This diagnosis is essentially a histologic one and traditionally requires one of two features: (1) the presence of at least 50% solid growth or (2) some solid growth pattern (grade 2) and conspicuous nuclear atypia, justifying upgrading to grade 3. Morphologic features of grade 3 endometrioid carcinomas can overlap with the other patterns, including serous, clear cell, undifferentiated, and neuroendocrine carcinomas.
USCs are derived via mutations in the TP53 tumor suppressor gene and (from a morphologic perspective) may arise from clonal expansions of TP53 mutation positive epithelium (p53 signatures), atypias (endometrial glandular dysplasia), and, in some cases, a preexisting endometrioid adenocarcinoma.
Since the early 1980s, USCs have been recognized as a distinct entity with aggressive behavior. Additional studies have essentially confirmed the integrity of this entity while expanding its spectrum to include two overlapping patterns:
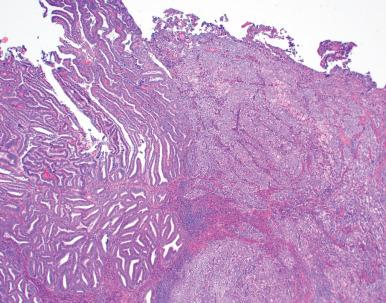
Serous carcinomas confined to the endometrial surface or endometrial polyps, so-called serous endometrial intraepithelial carcinomas (see Chapter 17 )
Invasive serous carcinomas, with or without the noninvasive component
Despite the absence of invasion and the small amount of neoplastic epithelium in some of these USCs, they have a strong tendency to spread to peritoneal surfaces, possibly by retrograde expulsion through the fallopian tubes. The discrete nature of these neoplasms underscores the abrupt transformation from benign to malignant seen in serous endometrial carcinomas and supports the concept that even the earliest noninvasive forms are capable of metastasizing and killing the patient.
Pure serous carcinomas have constituted as few as 1.1% of endometrial carcinomas in one study and as many as 10% in others. The actual frequency is elusive, subject to the vagaries of misclassification (e.g., the misinterpretation of villoglandular endometrioid tumors as serous, the underappreciation of mixed endometrioid and serous patterns) and the variable emphasis assigned to certain histologic and immunohistochemical (p53) parameters in making the diagnosis of a serous tumor.
For the purposes of this discussion, pure serous carcinomas almost always exhibit some element of papillary or micropapillary architecture or display the classic, slit-forming, glandular features, with a high nuclear grade. All serous tumors have features in common, which are high-grade nuclear atypia, a cuboidal to low-stratified epithelium and macronuclei, and prominent nucleoli in at least a portion of the lesion. The architectural patterns encountered vary from those that are obviously papillary to those that exhibit characteristic glandular features. Many of these patterns can be encountered in a single tumor. The patterns are as follows.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here