Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Marked hypoxemia in the newborn can be caused by parenchymal lung disease, pulmonary vascular disease, or congenital heart defects.
Events occurring at delivery, as well as the response to supplemental oxygen and to continuous positive airway pressure, can provide important clues to the pathophysiology of hypoxemic respiratory failure in the newborn.
The echocardiogram has become a vital tool in the clinical management of newborns with hypoxemic respiratory failure, primarily to assess for pulmonary hypertension and to interrogate shunts that may be contributing to hypoxemia.
Although gestational age is a critical variable in the relative frequency with which different respiratory disorders affect newborns, a broad spectrum of potential causes must be considered in an individual baby of any gestational age.
In extremely preterm newborns with respiratory distress, surfactant deficiency is the most common cause.
Transient tachypnea of the newborn is one of the most common causes of respiratory distress in the term newborn.
The evaluation and management of respiratory distress in the newborn poses unique challenges and remains one of the most vexing problems facing neonatal caregivers. Although some of the pathophysiologic features of respiratory disorders in the preterm infant are similar to those in term newborns, the incidence of disorders varies by gestational age. Table 42.1 lists the most common causes of respiratory distress in newborns and demonstrates the relative frequency of each diagnosis. The traditional perspective of categorizing hypoxemia and respiratory failure in the newborn, due to cardiac, pulmonary vascular, or air space (lung) disease, is insufficient because of the inevitable complex interactions among the three. For example, the problem of persistent pulmonary hypertension of the newborn (PPHN) is defined by severe pulmonary vasoconstriction leading to suprasystemic pulmonary artery pressure with extrapulmonary right-to-left venoarterial admixture across the fetal channels of the foramen ovale and the ductus arteriosus. However, PPHN rarely occurs without concomitant parenchymal lung disease and disturbances in cardiac performance.
| Cause | Frequency (%) |
|---|---|
| Respiratory distress syndrome | 46 |
| Transient tachypnea of newborn | 37 |
| Pneumonia/sepsis | 5 |
| Meconium aspiration syndrome | 2 |
| Congenital cardiac malformation | 2 |
| Chromosomal disorder/multiple congenital anomalies | 1.4 |
| Spontaneous pneumothorax | 1.2 |
| Perinatal asphyxia | 1.1 |
| Pulmonary hemorrhage | 1.0 |
| Persistent pulmonary hypertension | 0.8 |
| Diaphragmatic hernia | 0.8 |
| Apnea of prematurity | 0.6 |
| Pulmonary hypoplasia | 0.3 |
| Pulmonary dysplasia | 0.2 |
| Hydrothorax | 0.2 |
| Postsurgical diaphragmatic palsy | 0.2 |
In this chapter, we present an algorithm for evaluation of the newborn with hypoxemia and respiratory distress. We then focus on common causes of neonatal respiratory failure, progressing from those most frequently encountered in preterm infants to those more commonly diagnosed in near-term and term infants.
One of the most anxiety-provoking experiences for many clinicians (particularly those in training) is the initial evaluation and management of a newborn with hypoxemia and respiratory distress. Traditional textbooks provide a wealth of information about individual conditions once they have been identified. However, there are few sources designed to guide the clinician in an orderly fashion through a comprehensive diagnostic evaluation. In this section, we propose an approach to the evaluation of the hypoxemic newborn that may be useful in clarifying the cause of hypoxemia/respiratory distress and in determining the proper sequence of diagnostic and therapeutic interventions.
Marked hypoxemia in the newborn can be caused by parenchymal lung disease with ventilation–perfusion (V/Q) mismatch or intrapulmonary shunting, pulmonary vascular disease causing extrapulmonary right-to-left shunting (i.e., PPHN), or anatomic right-to-left shunting associated with congenital heart disease. Evaluation should begin with a history that includes assessment of risk factors for hypoxemic respiratory failure. A relevant history may include the results of prenatal ultrasound studies. Lesions such as congenital diaphragmatic hernia (CDH) and congenital cystic adenomatoid malformation are diagnosed prenatally with increasing frequency. Although many anatomic congenital heart defects can be diagnosed prenatally, vascular abnormalities (e.g., coarctation of the aorta, total anomalous pulmonary venous return) are more difficult to diagnose with prenatal ultrasonography. A history of a structurally normal heart by fetal ultrasonography should be confirmed by echocardiography in the newborn with cyanosis (see section on Echocardiography later).
Other historical information that may be important in the evaluation of the cyanotic newborn includes a history of severe and prolonged oligohydramnios causing pulmonary hypoplasia. Also important is a history of prolonged fetal bradyarrhythmia and/or tachyarrhythmia and marked anemia (caused by hemolysis, twin–twin transfusion, or chronic hemorrhage) that may cause congestive heart failure, pulmonary edema, and respiratory distress. Maternal illness (e.g., diabetes mellitus), medication use (e.g., aspirin or medications containing nonsteroidal anti-inflammatory drugs causing premature constriction of the ductus arteriosus, association of Ebstein malformation with maternal lithium use), and illicit drug use may contribute to acute cardiopulmonary distress in the newborn. Risk factors for infection that cause sepsis/pneumonia should be considered, including premature or prolonged rupture of membranes, fetal tachycardia, maternal leukocytosis, uterine tenderness, and other signs of intraamniotic infection. Additionally, the use of antenatal steroids should be ascertained in the event of a preterm delivery.
Events at delivery may provide clues to the cause of hypoxemic respiratory failure in the newborn. For example, if positive pressure ventilation is required in the delivery room, the risk of pneumothorax increases. A history of meconium-stained amniotic fluid is the sine qua non of meconium aspiration syndrome (MAS). Birth trauma (e.g., clavicular fracture, phrenic nerve injury) or acute fetomaternal or fetoplacental hemorrhage may cause respiratory distress in the newborn ( Box 42.1 ).
Prenatal ultrasound study results
History of oligohydramnios and duration
History of fetal brady/tachyarrhythmia
Maternal illnesses, drugs, medications
History of fetal distress or nonreassuring fetal heart tones
Risk factors for infection
Use of antenatal steroids
History of positive pressure ventilation in delivery room
Meconium-stained amniotic fluid
Hemorrhage (acute fetomaternal or fetoplacental hemorrhage; abruption)
Birth trauma
Low Apgar score
The initial physical examination provides important clues to the cause of cyanosis. Marked respiratory distress in the newborn (retractions, grunting, nasal flaring) suggests the presence of pulmonary parenchymal disease with decreased lung compliance. However, it is important to recognize that upper airway obstruction (e.g., Pierre Robin sequence or choanal atresia) and metabolic acidemia can also cause severe respiratory distress. In contrast, the newborn with cyanosis alone or cyanosis plus tachypnea (i.e., nondistressed tachypnea) typically has cyanotic congenital heart disease, most commonly transposition of the great vessels. Although pulmonary edema is a common complication of some forms of congenital heart disease and can lead to respiratory distress, this would be an unlikely presenting sign immediately after delivery when pulmonary vascular resistance (PVR) is still elevated.
The presence of a heart murmur audible in the first few hours after birth is an important sign in the newborn with cyanosis or respiratory distress. In this setting, it is unusual for the common left-to-right shunt lesions (patent ductus arteriosus, atrial septal defect, ventricular septal defect) to produce an audible murmur because PVR remains high and little turbulence is created across the defect. A murmur that sounds like a ventricular septal defect in the first few hours of life is most commonly caused by tricuspid regurgitation (associated with PPHN or an ischemic myocardium) ( Box 42.2 ).
A marked increase (to 100%) in arterial oxygen saturation (Sa o 2 ) with supplemental oxygen (by hood, mask, or endotracheal tube) suggests the presence of intrapulmonary shunt or V/Q mismatch caused by lung disease or reactive PPHN. The response to continuous positive airway pressure is also a useful discriminator between severe lung disease and other causes of hypoxemia. Most patients with PPHN have at least a transient increase in oxygenation in response to interventions such as high inspired oxygen concentration and/or initiation of mechanical ventilation. But if preductal Sa o 2 never reaches 100%, the likelihood of cyanotic heart disease is high ( Box 42.3 ).
The interpretation of preductal (right hand) and postductal (lower extremity) arterial oxygen saturation, measured by pulse oximetry, provides important clues to the cause of hypoxemia in the newborn. Right-to-left shunting across the ductus arteriosus (but not the patent foramen ovale) causes postductal desaturation (with a >5% preductal–postductal saturation difference). However, it is important to recognize that variability in oximetry readings may be related to differences in available devices and may be affected by local perfusion.
If the measurements of preductal and postductal arterial oxygen saturation are equivalent, this suggests either that the ductus arteriosus is patent and PVR is subsystemic (i.e., the hypoxemia is caused by parenchymal lung disease with intrapulmonary shunting or cyanotic heart disease with ductal-dependent pulmonary blood flow) or that the ductus arteriosus is closed (precluding any interpretation of pulmonary artery pressure without echocardiography). It is uncommon for the ductus arteriosus to close in the first few hours after birth in the presence of systemic or suprasystemic pulmonary artery pressures.
The most common cause of preductal–postductal gradients in oxygenation is suprasystemic PVR in PPHN causing right-to-left shunting across the ductus arteriosus (associated with MAS, surfactant deficiency/dysfunction, CDH, non-CDH pulmonary hypoplasia, or idiopathic pulmonary hypertension [without accompanying pulmonary parenchymal disease]). However, ductal-dependent systemic blood flow lesions (hypoplastic left-sided heart syndrome, critical aortic stenosis, interrupted aortic arch, coarctation) may also present with postductal desaturation. Moreover, anatomic pulmonary vascular disease (alveolar capillary dysplasia, pulmonary venous stenosis, anomalous venous return with obstruction) can cause suprasystemic PVR with right-to-left shunting across the ductus arteriosus and postductal desaturation.
Finally, the unusual occurrence of markedly lower preductal Sa o 2 compared with postductal Sa o 2 suggests one of two diagnoses: transposition of the great vessels (TGV) with pulmonary hypertension (PH) or TGV with coarctation of the aorta ( Table 42.2 ).
| Preductal Sa o 2 = postductal Sa o 2 |
|
| Preductal Sa o 2 > postductal Sa o 2 |
|
| Preductal Sa o 2 ≤ postductal Sa o 2 |
|
One of the most important tests to perform in the evaluation of the newborn with cyanosis is the chest radiograph (CXR). The CXR can demonstrate the classic findings of respiratory distress syndrome (RDS) (air bronchograms, diffuse granularity, underinflation), pneumonia (diffuse parenchymal lung disease), MAS (heterogenous parenchymal lung disease, often with adjacent areas of air trapping and atelectasis), or CDH. Perhaps the most important question to ask when one is viewing the CXR is whether the severity of hypoxemia is out of proportion to the radiographic changes. In other words, marked hypoxemia despite supplemental oxygen in the absence of severe pulmonary parenchymal disease radiographically suggests the presence of an extrapulmonary right-to-left shunt (idiopathic PPHN or cyanotic heart disease; Box 42.4 ).
Hypoxemia out of proportion to radiographic changes suggests congenital heart disease with ductal-dependent pulmonary blood flow or extrapulmonary right-to-left shunting with persistent pulmonary hypertension of the newborn.
Arterial blood gas: assess respiratory and metabolic acidemia
Complete blood count: for evidence of infection
Blood pressure: ductal-dependent systemic blood flow and closing of patent ductus arteriosus (e.g., coarctation)
Other essential measurements include measurement of arterial blood gas to determine the blood gas tensions and pH, a complete blood count to evaluate the newborn for signs of infection or evidence of symptomatic polycythemia, blood glucose to evaluate for symptomatic hypoglycemia, and blood pressure measurements in the right arm and a lower extremity to identify aortic obstruction (interrupted aortic arch, coarctation).
Echocardiography has become a vital tool in the clinical management of newborns with hypoxemic respiratory failure ( Box 42.5 ). The initial echocardiographic evaluation is important to rule out structural heart disease causing hypoxemia (e.g., ductal dependent pulmonary blood flow lesions, anomalous pulmonary venous return). Moreover, it is critically important to diagnose congenital heart lesions for which inhaled nitric oxide (iNO) treatment would be contraindicated. In addition to the lesions mentioned earlier, congenital heart lesions that can present with hypoxemia unresponsive to high inspired oxygen concentrations (i.e., dependent on right-to-left shunting across the ductus arteriosus) include critical aortic stenosis, interrupted aortic arch, and hypoplastic left-sided heart syndrome. Decreasing PVR with iNO treatment in these conditions could lead to systemic hypoperfusion, worsening the clinical course and delaying definitive diagnosis.
Right-to-left shunting at the arterial duct and/or foramen ovale is observed in infants with suprasystemic pulmonary hypertension.
If echocardiography demonstrates adequate left-ventricular performance, consider inhaled NO use after effective lung recruitment (see functional measurements below).
Inhaled NO use may be contraindicated in the presence of duct-dependent systemic blood flow (e.g., hypoplastic left heart syndrome).
Inhaled NO use may be contraindicated in the presence of left ventricular systolic/diastolic dysfunction (e.g., mitral insufficiency with left-to-right atrial shunting with right-to-left ductal shunting suggesting possible right ventricle–dependent systemic blood flow).
Echocardiographic evaluation is an essential component in the initial evaluation and ongoing management of the hypoxemic newborn. As noted earlier, hypoxemia can be caused by intrapulmonary right-to-left shunting or V/Q disturbances associated with severe lung disease. In unusual circumstances, right-to-left shunting can occur across pulmonary-to-systemic collaterals. However, extrapulmonary right-to-left shunting at the foramen ovale and/or ductus arteriosus (PPHN) also complicates hypoxemic respiratory failure and must be assessed to determine initial treatments and evaluate the response to those therapies.
PPHN is defined by the echocardiographic determination of extrapulmonary venoarterial admixture (right-to-left shunting at the foramen ovale and/or ductus arteriosus), not simply evidence of increased PVR (i.e., elevated PVR without extrapulmonary shunting does not directly cause hypoxemia). Echocardiographic signs suggestive of PH (e.g., increased right ventricular systolic time intervals, septal flattening) are less definitive.
Doppler measurements of atrial-level and ductal-level shunts provide essential information when one is managing hypoxemic respiratory failure in a newborn. For example, left-to-right shunting at the foramen ovale and ductus arteriosus with marked hypoxemia suggests predominant intrapulmonary shunting, and interventions should be directed at optimizing lung inflation.
Finally, the measurements made with echocardiography can be used to predict or interpret the response or lack of response to various treatments. For example, in the presence of severe left-ventricular dysfunction with pulmonary hypertension, the left-ventricular dysfunction may contribute to pulmonary venous hypertension, such as occurs in congestive heart failure. Pulmonary vasodilation alone (without improvement in cardiac performance) may be ineffective in increasing oxygenation. In this setting, the echocardiographic findings include right-to-left ductal shunting (caused by suprasystemic PVR) and mitral insufficiency with left-to-right atrial shunting. Efforts to reduce PVR should be accompanied by targeted therapies to increase cardiac performance and decrease left-ventricular afterload. As such, careful echocardiographic assessment will provide invaluable information about the underlying pathophysiology and help guide the course of treatment. Furthermore, serial echocardiography is important to determine the response to interventions (e.g., pulmonary vasodilators) and to reevaluate cases where specific interventions have not resulted in improvement or with progressive clinical deterioration.
As described previously, PPHN is a syndrome associated with diverse neonatal cardiac and pulmonary disorders that are characterized by high PVR causing extrapulmonary right-to-left shunting of blood across the ductus arteriosus and/or foramen ovale. (The syndrome of PPHN and the role of iNO are discussed in more detail in Chapter 47 .) However, because its relationship to respiratory failure in newborns is so vital to understanding the clinical pathophysiology and approaches to treatment, the current use of iNO and other approaches to PPHN treatment as they relate to specific respiratory diseases are discussed in the following sections.
It is important to acknowledge that although gestational age is a critical variable in the relative frequency with which each of these disorders affect newborns, a broad spectrum of potential causes must be considered in an individual baby of any gestational age. We will start with RDS, the respiratory disease most commonly associated with prematurity, and then proceed to those more commonly encountered in newborns of progressively advancing gestational age.
More than half of extremely low birth weight newborns will have some type of respiratory distress ( Fig. 42.1 ). In that population, RDS, historically known as hyaline membrane disease (HMD), is by far the most common diagnosis (51%), followed by transient tachypnea of the newborn (TTNB) (4%), and pneumonia/sepsis (2%). In preterm newborns at later gestational ages, the incidence of any type of respiratory distress is much lower, and the proportion with TTNB increases. Compared with the incidences of the three diagnoses featured in Fig. 42.1 , the incidence of other causes of respiratory distress (see Table 42.1 ) is very low in preterm infants, but the causes should be considered in those newborns with an atypical clinical course.
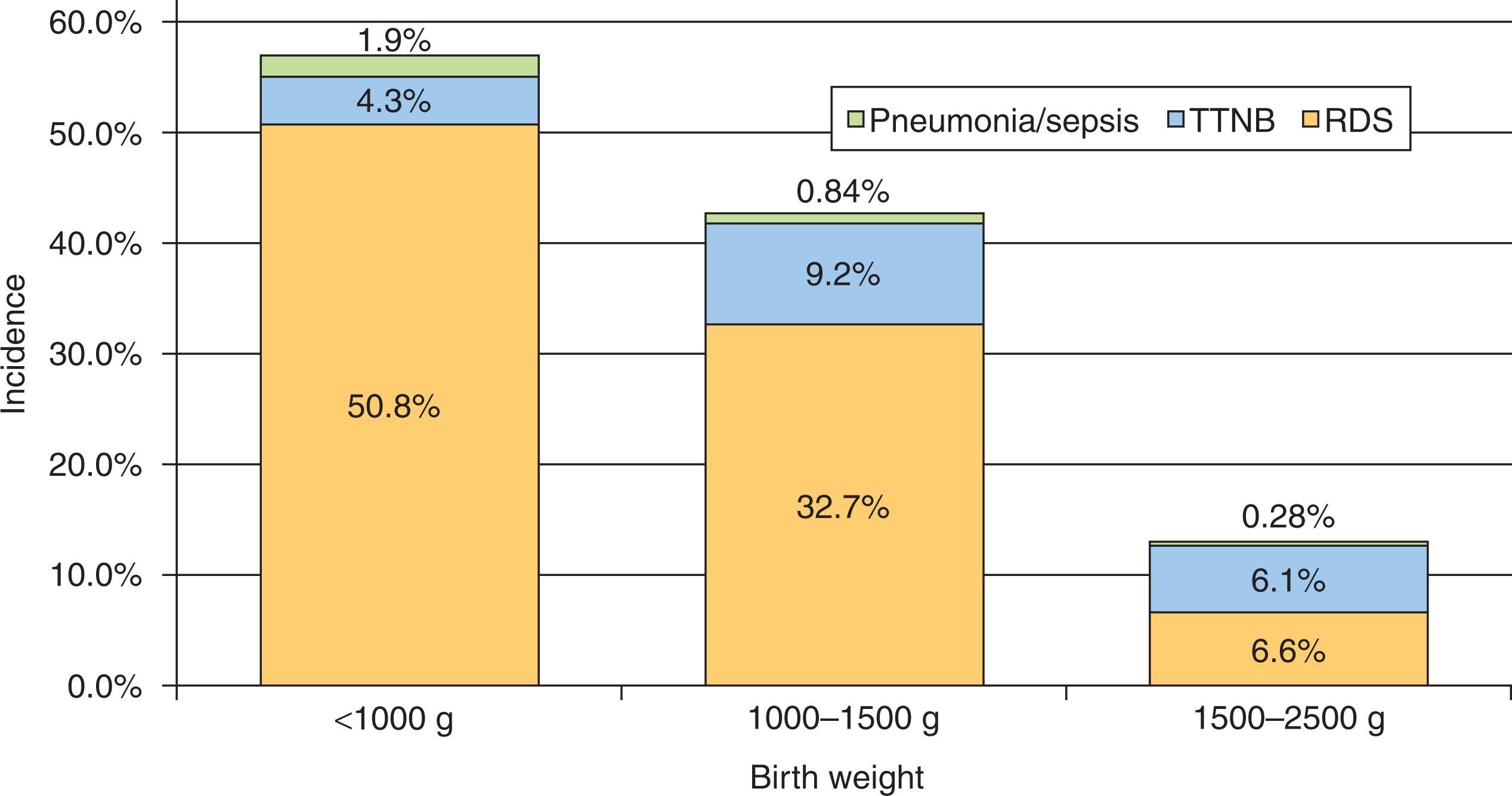
The main risk factor for RDS, by far, is prematurity (see Fig. 42.1 ). Other factors that increase the risk of RDS include perinatal asphyxia, maternal diabetes, absence of labor, absence of prenatal steroid administration to the mother, male sex, and white race. The central feature of RDS is surfactant deficiency.
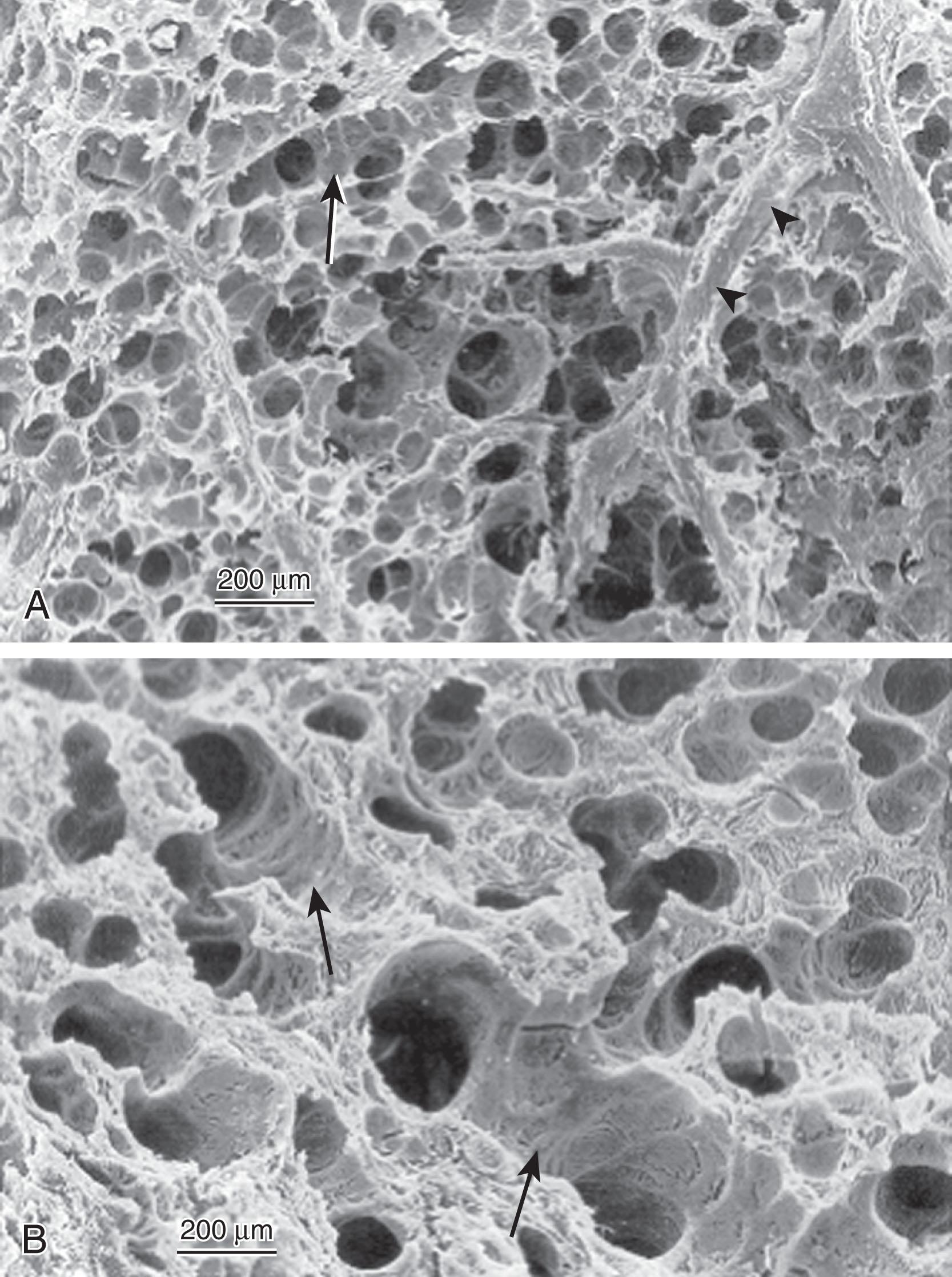
Because alveoli with insufficient (or dysfunctional) surfactant are unstable and tend to collapse, patients with RDS develop generalized atelectasis, ventilation–perfusion mismatching, and subsequent hypoxemia and respiratory acidosis. During breathing (either spontaneous or assisted), shear stress in the alveoli and terminal bronchioles occurs because of the repetitive reopening of collapsed alveoli and the overdistention of open alveoli. These forces can quickly damage the fragile lung architecture, leading to leakage of proteinaceous debris into the airways (i.e., hyaline membranes). This debris ( Fig. 42.2 ) may impair the function of what little surfactant is present, leading to a progression of respiratory symptoms and respiratory failure if not interrupted.
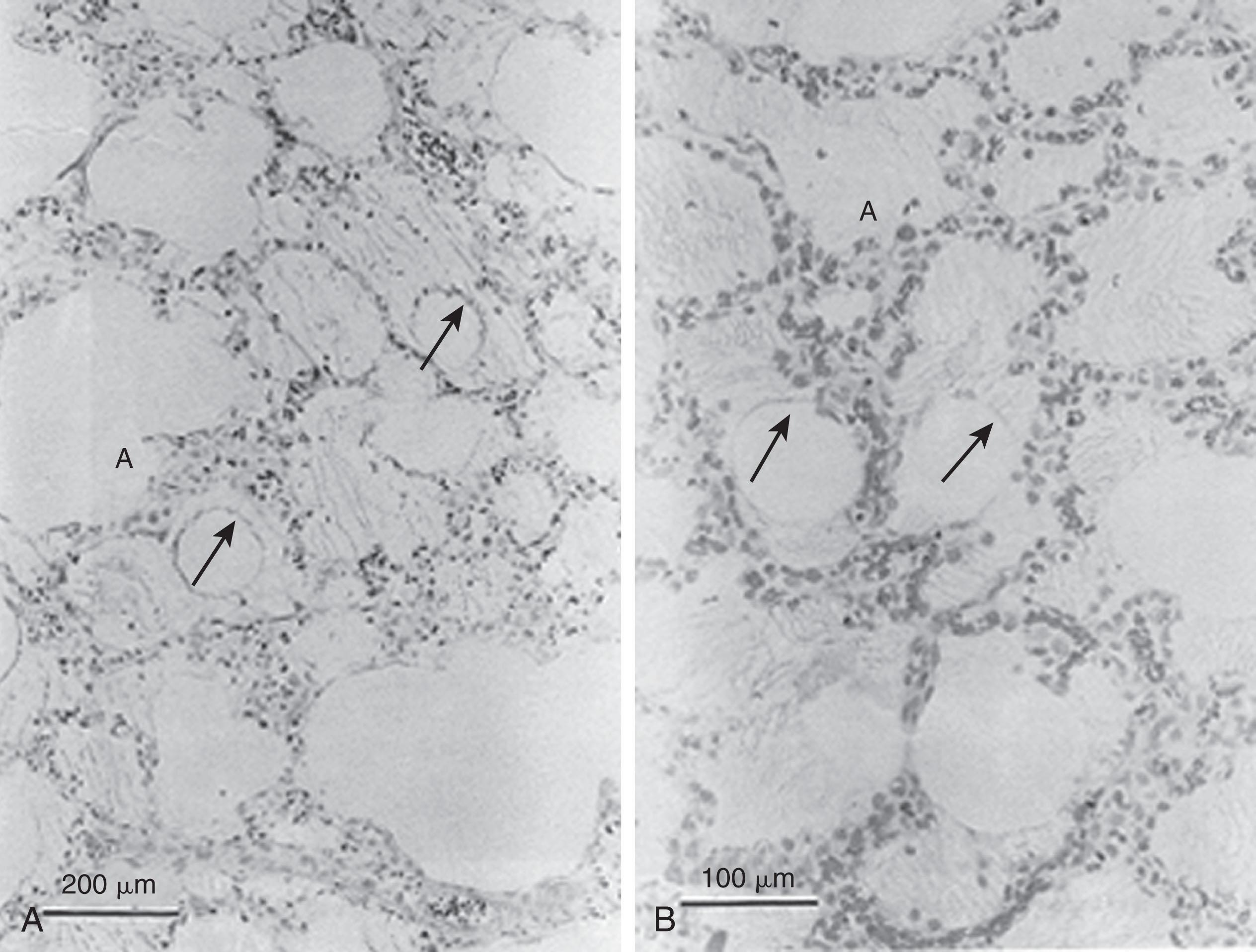
If supportive therapy is successful, the repair phase usually begins during the second day after birth with the appearance of macrophages and polymorphonuclear cells. Debris is phagocytosed, and the damaged epithelium is regenerated. Interstitial fluid is mobilized into lymphatics, leading to the diuretic phase of RDS characterized by high urine output.
With uncomplicated RDS, the patient’s condition improves by the end of the first week after birth. However, infants requiring high concentrations of oxygen and positive pressure ventilation for severe RDS may develop inflammation, progressive injury, and inappropriate repair of the growing lung, leading to bronchopulmonary dysplasia (BPD) (see Chapter 43 ).
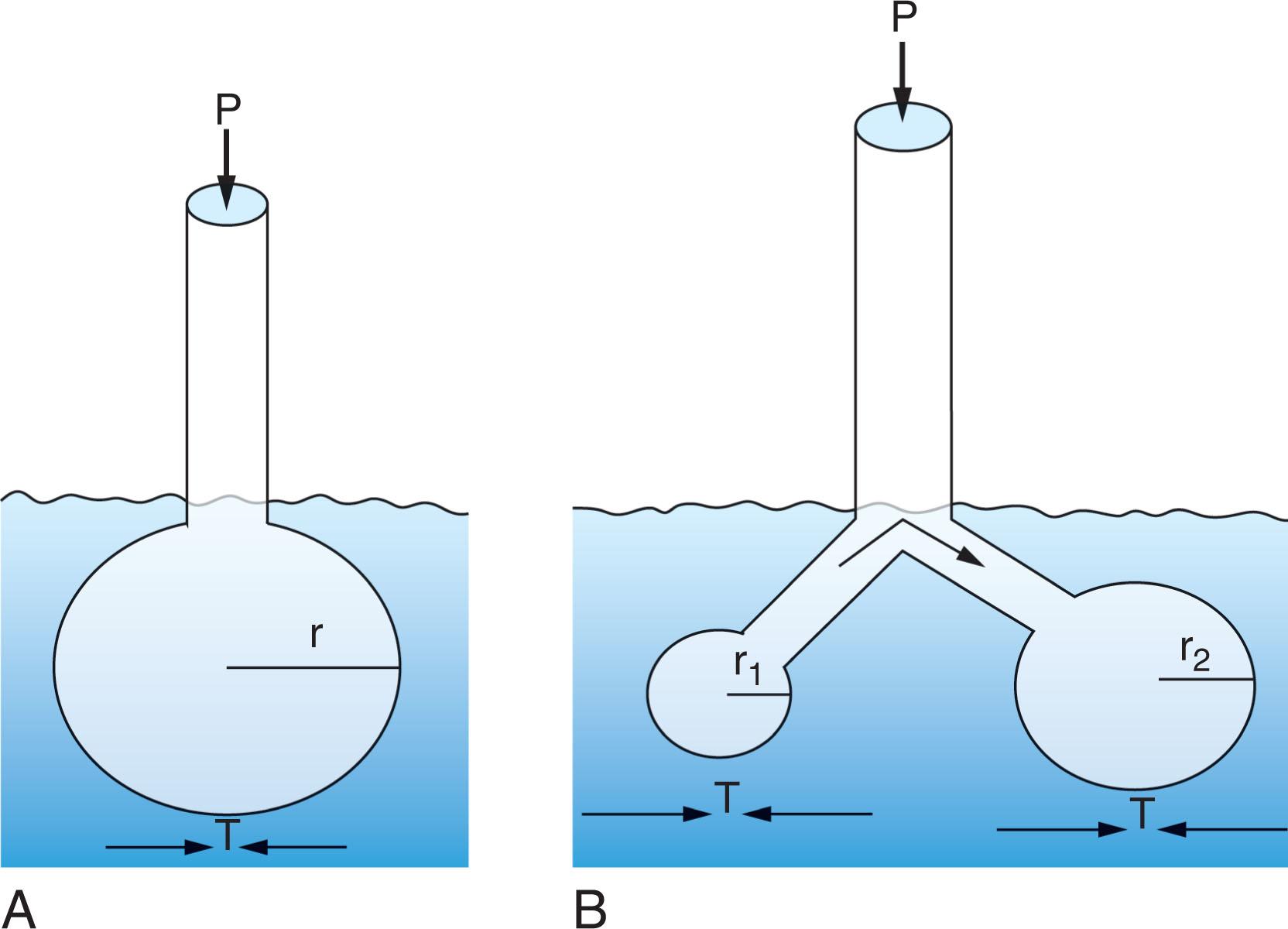
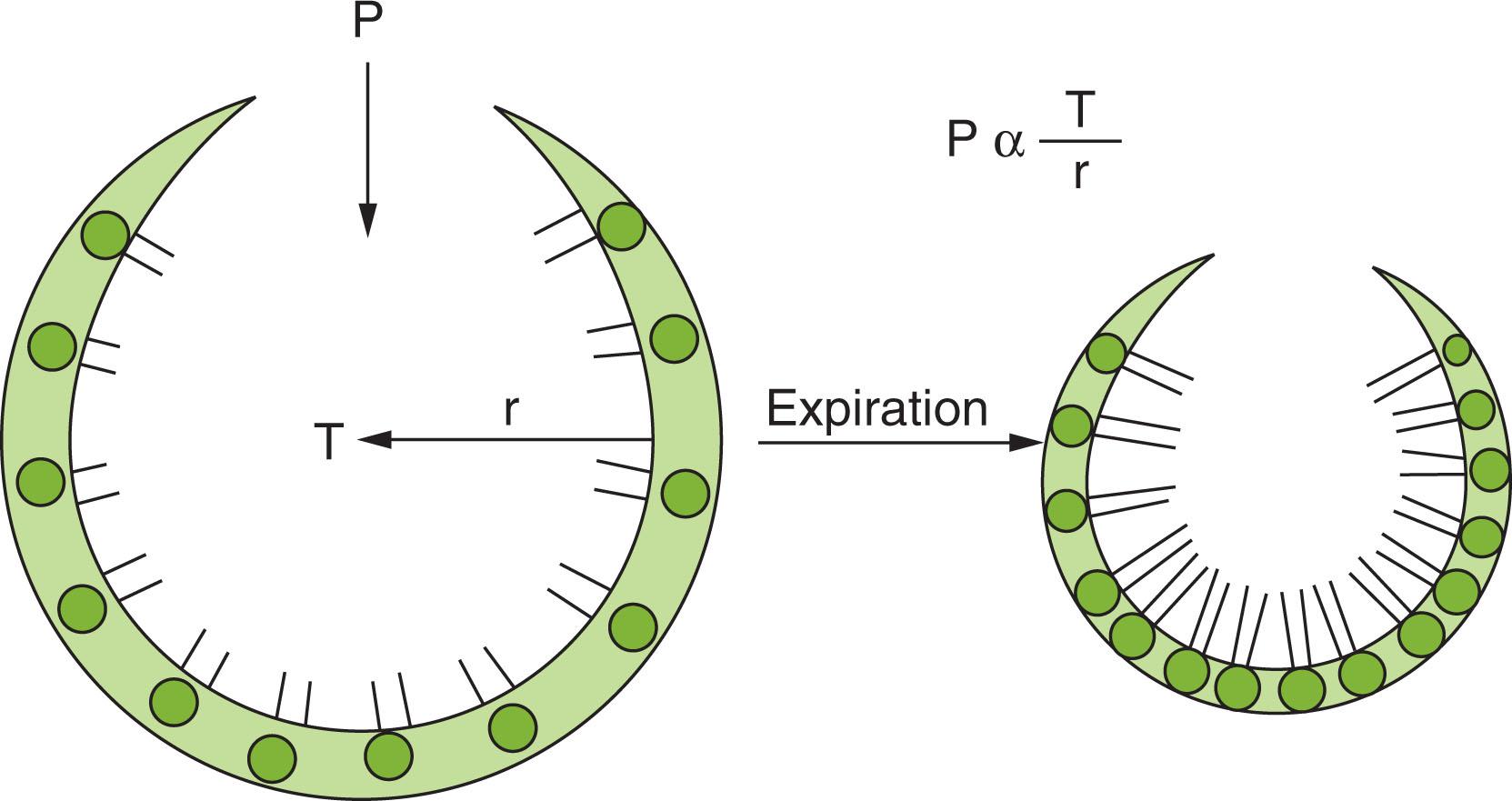
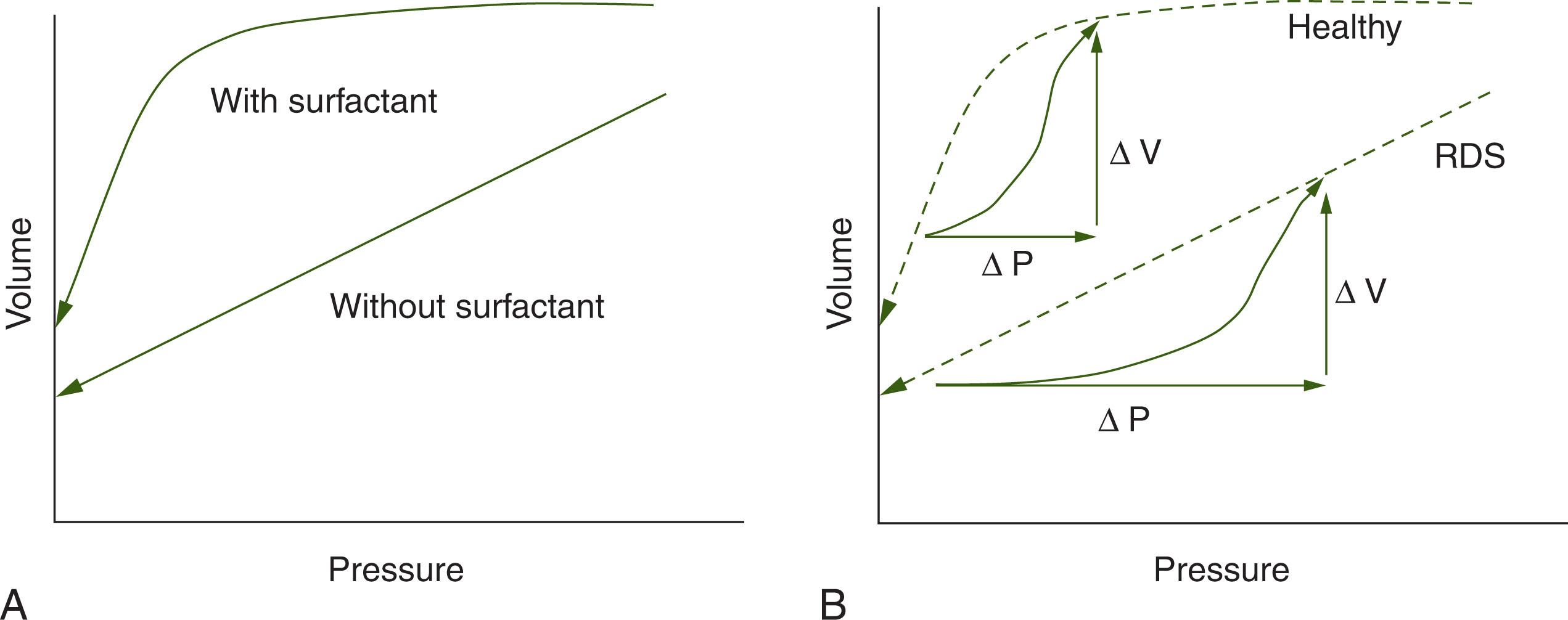
Surface tension is generated from molecular attractive forces within a liquid that oppose spreading; this is the reason that water “beads up” on a clean surface. According to Laplace’s law, the pressure within a sphere is proportional to its surface tension and inversely proportional to its radius. Surfactants are surface-active materials that lower surface tension. Lung surfactant, composed of phospholipids and proteins, reduces surface tension as the radius of the alveolus decreases. In the absence of surfactant, there is high surface tension, so the pressure required to keep smaller alveoli open would exceed the pressure keeping open larger alveoli, resulting in collapse of the small alveoli into larger ones, as shown in Fig. 42.3 . However, when lined with high-quality surfactant, the surface tension falls quickly as the alveolar radius gets smaller because the surfactant molecules become crowded during deflation ( Fig. 42.4 ). When the radius is very small, the surface tension falls almost to zero, and the pressure required to keep smaller alveoli open is negligible, preventing collapse. During inflation, as the radius of each alveolus increases, surface tension increases even faster. This means that the pressure in the larger alveoli will be higher than that in smaller ones; this pressure difference will cause flow to the smaller alveoli, thereby improving gas distribution.
The cumulative result of surfactant sufficiency is a nonlinear pressure–volume relationship with maintenance of an adequate functional residual capacity (FRC) during expiration, compared with alveolar collapse in the surfactant deficient lung ( Fig. 42.5A ). Furthermore, during inflation, more pressure is required in the surfactant-deficient lung to achieve a similar tidal volume (see Fig. 42.5B ), because of poor compliance (Δ V /Δ P ) from having to reopen collapsed alveoli. For instance, to achieve a tidal volume of 5 mL/kg, an infant with RDS may require a pressure increase of 25 cmH 2 O; dividing the volume change, Δ V , by the pressure change, Δ P , the calculated compliance is only 0.25 mL/kg per centimeter of water, approximately one-third of normal. For more information on surfactant, refer to Chapter 38 .
In the absence of adequate functional surfactant in the newborn lung, there is inhomogeneous lung inflation with widespread alveolar collapse and overdistention of open alveoli ( Fig. 42.6 ). Because reopening collapsed alveoli requires high pressure, the spontaneously breathing newborn with surfactant deficiency must generate highly negative intrathoracic pressure. Clinically, this is manifested by retractions of the chest wall and use of accessory muscles during inspiration. Because the rib cage in premature infants is so compliant, the sternum may deeply retract during inspiration. Newborns may also attempt to prevent alveolar collapse by “grunting.” This partial closure of the glottis during expiration helps maintain an end-expiratory pressure, preserving FRC. The respiratory rate in infants with RDS is elevated in response to hypercarbia and hypoxemia. Furthermore, a consequence of widespread alveolar collapse is intrapulmonary shunting of blood past nonaerated lung tissue, limiting exchange of oxygen and carbon dioxide. Desaturation results from inadequate oxygenation, and pallor results from acidosis due to poor elimination of carbon dioxide. In addition, lungs that are poorly inflated have partially collapsed intrapulmonary vessels leading to pulmonary hypertension. The elevated pulmonary artery pressures lead to right-to-left shunting of unoxygenated blood across the patent ductus arteriosus to the descending aorta causing a differential between pre- and post- ductal oxygen saturations (see Chapter 47 ). The combination of increased work of breathing, oxygen desaturation, and acidosis causes lethargy, disinterest in feeding, and eventually apnea ( Box 42.6 ).
Tachypnea
Grunting
Increased work of breathing (nasal flaring, retractions)
Cyanosis
Pallor
Lethargy
Disinterest in feeding
Apnea
On auscultation, there may be inadequate air entry from the fast inspiratory rate and low tidal volume, and fine inspiratory rales may be heard because of the reopening of collapsed, small airways. The onset of symptoms is always within hours after birth and, in severe cases, may occur almost immediately. In general, the respiratory distress from untreated RDS tends to worsen in the first 1 to 3 days after birth and then usually abates gradually thereafter (although the natural course may be interrupted by exogenous surfactant therapy or application of continuous positive airway pressure [CPAP]). This progressive worsening can be helpful in differentiating RDS from other newborn respiratory diseases.
Initially, the arterial blood gases will show hypoxemia. Pa co 2 is almost always elevated, but often to a lesser degree than anticipated because of tachypnea. As the infant tires, Pa co 2 will rise further and cause respiratory acidosis. With imminent respiratory failure, there may be progressive metabolic acidosis due to inadequate oxygen delivery to tissues from poor peripheral perfusion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here