Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute respiratory failure (ARF) is defined by the sudden onset of severe impairment of pulmonary gas exchange and is characterized by the inability of the lungs to meet the body’s metabolic needs for the transport of oxygen (O 2 ) into the blood and/or removal of carbon dioxide (CO 2 ) from the blood. The diagnosis of ARF is based on the measurements of arterial blood gas (ABG) parameters (i.e., partial pressure of oxygen [PaO 2 ], partial pressure of carbon dioxide [PaCO 2 ], and pH), and these values must always be interpreted in relation to the patient’s baseline status. As a final common pathway for a variety of illnesses, ARF is one of the most frequently encountered diagnoses in the intensive care unit (ICU), and its management represents one of the key challenges of critical care medicine. This chapter aims to tie the physiology of breathing to the pathologic processes that lead to ARF and will also discuss the clinical approach to the patient with ARF.
ARF is one of the most common reasons for admission to the ICU and accounts for ∼2 million admissions per year in the United States. More than half of all patients admitted to the medical ICU with stays >48 hours have ARF at some point during their hospitalization, with overall mortality rates of >33%. Mortality significantly increases with age, preexisting comorbidities, and the presence of shock or multisystem organ failure. With the aging population in the United States, the incidence of patients with ARF is expected to increase by 80% over the next two decades.
Understanding the process of respiration is a key step in understanding and managing ARF. Respiratory control is established by the tight coordination of three groups of neurons in the medulla oblongata: a dorsal respiratory center that controls inspiration, a ventral respiratory group that controls expiration, and a pneumotaxic center that controls the rate and depth of breathing. In addition to neurons in the brainstem, a peripheral chemoreceptor system is located outside the brain in the form of carotid bodies and aortic bodies that detect subtle changes in PaO 2 . The neural impulses from the central nervous system (CNS) traverse the spinal cord and motor neurons, reaching and activating the diaphragm and other respiratory muscles. Contraction of the inspiratory muscles creates negative pleural pressure by expanding the chest cavity and pushing the abdominal contents caudally. The negative pressure created in the thorax during inspiration leads to subatmospheric pressure in the alveoli, creating a gradient for the flow of inspired air toward the alveoli. Oxygen-rich inspired air allows the diffusion of O 2 from the alveoli to the blood through the alveolar–capillary membrane, where deoxygenated hemoglobin becomes saturated with O 2 to form oxyhemoglobin.
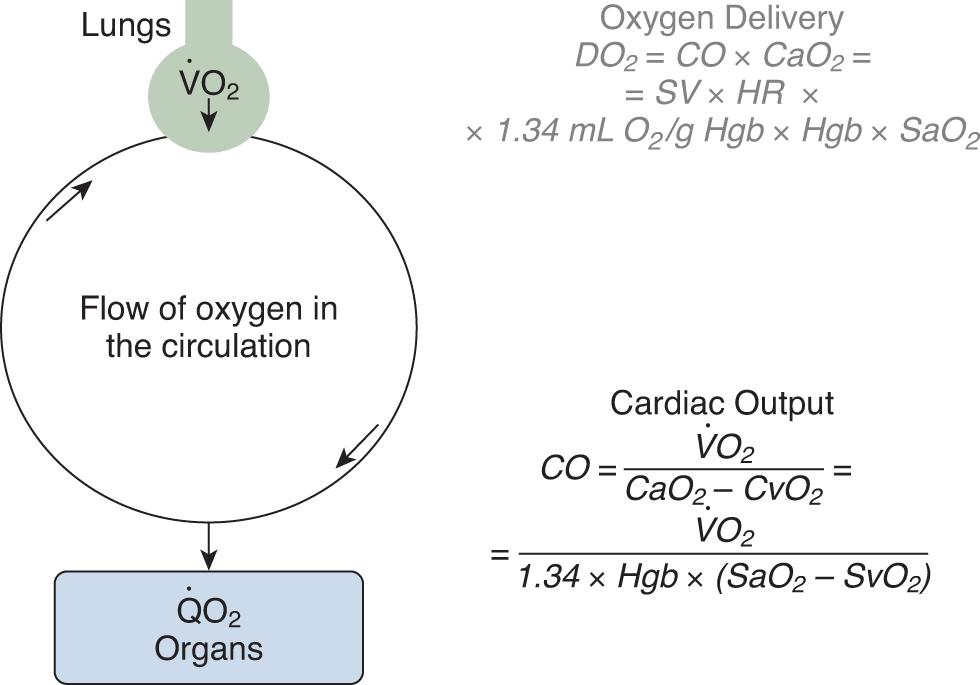
O 2 is consumed by all human tissues, and the ability to do so depends on gas exchange in the lungs. The average O 2 uptake of an adult is approximately 250 mL/min, although this rate depends on numerous factors. Most (∼98.5%) O 2 is carried to peripheral tissues via oxyhemoglobin, whereas the remainder is transported as O 2 dissolved in the fluid phase of blood. The total transport of O 2 by the arterial system is termed oxygen delivery (DO 2 ) and is normally several-fold higher than the O 2 demand of the peripheral tissues. However, O 2 utilization (VO 2 ) can become dependent on DO 2 in pathologic conditions such as ARF. In these states, the normally balanced relationship between oxygen delivery and oxygen demand can be disrupted by decreased O 2 delivery or increased O 2 demand ( Fig. 9.1 ).
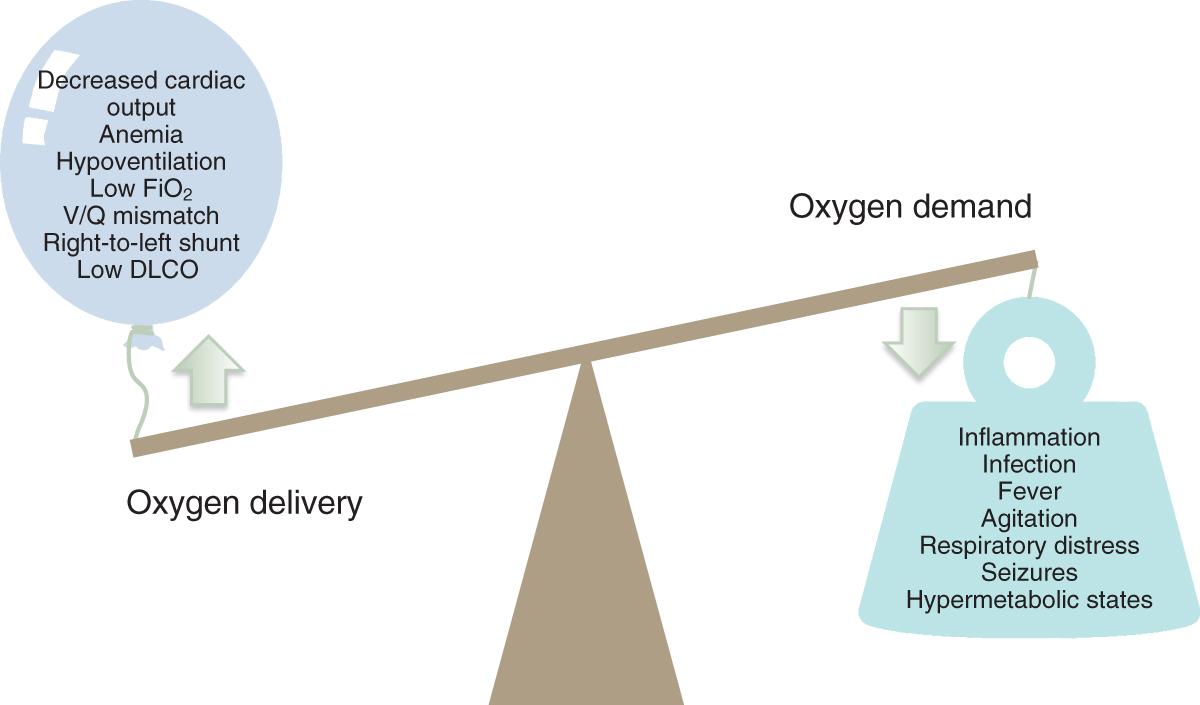
DO 2 is the product of cardiac output and arterial oxygen content (CaO 2 ), a value determined by the concentration of hemoglobin (Hgb) and oxygen saturation (SaO 2 ) ( Fig. 9.2 ). Adequate perfusion of capillaries in the peripheral tissues allows for the liberation of O 2 from oxyhemoglobin.
ARF can be a consequence of a wide range of tissue defects, which can have both pulmonary and extrapulmonary etiologies. It is important to understand the mechanisms leading to hypoxemia, as the best therapeutic approach may require targeting the appropriate etiology. This, however, is often difficult in the early stages of managing a patient with acute hypoxemia. Considering a broad differential diagnosis for ARF is crucial to appropriate management of the underlying condition ( Table 9.1 ).
|
Respiratory failure can be classified as acute or chronic. The clinical presentation of ARF is typically dramatic and obvious, often with profound derangements in ABG values. “Acute on chronic” respiratory failure represents an acute deterioration in the presence of preexisting chronic pulmonary disease and chronic respiratory dysfunction. Chronic respiratory dysfunction may present with markers of chronic hypoxemia (e.g., polycythemia, or cor pulmonale ) and may or may not require ICU care. Regardless of acuity, respiratory failure represents a potentially life-threatening group of disorders for which inadequate management may lead to rapid clinical deterioration.
ARF has been classically described as one of two types: hypoxemic or hypercarbic failure. More recent classifications categorize ARF into four different types, based on the mechanism of hypoxemia. Table 9.2 describes differences among the four types of ARF with regard to the mechanism of hypoxemia, location of the abnormality, and most commonly seen clinical syndromes. Despite these categories, considerable overlap exists in the different types of ARF. Furthermore, a given patient can have multiple types of ARF contributing to their clinical presentation.
| Type I | Type II | Type III | Type IV | |
|---|---|---|---|---|
| Mechanism of hypoxemia |
|
Hypoventilation |
|
Hypoperfusion or inadequate oxygenation of peripheral tissues |
| Location of pathologic process |
|
|
Alveolar–capillary unit collapse with regional hypoventilation |
|
| Clinical syndromes |
|
|
|
|
Type I ARF is the most common form of respiratory failure and at sea level is defined by PaO 2 <60 mm Hg, with normal or decreased PaCO 2 . The primary abnormality originates in one of three dysfunctions: (1) inadequately oxygenated alveoli (because of low fraction of inspired oxygen [FiO 2 ] and/or alveolar collapse and/or the presence of alveoli filled with fluid, cells, debris, or blood); (2) compromised transition of oxygen from the alveoli to the blood (because of interstitial processes or pulmonary vascular disease); or (3) compromised ability of the blood to oxygenate (because of obstructed blood flow, shunting, low Hgb concentration, or the presence of dysfunctional Hgb). The analysis of ABG values and calculation of the alveolar-arterial (A-a) gradient are important in the assessment of type I ARF.
Type II ARF (PaCO 2 >45 mm Hg) represents the failure of the lungs to remove a sufficient amount of CO 2 in a given time interval and is characterized by decreased alveolar minute ventilation. An increase in PaCO 2 leads to hypoxemia because CO 2 displaces O 2 and effectively reduces the alveolar partial pressure of oxygen (PAO 2 ). In contrast to some cases of type I ARF, hypoxemia in type II ARF is rather easily corrected with supplemental oxygen. This type of respiratory failure is frequently the result of acute or chronic neuromuscular dysfunction or the inability of the airways or lungs to ensure adequate ventilation and CO 2 exchange.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here