Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
the practice of pain management in children continues to advance. Since the early 1980s, clinicians have recognized that neonates and infants experience pain and process those learning experiences. Research has demonstrated long-term adverse consequences of unrelieved pain, including harmful neuroendocrine responses, disrupted eating and sleep cycles, and increased pain perception during subsequent painful experiences. Adequate pain control is second only to correct diagnosis when parents are surveyed about their priorities and concerns surrounding hospital admission. Disparities in pain treatment led organizations, such as the Agency for Healthcare Research and Quality (AHRQ) and the American Pain Society (APS), to provide guidelines and the Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations) to issue mandates that further enhanced pediatric pain management. The availability of reliable and valid pain assessment tools for children and governmental incentives encouraged the inclusion of children in analgesic drug trials. Many children's hospitals have dedicated multidisciplinary teams that manage acute and chronic pain. The increasing use of regional analgesia techniques led to the development of the Pediatric Regional Anesthesia Network (PRAN), a registry of practice patterns and complications of regional anesthetics in children. An enormous expansion of the breadth of techniques for acute pain management in children, the establishment of pediatric pain services, and the investigation and introduction of innovative modalities of therapy all attest to the importance accorded to this aspect of perioperative care.
Nociceptive pathways in the periphery, spinal cord, and brain develop in a series of stages through the second and third trimester in humans. By 26 weeks postconception age, there is sufficient maturation of peripheral and spinal afferent transmission for the late-gestation fetus or preterm neonate to respond to tissue injury or inflammation with withdrawal reflexes, autonomic arousal, and hormonal metabolic stress responses. There are also changes in responsiveness after injury or repetitive stimulation indicative of central sensitization. In general, preterm neonates have reduced thresholds for withdrawal to noxious thermal and mechanical stimuli and immature descending inhibitor pathways that are necessary for modulating the pain response compared with older infants and children. One mechanism that may contribute to these low-threshold responses involves projections of low-threshold peripheral afferents to superficial as well as deep laminae in the spinal dorsal horn; later in development these afferents project only to deeper dorsal horn laminae. Most of the neural pathways that conduct nociception from the periphery through the central nervous system (CNS) are present and functional at 24 weeks gestational age, although the central connections, particularly in the thalamocortical pathways that are involved in the integration and perception of conscious pain, are not as well developed. Controversy remains as to the meaning and implications of this neural immaturity. Opioid receptors and responses are present in the spinal cord at the time of birth, although spinal glial inflammatory mechanisms are immature. Because these mechanisms are central to the cyclooxygenase (COX-1 and COX-2) responses, this may imply that there is limited or no analgesic response to nonsteroidal antiinflammatory drugs (NSAIDs) and COX inhibitors in preterm infants or neonates, whereas opioid responses are active. γ-Aminobutyric acid (GABA) receptors and associated pathways, which play an important role in the effects of analgesics and anesthetics, can be either excitatory or inhibitory, depending on the stage of development. The neuroplasticity that is characteristic of these infants may be a double-edged sword. Animal models and some clinical evidence suggest that repeated noxious stimuli may result in heightened sensitivity to nociceptive input and adverse behavioral sequelae. On the other hand, nerve injury in infant animals may result in less pain than in older animals. In humans, the neural injury to the brachial plexus after shoulder dystocia during delivery rarely results in chronic pain. It may be that there are both vulnerable periods and periods of greater resiliency during development, so that the consequences of pain in young children may not be easily predictable.
Investigators have examined indexes suggestive of cortical activation, including near-infrared spectroscopy and electroencephalography, in response to noxious events. Using near-infrared spectroscopy, a unilateral heel stick (performed for clinical purposes) produces signal changes suggestive of contralateral cortical activation. Despite these lines of evidence, the nature of pain in neonates, viewed as conscious suffering, remains unknown. Other investigators have looked for long-term consequences of painful events (with or without treatment) in humans and in animal models. Despite attempts by these investigators to correct for confounding factors, in our view, the interpretation of these studies, especially in humans, should be quite cautious. Neonates who undergo painful procedures are commonly those who are more medically ill. It appears difficult to distinguish consequences of pain per se from the consequences of other factors, such as prematurity, critical illness (including episodes of hypoxia or ischemia), deprivation of tactile and social contact, and nutritional deprivation. Many clinicians and investigators have adopted the view that, in the absence of better information about either the nature of suffering experienced by neonates or the potential adverse consequences of pain in terms of long-term development, caregivers should err on the side of providing, rather than withholding, analgesia. Although this is a compelling perspective, it is important to highlight three concerns: (1) in general, available studies have had difficulty showing effects of routine administration of analgesia (e.g., morphine infusions) on immediate behavioral indexes of distress in neonates undergoing intensive care; (2) repeated or prolonged administration of anesthetics and sedatives in animal models have been shown to have deleterious effects on brain development, (the human implications of these animal studies remain unclear at this time [see also Chapters 7 and 25 ]) ; and (3) as detailed later, younger organisms develop tolerance to opioids and benzodiazepines more rapidly than older organisms, so that the management of tolerance and withdrawal has now become a nearly universal consequence of prolonged administration of these medications to critically ill neonates, infants, and children.
The International Association for the Study of Pain (IASP) has defined pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. The IASP and others have acknowledged that the inability to communicate verbally, as in the preverbal, nonverbal, or the cognitively impaired, does not preclude the possibility that an individual is experiencing pain and is in need of appropriate pain management. Table 44.1 summarizes various pain assessment tools in terms of appropriate age, target population, ease of use, and practicality; commonly used scales are discussed in more detail later.
| Self-Report Tools | Appropriate Age Groups | Comments |
|---|---|---|
| Faces Pain Scale | 3–18 years | Simple and quick to use; extensively validated in healthy school children with postoperative and cancer pain |
| Oucher | 3–18 years | Photographic for ≥3-year-olds, numeric 0–10 scale for ≥6-year-olds; less clinical utility and feasibility compared with other faces scales |
| Manchester Pain Scale | 3–18 years | Panda bear faces eliminate gender and ethnic bias; tested in emergency department setting |
| Computer Face Scale | 4–18 years | Offers option for continuous rather than categorical format; good construct validity; preferred by children over the Wong-Baker Faces Scale; further testing needed |
| Sydney Animated Facial Expression Scale (SAFE) | 4–18 years | Animated version of Faces Pain Scale; rated by children as easiest to use; no psychometric advantage compared with other scales |
| Visual Analog Scale (VAS) | 6–18 years | Simple and quick to use; requires the concepts of order, magnitude, and seriation (the ability to place or visualize in series); widely used across settings; preferred to other self-report tools by children ≥8 years old and adolescents |
| Numeric Rating Scale (NRS) | 7–18 years | Simplest and most commonly used in clinical as well as research settings |
| Observational/Behavioral Measures | ||
| Comfort Scale | 0–18 years | Developed for use in intensive care settings; useful in mechanically ventilated children and in the postoperative setting |
| Face, Legs, Activity, Cry, Consolability (FLACC) | 2 months–7 years | Excellent pragmatic and psychometric qualities; widely adopted in clinical and research settings; has been translated into several languages other than English |
| Children's Hospital of Eastern Ontario Pain Scale (CHEOPS) | 1–7 years | Good psychometric properties; lengthy with inconsistent scoring among categories; cumbersome; extensively used both in clinical and research settings |
| Cognitively Impaired Children | ||
| Revised FLACC | All ages | Allows for scoring individualized pain behaviors; good psychometric properties; highest clinical utility compared with the Non-Communicating Children's Pain Checklist–Postoperative Version (NCCPC-PV) and Nursing Assessment of Pain Intensity (NAPI) |
| Non-Communicating Children's Pain Checklist (NCCPC) | All ages | Requires 5-minute observation period; comprehensive but cumbersome; used in clinical and research setting |
| University of Wisconsin Pain Scale | All ages | Inconsistent scoring style compared with other clinical scoring systems; scoring style may permit flexibility but limits precision |
| The Pain Indicator for Communicatively Impaired Children | All ages | Useful for pain assessment in cognitively impaired children in the home setting. |
Consensus guidelines have been published with recommendations for appropriate tools to use for research studies, many of which are simple to use in routine clinical settings. It should be emphasized that pain intensity is only one of many factors that should be considered when performing a global pain assessment. Other important outcomes include functional recovery, patient satisfaction, adverse effects, emotional recovery, and economic factors. The Revised American Pain Society Patient Outcomes Questionnaire (APS-POQ-R) consists of 12 questions pertaining to multiple pain outcomes. A study is currently under way to validate a modified version in children. Although this tool is likely too cumbersome for daily use, it can be helpful for quality assurance and research studies.
Self-report metrics in which a patient is asked to quantify the severity of the pain between 0 (no pain) and 10 (maximum pain) most accurately reflect acute pain because pain is a subjective experience. Because many children lack the cognitive skills to use such scales, pain assessment metrics have been developed to include developmentally appropriate self-report tools, behavioral-observational tools, and physiologic-biologic measures (see Table 44.1 ). Given the multidimensional nature of the individual pain experience, and the complexity and inherent biases associated with self-report, the use of unidimensional numeric scales alone to reflect pain is overly simplistic. Therefore, regardless of the metric used, it must be emphasized that a complete pain assessment is more than just a number attempting to quantify the severity of pain. Estimating the impact of pain on the suffering and the quality of the individual's life and recovery process, targeting appropriate therapeutic metrics, and evaluating the efficacy and side effects of such measures are additional key components of a global and ongoing pain assessment and treatment strategy.
For children to use numeric scales, they must understand the concepts of magnitude and ordinal position—that is, they must be able to identify which of different-sized objects is bigger and place them in order from smallest to largest. They must also be able to arrange geometric figures or numbers in a series (seriation) . These skills are typically not present until about 7 years of age; thus pain assessment tools that use graphic facial displays representing different degrees of pain expression are used to facilitate self-report of pain in young children.
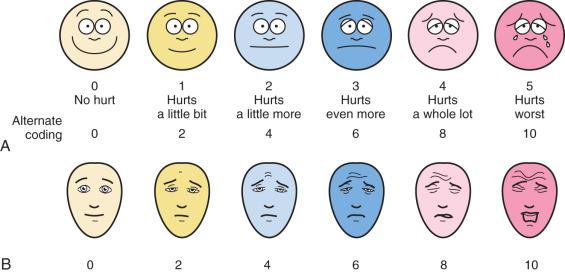
Faces pain scales consist of a series of line diagrams of faces with expressions of increasing distress. Some versions have a smiling face, whereas others, notably the Faces Pain Scale and Faces Pain Scale-Revised (FPS-R), have a neutral face to represent the “no pain” end of the scale ( Fig. 44.1 ). Unlike the numeric scales, the faces scales do not require the concept of magnitude or seriation and can therefore be used by preschool-aged children. The Wong-Baker Faces Pain Scale has been extensively studied and its reliability and validity confirmed in children 3 to 18 years of age. Strong correlations have been reported between the Wong-Baker Scale scores and other faces scales, the Visual Analog Scale (VAS), as well as nurses' ratings based on behavior. Data suggest that versions with the smiling face at the no-pain end of the spectrum, such as the Wong-Baker Scale, may overestimate pain because children without pain, but with distress from other sources, may be reluctant to choose the smiling face. The Wong-Baker Scale was preferred by children to the numeric rating scale, the graphic rating scale, and the Color Analog Scale. Overall, the FPS-R is the faces scale with the largest support for its validity. The International Association for the Study of Pain (IASP) has the FPS-R available in dozens of languages on its website ( http://www.iasp-pain.org/education ).
Several versions of the VAS are available, including horizontal and vertical lines, word anchors representing extremes of pain, and lines with divisions and numeric values ( Fig. 44.2 ). When using the vertical versions of this scale, the severity of the pain increases as one ascends the ladder. Although moderate to strong correlations have been reported between the VAS, faces pain scales, the Oucher ( E-Fig. 44.1 ), and ethnic versions of the Oucher ( E-Fig. 44.2 ), the effect of user age on VAS ratings are conflicting.
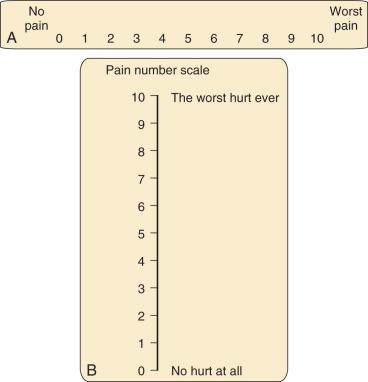
The Numeric Rating Scale (NRS) is the simplest and most commonly used numeric scale in which the child rates the pain from 0 (no pain) to 10 (worst pain). Its validity has been established with good correlations between NRS and FPS-R scores in children 7 to 17 years of age and NRS and VAS scores in children 9 to 17 years of age. The NRS also correlates well with perceived need for analgesia, pain relief, and patient satisfaction in children. An important caveat when using numeric scales is to verify the denominator that the child is using. For example, a pain score of 9 on a 0 to 100 scale would reflect mild pain and may not require treatment, whereas a score of 9 on a 0 to 10 scale would reflect severe pain that warrants aggressive treatment.
Selection of a self-report tool for a child requires careful consideration of the age and cognitive and developmental level. Fig. 44.3 depicts the percentages of children of different ages who can self-report their pain and the tools most appropriate for various age ranges. Children who are unable to use a self-report tool may be able to report their pain intensity using simple words, such as “small,” “medium,” and “big.” However, self-reports of pain are subject to the modulating influences of many factors, including the child's previous pain experience and response to treatment, psychosocial factors, and parental preferences and influences. Consequently, in many cases, it may be necessary to complement self-reported pain scores with behavioral observations, particularly in preschool-aged children. Regardless of the tool selected, assessment of postoperative pain is greatly facilitated by the introduction of the concept of rating pain and of the tool itself during the preoperative preparation of the child.
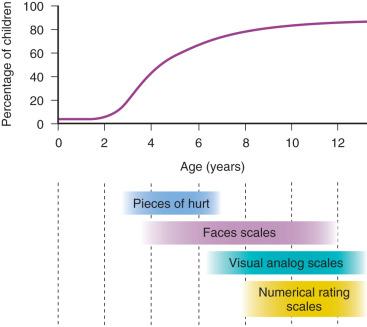
These self-report tools use a categorical format and the static faces do not allow for “fine-tuning” of the ratings before a final assessment regarding the severity of pain is reached. In recent years, there has been interest in developing computer-based self-report assessment tools that use a continuous rather than categorical format.
The Computer Face Scale allows the child to adjust the shape of the mouth of a cartoon face from smiling to frowning and simultaneously to adjust the eyes from completely open to completely closed. The suggested benefits of this scale include increased sensitivity (given the ability to select from a wide range of faces) and computerized storage of the results, with ready access and data display. Preliminary work with this scale has demonstrated its construct validity, and it was preferred by children over the Wong-Baker Faces Scale.
The Sydney Animated Facial Expression Scale (SAFE) is an animated version of the Faces Pain Scale and consists of a series of 101 faces. To administer this scale, the child pushes the left or right arrow key on a computer, causing the expression of the single face to change until it corresponds with the child's pain intensity ( http://www.usask.ca/childpain/research/safe ). At this point, a keystroke records a score between 0 and 100. The SAFE scale was rated to be easiest to use by children aged 4 to 16 years compared with other scales, including the Faces Pain Scale, the Color Analog Scale, and Pieces of Hurt, although it offered no psychometric advantage over the other scales. Further research with this tool is needed before its role can be clearly defined.
Despite the availability of several age-appropriate methods for self-reporting, assessing pain in children who are unable or unwilling to self-report depends on observations of their behaviors. Five behaviors shown to be reliable, specific, and sensitive when predicting analgesic requirements are facial expression, vocalization or cry, leg posture, body posture, and motor restlessness. Variations in these behaviors have been used in several observational pain tools. Table 44.2 describes the content validity of some commonly used observational tools. Behavior checklists provide a list of pain behaviors that are marked as present or absent, and the extent of pain is estimated on the basis of the number of behaviors present at the time of the assessment. Behavior rating scales also incorporate a rating of the intensity or frequency and duration of each behavior. Global rating scales provide a rating of the observer's overall impression of the child's pain.
| FLACC | CHEOPS | OPS | TPPPS | Büttner/Finke |
|---|---|---|---|---|
| Face | Facial expression | Facial pain expression | Facial expression | |
| Legs | Leg movement | Movement | Leg position | |
| Activity | Torso movement | Agitation | Bodily pain expression | Position of torso |
| Motor restlessness | ||||
| Cry | Cry | Cry | Vocal pain expression | Cry |
| Consolability | Touching of the wound | Blood pressure | Consolability | |
| Verbal report of pain | Verbal complaint and body language |
The Children's Hospital of Eastern Ontario Pain Scale (CHEOPS), one of the earliest behavioral rating scales ( Table 44.3 ), incorporates six categories of behavior scored individually from 0 to 2 or 1 to 3 and then sums them to provide a pain score ranging from 4 to 13. Scores of 6 or less indicate no pain. Its validity and reliability for brief painful events and for postoperative pain has been well established, with good to excellent correlations with faces pain scales and the VAS. However, the time required to complete the evaluation and inconsistent scoring among categories of the CHEOPS make it cumbersome and impractical to use in a busy clinical setting.
| Item | Behavioral | Score | Definition |
|---|---|---|---|
| Cry | No cry | 1 | Child is not crying. |
| Moaning | 2 | Child is moaning or quietly vocalizing silent cry. | |
| Crying | 2 | Child is crying, but the cry is gentle or whimpering. | |
| Scream | 3 | Child is in a full-lunged cry; sobbing; may be scored with complaint or without complaint. | |
| Facial | Composed | 1 | Child has neutral facial expression. |
| Grimace | 2 | Score only if definite negative facial expression. | |
| Smiling | 0 | Score only if definite positive facial expression. | |
| Child Verbal | None | 1 | Child is not talking. |
| Other complaints | 1 | Child complains, but not about pain, e.g., “I want to see mommy” or “I am thirsty.” | |
| Pain complaints | 2 | Child complains about pain. | |
| Both complaints | 2 | Child complains about pain and about other things, e.g., “It hurts; I want my mommy.” | |
| Positive | 0 | Child makes any positive statement or talks about others things without complaint. | |
| Torso | Neutral | 1 | Body (not limbs) is at rest; torso is inactive. |
| Shifting | 2 | Body is in motion in a shifting or serpentine fashion. | |
| Tense | 2 | Body is arched or rigid. | |
| Shivering | 2 | Body is shuddering or shaking involuntarily. | |
| Upright | 2 | Child is in a vertical or in upright position. | |
| Restrained | 2 | Body is restrained. | |
| Touch | Not touching | 1 | Child is not touching or grabbing at wound. |
| Reach | 2 | Child is reaching for but not touching wound. | |
| Touch | 2 | Child is gently touching wound or wound area. | |
| Grab | 2 | Child is grabbing vigorously at wound. | |
| Restrained | 2 | Child's arms are restrained. | |
| Legs | Neutral | 1 | Legs may be in any position but are relaxed; includes gentle swimming or discrete movements. |
| Squirming/kicking | 2 | Definitive uneasy or restless movements in the legs and/or striking out with foot or feet. | |
| Drawn up/tensed | 2 | Legs tensed and/or pulled up tightly to body and kept there. | |
| Standing | 2 | Standing, crouching, or kneeling. | |
| Restrained | 2 | Child's legs are being held down. |
a Recommended for children 1 to 7 years old; a score greater than 6 indicates pain.
The Face, Legs, Activity, Cry, Consolability (FLACC) scale was developed in an effort to improve on the pragmatic qualities of the existing behavioral pain tools by providing a simple framework for quantifying pain behaviors in children. This tool includes five categories of behaviors previously found to reliably correlate with pain in young children: facial expression, leg movement, activity, cry, and consolability ( Table 44.4 ). The acronym FLACC facilitates recall of these categories, each of which is scored from 0 to 2 to provide a total pain score ranging from 0 to 10. The FLACC tool has been extensively tested and determined to have good interrater reliability and excellent validity based on changes in pain scores from before to after analgesic administration and excellent correlation with the Objective Pain Scale (OPS), the CHEOPS, the Toddler Preschool Preoperative Pain Scale (TPPPS), and good correlation with self-reported pain scores using faces pain scales. The FLACC scale has been translated into several languages, including Spanish, Chinese, Swedish, French, Italian, Portuguese, Norwegian, and Thai.
| Categories | SCORING | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Face | No particular expression or smile | Occasional grimace or frown, withdrawn, disinterested | Frequent to constant frown, clenched jaw, quivering chin |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking, or legs drawn up |
| Activity | Lying quietly, normal position, moves easily | Squirming, shifting back and forth, tense | Arched, rigid, or jerking |
| Cry | No cry (awake or asleep) | Moans or whimpers, occasional complaint | Crying steadily, screams or sobs, frequent complaints |
| Consolability | Content, relaxed | Reassured by occasional touching, hugging, or being talked to, distractible | Difficult to console or comfort |
The Comfort scale ( Table 44.5 ), developed for use in an intensive care setting, consists of six behavioral and two physiologic measures, each of which has five response categories, thereby allowing detection of subtle changes in the child's distress. Initial evaluation of the Comfort scale found acceptable interrater reliability and good correlations with VAS scores in 37 mechanically ventilated infants. Another study evaluated the reliability and validity of the Comfort scale as a postoperative pain instrument in children after thoracic or abdominal surgery. This study found good to excellent interrater agreement for all categories except respiratory response, for which there was moderate agreement. Additionally, strong correlations between Comfort and VAS pain scores support the use of the Comfort scale as a postoperative pain assessment instrument in children.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Alertness | Deeply asleep | Lightly asleep | Drowsy | Fully awake and alert | Hyper-alert |
| Calmness or Agitation | Calm | Slightly anxious | Anxious | Very anxious | Panicky |
| Respiratory Response | No coughing and no spontaneous respirations | Spontaneous respiration with little or no response to ventilation | Occasional cough or resistance to ventilator | Actively breathes against ventilator or coughs regularly | Fights ventilator; coughing or choking |
| Physical Movement | No movement | Occasional, slight movement | Frequent slight movement | Vigorous movement limited to extremities | Vigorous movement including torso and head |
| Blood Pressure | Less than baseline | Consistently at baseline | Infrequent increases of 15% or more (1–3 episodes during observation period) | Frequent increases of 15% or more (>3 episodes) | Sustained increase >15% |
| Muscle Tone | Muscles totally relaxed; no muscle tone | Reduced muscle tone | Normal muscle tone | Increased muscle tone and flexion of fingers and toes | Extreme muscle rigidity and flexion of fingers and toes |
| Facial Tension | Facial muscles totally relaxed | Facial muscle tone normal; no facial muscle tension evident | Tension evident in some facial muscles | Tension evident throughout facial muscles | Facial muscles contorted and grimacing |
| Heart Rate | Below baseline | Consistently at baseline | Infrequent elevations of 15% or more above baseline | Frequent elevations of 15% or more above baseline | Sustained elevation of 15% or more above baseline |
After a systematic review of observational pain measures, the FLACC and the CHEOPS scales were recommended for assessment of pain associated with medical procedures, the FLACC for postoperative pain, and the Comfort scale for pain in children in critical care. Despite the extensive science supporting the use of behavioral tools, it may be difficult to separate behaviors caused by pain from those caused by other sources of distress in some children. Accurate pain assessment therefore requires careful consideration of the context of the behaviors. Input from the parents or caregivers may be valuable as proxy measures, although some parents may lose objectivity in such a situation. Similarly, a regular caregiver may best assess older children with significant developmental delay. When in doubt regarding the source of distress, a trial of analgesics is appropriate and may be both diagnostic and therapeutic.
The Parents' Postoperative Pain Measure (PPPM) is a 15-item yes/no questionnaire that is completed by a parent/caregiver and designed specifically for use at home without involvement of a health professional ( E-Table 44.1 ). A score of 6 or greater correlates with clinically significant pain. This tool has been validated for children 2 to 12 years old and is useful for research and quality improvement projects, especially as more operations are done on an outpatient basis.
|
a Answering “yes” to 6 or more questions indicates clinically significant pain.
It remains unclear whether integration of routine pain assessment into clinical practice improves patient outcomes. A critical review of the studies that addressed this question determined that in two of six studies, children experienced a reduction in pain intensity when a standardized pain assessment tool was used; in two studies there was no change in pain intensity; and in two studies pain intensity decreased when pain assessment was combined with pain management interventions. Studies that examined sustainability of the benefits over time reported conflicting results, and most studies were identified as having major methodologic problems. Additional investigation is required to determine whether routine pain assessment has any effect on pain outcomes.
Despite the large body of evidence supporting the psychometric properties of numerous structured pain assessment tools, there remains considerable variability in the interpretation of the clinical relevance of pain scores. Attempts have been made to define what range of pain scores is associated with a perceived need for medicine or what magnitude of change in pain score is associated with a perception of better or worse pain. A survey of 6- to 16-year-old hospitalized children found that a median pain score of 3 on a 0-to-6 Faces Pain Scale was associated with the child's perceived need for medicine. Others have reported that a 10-mm change in a 0-to-100-mm VAS score was the minimum difference whereby children in the emergency department perceived their pain to be slightly better or slightly worse. In the postoperative period, children with a median pain score of 6 on a 0-to-10 NRS scale perceived the need for an analgesic, whereas those with a score of 3 felt there was “no need” for treatment. In addition, children felt “a little better” or “worse” if the NRS scale changed by at least 1. Despite these findings, there was large variability and overlap in scores associated with these outcomes.
Evaluations of the effectiveness of pain treatment algorithms based on numerical pain scores have yielded conflicting results. One study reported increased prescription of opioid and nonopioid analgesics, an increased administration of nonopioids, and reduced pain scores in children who received postoperative pain treatment based on a pain score–based algorithm. Children whose pain management was algorithm-based experienced more nausea, but no other adverse effects. In contrast, hospitalized adults whose pain management was based on a numerical pain treatment algorithm experienced a 2-fold increase in episodes of oversedation and a 49% increase in opioid-related adverse drug events. This latter study highlights the potential for harm when numeric pain scores alone are used to guide decisions regarding pain treatment. A comprehensive approach to pain assessment that includes consideration of the child's self-reporting (when available), combined with behavioral observation and the overall clinical context, is required to direct treatment decisions.
It has been suggested that the widespread adoption of a pain score as the fifth vital sign contributes to the overprescribing of analgesics and sedatives. National survey data demonstrate that approximately 276,000 adolescents in the United States abused prescription pain relievers in 2015, second only to marijuana. Unfortunately, even prescription use of opioids in the teenage years has been identified as an independent risk factor for nonmedical use of opioids in early adulthood. Adult data show that many patients use only a fraction of their prescribed opioid after common outpatient procedures. At one author's institution (BW), quality improvement data showed that children used only 10% to 20% of the opioid prescribed to them after tonsillectomy, and data from other institutions demonstrate that significant amounts of opioid are prescribed regardless of patient age or weight. These data highlight the need for procedure-specific opioid prescribing guidelines for children, as well as the importance of patient and family education regarding proper use, storage, and disposal of prescription opioids.
Children who are cognitively impaired experience pain more frequently than cognitively intact children because of many inherent conditions, such as spasticity, muscle spasms, the need for assistive devices for positioning and mobility, and the need for invasive surgical procedures. Indeed, as many as 60% of children with cerebral palsy undergo orthopedic surgery by 8 years of age, and many of them require repeated procedures. Yet both children and adults who are cognitively impaired receive fewer analgesics than those who are cognitively intact with similar painful conditions. Barriers to effective pain management in the cognitively impaired include the complexity of pain assessment in those who cannot verbalize their pain, outdated beliefs that these children have altered or blunted pain perception, limited evidence for the safety and efficacy of analgesic regimens, and an exaggerated concern regarding opioid adverse effects, particularly respiratory depression. Although there is also evidence of cognitive impairment as an independent risk factor for needing a rescue intervention when patient-controlled analgesia (PCA) by proxy is used, this may reflect challenges in thorough assessment of these patients rather than inherent differences in pain tolerance or opioid metabolism. Difficulties with pain assessment have led to the virtual exclusion of these children from clinical drug trials, leading to deficits in our knowledge of how to effectively manage their pain. A survey of clinicians who treat children who are cognitively impaired identified inadequate pain assessment tools and inadequate training and knowledge of providers as significant barriers to effective pain management, despite respondents' beliefs that children who are cognitively impaired perceive pain to a similar extent as cognitively intact children.
Initial evaluation of the FLACC tool in children with cognitive impairment found a good correlation between scores assigned independently by different observers and by parent global ratings of pain. Although measures of exact agreement between observers were acceptable for the face, cry, and consolability categories, measure of agreement for the legs and activity categories were less acceptable, likely because of coexisting motor impairments such as spasticity. The FLACC tool was therefore revised to incorporate additional descriptors of behaviors most consistently associated with pain in children with cognitive impairment ( Table 44.6 ). Interrater reliability for the total FLACC scores, as well as for each of the categories, improved when the evaluation included the revised FLACC (r-FLACC) in 52 cognitively impaired children. Also, good correlation among r-FLACC, parent, and child scores supported its criterion validity. r-FLACC scores were noted to decrease after an opioid was administered, supporting the construct validity of the tool. The pragmatic attributes of the r-FLACC were compared with those of the Nurses' Assessment of Pain Intensity (NAPI) and the Non-Communicating Children's Pain Checklist (NCCPC-PV) ( E-Tables 44.2 and 44.3 ). Clinicians using these tools to score pain rated the complexity as less and the relative advantage and overall clinical utility of the FLACC and the NAPI to be greater compared with the NCCPC-PV, suggesting that these tools may be more readily adopted into clinical practice.
| 0 | 1 | 2 | |
|---|---|---|---|
| Face | No particular expression or smile | Occasional grimace/frown; withdrawn or disinterested (Appears sad or worried) | Consistent grimace or frown; frequent/constant quivering chin, clenched jaw (Distressed-looking face; expression of fright or panic) |
| Legs | Normal position or relaxed | Uneasy, restless, tense (Occasional tremors) | Kicking, or legs drawn up (Marked increase in spasticity, constant tremors or jerking) |
| Activity | Lying quietly, normal position, moves easily | Squirming, shifting back and forth, tense (Mildly agitated [e.g., head back and forth, aggression]; shallow, splinting respirations, intermittent sighs) | Arched, rigid, or jerking (Severe agitation head banging; shivering [not rigors]; breath-holding, gasping or sharp intake of breath; severe splinting) |
| Cry | No cry (awake or asleep) | Moans or whimpers, occasional complaint (Occasional verbal outburst or grunt) | Crying steadily, screams, or sobs; frequent complaints (Repeated outbursts, constant grunting) |
| Consolability | Content, relaxed | Reassured by occasional touching, hugging, or “talking to”; distractible | Difficult to console or comfort (Pushing away caregiver, resisting care or comfort measures) |
a Revised descriptors for children with disabilities shown in parentheses.
| Vocal | Social |
| Moaning, whining, whimpering (fairly soft) | Not cooperating; cranky, irritable, unhappy |
| Crying (moderately loud) | Less interaction, withdrawn |
| Screaming or yelling (very loud) | Seeks comfort or physical closeness |
| A specific sound or vocalization for pain | Difficult to distract; not able to satisfy or pacify |
| Facial | Activity |
| Furrowed brow | Not moving, less active, quiet; jumping around, agitated, fidgety |
| Change in eyes, including squinting, eyes opened wide, eyes frown | |
| Turn down of mouth, not smiling | |
| Lips pucker up, tight, pout, or quiver | |
| Clenches or grinds teeth, chews, thrusts tongue out | |
| Body and Limbs | Physiologic Signs |
| Floppy | |
| Stiff, spastic, tense, rigid; gestures to or touches part of body that hurts; protects, favors, or guards part of body that hurts; flinches or moves away part of body that hurts; moves in specific way to show pain | Shivering |
| Change in color, pallor; sweating, perspiring; tears | |
| Sharp intake of breath, gasping; breath-holding |
|
Pain is a complex phenomenon that occurs because of the transmission of nociceptive stimuli from the peripheral nervous system through the spinal cord to the cerebral cortex. Pain perception is further influenced by emotions, behavior, and previous pain experiences via multiple synapses in the limbic system, frontal cortex, and thalamus. Given the complexity of the pain mechanism, effective treatment of pain requires the use of multimodal therapies that target multiple sites along the pain pathways, as illustrated in Fig. 44.4 . Multimodal treatment includes many nonpharmacologic techniques that are used more commonly for brief procedures (e.g., comfort position, cold/vibration, distraction) in addition to pharmacologic interventions (see further). The primary goal of this approach is to minimize opioid needs and opioid-related side effects.
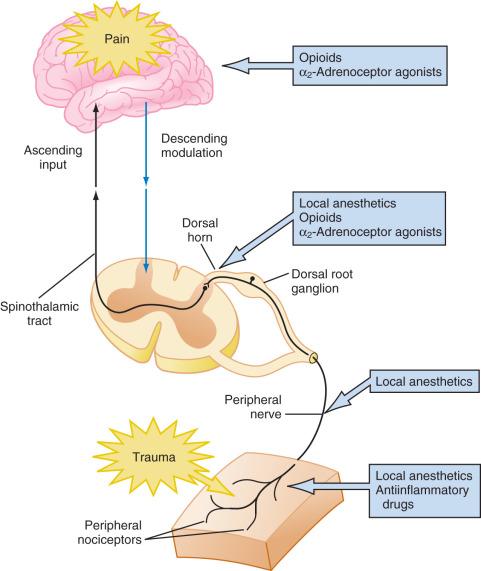
Analgesics with additive or synergistic activity and different adverse effect profiles should be selected so that adequate analgesia can be provided with fewer adverse consequences. Thus pain can be treated at the peripheral level using local anesthetics, peripheral nerve blockade, NSAIDs, antihistamines, or opioids. At the spinal cord level, pain can be treated with local anesthetics, neuraxial opioids, α 2 -adrenoceptor agonists, and N- methyl- d -aspartate (NMDA) receptor antagonists. Finally, at the cortical level systemic opioids, α 2 -agonists, and voltage-gated calcium channel α 2 δ proteins (targets for anticonvulsants) can be used. Most cases of moderate to severe pain are best treated with a multimodal approach.
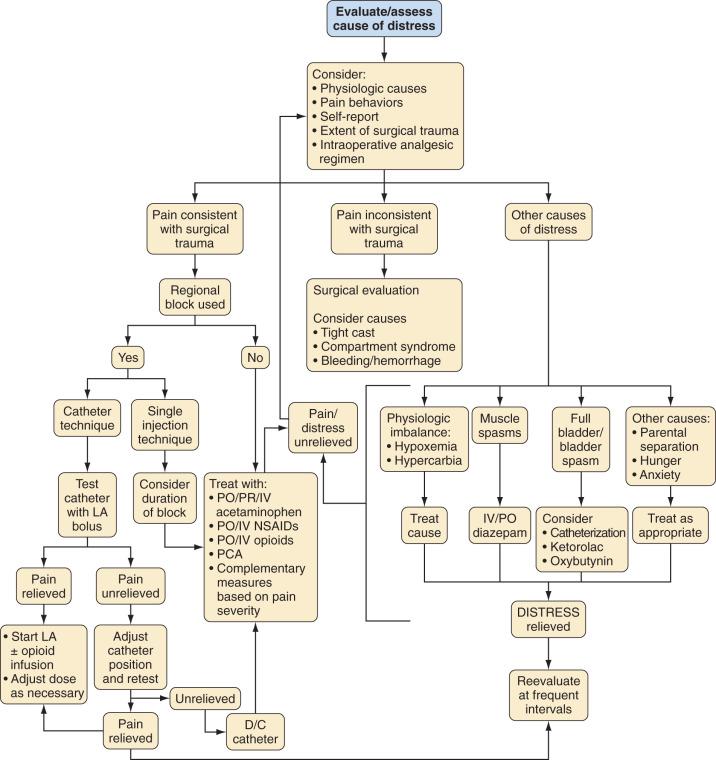
The strategy for postoperative pain management is an integral part of the preanesthetic plan, so that informed consent for procedures, such as placement of peripheral or regional blocks, can be obtained (see Chapters 42 and 43 ). Additionally, appropriate teaching of techniques such as PCA should begin in the preoperative period. An honest discussion with the child that, although some discomfort is inevitable, every effort will be made to minimize pain after surgery, decreases the anxiety related to the perioperative experience. This, together with the use of nonpharmacologic techniques, may even reduce the need for opioids and other analgesics. Selection of an analgesic regimen requires careful consideration of many factors, including scope and requirements of the surgical procedure, age and cognitive abilities of the child, the child's previous pain experience and response to treatment, underlying medical conditions that might alter the response to pain medications, and child and family preferences. The goal should be for the child to emerge from anesthesia in reasonable comfort, because it is generally easier to maintain analgesia in a pain-free child than to achieve analgesia in a child with severe pain. Fig. 44.5 presents a flowchart describing strategies for assessment and management of acute postoperative pain in a child.
The scope and requirements of the surgical procedure, as well as specific postoperative issues, should be discussed with the surgical team before choosing an analgesic regimen, particularly if a regional technique is planned. For example, the site of placement of an epidural catheter and choice of epidural solution will differ in a child with a vertical midline incision from a child with a transverse suprapubic incision. With certain procedures, an epidural catheter may intrude into the surgical field or access to the catheter site in the postoperative period may be obscured by a cast or dressing. In such cases, the catheter may be tunneled subcutaneously away from the surgical field. Alternatively, one or more epidural catheters may be placed under direct vision by the surgeon at the end of the procedure (e.g., spinal fusion or selective dorsal rhizotomy). Postoperative pain is managed using an infusion of local anesthetic and/or opioid solutions through the catheter. Painful muscle spasms after certain procedures are often ameliorated by continuous regional analgesia. This effect is partly related to the density of blockade and may also require supplemental oral or parenteral benzodiazepines if the block alone is inadequate. Refractory spasms of the bladder, which can be quite problematic after some surgeries (e.g., ureteral reimplantation), can also be effectively treated with NSAIDs (e.g., ketorolac) or anticholinergics. Intravesical bupivacaine has also been used to manage bladder spasm, although close attention must be paid to dosing to avoid toxicity. Epidural blockade may favorably alter diaphragmatic mechanics after thoracotomy and upper abdominal surgery. This effect is likely a result of the motor blockade of the intercostal muscles and alteration in the resting length of the diaphragm and is not solely a result of reversal of diaphragmatic inhibition. However, it remains uncertain whether analgesia alone, achieved by systemic opioids or central neuraxial blockade, diminishes postoperative diaphragmatic inhibition or significantly improves postoperative pulmonary function. Effective analgesia, however, does improve child compliance with measures such as deep breathing and early mobilization, thereby reducing the incidence of postoperative complications.
Analgesic techniques, such as infiltration of the wound with local anesthetics, peripheral nerve blocks, or regional blockade that minimize the use of opioids and central respiratory depressants, may be ideal for preterm or very young infants with impaired central respiratory drive. Acetaminophen can be a useful adjunct, because when used within its recommended dose range, it has a large therapeutic window with few untoward effects. Although the judicious use of opioids is not contraindicated, preterm or term infants younger than 1 month of age who receive these medications require careful observation and monitoring to detect respiratory depression. The use of local anesthetics in infants younger than 6 months of age also requires more careful attention to dose because decreased protein binding of local anesthetics puts them at increased risk for toxicity.
Older infants and toddlers who are expected to experience moderate to severe pain may be adequately treated with oral opioids when oral intake resumes. Alternatively, low-dose continuous opioid infusions, nurse-controlled analgesia (NCA), or regional blockade may be required in those undergoing extensive surgery. Nonpharmacologic techniques that focus on distraction, such as child-life therapy and the presence of a comforting parent, can augment the analgesic therapy.
Preschool- and school-aged children have greater fears and better understanding of the postoperative experience than do their younger counterparts. Most cognitively intact children 7 years of age or older are able to understand the concept of PCA, which may help to give a sense of control back to the child during a period in which all other aspects of control are removed. Such issues of control and dependency assume even greater importance in adolescents; allowing them to participate in decision making will contribute to the success of any analgesic technique. Regional techniques are excellent for providing analgesia in all age groups and are associated with a reduced incidence of adverse effects compared with systemic opioids (e.g., nausea, vomiting, excessive sedation, dysphoria, and respiratory depression). Children with significant developmental delay require special consideration of their physical disability, as well as cognitive abilities, although in most cases the pharmacologic actions of the drugs are not altered.
Regardless of the child's age, a detailed history regarding the child's previous pain experience, analgesic history, response to treatment, and adverse effects from previous analgesic regimens should be carefully considered when selecting a pain management technique. An opioid-naive child undergoing surgery for the first time requires smaller doses of opioids for a shorter duration compared with a child with chronic pain who has developed opioid tolerance because of long-term or repeated opioid use. Analgesic selection should also be modified based on the effectiveness of analgesics for that particular child in the past. As the use of genomic testing becomes more available and clinically feasible, identification of target polymorphisms, particularly in the cytochrome P450 enzyme system, may be used to tailor drug selection and dosing.
Nonopioid analgesics are used as sole agents for mild pain and as important adjuncts for multimodal treatment of moderate to severe pain. Although most nonopioid analgesics produce dose-dependent responses, they are limited by a ceiling effect in the analgesia achieved—that is, larger doses of the medication provide no additional analgesia. Hence, more severe pain is resistant to therapy from these medications alone. Ideally, nonopioid medications ( Table 44.7 ) are delivered on a scheduled basis for at least the first few days postoperatively, while opioids are available pro re nata (PRN) for breakthrough pain.
| Medication | Individual Dose for Children <60 kg (mg/kg) | Individual Dose for Children ≥60 kg (mg) | Dosing Interval (hours) | Maximum Daily Dose for Children <60 kg (mg/kg) | Maximum Daily Dose for Children ≥60 kg (mg) |
|---|---|---|---|---|---|
| Acetaminophen | 10–15 | 650–1000 | 4–6 | 75 a | 3000 |
| Ibuprofen | 5–10 | 400–600 | 6 | 40 | 2400 |
| Naproxen | 5–6 | 250–375 | 12 | 10 | 1000 |
| Diclofenac | 1 | 50 | 8 | 3 | 150 |
| Ketorolac b | 0.5 | 30 | 6–8 | 2 | 120 |
| Tramadol | 1–2 | 50 | 6 | 8 | 400 |
a See text for new age-related dosing.
b Ketorolac should be administered for a maximum of 5 days or 20 doses up to 15 mg per dose for children <60 kg or 30 mg per dose for children >60 kg.
Acetaminophen is the most common antipyretic and nonopioid analgesic used in children. Despite years of study, the predominant mechanism of its analgesic action remains unclear. It may exert its analgesic effects by blocking central and peripheral prostaglandin synthesis, reducing substance P–induced hyperalgesia, and modulating the production of hyperalgesic nitric oxide in the spinal cord. In addition, acetaminophen might produce analgesia via activation of descending serotonergic pathways. One putative site of action may be inhibition of prostaglandin H 2 synthetase at the peroxidase site. Effective analgesia and antipyresis have been described with plasma concentrations of 5 to 20 µg/mL ; a target effect-site (similar to cerebrospinal fluid) concentration of 10 µg/mL reduces pain after tonsillectomy by 3.6/10 pain units. The total daily dose of acetaminophen via any route is age- and weight-based, but should not exceed 75 mg/kg for children, 60 mg/kg for neonates 32–44 weeks postconceptual age, and 40 mg/kg for preterm neonates 28–32 weeks.
The recommended dose for oral administration is 10 mg/kg every 4 hours or 15 mg/kg every 6 hours, or a total daily dose of 60 mg/kg per day, which is less than the upper daily limit of 75 mg/kg. Acetaminophen has a wide margin of safety when administered in the recommended therapeutic dose range. However, hepatotoxicity has been reported with doses only slightly above the recommended dose, suggesting that acetaminophen may have a narrow therapeutic index in some children. Because of these reports and on the advice of a U.S. Food and Drug Administration panel, the manufacturers have reduced the maximum daily dose to 3 g. Acetaminophen is available in a wide variety of formulations, alone or in combination with decongestants, for oral use in a variety of cold remedies, and with opioids for the treatment of moderate to severe pain. There are currently more than 600 over-the-counter acetaminophen-containing products, increasing the risk of an overdose because children may take more than one formulation that contains the drug. Frequent review of medications and parental education is needed to minimize the risk of overdose. In the past, pediatric liquid formulations of acetaminophen as in infant drops were commonly supplied in larger concentrations than that in elixirs, resulting in dosing errors. As a result, the 80-mg/mL concentration has been removed from the U.S. market by the manufacturer, and liquid formulations have been standardized to 32 mg/mL. This concentration equals approximately 0.5 mL/kg of acetaminophen if given as a 15-mg/kg dose. Since both gastric fluid volume and pH are unchanged after liquid acetaminophen (40 mg/kg of a 50 mg/mL elixir, 0.8 mL/kg) administered orally 90 minutes before induction of anesthesia, it is feasible to use this preoperatively. Whether these results are applicable to acetaminophen that is administered closer to the time of induction is not known.
Intravenous (IV) formulations of paracetamol (acetaminophen) have been used in Europe and Australia for many years and are now available in the United States. IV acetaminophen is available as a 10-mg/mL solution and should be infused over ~15 minutes in a dose of 12.5 mg/kg every 4 hours or 15 mg/kg every 6 hours, with a total daily limit of 75 mg/kg (although at the time of writing, the IV manufacturer continues to recommend a 4 grams per day limit, unlike the recommendations for oral acetaminophen). In neonates between 32 and 44 weeks postconception age, a loading dose of 20 mg/kg should be followed by 10 mg/kg every 6 hours (every 12 hours in 28–31 weeks postconception age). Each dose of IV acetaminophen must be documented in a timely manner to avoid overdoses from multiple dosing; several cases of frank overdoses have occurred in young infants who received excessive doses of IV acetaminophen on the ward. Dosing recommendations for premature and full-term neonates continues to be an active area of research, and one study has shown weight to be a better predictor of individual pharmacokinetics than either postconception or gestational age.
After IV administration of acetaminophen, analgesic onset occurs within 15 minutes and antipyresis within 30 minutes. IV acetaminophen rapidly penetrates the blood-brain barrier, yielding detectable concentrations in the cerebrospinal fluid (CSF) within 5 minutes of administration, and peak CSF concentrations within 57 minutes after injection (compared with 2 to 3 hours after rectal or oral administration). This explains the fast onset of its analgesic and antipyretic effects. A large multicenter trial reported that 1 gram of IV paracetamol and 2 grams of IV propacetamol (equivalent to 1 gram acetaminophen) provided superior analgesia with a reduced need for morphine compared with placebo in adults after lower extremity joint replacement. The propacetamol group experienced a greater incidence of local skin reactions and pain on injection compared with the IV paracetamol group. A controlled randomized trial reported that both rectal acetaminophen, 40 mg/kg, and IV acetaminophen, 15 mg/kg, administered after induction of anesthesia in children undergoing adenotonsillectomy provided good analgesia for the first 6 hours after surgery. However, children who received acetaminophen rectally had a greater duration of analgesia and did not require rescue analgesia as early as those in the IV group. This is attributable to the slow absorption of rectal acetaminophen, causing sustained effective concentrations (see also Fig. 4.2 ). When compared with oral acetaminophen, there was evidence of lower intraoperative opioid requirements (again attributable to differing time to peak effect-site concentration), but no difference in postoperative opioid consumption in young children undergoing cleft palate repair when children received scheduled acetaminophen via the oral or IV route.
Slow and unpredictable absorption of acetaminophen after rectal administration, however, results in variable blood concentrations, with peak concentrations reached between 60 and 180 minutes after administration ( Fig. 4.2 ). This unpredictability was illustrated in a series of studies from the same group of investigators examining opioid-sparing with rectal and IV acetaminophen in infants undergoing major abdominal and thoracic surgery. In the rectal study, no opioid-sparing effect was detected, and plasma concentrations of acetaminophen varied 50-fold among patients. In a follow-up study in the same population using IV acetaminophen, opioid consumption was reduced by 60% and the frequency of apnea was also reduced. In children undergoing orthopedic surgery, a loading dose of 40 mg/kg rectal acetaminophen followed by 20 mg/kg every 6 hours yielded serum concentrations of 10 to 20 µg/mL in half of the patients, with no evidence of accumulation over a 24-hour period. This dosing scheme is now the one most commonly recommended when the rectal route is used, but it should be noted that scheduled dosing with this scheme would result in a dose of 80 mg/kg per day (or 100 mg/kg on the first day with the loading dose). Contrary to previous assumptions, acetaminophen is relatively evenly distributed in most suppositories, allowing them to be split to achieve a desired dose, although accuracy of dosing is problematic.
In conclusion, acetaminophen has opioid-sparing potential with very few adverse effects and a low risk of toxicity with appropriate dosing. The oral and IV routes produce equivalent analgesia when differences in onset time are considered. The rectal route should be considered a “last resort” because of unpredictable absorption and limited dosing options. Financial considerations have limited the routine use of IV acetaminophen at many centers in the United States.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here