Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Major burn injury results in pathophysiologic changes affecting virtually all organs from the onset of injury until wounds are healed. Anesthesiologists are often called on to care for burn injury patients through their hospitalization including acute airway management and resuscitation, intraoperative anesthetic care, intensive care, and management of postoperative pain.
Burn shock is a paradigm of ischemia/reperfusion injury. The initial ischemic (ebb) phase of burn shock is a hypodynamic, hypovolemic state, with rapid loss of fluid from the intravascular space and decreased cardiac output, which typically lasts for the first 24 to 48 hours following injury. Approximately 48 hours after successful resuscitation, a hyperdynamic, hypermetabolic (flow) phase ensues consisting of tachycardia, increased cardiac output, hyperthermia, hyperglycemia, and increased protein catabolism.
The goal of fluid resuscitation is to maintain organ perfusion by replenishing the massive loss of fluid from intravascular to extravascular compartments. Multiple fluid resuscitation formulae exist for estimating fluid needs and differ somewhat in their recommendations for the amount of crystalloid and colloid replacement. No matter which formula is used, it should serve only as a guideline, and fluid resuscitation should be titrated to physiologic endpoints.
Inhalation injury is a major prognostic factor for morbidity and mortality after burn injury. Management of inhalation includes observation and monitoring. Endotracheal intubation or tracheostomy is indicated if airway patency is threatened.
Patients with severe burn injury often suffer from nonthermal traumatic injuries. Failure to diagnose these associated injuries during initial evaluation can lead to serious morbidity and mortality. All burn patients should be approached initially as multiple-trauma patients.
The magnitude of burns is classified according to percentage of total body surface area (TBSA) involved, depth of the burn, and the presence or absence of inhalational injury. Accurate estimation of burn magnitude is needed to guide the initial resuscitation strategy, make the referral to a burn center, ascertain the need for surgery, and to estimate prognosis. Three of the most commonly used methods to estimate %TBSA are the “rule of nines,” palmar surface area, and the Lund-Browder diagram.
“Fluid creep” refers to the trend of over-resuscitation in burn injury patients. Overly aggressive fluid administration may result in pulmonary edema, compartment syndromes, multiorgan failure, nosocomial infection, and increased mortality as well as the extension of the burn injury because of excessive local edema. Factors that contribute to “fluid creep” include overestimation of the burn size, use of supra-physiologic hemodynamic targets, increased opioid utilization, and failure to reduce the rate of fluid administration in patients with evidence of adequate tissue perfusion.
Electrical burns can have acute and chronic effects not occurring with other types of burn injury, and with morbidity far higher than expected based on burn size estimation alone. High-voltage injuries are typically associated with loss of consciousness, arrhythmias, myoglobinuria, and extensive deep tissue damage that can result in compartment syndromes. Patients suffering from electrical injury should be evaluated for associated traumatic injury, rhabdomyolysis, and compartment syndromes. For treatment of these complications, patients may come to the operating room within 24 hours of injury.
Airway management in the burn-injury patient may be challenging and warrants particular consideration. Key features of airway assessment include preexisting airway abnormality, current airway injury (i.e., inhalation injury), and signs of glottic obstruction. The type of airway abnormalities may vary depending on the stage of the injury. In the acute burn setting, mandibular mobility and mouth opening may be limited because of edema or, in later care, may have significant scarring and contractures in the face, mouth, nares, neck, and chest which can make airway management difficult.
Burn-injured patients develop tolerance to most narcotics and sedatives, thereby requiring substantially higher doses than patients without thermal injury. Sedatives and narcotics should be titrated to effect while the patient is carefully monitored. Adverse effects of opioids, such as respiratory depression, acute opioid tolerance, and hyperalgesia, particularly with the need for rapidly escalating doses, have generated increasing attention to multimodal strategies.
Muscle relaxant pharmacology is significantly and consistently altered after burn injury. Exposure to succinylcholine can result in an exaggerated hyperkalemic response, which can induce cardiac arrest. The current recommendation is to avoid succinylcholine administration in patients 48 to 72 hours after burn injury. The duration of this dangerous response to succinylcholine after burn injury is unknown.
Surgical excision of burn wounds is often associated with substantial bleeding. It is not uncommon for the surgical team to remove eschar so rapidly that the patient becomes hypovolemic and hypotensive. Clinical judgment remains a vital component for intraoperative resuscitation, using markers of perfusion, erythrocyte mass, and coagulation, pulse, or arterial waveform as key assessment tools. Good communication between the surgical and anesthesia teams as well as limiting the operative duration and extent of excision are also essential.
Patients with major burn injury have an impaired ability for thermoregulation and therefore require close monitoring of body temperature. Multiple strategies are used to maintain body temperature in the operating room, including use of forced-air warming blankets, thermal water mattresses, blood/fluid warmers, minimizing skin surface exposure, and wrapping the head and extremities with plastic or thermal insulation.
Postoperatively, burn-injured patients are likely to be less stable physiologically compared with the preoperative period. Continued bleeding may be concealed by dressings, the patient may be more prone to hypothermia, emergence may be associated with delirium, and analgesic requirements will be greater. During this period of exaggerated physiologic fragility, it is important to be especially vigilant during transfer of the monitors and respiratory and hemodynamic support equipment to the intensive care unit staff.
Burn injury leads to increased susceptibility to infection due to decreased immunity through multiple mechanisms including loss of the physical barrier of intact skin, damage to lining of the respiratory tract from inhalation injury, and altered gut permeability and function. Preventative measures against infection are critical for the burn-injury patient and include early excision of burn eschar to improve local perfusion and prevent microbial colonization, prudent use of invasive devices, application of antimicrobial burn dressings, and diligent compliance with infection control.
Nearly all aspects of burn care (e.g., dressing changes, excision and grafting procedures, physical therapy, and line insertion) are associated with pain. There can be ongoing background pain, periodic breakthrough pain, procedure-related pain, and eventually, chronic pain can develop. Standardized pain and anxiety guidelines are used to provide appropriate, consistent patient comfort.
Burn injuries are among the leading causes of injury and death worldwide, with about 11 million seeking medical care and over 265,000 deaths annually. Over 95% of these occur in low- and middle-income countries. Approximately 486,000 burn injuries are treated at U.S. medical facilities each year of which 40,000 require hospital admission with approximately 3275 deaths.
Major burn injury produces pathophysiologic changes that affect virtually all organs from the very onset of injury until after the wounds heal. Pathophysiologic effects may persist for years especially in patients with major injuries and include insulin resistance, neuromuscular dysfunction, pruritus, pain, and more frequent hospital admissions related to infections and cardiomyopathies. Severe burn injury patients are different from other intensive care patients as they pose challenges regarding fluid resuscitation, metabolic stress, perioperative demands, and other specific burn injury–related complications. Most burn injury patients present to emergency rooms in community hospitals, which do not have a designated burn center. After initial acute care, these patients are usually transferred to tertiary care facilities with a specialized burn center. As a result, anesthesiologists staffing these peripheral hospitals with emergency rooms must be familiar with the pathophysiology of acute burn injury and resuscitation. In addition, treatment of a burn injury patient requires multiple operations, frequent dressing changes, and prolonged hospital stay with extensive rehabilitation needs. In burn care facilities, anesthesiologists need to have expertise in the specific management of the pathophysiologic changes affecting these victims and particularly the unique features of perioperative management of this patient population. Therefore there is a continuous need for specific teaching, training, and maintenance of specialized skills in this field.
Although morbidity from burn-related injuries remains high, advanced methods of resuscitation, early excision and grafting of burn wounds, better methods of wound coverage, improved anesthesia and intensive care techniques, early diagnosis and aggressive treatment of infections, as well as enhanced nutritional support and mental health–care methods have led to significant decrease in burn injury–related morbidity and mortality. Other factors, including immediate prehospital care, early emergency treatment with advanced life support capability, and secondary transfer to a specialized burn unit have also contributed to improved survival. Despite significant advances in therapeutic strategies, care of the burn injury patient continues to pose multiple challenges for clinicians.
Burn injury can cause massive tissue destruction and result in activation of an inflammatory response that leads to profound pathophysiologic effects at sites both local and distant from the injury. Understanding the pathophysiologic alterations and their time course is essential for providing appropriate resuscitation and perioperative care.
Burn shock is a paradigm of ischemia/reperfusion injury. The initial ischemic (ebb) phase of burn shock is a hypodynamic, hypovolemic state, with rapid loss of fluid from the intravascular space and decreased cardiac output, which typically lasts for the first 24 to 48 hours following injury. A large volume fluid resuscitation is required to maintain intravascular volume for organ reperfusion, acutely diluting plasma proteins. Approximately 48 hours after successful resuscitation, a hyperdynamic, hypermetabolic (flow) phase ensues due to the systemic inflammatory responses from the trauma-induced release of damage-associated molecular patterns (DAMPS), even in the absence of overt infection. This is characterized by increased cardiac output, oxygen consumption, muscle protein catabolism, and body temperature, lasting for many months to years after healing of the burn wounds. When burn shock is left untreated, physiologic exhaustion ensues and is fatal.
The goal of fluid resuscitation is to maintain organ perfusion by replenishing the massive loss of fluid from intravascular to extravascular compartments. There are two main reasons for this loss, both of which have been extensively investigated and have clear temporal patterns. First, there is the negative imbibition pressure in tissues injured by a burn and, second, there is an increase in vascular permeability with loss of fluid from the vasculature as well as from the injured areas. In the injured areas, there is protein loss from the vasculature in addition to crystalloid loss. At the uninjured distant sites, there is capillary sieving of protein with loss of crystalloid only.
When fluid losses were investigated in experimental models they could not be explained completely by an increase of permeability, and it was then postulated that there must be another mechanism that would explain these losses. In 1960, Gösta Arturson suggested that the loss from the blood could be explained by a reduction in the interstitial tissue pressure. Subsequently, Lund et al. using in vitro models showed that there is a build-up of strong negative interstitial pressure within injured tissue causing a negative interstitial pressure in the range of –25 to –50 mm Hg ( Fig. 87.1 ). This negative gradient, called negative imbibition pressure, explains most of the early total fluid loss. Imbibition pressure is a negative pressure where water or crystalloid is absorbed causing large increases in volume and is different from hydrostatic and osmotic pressure. More recent in vivo studies by Kinsky and associates have confirmed the previous in vitro findings on imbibition pressure. This negative imbibition pressure is most pronounced immediately after the burn and remains for several hours. Intriguingly, the fluids provided during resuscitation seem to have an adverse effect on the negative imbibition pressure (see Fig. 87.1 ). Administering a greater amount of fluid results in larger negative tissue imbibition pressure, and correspondingly larger fluid leak and overall demand for fluids. The mechanism underlying the development of negative tissue pressure is not well understood, but probably results from the effect of thermal energy on the tissue integrins (components important for the regulation of hydrostatic pressure in the interstitium), which lose the ability to maintain their beneficial effect on imbibition pressure. The magnitude of the burn seems to affect this defect; the larger the burn injury, the more pronounced is the negative pressure in the tissue. This is the reason for early hypovolemia despite fluid treatment therapy. Importantly, most of the intravascular fluid loss disappears within 24 to 48 hours. The resorption of the extravascular edema fluid, however, takes much longer as detailed later in the Fluid Resuscitation Strategies section.
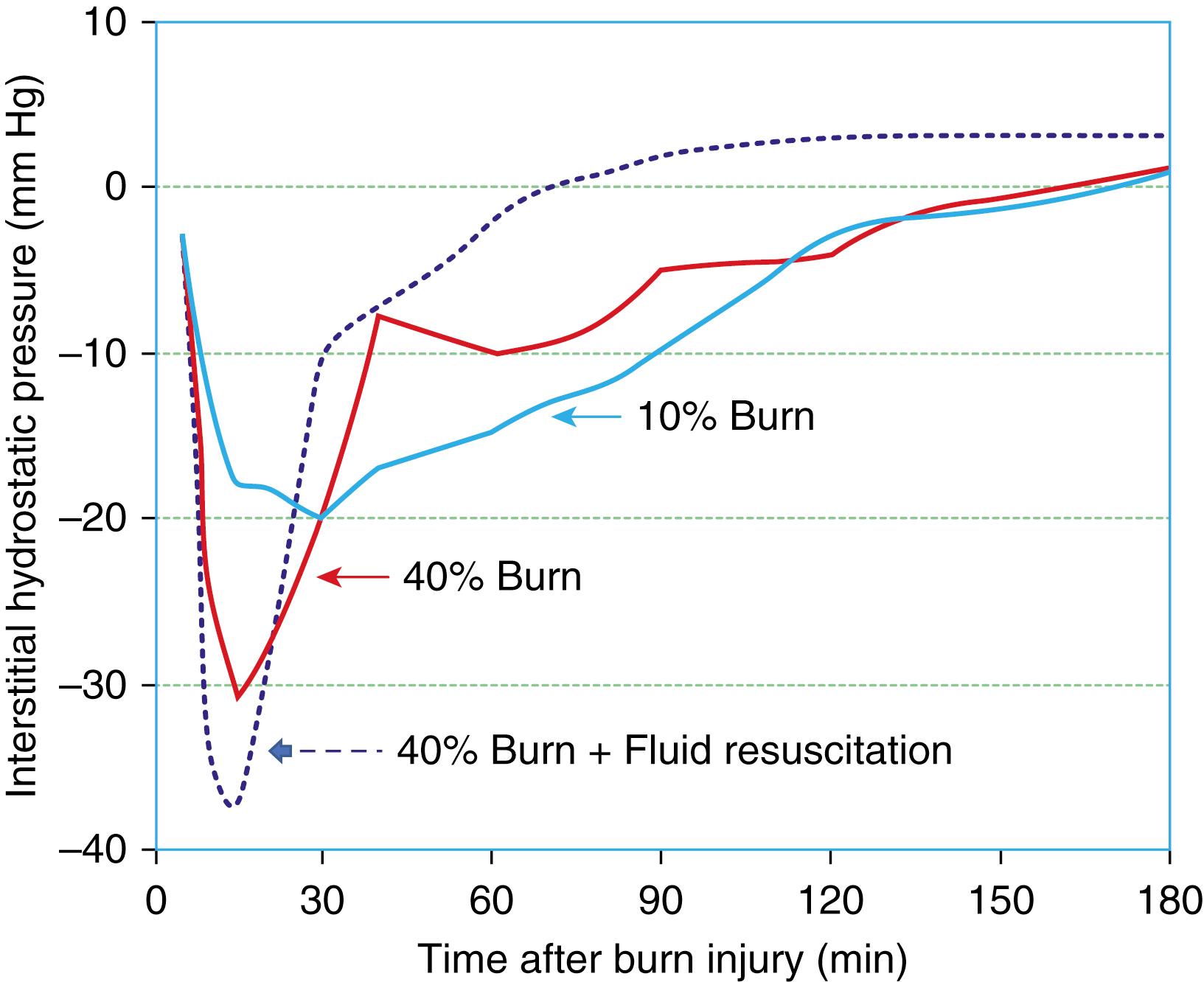
The loss of fluid from the intravascular space is also due to the increase in vascular permeability. This effect is obvious even in a small 5% total body surface area (TBSA) burn and occurs soon after the injury, explaining the blister formation even with small burn injury. Detailed mechanisms as to how even a small burn causes leakage of fluid is unclear, but there seem to be a number of putative mediators (see also section on Mediators Important in Fluid Loss). Another important aspect of vascular permeability is the onset of vasodilatation in most vascular beds as a result of the continued liberation of proinflammatory cytokines into the injured tissues. This vasodilatation increases the hydrostatic pressure in the microcirculation, which leads to further loss of fluid into the interstitial compartment. The Starling equation given here further describes the different factors that play a role in fluid filtration:
where J v is the fluid volume, Kf the filtration coefficient, P c the capillary hydrostatic pressure, P i the interstitial hydrostatic pressure, σ the reflection coefficient, π c is the capillary osmotic pressure, and π i the interstitial osmotic pressure. In particular, the coefficient of filtration increases dramatically, often to a range 20-fold greater; for the latter parts of the formula, there is an increase in hydrostatic pressure in the capillaries due to vasodilatation; decrease in interstitial pressure (the negative imbibition pressure) together with a decrease in colloid osmotic pressure within the capillaries as the result of a capillary leak, onto which also the increased osmotic effect of proteins lost into the interstitium, is added.
Although these changes affect most vascular compartments, the effect at the venular-end seems more important from a quantitative perspective. Most of the proteins lost across the vascular wall are smaller molecules; a few larger proteins are also lost. This large protein loss is important to note, as it underlies the arguments in favor of so-called “colloid rescue” fluid resuscitation in larger burns, which reduces the total colloid loss (and corresponding total fluid loss) with decreased risk of compartment syndromes. It is important to stress that the loss of fluid from the intravascular space is due to alterations in all the factors previously described, which are important for the transport of fluid across the capillary and venular walls, and therefore contribute to the large fluid volume loss after burn injury.
In clinical practice, the increase in permeability, dilution effects of the resuscitation, and loss of protein manifest as a decrease in the concentration of serum albumin. The intravascular colloid osmotic pressure during the acute phase of burn injury is dependent not only on albumin but also on the newly synthesized acute phase proteins. Uncertainties in the temporal pattern of the vascular leak of protein locally means that the optimal timing of colloid administration is unclear. Thus the intense debate as to when colloids can safely be started during fluid resuscitation continues. Today most burn clinicians would agree that it is appropriate to start colloids 8 to 12 hours postburn to reduce the total fluid volume (see section Colloid Rescue Treatment ). It is important to understand, however, that early colloid administration may lead to its extravasation into the extravascular space with a concomitant increase in tissue edema. It must also be noted that even in the absence of burn injury only 20% of the administered crystalloid fluid volume stays within the vasculature, and that large volumes of crystalloid alone will decrease intravascular colloid osmotic pressure and cause a further loss of fluid from this compartment. These interrelated effects must be appreciated when fluid resuscitation for a burn injury patient is initiated. However, the previous dictum that the first 24 hours after burn should consist of only crystalloids does not hold anymore.
When caring for burn injury patients, it is important to discuss the temporal aspects of the fluid loss, and the recommended fluid management. This is particularly important, as recent investigations have shown that in present guidelines there is a clear temporal mismatch between fluid loss and fluid volume protocols. Most of the fluid that is lost from negative imbibition pressure is lost within the first 3 to 4 hours after the burn. The picture is somewhat different for the fluid that is lost from the increase in permeability. The most reliable data in humans has suggested that this fluid is lost at the time of the injury, and up to 8 to 10 hours later. The permeability effects continue even after 48 hours due to the continuous systemic inflammatory response that is ongoing after the burn, although its magnitude is significantly less, unless complicated by sepsis.
More important is that the present guidelines for fluid resuscitation, particularly if they are based purely on crystalloids, do not fully account for this early loss of fluid. The patient may therefore be claimed to be in a controlled hypovolemic state during the first 12 to 16 hours after the burn injury. The tissue edema reaches its maximum between the first 24 to 48 hours after the injury, and thereafter the added fluid volume is slowly returned to the circulation and excreted as urine, often until 7 to 14 days after the burn depending on the magnitude of the injury. This is also the time when lung dysfunction can occur because of hypervolemia from the reabsorbed fluid.
Many mediators have been thought to be important in the underlying mechanisms of fluid loss in burns, and there are probably several that contribute in different ways. The most important are: serotonin, nitric oxide, thromboxanes, prostaglandins, and several others including reactive oxygen species and proinflammatory cytokines. But molecular mediators are not the only substances implicated in the permeability effects; white blood cell–related effects have also been suggested. Coagulation and the complement cascade are thought to be activated early, and also play an important part. Interest in the mediators and their possible role in the generation of fluid losses is driven by the hope of finding a treatment that can stop or reduce the process. Some attempts have been made and the most successful has been the use of high-dose vitamin C (as a scavenger of oxygen radicals) which, in randomized trials in both animals and humans, showed a reduction in fluid loss in the treatment arm. In addition, direct effects were seen on the negative imbibition pressure with vitamin C administration.
Burn shock can result in profound hemodynamic alterations associated with organ dysfunction. Severe burn shock is both distributive and hypovolemic in nature. The increased systemic vascular resistance (SVR) (due to release of catecholamines, antidiuretic hormone, and hemoconcentration) compounds the shock phase adverse effects.
A reduction in cardiac output also often occurs with major thermal injury even before any detectable reduction in plasma volume and may continue even when hypovolemia is alleviated. The cardiac dysfunction is characterized by slowed isovolemic relaxation, impaired contractility, and decreased left ventricular diastolic compliance and often continues for 24 to 36 hours. Burn-related left ventricular contraction and relaxation defects increase with burn size, achieving a nadir with 40% TBSA burns. This cardiac dysfunction has been identified as a major cause of multiple organ dysfunction syndrome (MODS) and mortality.
A hyperkinetic and hypermetabolic state develops 48 to 72 hours after burn injury, and is characterized by a decrease in vascular permeability, increased heart rate, and decreased SVR resulting in an increase in cardiac output. Cardiac output is often increased to more than 1.5 times that of a nonburned, healthy patient 3 to 4 days following the injury. The metabolic rate is increased, approximately 1.5 times that of normal basal rate. This increase in cardiac output is associated with increased liver and kidney blood flow, which has implications for elimination of blood flow–dependent drugs including some antibiotics and anesthetic drugs. The onset of sepsis may further increase cardiac output and decrease SVR.
In addition to age and the extent of burns, inhalation injury is a major prognostic factor for morbidity and mortality after burn injury. Inhalation injury can be classified under three subcategories: direct thermal injury to the upper airway; chemical irritation to the lower (subglottic) airway and even lung alveoli; and systemic chemical or metabolic injury caused by specific noxious combustion chemicals, or a combination of these factors. Direct heat and steam injury to the upper airway can lead to marked swelling of the face, tongue, epiglottis, and the glottic opening resulting in an upper airway obstruction. Because airway swelling may not occur immediately but may develop over a period of hours (especially with, and complicated by, concurrent fluid resuscitation), a high index of suspicion and frequent reevaluations of the respiratory status are essential. A scald injury of the epiglottis may mimic symptoms of epiglottitis.
Thermal injury to the lower airway is uncommon due to a highly efficient heat exchange system in the oropharynx and nasopharynx and the low specific heat of steam in conjunction with the laryngeal closure reflex due to the irritation. Damage to the lower airways and lung parenchyma following smoke inhalation tends to be chemical rather than thermal. Toxic substances in smoke damage the epithelium and capillary endothelial cells of the airway resulting in the release of inflammatory mediators, increased vascular permeability, and edema of distal bronchi and alveoli. Many of the previously listed mediators are relevant here as well. Damaged mucosal cells produce excess exudates rich in protein and necrotic debris. Chemicals in smoke promote the formation of neutrophil-generated oxygen radicals leading to inflammation. Destruction and damage to the airway’s ciliary transport function leads to the accumulation of casts, airway plugging, and impaired clearance of bacteria and debris. Alveolar collapse and atelectasis can occur because of loss of surfactant production or from plugging of small airways by mucosal debris. Over time, these changes can result in bronchospasm, airway obstruction, atelectasis, and pneumonia, which cause ventilation perfusion mismatch, impaired gas exchange, and decreased pulmonary compliance. The severity of inhalation injury may not be directly proportional to the degree of exposure to smoke alone. Rather, the severity of injury is probably due to the composition of the inhaled material and combustion substances together with differences in the individual host response as well as added effects from the cutaneous burn.
Injury to the airways and lung can also occur with severe cutaneous burns in the absence of inhalational injury. Mechanisms include the effects of inflammatory mediators from the burn-injured area, and the effects of fluid resuscitation and infection. For example, acute lung injury can occur in patients with scald injury without smoke exposure where bronchoscopic features can mimic smoke-induced airway injury.
While the gas-phase components of smoke do not produce direct injury to the respiratory tract, they can produce systemic effects. Among the most toxic gas-phase components are carbon monoxide (CO) and cyanide inhalation, which can lead to major morbidity and mortality following inhalation injury. CO is an odorless, colorless gas that has a 200-fold higher affinity than oxygen to the same binding sites on hemoglobin. CO shifts the oxyhemoglobin dissociation curve to the left and alters its shape. In addition, CO binds to cytochrome oxidase, impairing mitochondrial function and reducing adenosine triphosphate (ATP) production. CO thus reduces both the oxygen-carrying capacity of blood and oxygen dissociation at a tissue level, as well as disrupting oxidative cellular respiration. The clinical manifestations with patient symptoms of CO poisoning appear when carboxyhemoglobin (HbCO) levels are more than 15%. The symptoms are typical of tissue hypoxia, most notably neurologic impairment and myocardial dysfunction (the organ systems most vulnerable to hypoxia). There is no set combination of symptoms that confirms or rules out diagnosis of CO poisoning. The intensity of clinical manifestations varies and does not correlate closely with HbCO levels. Early signs tend to be neurologic. Central nervous system (CNS) injury can lead to progressive and permanent damage. Severe myocardial dysfunction may occur, especially in patients with preexisting coronary disease. The clinical diagnosis of HbCO poisoning should be confirmed by demonstrating its elevated levels. Elevated HbCO levels indicate significant exposure to smoke, which points to the likelihood of chemical airway injury. Low HbCO levels do not always mean minimal exposure, as oxygen therapy in the early stages can reduce levels during transport to the emergency department. Hypoxemia caused by CO poisoning is not detected by pulse oximetry or by partial pressure of oxygen (PaO 2 ) measurements, and patients may appear “cherry pink” rather than cyanotic. CO-oximetry is required to make the diagnosis. The binding of CO to hemoglobin is stable, with a half-life of 4 hours for a person breathing air. Increasing the arterial PaO 2 accelerates the CO displacement from the hemoglobin molecule; administration of 100% oxygen at atmospheric pressure shortens the half-life to an average of 74 minutes. Hyperbaric oxygen therapy has been suggested as a therapy to reduce the neurologic sequelae from CO toxicity. Hyperbaric oxygen therapy achieves faster CO displacement and may be more useful in cases of prolonged exposure, when it also may be assumed that it is harder to displace the toxin bound to the cytochrome system (e.g., mitochondria). The drawback of hyperbaric oxygen therapy is the need to transfer the burn injury patient to a treatment facility equipped with a hyperbaric chamber during the critical period of hemodynamic and pulmonary instability. For these reasons, it may be considered in patients with severe neurologic involvement and levels of HbCO greater than 50%, without extensive burns or severe lung damage, and whose symptoms fail to improve despite a high flow of oxygen. The lack of availability of hyperbaric oxygen poses a barrier to its use in many tertiary care centers.
Cyanide (CN) is released by the combustion of nitrogen-containing compounds, which are present in plastics, fabrics, and paper. CN acts by binding to cytochrome oxidase, thereby inhibiting the mitochondrial respiratory chain, cell metabolism, and tissue ATP production, resulting in cytotoxic hypoxia and metabolic acidosis. CN toxicity may have a synergistic effect with CO in producing tissue hypoxia. Concentrations of CN greater than 20 ppm are considered dangerous and concentrations of 100 ppm can lead to seizures, coma, respiratory failure, and death. A rapid diagnostic test for CN poisoning is not widely available; as a result, the treatment of CN poisoning is generally based on clinical suspicion. CN poisoning should be suspected in any patient with a history of smoke inhalation injury with an anion gap metabolic acidosis in the presence of apparently adequate oxygen delivery. Lactic acidosis with a high anion gap can indicate CN poisoning, as can an arteriovenous oxygen saturation difference of less than 10 mm Hg because of the inability of the mitochondria to use tissue oxygen. Lactic acidosis in burn victims may, however, be due to several causes and is not specific for cyanide toxicity. CN’s short blood half-life (∼1 hour) makes accurate determination of CN poisoning difficult and is hampered by delayed blood sampling. Blood CO concentration is highly correlated with CN levels and as such, may be considered as an indicator of CN poisoning. Empirical treatment involves administration of high-flow oxygen. Specific antidotes are advocated, especially hydroxocobalamin, which binds to CN and is relatively nontoxic; but administration must be immediate for any effect to be useful. The deleterious effects of CN can also be neutralized by the administration of thiosulfate converting CN to thiocyanate, which is excreted in the urine. Exogenous thiosulfate has a slower onset than hydroxocobalamin. The treatment of CN toxicity by administration of nitrites (e.g., amyl nitrite) has generated controversy as this treatment in itself can be hazardous. Nitrites induce methemoglobinemia which, together with HbCO, may interfere with oxygen transport, thus contributing to hypoxia.
The diagnosis of inhalation injury is based on a combination of clinical findings such as patient history, physical examination, and HbCO levels. Patient history should include the duration of exposure such as whether the patient was found in an enclosed space, unconscious at the scene, or has extensive cutaneous burns. Physical findings include facial burns, singed nasal hair, signs of upper airway injury (hoarseness, stridor, carbonaceous sputum, erythema, and swelling of the oropharynx), and signs of lower airway involvement (dyspnea, tachypnea, wheezing, decreased O 2 saturations). Stridor, dyspnea, increased respiratory effort, and cyanosis appear only once critical narrowing of the airway occurs.
Chest radiographs lack the necessary sensitivity to detect lung damage for inhalation injury in the early stages, but their use is helpful as a baseline for determining future changes. Fiberoptic bronchoscopy (FOB) offers a potential means by which to evaluate severity of inhalation injury although it may underestimate the presence of parenchymal disease and controversy exists over whether or not the visualized severity of the mucosal injury predicts clinically meaningful outcomes. FOB is unnecessary if subglottic pathology is unlikely. In patients with clinical signs suggestive of thermal airway injury, a normal endoscopic appearance is reassuring although alveolar damage would not be revealed. FOB can be repeated at intervals or if there is clinical deterioration. The presence of soot, mucosal edema, mucosal hyperemia, and pooling of secretions indicates inhalation injury, and suggests the need for close observation with repeated assessment; more ominous signs include narrowing of the laryngeal inlet, mucosal erosion, ulceration, and exudation. Virtual bronchoscopy is an alternative diagnostic modality to identify inhalation injury; however this is not widely practiced. Other diagnostic methods include xenon scanning, pulmonary function testing, and computed tomography (CT). The most reliable indicator of the impact of inhalation injury is the arterial partial pressure of oxygen to inspired oxygen fraction (PaO 2 /FiO 2 ) ratio after the resuscitation has started.
Laryngeal injuries are common in burn injury patients and can be associated with long-term morbidity. Early recognition of laryngeal injury and consultation with a laryngologist can influence treatment choices (e.g., tracheostomy) and limit morbidity. Because anesthesiologists are most likely to view the larynx of patients with acute burns, it is important to make laryngeal examination part of the initial intubation whether during resuscitation or induction of general anesthesia. Any pathologic laryngoscopic findings should be documented.
Treatment of upper airway burns secondary to smoke inhalation includes observation and monitoring.
Endotracheal intubation or tracheostomy is indicated if airway patency is threatened. Thermal injury to the oral cavity and supraglottic structures can cause edema; with severe injury, airway obstruction may result as a consequence of edema of the supraglottic airway. Clinically significant obstruction can also occur following fluid resuscitation, with maximal edema typically presenting hours after the initial insult and lasting for several days. There can be no substitute for patience and repeated airway assessment by an experienced anesthesiologist while minimizing edema formation by upright positioning and the avoidance of excessive fluid therapy. As a general rule, when indicated, it is safer to intubate the patient early than risk a difficult intubation after airway swelling has occurred. Although preemptive intubation of patients with inhalation injury can be lifesaving, it should be performed for clear indications. Reasons for intubation include: protection against anticipated airway swelling, treatment of impaired oxygenation and/or ventilation due to lung injury, and to ensure airway protection and optimal oxygenation in cases of hypoxia or CO poisoning with neurologic impairment. The treatment of pulmonary parenchymal injury is inherently more complex than treatment for cutaneous burns. Necrotic skin can be excised, and healing can be observed directly. In contrast, injured lung involves measures to prevent further injury to allow host mechanisms to repair injured tissues. Healing of pulmonary injury is followed indirectly by observations of blood gas analysis and radiographs/CT scans. Adequate oxygenation must be maintained and bronchial hygiene facilitated. Some patients may benefit from noninvasive ventilation in the absence of usual contraindications. Endotracheal intubation may be necessary if the patient has increased work of breathing or if gas exchange is compromised.
Patients with both cutaneous burns and inhalation injury may require larger fluid volumes for resuscitation than those without inhalation injury. Additional fluid resuscitation measures beyond titrating fluid input to maintain adequate urine output (generally considered 0.5-1 mL/kg/h) are not necessary and there are at least theoretical issues that the lung should be maintained “dry” to optimize gas exchange.
Moderate elevation of the head of the bed allows gravity to help reduce airway edema by facilitating venous and lymphatic drainage, and is therefore a sensible, critical standard practice. The patient should be given oxygen by mask to maintain adequate arterial oxygen saturation. Suction should be used to keep the airway clear of debris and secretions. Children are at greater risk of obstruction because of their smaller airways, as are patients whose burns include circumferential burns to the neck. Other early signs and symptoms of respiratory dysfunction may be more suggestive of a more severe inhalation injury.
Respiratory failure is a consequence of inhalation injury; however, the severely burn-injured patient often has multiple mechanisms contributing to lung injury, systemic inflammation in response to burn injury, pulmonary edema from fluid resuscitation, and sepsis. Thus the extent to which inhalation injury impacts burn patient outcome is difficult to separate from the contributions of other injury drivers, which affect the lungs. Management of respiratory failure commonly consists of mechanical ventilation and effective and repetitive pulmonary toilet. A myriad of ventilation strategies exists, and consensus regarding the most appropriate way to ventilate patients with inhalation injury has not been reached. In all cases, the goal of mechanical ventilation should be to optimize oxygenation and ventilation while minimizing potential ventilator-induced lung injury. The mechanisms of ventilator-induced lung injury include: high airway pressures causing barotrauma, over-distension of alveoli leading to volutrauma, repetitive opening and closing of alveoli causing atelectrauma, and lung inflammation caused by the release of proinflammatory cytokines that produce biotrauma. The use of lung-protective ventilation strategies (i.e., tidal volume of 5-8 mL/kg predicted body weight, limitation of plateau pressure to less than 28 cm H 2 O, and application of sufficient positive end-expiratory pressure to maintain alveolar patency and adequate oxygenation) is recommended as the initial approach if invasive ventilation is required. Studies have shown that lung protective ventilation with low tidal volumes are associated with lower mortality in patients with ARDS and are therefore also recommended in burn injury patients. As in other critically ill patients, prone positioning has been shown to improve oxygenation in burn injury patients with severe ARDS. Permissive hypercapnia, where blood carbon dioxide partial pressure (PaCO 2 ) is allowed to rise (<60 mm Hg) should be considered to limit plateau pressures unless there is a concomitant neurologic injury with suspected intracranial hypertension. These lung-protective ventilation strategies are generally considered to also apply to pediatric patients, although some evidence exists to challenge this presumption. Other strategies used by some burn centers for management of inhalation injury include high frequency percussive ventilation or high frequency oscillation ventilation, either of which may facilitate the clearance of airway debris and secretions. High frequency percussive ventilation and high frequency oscillation ventilation may be considered as “rescue modes” in very severe lung disease, though a benefit on outcome remains unproven. Extracorporeal membrane oxygenation (ECMO) is increasingly used as a rescue treatment for patients with refractory hypoxemia but there is inadequate evidence to support its benefit in inhalation injury at this time.
The consensus recommendations for mechanical ventilation also apply in this context, as do strategies for the prevention of ventilator-associated pneumonia. Bronchodilators may be administered to help optimize ventilation in the event of bronchospasm. Bronchoscopy may improve pulmonary hygiene and patient prognosis by clearing secretions and sloughed epithelial cells. Although not yet in routine clinical use, there are several promising experimental pharmaceutical adjuncts that address physiologic changes associated with inhalation injury. Aerosolized racemic epinephrine serves as a bronchodilator, vasoconstrictor, and mucolytic agent to alleviate wheezing and bronchospasm caused by the chemical tracheobronchitis. An aerosolized N-acetylcysteine/heparin combination therapy, which acts as both an oxygen free-radical scavenger and a mucolytic agent, has also been successfully used in children and adults with inhalation injury. Inhaled nitric oxide causes selective vasodilation in ventilated lung segments, and may improve oxygenation and pulmonary hemodynamics.
Noninvasive positive pressure ventilation (NIV) has been successfully used to avoid endotracheal intubation in select patients with mild inhalation injury with little evidence of edema, or as respiratory support strategy following extubation. The potential benefits of NIV are numerous including allowing the patient to communicate freely, require less sedation, allow cough and expectoration of secretions, and avoid other potential complications of intubation such as oropharyngeal trauma, mucosal ulceration, and ventilator-associated pneumonia. However, very strict case selection is essential. NIV requires compliant patients who can cough and protect their own airway and it must not be used where there is a risk of airway obstruction, or in patients with facial burns because of the tight mask required. In addition, there are legitimate concerns that NIV may conceal signs of progressive airway obstruction in the setting of an inhalation injury. The use of opioid and sedative drugs, often needed in burn injury patients, may complicate the use of NIV as a consequence of their respiratory depressant effects.
High-flow nasal cannula is a mode of respiratory support increasingly used in the management of acute respiratory failure. This mode delivers humidified gas via a nasal cannula at flow rates exceeding minute ventilation. The benefits include the reduced work of breathing and improved gas exchange by nasopharyngeal dead-space washout; decreasing the energy required to humidify and heat respiratory gases; and providing a degree of positive distending pressure. There are limited reports of its use in patients with inhalation injury.
Optimal management of burn injury patients begins at the scene of injury and continues in the emergency department and with transfer to a specialized burn unit. The primary survey should be performed using a systematic approach that first seeks to identify the greatest threats to life as provided in the Advanced Trauma Life Support (ATLS) and Advanced Burn Life Support (ABLS) guidelines. This approach requires a combined strategy of airway assessment and protection, initiation of resuscitation, and evaluation for coexisting injuries. Protecting the airway of a thermally injured patient is of the utmost priority. Early intubation is indicated in patients with symptomatic inhalation injury, or any thermal injury to the face, mouth, or oropharynx that threatens airway patency (stridor, swelling on laryngoscopy, upper airway trauma, altered mentation, and respiratory distress). The current criteria for prehospital intubation has been questioned because of the high incidence of over-intubation and early extubation posthospital admission. Despite this concern, it seems safer to intubate and modify therapy once in hospital rather than be afflicted by airway distress during prolonged transport. Oropharyngeal burns can rapidly cause obstruction; other causes of critical respiratory failure, such as coma, require immediate diagnosis and treatment. Once the airway is secure, breathing assessment follows. Auscultation of breath sounds and determination of respiratory rate and depth are essential for assessing the status of the lungs, chest wall, and diaphragm, and to evaluate the patient’s ability to adequately ventilate and oxygenate. Circumferential burns of the trunk or neck may impair respiration and require bedside escharotomy. Continuous monitoring of heart rate, blood pressure, pulse oximetry; and clinical assessment of unburned skin color should be used as parameters to assess circulatory status. An elevated heart rate (100-120 beats/min) is considered within normal limits for adults with burn injury; a higher heart rate should raise suspicion for hypovolemia, other trauma, and inadequate pain management. Circulatory assessment requires evaluation of perfusion of all extremities, paying particular attention to any circumferentially burned extremities. If perfusion is compromised, escharotomy is indicated. Intravenous access should be obtained via peripheral, central, and/or intraosseous routes and may safely be placed through burned tissue if necessary. Adjuncts such as ultrasound may be helpful in placing peripheral intravenous catheters. Large-bore peripheral access is preferred because smaller catheters do not allow faster fluid administration, especially in larger burns.
Fluid management based on weight and burn size should be addressed once total assessment of the injury has been established. Patients who have sustained a thermal injury often present with altered mental status and the possibility of associated injury, substance use, hypoxia, inhalation injury, or a preexisting condition that should always be addressed. As with trauma patients, the Glasgow Coma Scale, which utilizes verbal, motor, and eye measurements, can be used to establish a baseline mental status. Providing adequate environmental temperature control is essential for burn injury patients as they lose their ability to thermoregulate. The patient must be completely exposed to assess injury and to remove any contaminants that might prolong contact with chemicals or heat sources. A warmed environment and immediate coverage with clean blankets can limit hypothermia during the examination. More than 5% of patients admitted to burn centers have also sustained nonthermal traumatic injuries. Therefore all burned patients should be approached initially as multiple trauma patients. Whole-body CT imaging and focused assessment with sonography in trauma-echocardiogram exam should be performed when associated injuries are suspected.
Indicated imaging, laboratory analyses, and adjunctive measures such as urethral catheters and nasogastric tubes should be completed at this time. It is mandatory to perform a rapid primary evaluation and immediately correct any problems found. Once these steps are completed, a more thorough assessment of thermal injury may ensue. The patient’s full history should be taken including: detection of the mechanism of injury, consideration of abuse, height and weight, possibility of CO intoxication, and facial burns. In addition, history of previous diseases should be obtained, if possible (allergies, medications, past medical history, events).
Once the primary and secondary surveys have ensured stabilization of the thermally injured patient, transfer to a facility capable of providing the care necessary to support a burn patient is initiated. The American Burn Association has criteria for referral to a specialized burn center that includes both patient and burn characteristics such as size, depth, and etiology ( Box 87.1 ). Patients who should be referred to a higher level of care for burns include those with partial thickness (second degree) burns greater than 10% TBSA; those with burns of the face, hands, feet, genitals, perineum, or across major joints; and those with full thickness (third degree) burns of any size. Evidence suggests that burn injury patients have improved outcomes if transferred early to a facility capable of providing an advanced level of burn care. Therefore it is important to accurately identify those patients with burns severe enough to merit transfer so that outcomes will be optimized.
Second- and third-degree burns on >10% of TBSA in patients age <10 or >50 years
Second- and third-degree burns on >20% of TBSA in other age groups
Second- and third-degree burns that involve the face, hands, feet, genitalia, perineum, and major joints
Third-degree burns on >5% TBSA in any age group
Electrical burns, including lightning injury
Chemical burns
Inhalation injury
Burn injury in patients with preexisting medical disorders that could complicate management, prolong recovery, or affect mortality
Any patients with burns and concomitant trauma (such as fractures) in which the burn injury poses the greatest risk of morbidity or mortality; in such cases, if the trauma poses the greater immediate risk, the patient may be treated initially in a trauma center until stable before being transferred to a burn center
Hospitals without qualified personnel or equipment for the care of children with burns should transfer the patient to a burn center with these capabilities
Burn injury in patients who will require special social/emotional and/or long-term rehabilitative support, including cases involving suspected child abuse and substance abuse
TBSA, Total body surface area.
Burn centers have been developed to standardize and optimize the overall quality of care delivered to burn-injured patients. Burn centers provide acute care using a multidisciplinary team that includes burn surgeons, anesthesiologists with special interest in burns, critical care physicians, burn-trained nurses, physical and occupational therapists, pharmacists, and dietitians. In addition, improvements in burn survivors’ long-term functional and psychological outcomes and quality of life have resulted from burn units having integrated relationships with physiatrists and rehabilitation facilities as well as burn psychologists and exercise therapists. Since an important part of functional recovery includes returning to work or school, newer additions to the burn team include vocational counselors, recreational therapists, child life specialists, and teachers.
The magnitude of burn injury is classified according to the percentage of total body surface area (%TBSA) involved, depth of the burn, and the presence or absence of inhalational injury. Accurate estimation of burn magnitude is needed to guide the initial resuscitation strategy, make the referral to a burn center, ascertain the need for surgery, and to estimate prognosis. Whereas a detailed evaluation of the extent of the thermal injury is assessed during the secondary survey, an early estimate of burn size and depth is needed during the primary survey to calculate initial resuscitation fluid requirements for circulatory support. Three of the most commonly used methods to estimate %TBSA are the “rule of nines,” palmar surface area, and the Lund-Browder diagram. The rule of nines is used in adults but its use in children is not appropriate as the body surface area relationship varies with age. In children, the correct method for burn size estimation is to use of the Lund-Browder table and diagram, which account for the varying proportions in surface area with age. In adults, the rule of nines divides the body into surface areas of 9% (head, each upper limb, the upper and lower parts of front and back of the trunk, the front and the back of each lower extremity as depicted in the diagram on the left. The surface area of the patient’s palm (excluding the fingers), approximately 0.5% of the TBSA, is used to estimate small (<10% TBSA) burns. However, this method is inaccurate for larger burns. The Lund-Brower diagram is considered the most accurate, if used correctly ( Fig. 87.2 ). It allows for the variation in body proportions with age and is used especially in children. Computerized methods have evolved and demonstrate high correlation and reproducibility.
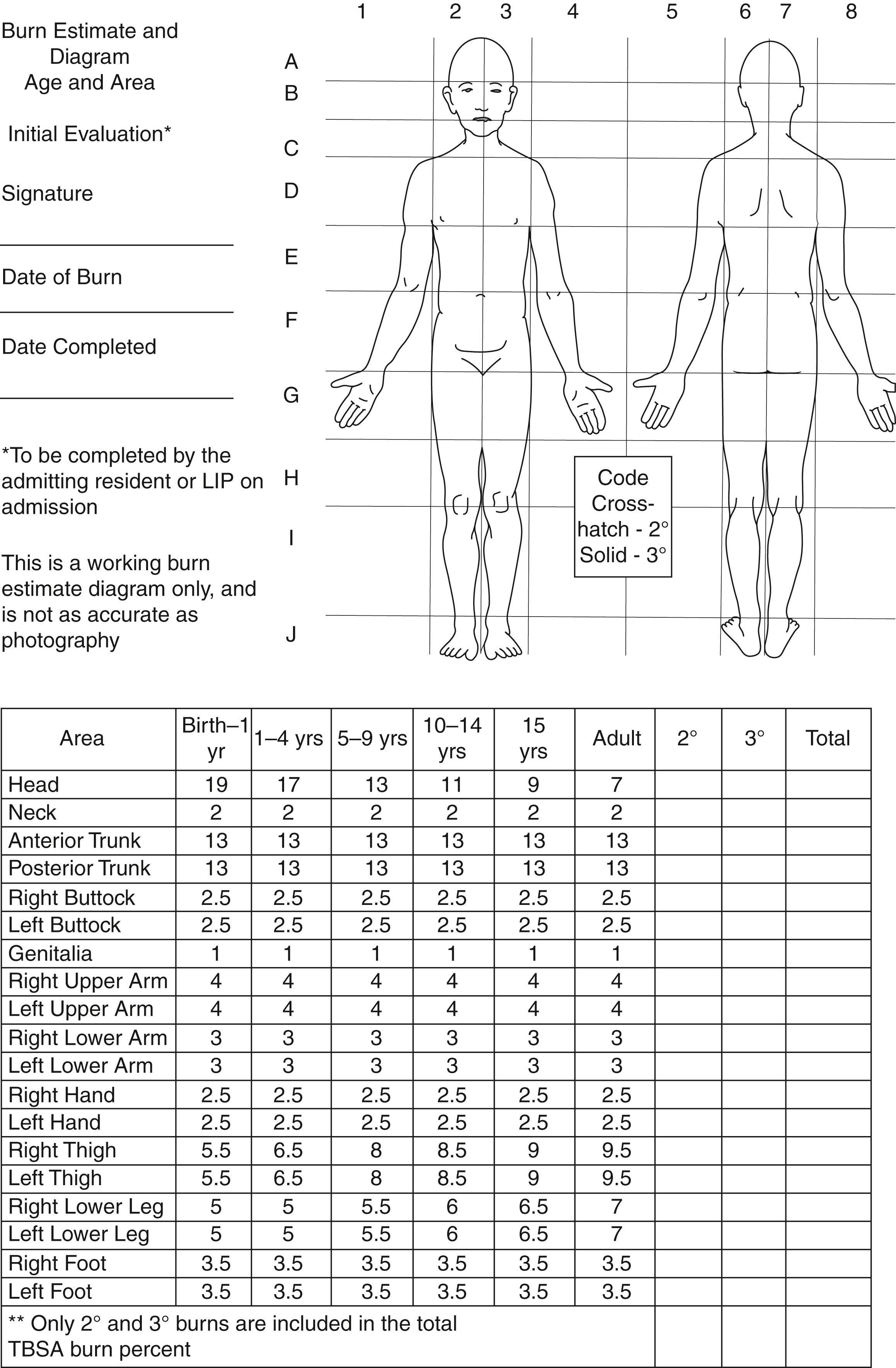
Burn depth is also considered an important determinant of outcome. First-degree burns are limited to the outer layer or epidermis of the skin. The skin usually appears red and dry and is very painful to touch. Healing takes place in 3 to 5 days. Second-degree burns are further categorized into superficial and deep partial thickness burns. A superficial partial thickness burn extends into the superficial papillary dermis and appears red in color with significant weeping and blisters. It will also blanch when pressure is applied, and generally it takes less than 2 weeks to heal. Deep partial thickness burns extend into the reticular dermis and appear yellow or white and dry and often are extremely painful; however, in some cases, the sensation in the deep partial thickness may become diminished. Full thickness or third-degree burns extend through the entire thickness of the dermis. These may appear dry, leathery, black, or white and are usually painless since nerves and endings are destroyed. They do not blanch under pressure. Although initially painless, the subcutaneous inflammation associated with deep dermal burn often becomes more painful than more superficial burns. The designation, fourth-degree burns, is used to describe those that have injured deeper structures, such as muscle, fascia, and bone. Deep second-, third-, and fourth-degree burns require surgical debridement and grafting, whereas more superficial burns do not. Since the area of injury may progress over the first 2 to 3 days after the initial insult due to the effects of coagulation and ischemia, burn depth estimation may be greater when examined later compared to the initial evaluation. Close reevaluation may be required to determine the actual burn size and depth.
Current fluid therapy is based on knowledge gained over the last century. Major breakthroughs in fluid management were made by Underhill, who described the pathophysiology of burn injury in detail in the 1920s. In 1940, after the Coconut Grove night club disaster in Boston, Massachusetts, the first attempts were made to use intravenous fluids to treat a large group of burn injury patients, and the result was that the mortality was significantly lower than expected. In 1953, the first fluid formula based on the size of the burn and the patient’s weight was introduced by Evans. The formula most widely used today is the one that was published in 1974 by Charles Baxter, who was then working at the Parkland Memorial Hospital in Dallas, Texas. The Parkland formula calls for 4 mL/kg/%TBSA of Ringer lactate solution given over the first 24 hours, half of which is given within the first 8 hours from the time of injury. The main advantages of the Parkland formula are use of an easily obtainable fluid (Ringer lactate), low cost, and a strategy that is easy to start and follow. A number of other formulations have been reported over the years, but none has the global impact of the Parkland formula. Some of the more common options are listed in Table 87.1 .
| Formulation | Contains |
|---|---|
| Parkland | Ringer lactate solution 2-4 mL/kg/TBSA% Half the fluid volume given during the first 8 h and the remainder during the next 16 h |
| Modified Brooke | Ringer lactate solution 2-4 mL/kg/TBSA% |
| Brooke | Ringer lactate solution 1.5 mL/kg/TBSA% + colloid 0.5 mL/kg + 5% glucose solution 2000 mL/24 h. The PiCCO instrument measures colloid can be albumin or fresh frozen plasma |
| Hypertonic Solutions | |
| Monafo | Ringer lactate containing sodium 250 mmol/L. Volume sufficient to produce urine 30 mL/h. Not commonly used in view of the hyperosmolar effects |
Today, few centers in Europe or the United States use formulations other than the Parkland initially. Appropriate resuscitation should be initiated promptly and tailored based on patient parameters to avoid over- and under-resuscitation. Delayed or inadequate fluid replacement results in hypovolemia, tissue hypoperfusion, hypovolemic shock, and multiple organ failure. Morbidities associated with over-resuscitation include pulmonary edema, compartment syndromes (muscle compartments, abdomen, and the orbits), and even cerebral edema. As a general rule, burns of less than 15% TBSA can be managed with oral or intravenous fluid administered at 1.5 times maintenance rate ( Box 87.2 ) and careful attention to hydration status. Maintenance fluids, including a source of glucose, should be added to pediatric patient resuscitation fluid as hepatic glycogen stores will be depleted after 12 to 14 hours of fasting.
100 mL/kg up to a body weight 10 kg
Above 10 kg weight, 50 mL/kg are added in the weight range of 11-20 kg
Above 20 kg weight, add 20 mL/kg for every kg
Example: Maintenance fluid for a child of 28 kg:
1000 mL + 500 mL + 160 mL, i.e., total 1660 mL/24 h
Later, when insulin resistance and associated hyperglycemia develops, glucose infusions should be modulated. Colloids have the potential to increase oncotic pressure and thereby reduce fluid shifts and losses. Controversy remains as to the ideal time for initiation of colloid therapy in burn resuscitation. There is a general trend now to initiate colloids earlier than the previously recommended time of 24 hours. All the formulae guide resuscitation with the goal of titrating fluids to obtain a urine output of 0.5 mL/kg/h in adults and 1.0 mL/kg/h in children. The reasons for using hourly urine output are that it is easily measured (once a Foley catheter has been placed), it reflects glomerular filtration rate and renal blood flow, and it is a surrogate for end-organ perfusion and an indirect correlate of cardiac output.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here