Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute lower extremity ischemia (ALI) is a serious condition with substantial morbidity and mortality. ALI refers to recent onset of lower extremity pain; long-standing lower extremity ischemia is dealt with elsewhere in this book. If ALI is severe and prolonged, the patient may develop progressive paresthesia, motor dysfunction, and eventually tissue infarction in the lower extremity. The rapidity plus severity of the onset of symptoms depend on the location, extent, and degree of collateral pathways around the obstruction. The estimated incidence of ALI is approximately 1.4–2.6 cases per 10,000 patients per year. ALI accounts for approximately 20% of inpatient peripheral vascular cases and amputation rates of 10%–30%. After the presenting event, the 1-year mortality rates of ALI are approximately 15%–20% along with low amputation-free survival rates of approximately 50%–65%. The two principal causes of ALI are (1) embolic, in which an artery may become occluded when an embolus becomes dislodged from a proximal source, and (2) thrombotic, in which occlusion of a native artery occurs in the setting of severe atherosclerotic disease.
A thorough clinical workup, which includes a physical examination, noninvasive imaging, and laboratory work is paramount when evaluating ALI. The physical examination should start with visual inspection of the extremity in question to ascertain the level of the ischemia (e.g., foot, ankle, knee, above the knee). A mottled versus a pink extremity are at opposite ends of the risk spectrum. The examination is continued with evaluation of the sensory and motor systems, which can further reveal the severity and duration of the ischemia and guide clinicians to appropriate treatment. Additionally, palpation and/or Doppler examination of the peripheral pulses can help the clinician target the level of the occlusion. The clinical presentation of ALI has classically been stated as the six Ps: pain, pallor, pulselessness, paresthesia, paralysis, and poikilothermia (inability to regulate body temperature).
After a thorough physical examination, noninvasive imaging is performed to supplement the evaluation. Available modalities include pulsed volume recording (PVR), duplex ultrasonography, computed tomographic angiography, and magnetic resonance angiography. In our practice, we start with duplex ultrasound and PVR, adding computed tomographic and magnetic resonance angiography as needed.
Laboratory evaluation is also critical in the evaluation and workup of ALI. Serum urea nitrogen and creatinine values provide baseline renal function and can guide clinicians on the appropriate therapy. Prothrombin time, partial thromboplastin time (PTT), and international normalized ratio are obtained for a coagulation baseline.
An acute, profoundly ischemic limb is a surgical emergency. Cell death begins within 4 hours of total ischemic time and is irreversible after 6 hours. Revascularization in this setting may result in sudden tissue reperfusion and lead to a systemic acid–base disorder and impaired cardiopulmonary function.
Once the diagnosis of ALI has been made, medical management with systemic anticoagulation using a heparin or an argatroban (in patients with heparin-induced thrombocytopenia) infusion is initiated. It is thought that prompt anticoagulation limits propagation of clot and decreases microvascular thrombosis.
Over the years, the treatment options for ALI have evolved to include medical management, surgical revascularization, and most recently endovascular revascularization. Current endovascular options include catheter-directed thrombolysis (CDT), percutaneous mechanical/pharmacomechanical thrombectomy (PMT), and vacuum-assisted thrombectomy (VAT).
In the 1970s, intravenous thrombolytic agents (e.g., streptokinase) were shown to restore patency in acutely occluded arteries in 75% of cases. However, systemic infusion of thrombolytic agents carries a high risk of remote bleeding complications and has largely been abandoned in favor of CDT. Local intraarterial thrombolytic therapy has gained popularity over the past 20 years and in many cases, barring contraindications, is the initial intervention of choice. Thrombolytic agents are infused directly into the occluding thrombus in a concentrated fashion so systemic drug concentration and the total dose required for the procedure are reduced. As flow is restored, the underlying culprit lesion is usually unmasked and managed by interventional or surgical techniques.
In recent years, PMT and VAT have emerged as either an adjunct or stand-alone therapeutic option in ALI. In PMT, the clot is endovascularly macerated and removed, reestablishing flow in the artery. VAT, on the other hand, relies on vacuum-assisted aspiration of clot from the artery.
A classification system was devised by the Society for Vascular Surgery (SVS, United States) and updated by the Trans-Atlantic Inter-Society Consensus (TASC) II guidelines in 2007 for describing the degree of acute limb ischemia ( Table 11.1 ). The main objective of this categorization is to stratify the acuity of limb ischemia into defined groups for decision-making purposes. The TASC II guidelines also suggest an algorithm for the diagnosis and treatment of acute limb ischemia ( Fig. 11.1 ).
| Category | Definition | Prognosis | Physical Examination | Doppler Signals | ||
|---|---|---|---|---|---|---|
| Sensory Loss | Muscle Weakness | Arterial | Venous | |||
| I | Viable | Not immediately threatened | None | None | + | + |
| II | Threatened | |||||
| II-a | Marginally | Salvageable with prompt treatment | Minimal (toes) | None | Occasional +, frequently − | + |
| II-b | Immediately | Salvageable with immediate treatment | More than toes, pain at rest | Mild to moderate | Rare +, usually − | + |
| III | Irreversible | Major permanent tissue loss | Anesthetic | Paralysis | − | − |
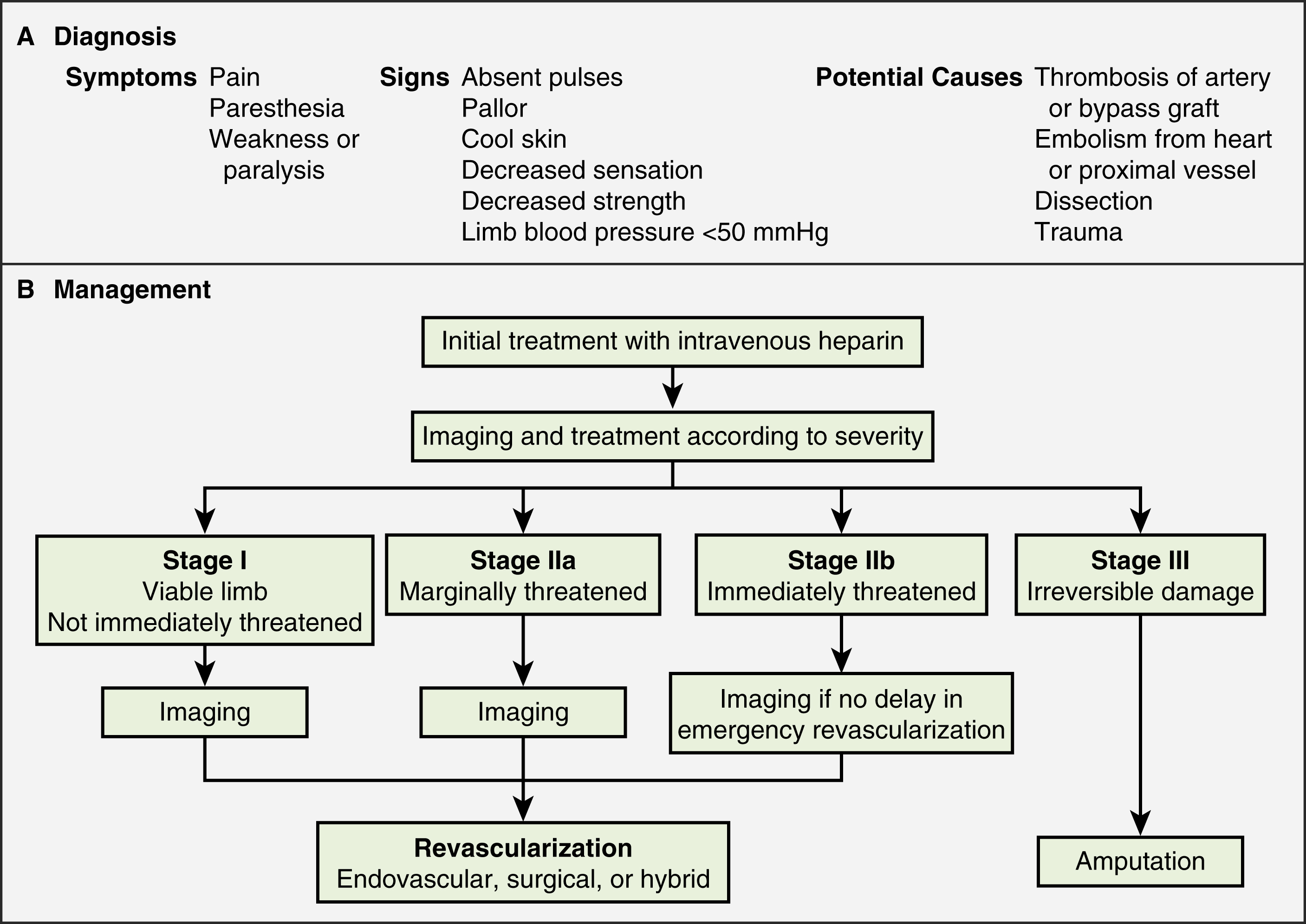
Endovascular therapy is indicated in patients who have acute-onset claudication (SVS I, the limb is generally viable) or limb-threatening ischemia (generally SVS II-a, but also SVS II-b) due to thrombotic or embolic arterial occlusion.
Relative and absolute contraindications for CDT are listed in Table 11.2 . Absolute contraindications include active internal bleeding, cerebrovascular events such as a recent (within 6–12 months) cerebrovascular accident or a transient ischemic attack (within 2 months), known intracranial neoplasm, or craniotomy within 2 months.
| Absolute |
| Active internal bleeding Irreversible limb ischemia (severe sensory deficits, muscle rigor) Recent stroke (arbitrary guidelines: TIA within 2 months or CVA within 6–12 months) Intracranial neoplasm or recent (2 months) craniotomy Protruding mobile left-sided heart thrombus |
| Relative |
| History of gastrointestinal bleeding Recent (10–14 days) major surgery, including biopsy Recent trauma Recent CPR Severe uncontrolled high blood pressure Emboli from a cardiac source Subacute bacterial endocarditis Coagulopathy Pregnancy and the postpartum period Severe cerebrovascular disease Diabetic retinopathy |
Irreversible ischemia (SVS III) accompanied by severe sensory loss, along with the onset of muscle rigor and absent arterial and venous signals on Doppler interrogation, puts the patient at high risk for the consequences of acute reperfusion. In this setting, endovascular therapy is associated with a high rate of amputation (25%) and mortality (13%). A surgical consultation is recommended in these scenarios.
Most relative contraindications to CDT are aimed at minimizing the risk of bleeding. The benefits of CDT should be weighed against this risk. Relative contraindications include recent major surgery, organ biopsy, trauma, gastrointestinal bleeding, pregnancy, postpartum status, uncontrolled hypertension, and diabetic hemorrhagic retinopathy. Of note, in elderly patients with uncontrolled hypertension who were treated for acute myocardial infarction with systemic thrombolytic therapy, a significantly greater risk for intracranial hemorrhage has been reported. The presence of a mobile intracardiac thrombus on echocardiography is predictive of a higher risk of embolization and should therefore be considered a relative risk for lytic therapy.
On the other hand, most of these contraindications are eliminated with the use of PMT and VAT. These therapies mechanically debulk or aspirate thrombus, reducing if not eliminating the use of intraarterial thrombolytics.
There are several devices on the market that facilitate infusion of thrombolytic therapy, but any standard catheter, 5F or under, can be used for an end-hole infusion. Multi-sidehole catheters allow for distribution of the lytic agent along the axis of the catheter once the catheter is well embedded within the thrombus. These catheters come with different effective infusion lengths (segment of distribution of the side holes) to match the length of the thrombus, and most can be used for both slow continuous and pulse-spray infusion. One such catheter has pressure-responsive side slits through which jets of fluid lace the thrombus during pulse-spray infusion, in addition to allowing slow continuous infusion (Uni-Fuse, SpeedLyser, and Pulse-Spray Infusion Systems [AngioDynamics Inc., Queensbury, NY]). An approach increasingly used is the EkoSonic Endovascular System (Ekos Corporation, Bothell, WA). The system consists of a multi-sidehole catheter through which a wire is placed that emits high-frequency, low-power microsonic energy. By exposing the thrombus to an energy source while infusing a thrombolytic, the interventionalist can achieve improved efficacy in a shorter period of time.
Although there is a large choice of potential delivery catheters, there is no compelling evidence that any of the more complex catheters offer any advantage over a single end-hole catheter for the delivery of lytic agent.
There are a multitude of devices for the use of PMT and VAT in the market today. The AngioJet (Boston Scientific, Quincy, MA) is one of the most widely used devices for PMT, which utilizes an infusion of thrombolytics via pulse-spray as well as mechanical thrombectomy. Other devices for PMT include the Inari FlowTriever and ClotTriever (Inari Medical, Aliso Viejo, CA). In terms of VAT, the Penumbra Indigo Mechanical Thrombectomy System (Penumbra, Alameda, CA) delivers high-level, continuous vacuum suction to remove thrombus from the artery. The Penumbra system can generate a continuous vacuum aspiration of −29 mmHg and consists of three components: pump, catheter, and separator.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here