Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The heart contains four one-way valves that regulate the directional flow of blood through its chambers. Effective cardiac pumping activity is dependent on the proper functioning of these valves. The atrioventricular (mitral and tricuspid) valves close during systole to allow for stepwise pressure gradients to be maintained between the atria and ventricles, while the semilunar (aortic and pulmonic) valves likewise close during diastole to maintain pressure gradients between the ventricles and great arteries.
The heart on average beats 100,000 times per day and more than 2.5 billion times over an average lifespan. Given the great number of open-close cycles the heart valves are subjected to and the relative infrequency of cardiac valvular heart disease - with a reported prevalence of less than 2% of the population, it must be concluded that the valvular structures are exceedingly well adapted to meet these physical demands.
The cardiac valves may, nevertheless, succumb to injury or degeneration due to a variety of pathophysiologic processes, and valvular dysfunction can result in significant morbidity and mortality. The advent in the past century of open surgical valve repair and replacement procedures have delivered extended life and improved health to millions of individuals with cardiac valve disease. Today, for example, approximately 90,000 patients in the United States and 280,000 worldwide undergo valve replacement each year. More recently, percutaneous interventions to repair or replace damaged valves are increasingly utilized to deliver these benefits without requiring open-heart surgery or cardiopulmonary bypass.
Notwithstanding investigations in the nineteenth century into the treatment of rheumatic heart disease, the modern history of heart valve surgery can be traced back to Sir Thomas Lauder Brunton, a Scottish physician, who in 1902 proposed a technique for closed repair of stenotic rheumatic mitral valve disease, with access to the valve gained by passing a dilator through the left ventricular (LV) wall. Unfortunately, this idea was shunned by Brunton’s colleagues as reckless and was never attempted clinically. Fortunately, however, Elliot Cutler and Peter Levine further developed Brunton’s early theory, and successfully performed the first surgical correction of the mitral valve in 1923 after their extensive experimentation in the research laboratories of the Peter Bent Brigham Hospital in Boston.
Henry Souttar of England adapted the Cutler and Levine technique and in 1925 reported the first successful case of closed digital commissurotomy, inserting the surgeon’s index finger through the left atrial (LA) appendage to accomplish mechanical mitral valve (MV) dilatation. This procedure was not widely adopted until 1948, following reports by Charles Bailey of Philadelphia and Dwight Harken of Boston of clinically successful closed digital mitral commissurotomies ( Fig. 61.1 ).
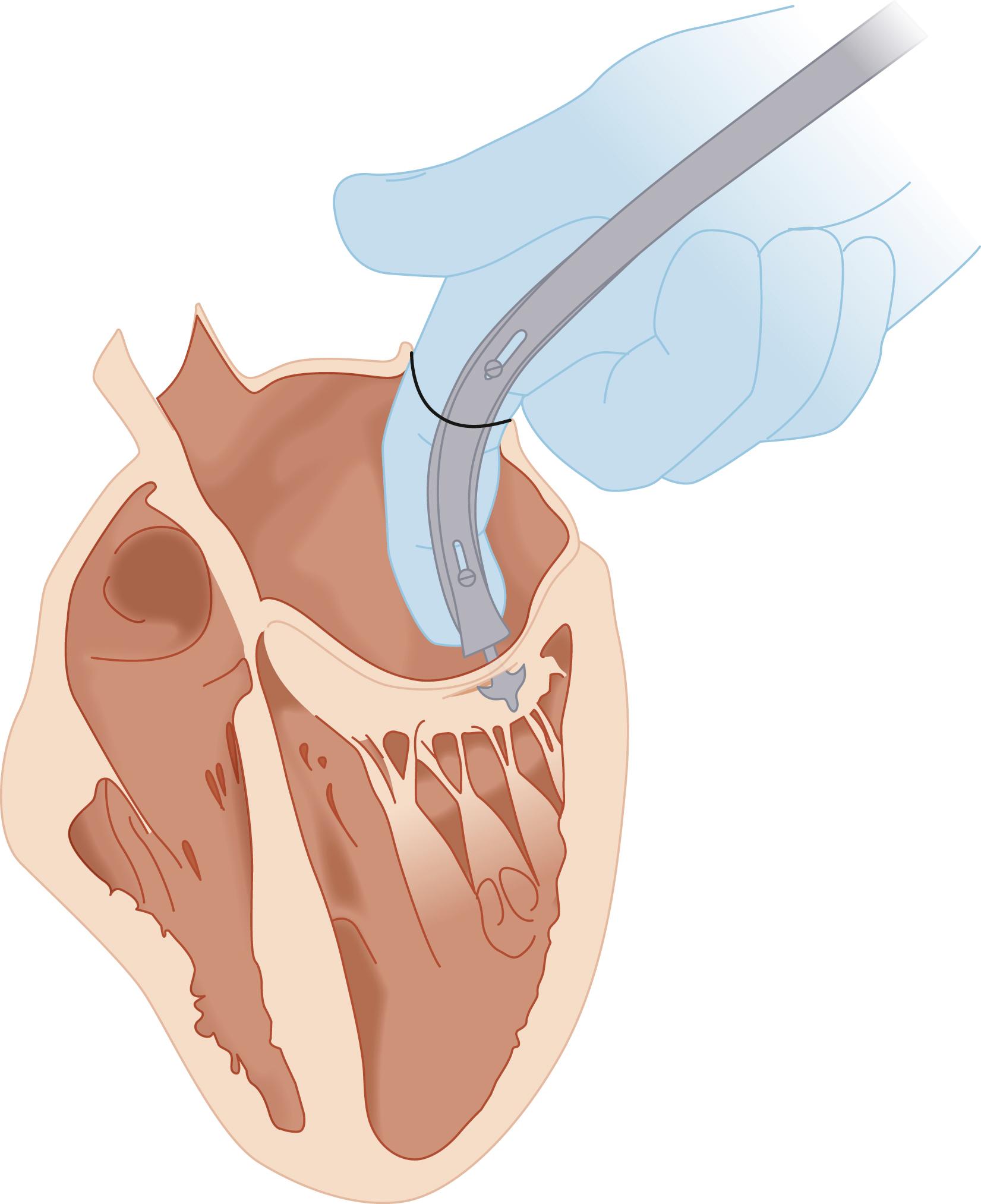
Blinded aortic valvular surgery followed a similar course. In 1912, Theodore Tuffier of Paris reported the first clinical attempt to dilate a stenotic aortic valve, pushing his finger against the aorta and invaginating the aortic wall through the valve to approach the valve. Mechanical dilation using an instrument passed retrograde through the innominate artery was reported by Russell Brock of London in 1940. Neither of these efforts gained acceptance, but paved the way for Horace Smithy of Charleston, South Carolina, who in 1948 performed the first successful aortic valvotomy. Three years later, Charles Bailey of Philadelphia reported the first successful aortic valvotomy using a transventricular expanding dilator.
Two major landmarks occurring in the twentieth century marked the beginning of the modern era of “open” heart valve surgery: the development of a prosthetic valve, and the advent of cardiopulmonary bypass. The first successful implantation of a prosthetic valve was performed without bypass and was reported by Charles Hufnagel of Georgetown University in 1952. Given the inability to access the aortic valve in situ, Hufnagel implanted a caged-ball valve into the descending aorta in patients with aortic insufficiency. Following the first successful clinical use of cardiopulmonary bypass by Gibbon in 1953, Harken in 1960 completed the first successful in situ aortic valve replacement using a caged-ball device inserted in place of an excised aortic valve. That same year, Albert Starr and Lowell Edwards in Oregon replaced the mitral valve using a similar caged-ball prosthesis of their own design. What followed was an explosion of improvements in prosthetic valve design and surgical implantation techniques allowing for progressive improvement in outcome after valve replacement, and more recently, valve repair.
The four human heart valves follow similar early embryologic development, beginning at four weeks of gestation with formation of the valve primordia in the primitive heart tube. This development is closely linked to the division of the heart tube into its chambers, including septation of the outflow tract (truncus arteriosus) and fusion of the atrioventricular canal cushions. Most of the cells, which migrate to form the valve primordia originate from the endocardial cushion, although epicardial and neural crest cells also appear to contribute. The valve primordia grow and elongate between 20 and 39 weeks of gestation, thinning to form leaflets and cusps. In late gestation and early after birth, these structures become stratified into highly organized layers to differentiate into formal valve leaflets. Valve maturation and remodeling continues into the juvenile stages of life.
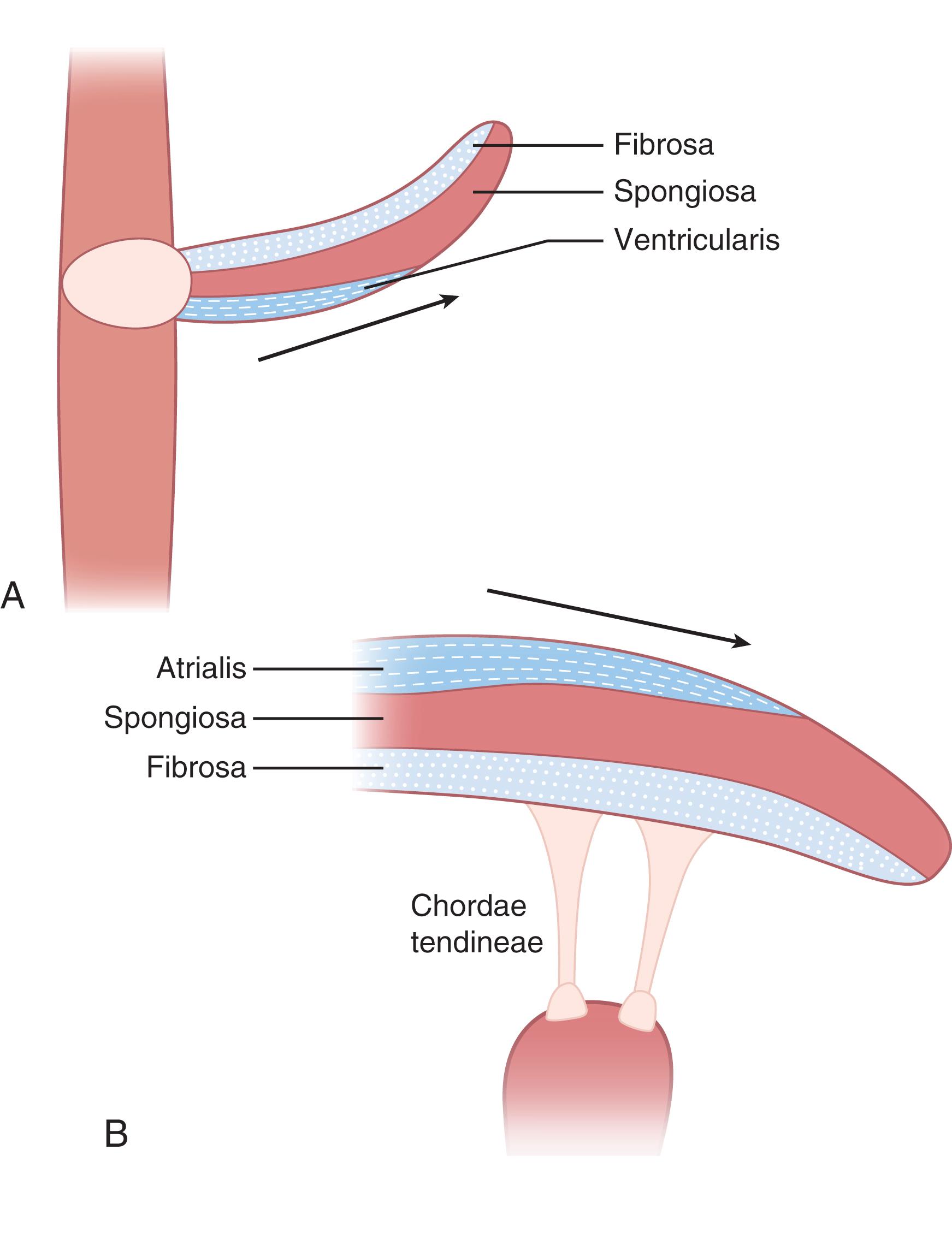
All four cardiac valves are supported by internal plates of dense collagen-, proteoglycan- and elastin-rich connective tissue that are continuous with the fibrous skeleton at the base of the heart ( Fig. 61.2 ). The extracellular matrix of each valve is organized into three layers: the fibrosa, made of fibrillar collagen; the spongiosa, made of proteoglycans; and an elastin layer termed the ventricularis on the semilunar valves or the atrialis on the atrioventricular valves, in reference to the heart chamber, the layer faces ( Fig. 61.3 ). Covered in a thin layer of epithelium, the atrialis or ventricularis form the surface over which blood flows through the valve, with the underlying spongiosa and fibrosa contributing structural support.
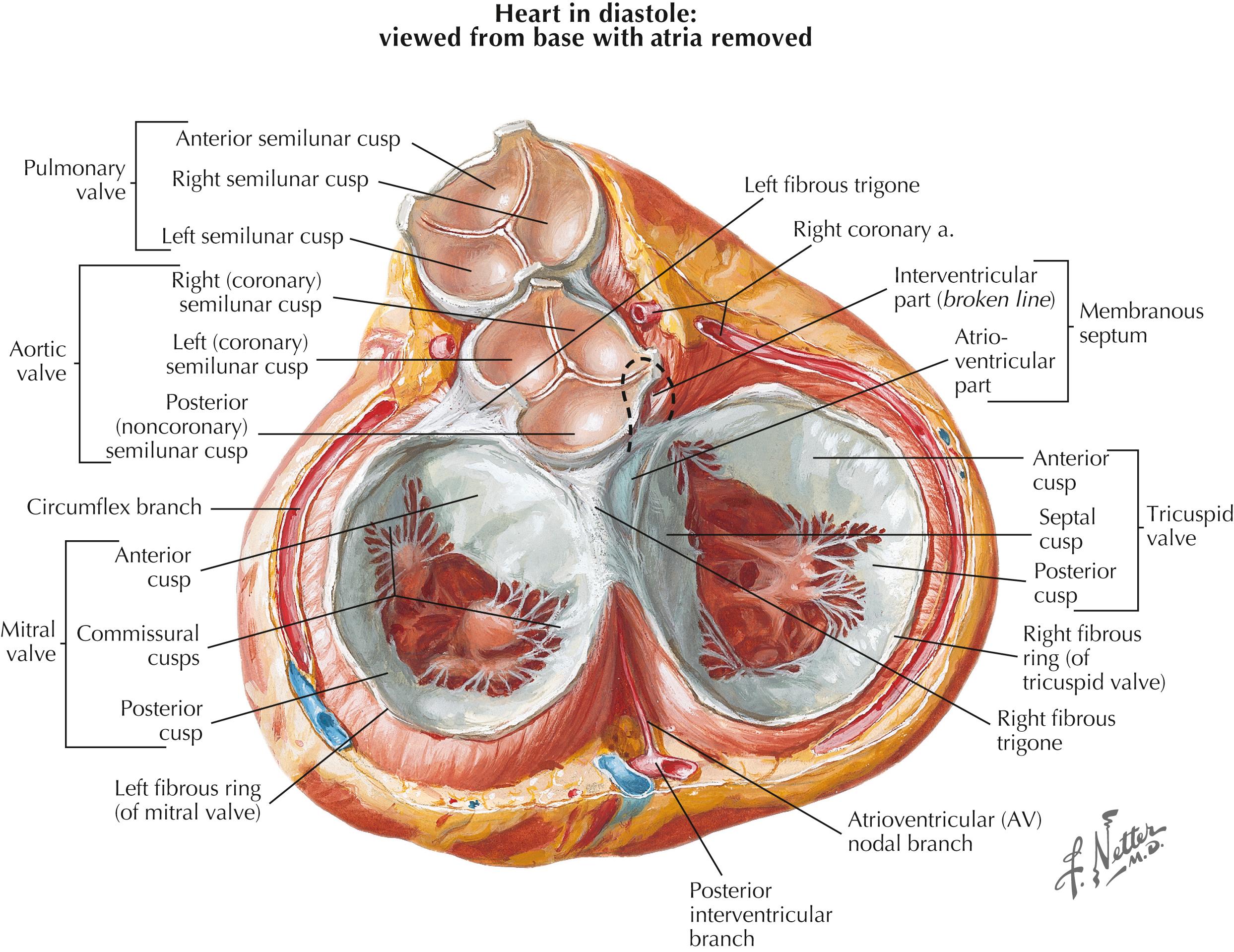
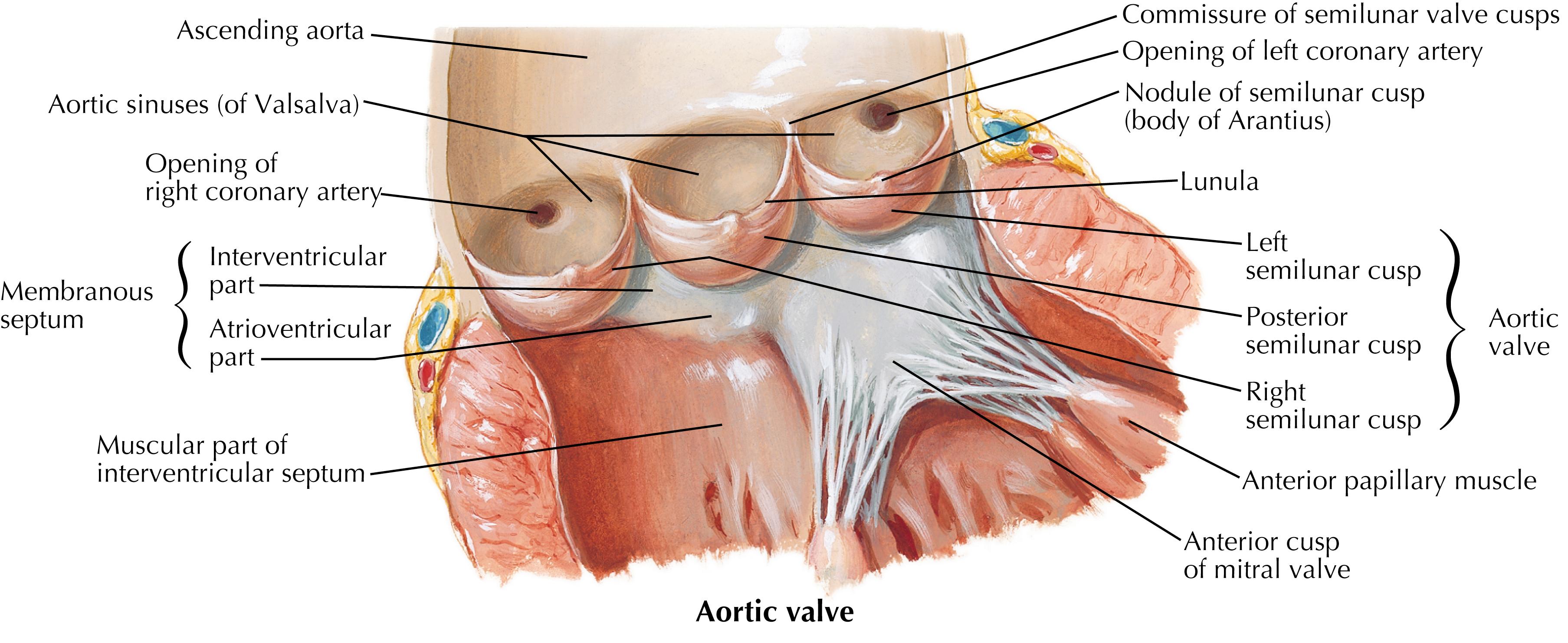
The aortic and pulmonic semilunar valves are freestanding structures seated atop the outflow tracts of their respective ventricles. They do not have discrete annuli but are instead attached in a curvilinear fashion to the wall of the aorta or pulmonary artery at their junction with the left or right ventricular (RV) outflow tracts, respectively. These valves have three cusps with a semilunar shape from which they derive their name ( Fig. 61.4 ). Each cusp is in turn made up of four components: the hinge region where the cusp connects to the annulus; the belly, which make up the majority of the cusp; the coapting surface at the cusp periphery; and the lannulae, which are thin, crescent shaped segments of the cusp surrounding a central fibrous nodule at the midpoint of its free edge (termed the nodes of Aranti in the aortic valve).
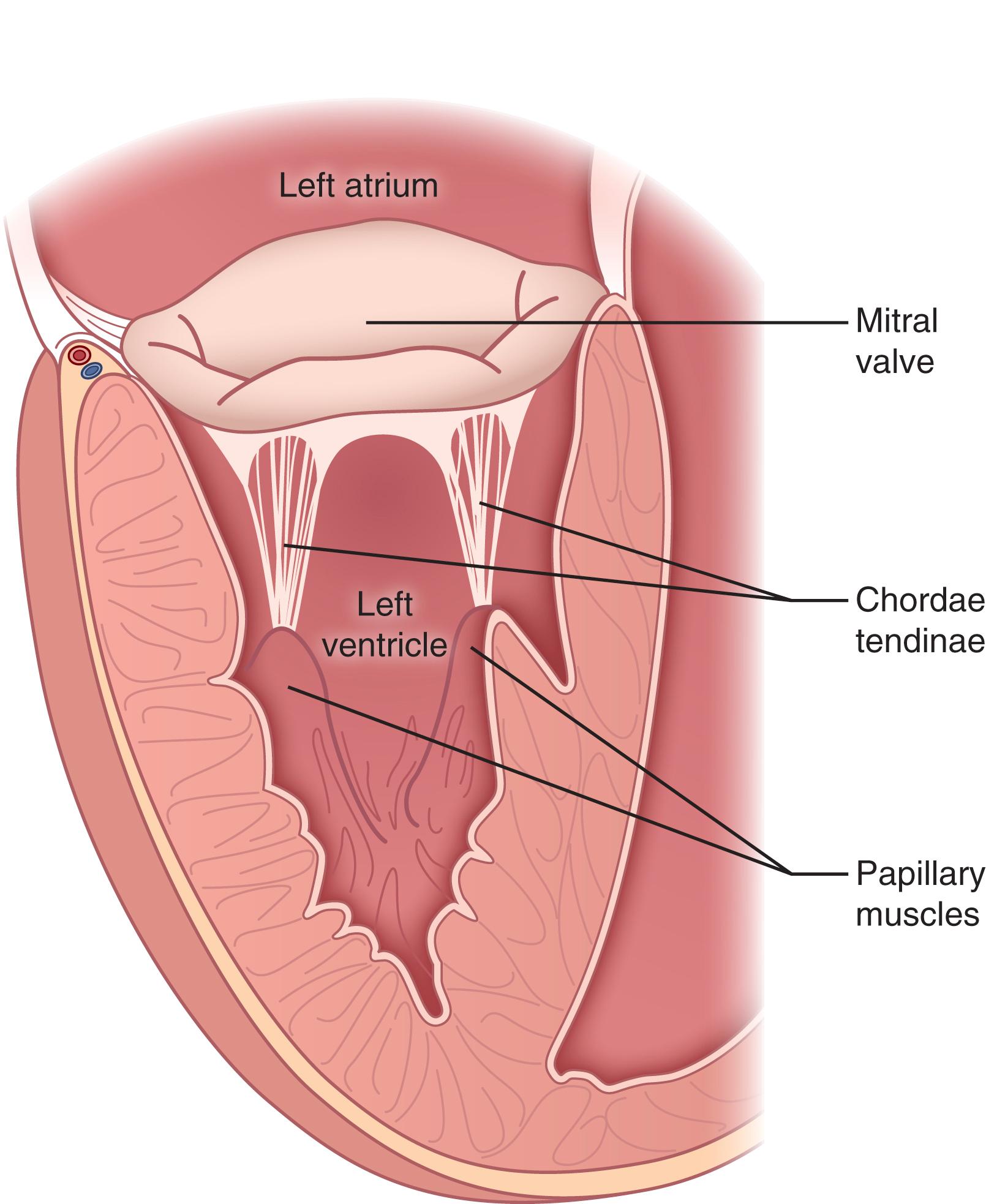
In contrast to the freestanding semilunar valves, the mitral and tricuspid valves exist within a functional valve complex composed of discrete, fibrous annular rings (the annuli fibrosis), the valve leaflets, fibrous chordae tendinae, and papillary muscles. The fibrous chordae tendinae arise from the leaflet free edges (marginal or primary chordae) or undersurface (intermediate or secondary chordae), with basal (tertiary) chordae also arising from the posterior leaflet base and annulus. These chordae attach to intraventricular papillary muscles, which in turn arise from the ventricular myocardium. These additional support structures allow the atrioventricular (AV) valves to maintain the high transvalvular pressures to which they are exposed during systole.
The mitral valve proper has two leaflets possessing approximately equal surface area ( Fig. 61.5 ). The square-shaped anterior leaflet originates from approximately the anterior one third of the valve annulus. The posterior mitral leaflet is less wide (“tall”) but is longer than the anterior leaflet, attaching to approximately two thirds of the annulus. The anterior and posterior leaflets each have three scallops (e.g., A1, P1, etc.), based upon indentations found in the posterior leaflet ( Fig. 61.5 ). In comparison, the tricuspid valve is comprised of anterior, posterior, and septal leaflets, of which the anterior leaflet is the largest and the septal leaflet the smallest.
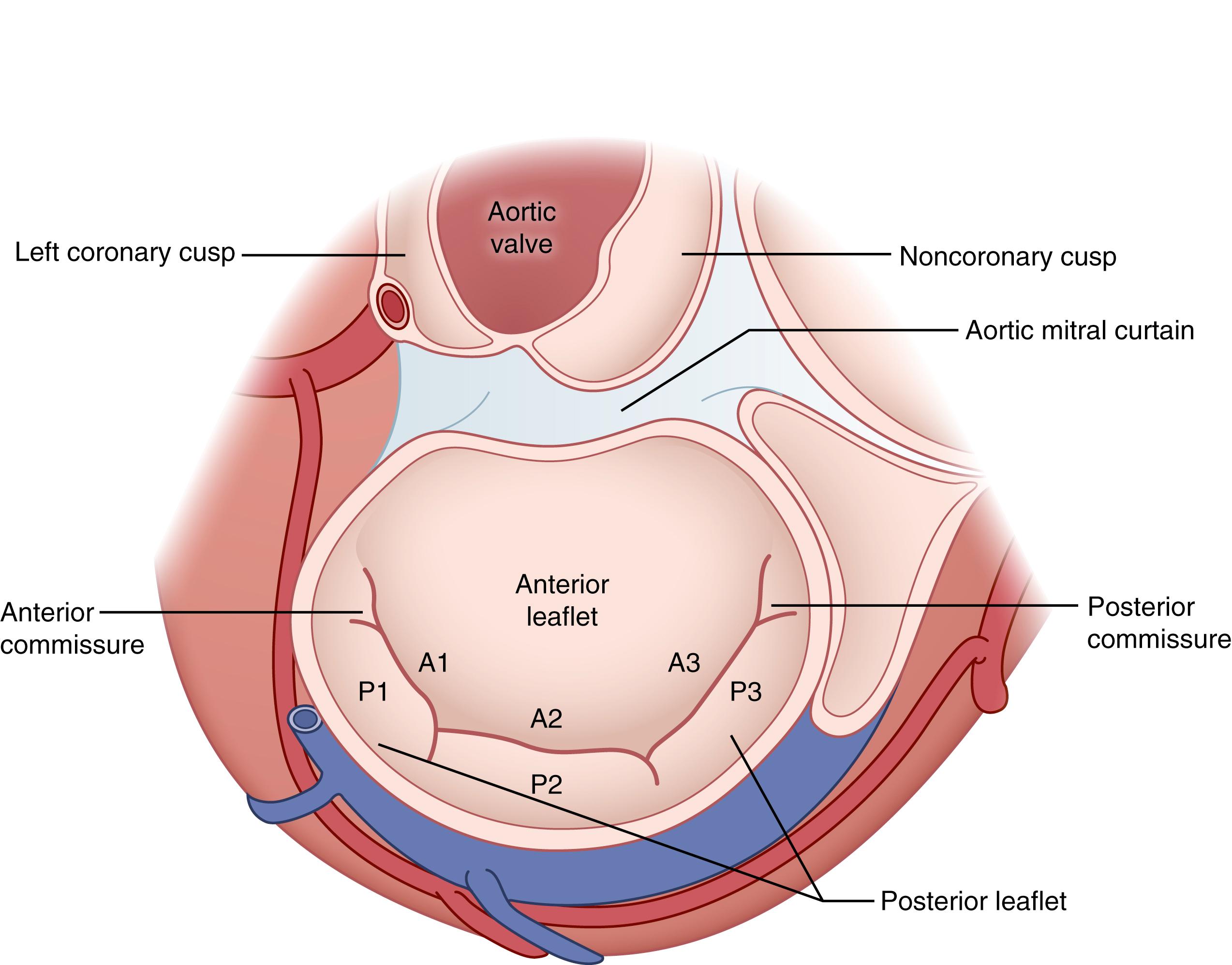
The mitral valve is supported by an anterior and posterior papillary muscle that each sends chordae to the anterior and posterior leaflets. In comparison, the tricuspid valve is supported by a large anterior papillary muscle that sends chordae to the anterior and posterior leaflets, and a variable medial or posterior papillary muscle that provides chordae to the posterior and septal leaflets. The RV septal wall also provides chordae to the anterior and septal tricuspid leaflets, but there is no formal septal papillary muscle.
As suggested by their anatomy, the semilunar and AV valves differ in the mechanisms employed to maintain coaptation. The semilunar valve cusps depend largely upon mechanisms intrinsic to the cusps themselves. During diastole, the cusps falling passively to the center and seal the orifice by coapting with the corresponding midpoint nodules on adjoining cusps. The AV valves, on the other hand, are tethered in their closed position by their chordal attachments to the papillary muscles, which contract during systole to maintain leaflet coaptation and prevent leaflet prolapse into the atria ( Fig. 61.6 ).
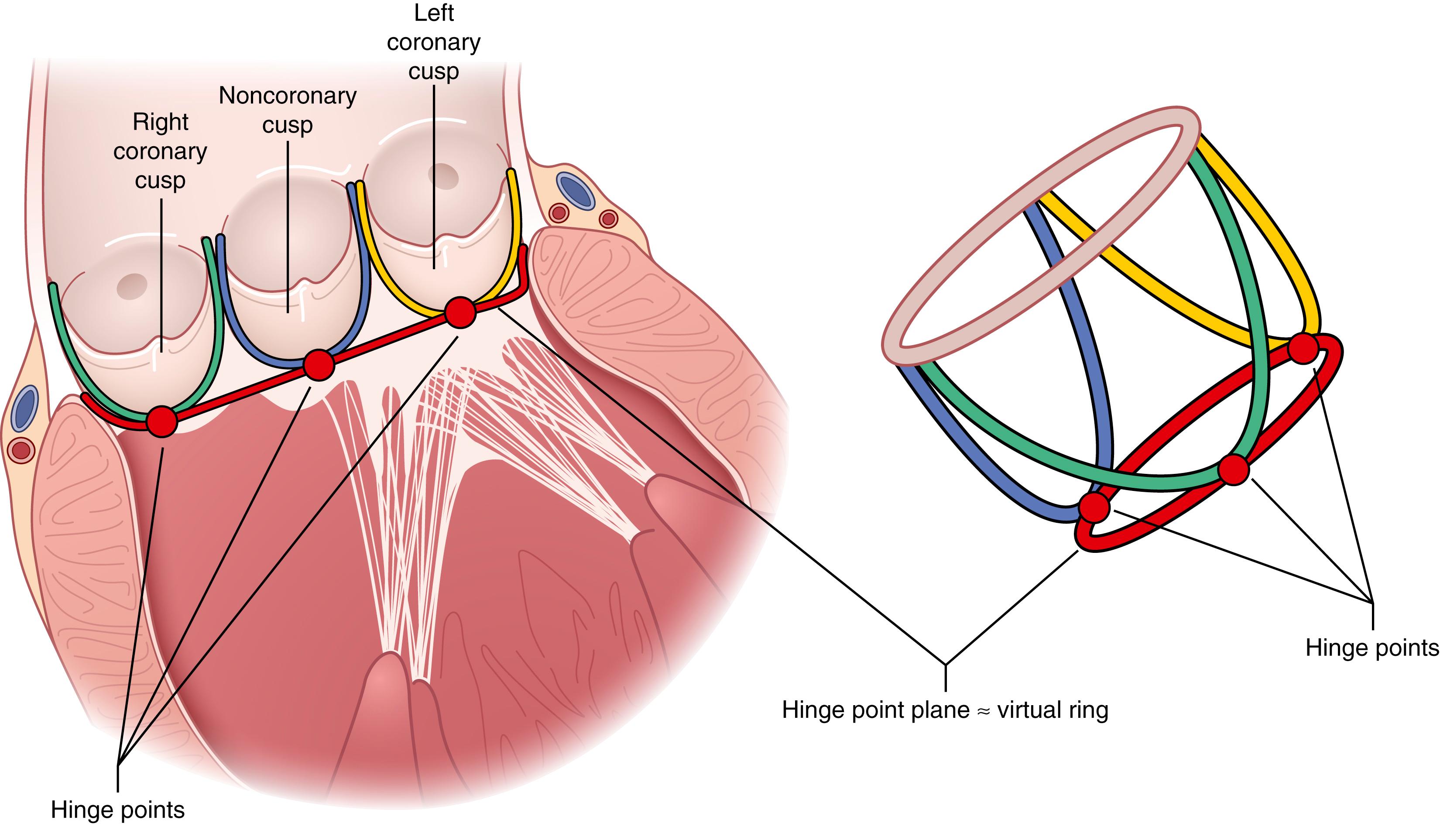
While the pulmonary valve is relatively easily accessed and isolated at the anterior of the heart, the aortic and atrioventricular valves are closely invested with each other at the base of the heart, with both the electrical conduction and coronary arterial systems in close proximity and at risk during cardiac valve procedures ( Fig. 61.7 ). The central location of the aortic valve at the base of the heart in particular imparts complex anatomic relationships to the other cardiac chambers and valves. The aortic valve cusps, for example, are named for their intimate relationship with the coronary arteries (i.e., the right coronary, left coronary, and noncoronary [posterior] cusps), which arise from the coronary ostia in the sinuses of Valsalva, gentle dilatations of the aorta just distal to the valve which direct flow into the ostia ( Fig. 61.7 ).
In direct continuity with the left and noncoronary cusps of the aortic valve, from about the 5 to the 8 o’clock position in the traditional surgical perspective, is the anterior leaflet of the mitral valve ( Fig. 61.8 ). The noncoronary cusp of the aortic valve and the anterior leaflet of the mitral valve are therefore at risk of injury during mitral and aortic valve surgery, respectively. Likewise, the atrioventricular node lies embedded in the top of the ventricular membranous septum, just beneath the commissure between the noncoronary and right coronary aortic leaflets, from the 3 to the 5 o’clock position in the surgical perspective. Only the remaining circumference of the aortic annulus is relatively free anatomically from surgical injury during aortic valve surgery.
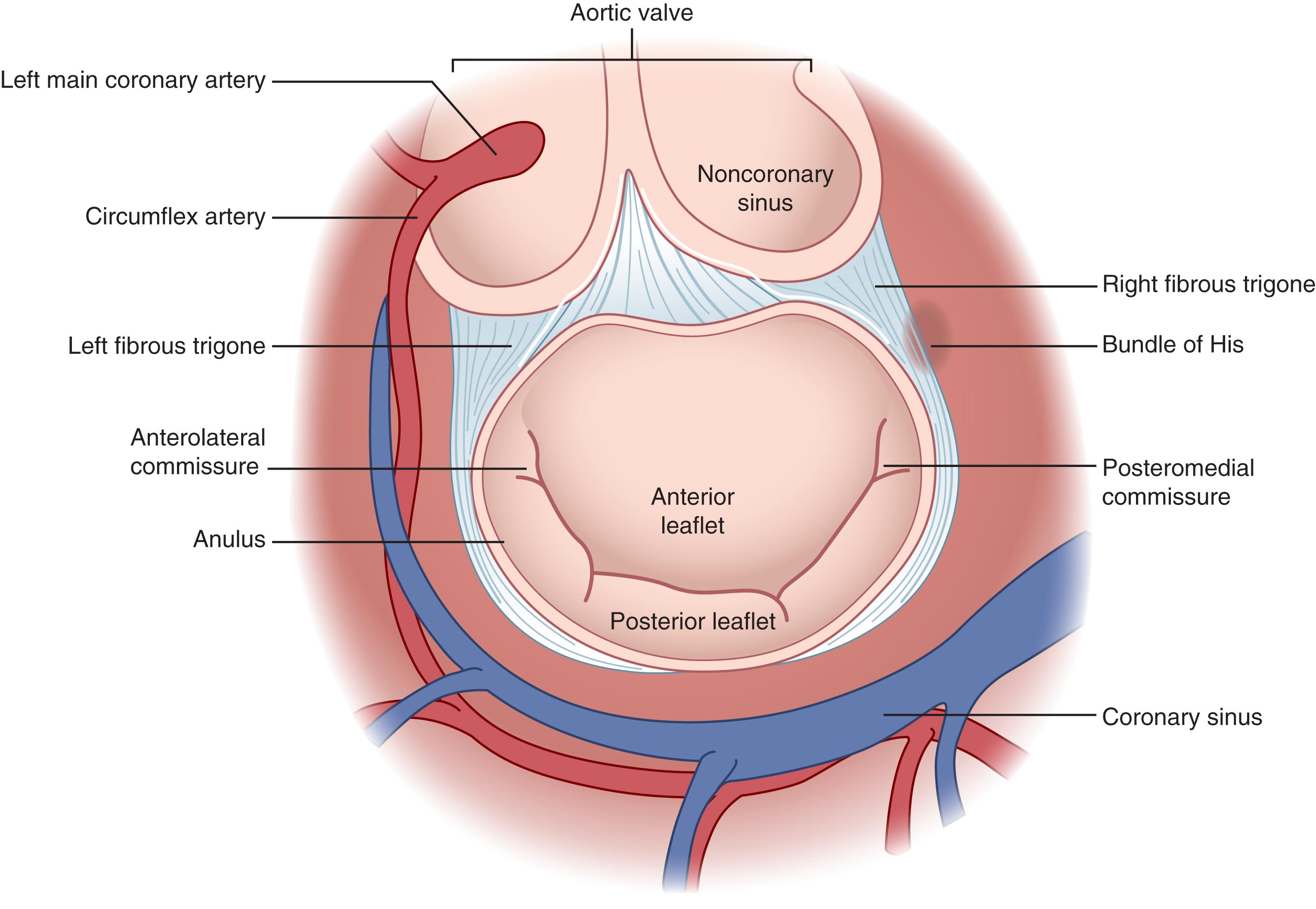
The mitral valve bears two important surgical anatomic relationships. The posterior (mural or lateral) leaflet is in continuity with the posterior LV wall. Deep (posterior) to the posterior leaflet lies the AV groove, within which lies the circumflex coronary artery and the coronary sinus, which are thereby at risk of surgical injury. The atrioventricular node and bundle of His likewise lie deep to the posteromedial commissure of the mitral valve, with errant sutures having potential to cause complete atrioventricular conduction block ( Fig. 61.9 ).
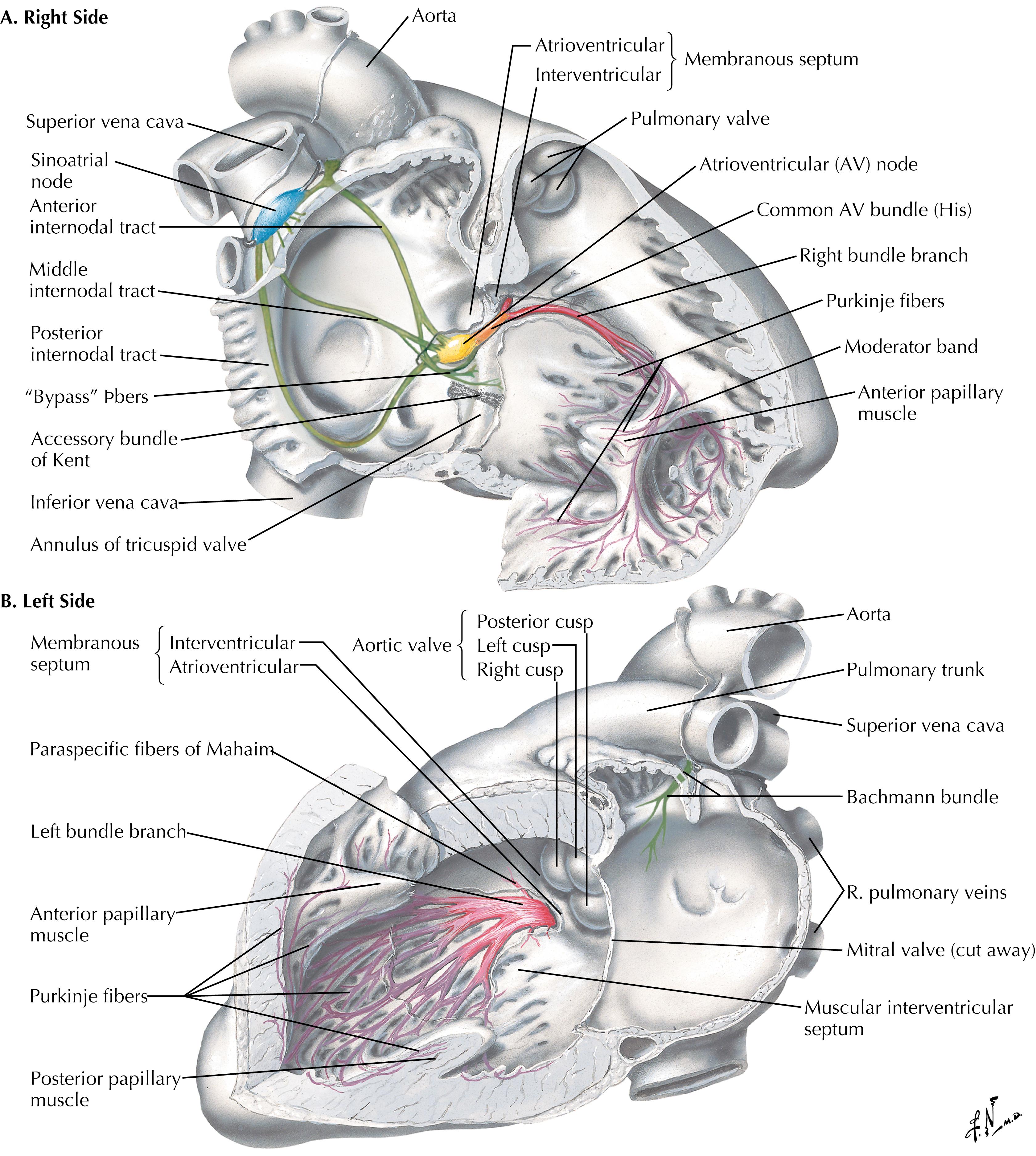
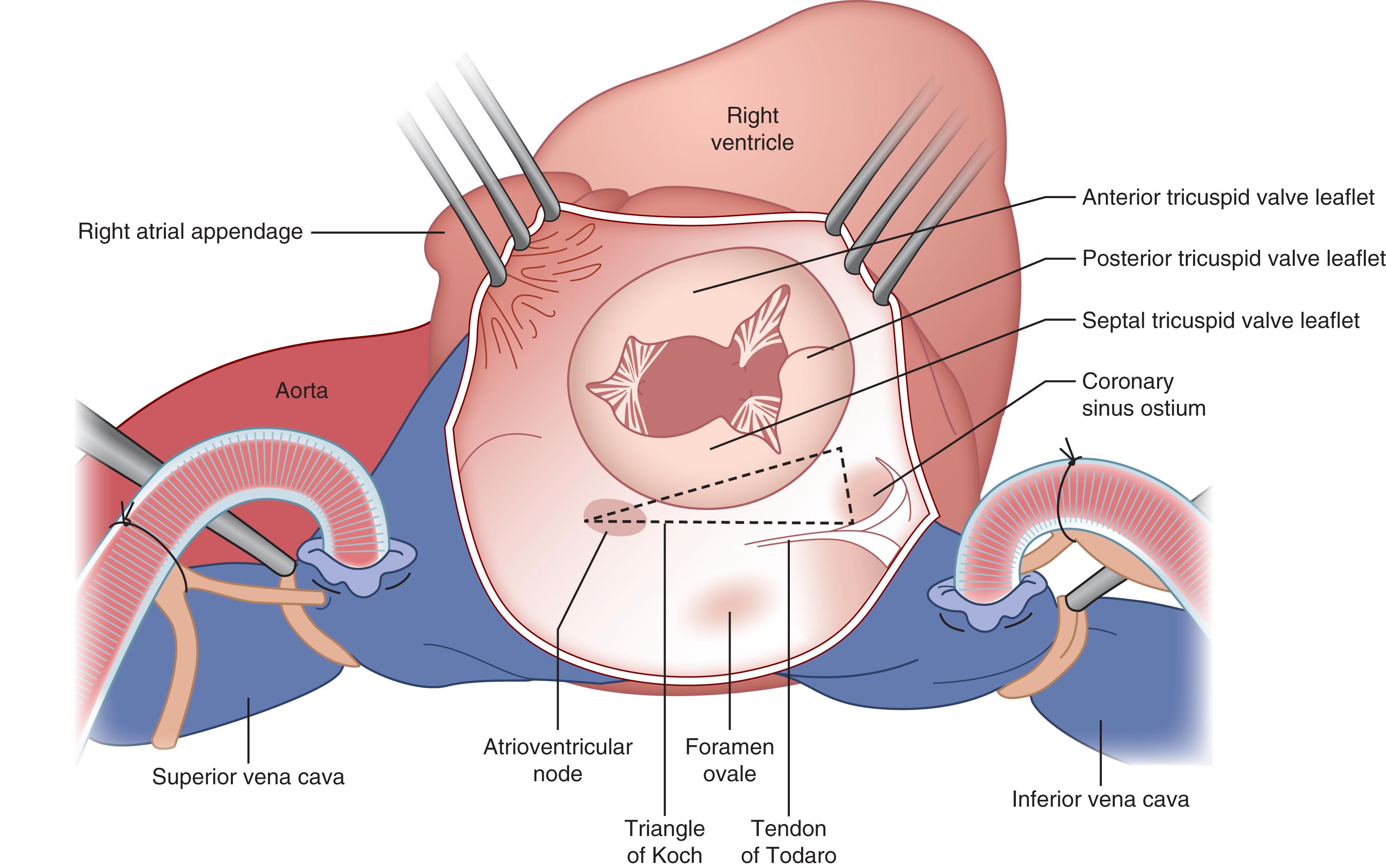
Tricuspid valve surgery also entails risk to the atrioventricular node, as the tricuspid annulus is positioned on the opposite side of the membranous septum from the mitral valve. The atrioventricular node lies in the apex of “Koch triangle,” which is bounded by the septal leaflet of the tricuspid valve anteriorly, the tendon of Todaro posteriorly, and the central fibrous body containing the bundle of His superiorly, leading to the coronary sinus inferiorly ( Fig. 61.10 ). Additionally, the coronary sinus ostium lies adjacent to the commissure of the septal and posterior leaflets of the valve and can be inadvertently oversewn if it is not carefully identified.
The cardiac valves may become impaired through two fundamental forms of dysfunction, stenosis and insufficiency. Valvular stenosis is an obstruction to forward flow due to incomplete opening of the valve. Valvular insufficiency, also referred to as regurgitation or incompetence, describes backward flow through a valve when its cusps or leaflets are unable to obtain or maintain coaptation. Both stenosis and insufficiency may exist simultaneously in any one valve.
Valvular stenosis is almost always caused by a primary abnormality of the cusp or leaflet from a chronic disease process. Regurgitation may be caused by an acute or chronic disease process affecting the valve itself or may be secondary to a structural abnormality of associated supporting structures, such as the great arteries, annuli fibrosi, chordae tendinae, papillary muscles, or ventricular myocardium ( Table 61.1 ).
| Leaflets | Annulus | Chordae Tendinae | Ventricular Wall/ Papillary Muscle (PM) | Aortic Root | |
|---|---|---|---|---|---|
| Mitral Stenosis | |||||
| Rheumatic valve disease | ++ | ++ | ++ | +/- (PM fusion/ shortening) | NA |
| Endocarditis (vegetation) | +/- | - | - | - | NA |
| Congenital ∗ | ++ | - | - | +/- | NA |
| Supravalvular (thrombus, myxoma) | ++ | - | - | - | NA |
| Mitral Regurgitation | |||||
| Mitral valve prolapse (myxomatous/connective tissue disorder) | ++ | ++ | ++ | - | NA |
| Rheumatic fever | ++ | - | +/- | - | NA |
| Endocarditis | ++ | +/- | ++ | + | NA |
| Congenital anomaly ∗∗ | ++ | - | +/- | - | NA |
| Systemic lupus erythematosus ∗∗∗ | ++ | +/- | +/- | +/- (PM) | NA |
| Mitral annular calcification (MAC) | =/- | ++ | - | - | NA |
| Myocardial ischemia/infarction | - | +/- | - | ++ | NA |
| Hypertrophic cardiomyopathy | ++ | NA | |||
| Aortic Stenosis | |||||
| Degenerative disease (trileaflet) | ++ | + | NA | - | - |
| Bicuspid valve disease | ++ | + | NA | - | - |
| Rheumatic valve disease | ++ | + | NA | - | - |
| Endocarditis (vegetation) | ++ | + | NA | - | - |
| Other congenital anomaly ^ | ++ | + | NA | ++ | + |
| Hypertrophic cardiomyopathy | - | - | - | ++ | - |
| Aortic Insufficiency | |||||
| Degenerative/connective tissue disease ^^ | ++ | ++ | NA | - | ++ |
| Rheumatic disease | ++ | - | NA | - | - |
| Inflammatory disease ^^^ | + | - | NA | - | ++ |
| Endocarditis | ++ | + | NA | - | + |
| Congenital (bicuspid, unicuspid) | ++ | - | NA | - | + |
| Aortic dissection/ aortic aneurysm # | - | ++ | NA | - | ++ |
∗ Parachute mitral valve, supramitral ring.
∗∗ Cleft leaflet, endocardial cushion defect, parachute mitral valve.
^ Unicuspid/unicommisural valve, hypoplastic annulus/root, subaortic membrane/stenosis.
^^ Marfan syndrome, myxomatous degeneration, osteogenesis imperfecta, Ehlers-Danlos syndrome.
^^^ Ankylosing spondylitis, Reiter syndrome, Takayasu disease, giant cell aortitis.
Although degenerative disease is a more common cause of valve disease in the developed world, rheumatic fever and ensuing rheumatic heart disease (RHD) likely remains the most common (albeit decreasing) cause of valvular dysfunction worldwide.
Mitral stenosis (MS) is by far most commonly caused by RHD and RHD most commonly involves the mitral valve. It is highly uncommon to have valvular RHD without mitral involvement. For reasons that remain incompletely understood, the prevalence of RHD is approximately twice as great in women as men. Other less common causes of MS include endocarditis, mitral annular calcification, and congenital anomalies such as parachute mitral valve and as a component of Shone complex, which is associated with supramitral rings.
Acute rheumatic fever develops because of a cross-reactive host immune response exhibited by genetically susceptible individuals in response to group A beta-hemolytic streptococcal infection acquired during the first decade of life, driven by molecular mimicry between streptococcal proteins and host cardiac proteins such as laminin. In some patients, this initial response causes clinically evident inflammation in the cardiac valves and/or conduction system several weeks after the streptococcal infection.
Rarely, acute rheumatic valvulitis can result in severe acute mitral regurgitation (MR) and may be associated with other manifestations of carditis such as atrioventricular conduction abnormalities or pericarditis. More typically, chronic RHD silently develops as ongoing valvular damage occurs due to repeated exposures to streptococcal infection and ongoing hemodynamic damage to a deformed valve in adolescence and adulthood.
Chronic RHD most commonly presents as either mitral valve stenosis or mixed valve lesions (stenotic and regurgitant). Mixed stenotic/regurgitant pathology takes the form of a pathognomonic “fish mouth” funnel valve lesion with associated fusion and shortening of the leaflets and chordae tendinae ( Fig. 61.11 ). Sclerosis induced from chronic inflammation reducing leaflet mobility and prevents both complete opening of the valve during diastole and adequate coaptation during systole. Clinically apparent heart failure typically develops from these RHD lesions in the third or fourth decade of life due to progressive valve dysfunction and/or exhaustion of compensatory mechanisms.
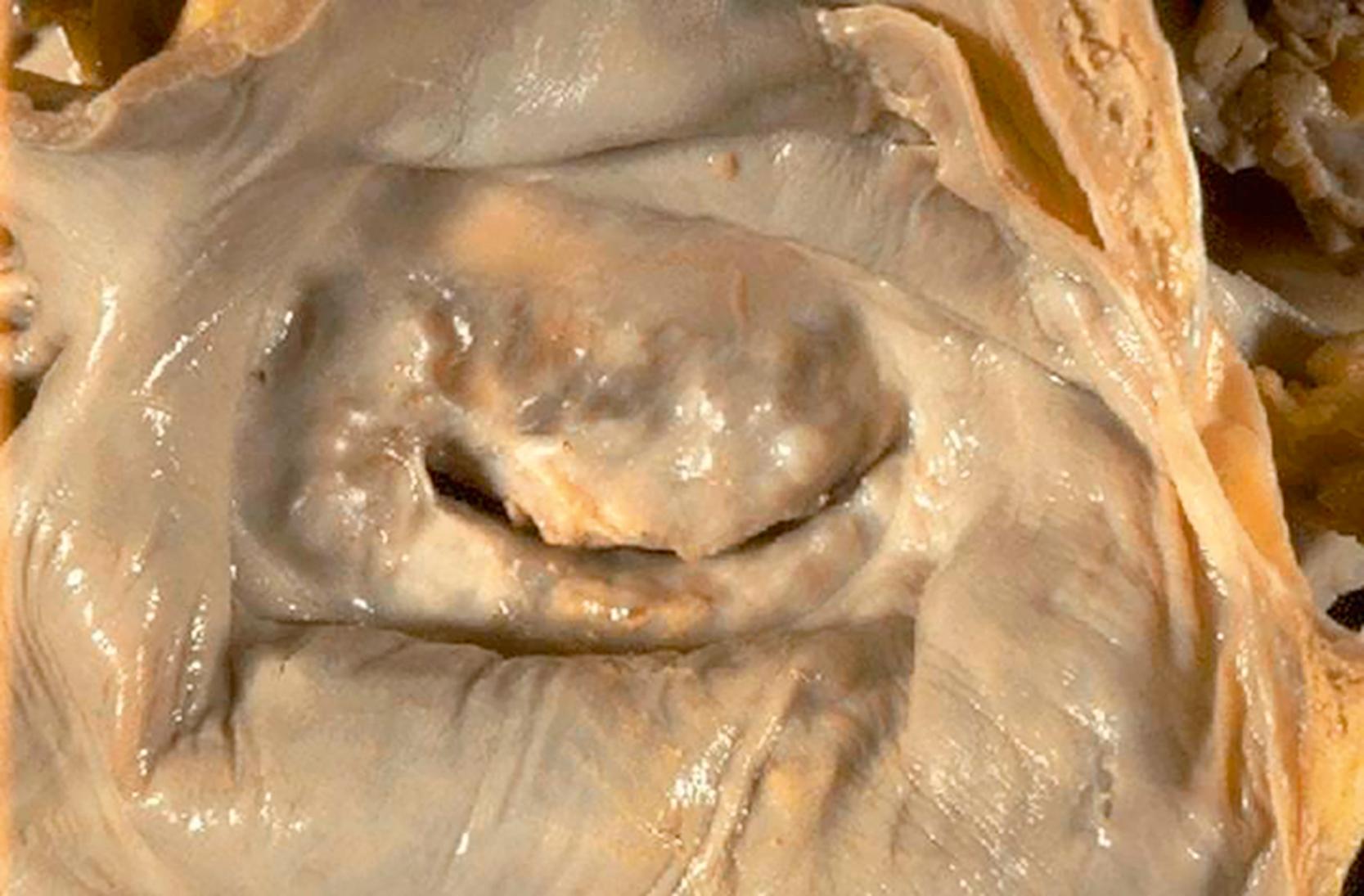
RHD next most frequently leads to aortic valve stenosis through a similar pattern of chronic inflammation with resultant sclerosis impairing valve opening leading to a narrowed orifice. Tricuspid regurgitation (TR) is a common secondary valvular abnormality in RHD due to upstream hemodynamic stresses caused by MS, with resultant pulmonary hypertension and dilation of the pulmonary artery compromising valvular coaptation.
In the developed world, aortic valvular stenosis (AS) is the most common form of valvular heart disease requiring surgical intervention. AS is almost always acquired, resulting from degenerative changes to the aortic valve, but unicuspid aortic valves or other congenital anomalies of the LV outflow tract may also present as early in life. Because of the aging population, the prevalence of AS continues to rise, and is now found in 4% of the population over 85-years old.
Stenotic disease of the aortic valve is relatively equally divided between that affecting initially normal trileaflet valves and that arising in congenitally bicuspid valves. Bicuspid aortic valves are present in 1% to 2% of the population, making this anomaly the most prevalent of the congenital valve lesions, and are more commonly found in males than females.
Stenosis develops through a degenerative process with prevalence increasing with age, with over 80% of patients requiring surgery for trileaflet AS aged 60 years or older. The progression of degenerative (calcific) aortic stenosis (previously termed “senile” or “wear and tear” disease) is now thought to be an actively regulated phenomenon related to atherosclerotic disease, wherein turbulent blood flow at leaflet attachment points induces endothelial injury and leads to accumulation of lipids, infiltration of macrophages and T-cells, and transformation of cells to an osteoblastic phenotype. , It is believed that this process leads to aortic leaflet calcification and may involve the aortic and mitral annuli as well as the mitral leaflets.
Degenerative disease of bicuspid aortic valves presents as about two decades earlier than it does with tricuspid valves, peaking in the fifth and sixth decades of life. AS occurs in 20% to 30% of patients born with bicuspid aortic valves. Increased hemodynamic stresses associated with the abnormally configured bicuspid valve leaflets are thought to accelerate degenerative valve changes in this anomaly.
MR is the most frequent form of valve dysfunction overall, with at least trivial MR present in most healthy adults, and is the second most common form of valvular heart disease requiring surgical intervention. The diagnosis and treatment of MR was greatly facilitated by the work of Carpentier, who in innovating surgical approaches to repair the mitral valve also described a functional classification of MR based on abnormal patterns of leaflet motion ( Table 61.2 ). Degenerative changes to the mitral valve apparatus are the most common cause of MR and are typically found in young patients ( Table 61.1 ). Surgical intervention for MR is related to degenerative disease in 60% to 70% of cases, to ischemic disease in 20% of cases, and to endocarditis or rheumatic disease in 2% to 5%.
| Carpentier Classification | Dysfunction | Lesions | Etiology |
|---|---|---|---|
| Type I | Normal leaflet motion | Annular dilatation Leaflet perforation/tear |
Dilated cardiomyopathy Endocarditis |
| Type II | Excessive leaflet motion (prolapse) | Elongation/rupture of chordae Elogation/rupture of papillary muscle |
Degenerative valve disease Fibroelastic deficiency Barlow disease Marfan disease Rheumatic (acute) Endocarditis Trauma Ischemic cardiomyopathy |
| Type IIIa | Restricted leaflet motion (diastole and systole) | Leaflet thickening/retraction Leaflet calcification Chordal thickening/retraction/fusion Commissural fusion |
Rheumatic (chronic) Carcinoid heart disease |
| Type IIIb | Restricted leaflet motion (systole) | Left ventricular dilatation/aneurysm Papillary muscle displacement Chordae tethering |
Ischemic/dilated cardiomyopathy |
A wide variety of connective tissue diseases may lead to degenerative disease of the mitral valve, typically causing annular dilation (Carpentier Type I lesion) and/or leaflet or subvalvular deformations resulting in excessive leaflet motion (Carpentier Type II lesion). The most prevalent of these diseases is Marfan syndrome. “Myxomatous disease” describes a common pathologic end point of these degenerative diseases, which typically presents as MR in the third or fourth decade of life. This process is characterized by glycosaminoglycan infiltration of the valve leaflets, thickening of the spongiosa, and separation of collagen bundles in the fibrosa.
Myxomatous disease of the mitral valve typically leads to redundant, “billowing” valve leaflets, annular dilatation, and/or chordae enlargement or elongation with consequent abnormal systolic leaflet prolapse into the atrium. This syndrome typically occurs in young women and is also known as Barlow disease after the clear identification in 1963 of the etiology of this “click-murmur syndrome.” Mitral disease is the most common manifestation of myxomatous disease, but it may also present as aortic and tricuspid valve disease.
Myxomatous disease should be differentiated from fibroelastic deficiency syndrome, which is typically characterized by thinned leaflets and chordal rupture and presents in older patients. Prolapse or flail of the middle posterior leaflet cusp (P2) due to chordae rupture is a common manifestation of fibroelastic deficiency syndrome.
Mitral annular calcification is an extremely common degenerative change found in older patients that is typically without functional sequelae. It may be associated with similar changes involving the aortic or mitral valves. Mitral annular calcification can, however, on occasion produce MR by reducing annulus pliability and systolic contraction, which prevent appropriate leaflet coaptation. Less frequently, mitral annular calcification disorders manifest through a more widespread degenerative pathology.
Coronary ischemia causing rupture of a papillary muscle or acute myocardial infarction, particularly in the inferior distribution, can also lead to significant MR. This typically involves the posterior papillary muscle leading to prolapse or flail of the posterior leaflet, because the blood supply to the posterior papillary muscle is from a single (terminal) branch of the posterior descending coronary artery, compared to dual blood supply to the anterolateral papillary muscle from the left anterior descending and circumflex arteries.
In contrast to the causes of primary MR noted above, secondary or “functional” MR, is not caused by abnormalities of the valve itself, but rather by distortion of the subvalvular apparatus and ventricle. Typically, functional MR is a result of myocardial ischemic events and/or cardiomyopathy-induced ventricular dilatation. Dilation of the ventricle results in outward (lateral) and apical (inferior) displacement of the posteromedial papillary muscle, causing tethering of the valve leaflets and loss of central coaptation (Carpentier Type IIIb lesion).
In addition to these and other acquired forms of MR, including those that limit systolic motion of the mitral leaflets (Carpentier IIIa lesion), congenital anomalies such as cleft leaflets and atrioventricular canal/endocardial cushion defects may lead to MR as well.
Aortic valve insufficiency (AI) may be caused by myxomatous disease leading to thinning, enlargement, perforation and/or prolapse of the aortic valve cusps themselves. AI may also be caused by chronic or acute dilatation of the aortic root, which prevents proper aortic valve coaptation by increasing intravalvular closing distances. Root enlargement is typically caused by hypertension and/or connective tissue disorders such as cystic medial necrosis, Marfan syndrome, Ehlers-Danlos syndrome, or Loeys Dietz syndrome, either directly or as a result of acute or chronic aortic dissection.
Importantly, (Mendelian) inheritance of a bicuspid aortic valve anomaly is also associated with dilation of the proximal ascending aorta in up to 50% of patients, which can lead to bicuspid-related AI. This association is hypothesized to be due to a currently unidentified common genetic defect causing abnormalities in aortic wall elasticity, or through hemodynamic “blast” effects of abnormal flow through the bicuspid valve orifice. AI due to bicuspid valve-related root enlargement typically develops at a much younger age than AS that develops from accelerated degeneration, and with a lower rate of progression requiring surgical intervention.
Less common causes of aortic root enlargement and/or aortic dissection include trauma, syphilitic aortitis, rheumatoid arthritis, lupus erythematosus, or other systemic vasculopathies such as Takayasu and giant cell aortitis, and osteogenesis imperfecta.
Endocarditis is a relatively common cause of AI, and a frequent cause of valve pathology overall. The incidence of endocarditis varies from 3 to 10 episodes per 100,000 person-years. Endocarditis typically causes valvular insufficiency by progressive inflammatory destruction of the affected valve. Less frequently, endocarditis can cause functional valve stenosis, with valve orifice obstruction developing from endocarditic vegetations–masses of platelets, fibrin, microcolonies of microorganisms, and inflammatory cells.
Although endocarditis is typically relatively indolent, it is the most common cause of death secondary to acute AI in the adult population and carries a relatively high mortality compared to other valve lesions. Endocarditis may affect previously normal valves, but it typically affects valves deformed by congenital or rheumatic disease, degenerative processes such as calcification, or previously replaced prosthetic valves. Infectious endocarditis is usually left-sided, reflecting the normal distribution of such preexisting valvular disease.
The pathophysiology of endocarditis is typically initiated by platelets and fibrin deposition on normal or deformed valves occurring as part of a normal healing process following normal disruptions of the valvular endothelium, which are in turn caused by hemodynamic or metabolic injuries. Endocarditis results from subsequent seeding onto thus damaged valves of microbiologic organisms after bacteremia or fungemia episodes; most commonly staphylococci, streptococci, or enterococci.
Acute endocarditis, increasingly affecting normal valves, may follow an aggressive course with valvular perforation or more extensive destruction of the leaflet and/or surrounding support structures, resulting in acute valvular regurgitation. With subacute or chronic presentations of endocarditis, valve insufficiency may result from residual leaflet deformities caused by fibrotic healing of endocarditic lesions. The growth of large vegetations may also uncommonly lead to improper leaflet coaptation and AI as well.
Nearly all the pathophysiologic mechanisms causing left-sided valve disease may analogously lead to primary right-sided valve disease; however, the most common presentation of right-sided valvular disease is functional TR caused by RV failure (which itself is typically secondary to left sided dysfunction and pulmonary hypertension). Less commonly, functional TR may also be caused by RV infarction or ischemia. Transvalvular pacemaker or cardioverter-defibrillator leads can also uncommonly cause mild or even higher-grade TR.
Tricuspid stenosis (TS) occurs infrequently in developed countries since rheumatic disease accounts for over 90% of such lesions. Carcinoid syndrome is the most common of a group of unusual disorders that lead to the deposition of pathologic materials in the tricuspid and/or pulmonic leaflets as a far less frequent cause of primary TR, TS, or pulmonic valve disease.
Congenital anomalies causing pulmonic valve stenosis, often associated with tetralogy of Fallot; tricuspid atresia; and TR occurring as part of the Ebstein anomaly are three of the most common of the congenital disorders leading to right-sided valve disease.
Two fundamental pathophysiologic derangements may affect the heart valves: stenosis and insufficiency. The hemodynamic hallmark of cardiac valve stenosis is the occurrence of an increased pressure gradient between an upstream pumping chamber and downstream receiving chamber or great artery. This increased gradient is necessary to maintain the baseline flow rate through the valve due to the increased resistance to laminar flow introduced by the smaller effective cross-sectional area of the stenotic valve orifice, as described by Poiseuille law (flow α Δp/resistance, where Δp signifies pressure gradient). The hemodynamic hallmark of regurgitant valvular disease is the retrograde flow of blood from downstream structures (ventricle or great vessel) into an upstream chamber during the diastolic interval during which the malfunctioning valve should normally be closed. Uncompensated, both stenotic and regurgitant lesions cause an increase in upstream chamber afterload and consequent wall stress – predominating either during ventricular systolic ejection against the resistance of stenotic valves, or with increased chamber filling by regurgitant volumes peaking at the end of diastole, respectively.
Two compensatory mechanisms provide robust reserves in cardiac function before the volume and pressure overload stresses of valve disease translate into significant cardiac physiologic derangements. The first, described by the Frank-Starling law ( Fig. 61.12 ), produces increases in ventricular contractile force as a function of end-diastolic volume (EDV), or preload, which enhances stroke volume and ventricular emptying. The second involves stress-induced ventricular hypertrophy, which leads to increased wall thickness. By decreasing chamber radius (volume) or increasing wall thickness, respectively, each of these processes can improve wall stress, as described by Laplace law: (wall stress α (pressure × radius)/(2 × wall thickness). Decreased wall stress in turn translates into decreased myocardial work and oxygen demand and improved cardiac function.
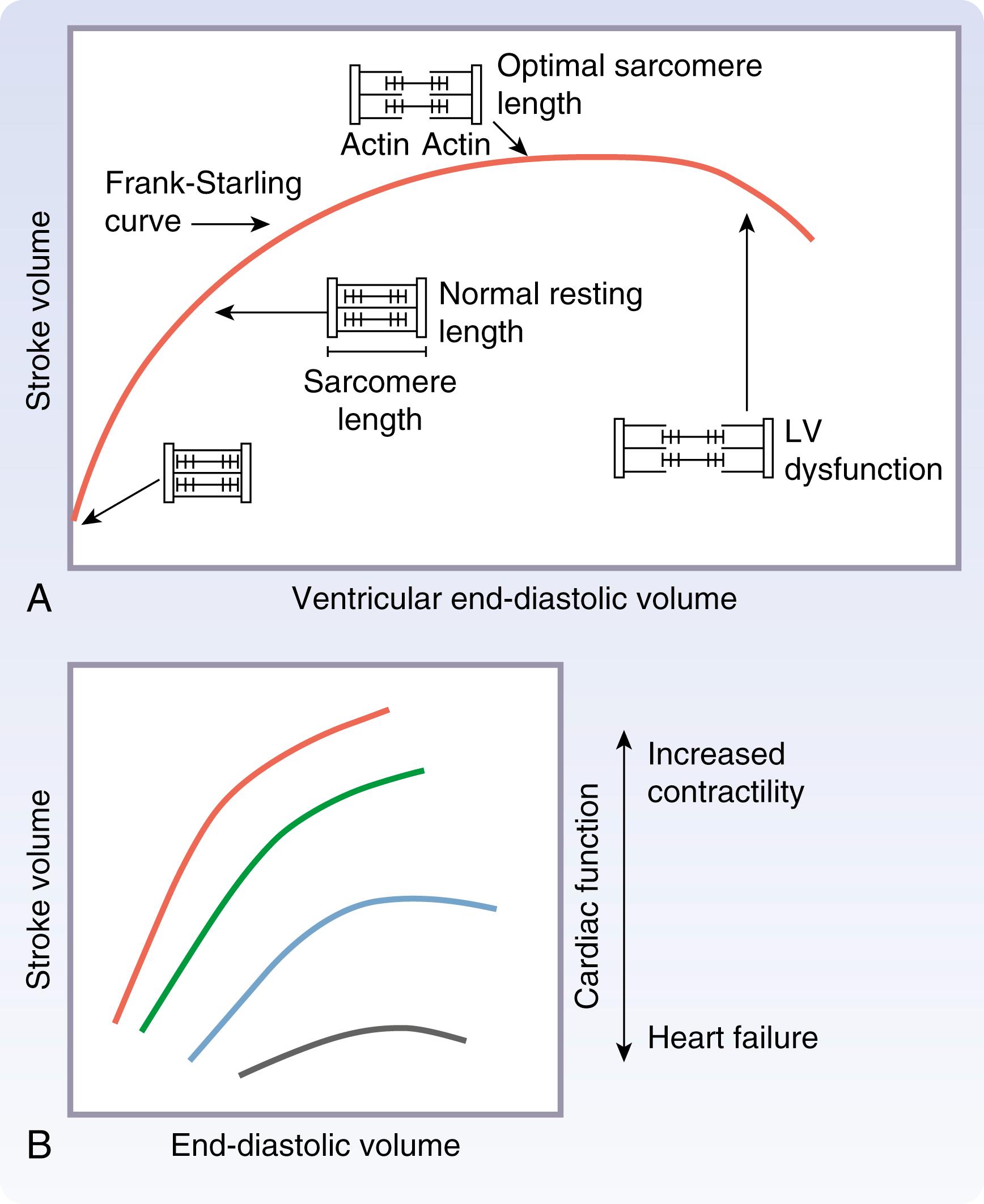
The microanatomic basis of the Frank-Starling relationship is the orientation between actin and myosin fibers of the cardiomyocyte sarcomere, which become ideally aligned to generate contractile force as cardiac muscle is stretched from a “zero-load” status. Ideal actin-myosin alignment occurs at a sarcomere length of 2.2 micrometers, at which point contractile proteins become optimally sensitized to calcium fluxes, resulting in maximized sarcomere contractility as well as rates of contraction and relaxation. As cardiomyopathy progress, contractile function diminishes at a given level of cellular stretch, and eventually heart failure ensues.
Patients with valvular heart disease typically progress through a series of disease stages, from Stage A (“at risk”) to Stage D (“symptomatic severe”). , Asymptomatic patients demonstrating mild to moderate valve pathology are classified as Stage B and asymptomatic patients with severe valve pathology are classified as Stage C, either with compensated (C1) or decompensated (C2) left or RV function. Regular assessment is recommended for patients with valvular heart disease (i.e., echocardiograms every 3–5 years for Stage B patients, increasing to every 6–12 months for Stage C patients). This surveillance should allow appropriately timed intervention to minimize valve-related symptomatology, sequelae (e.g., atrial fibrillation, stroke, pulmonary dysfunction) and mortality risk, while balancing the risks of intervention with the natural history of advanced disease.
There is typically no pressure gradient across a normally-sized mitral valve (4–6 cm 2 cross-sectional area) and “upstream” LA pressure is typically less than 10 to 15 mm Hg. As the mitral valve orifice narrows to a cross sectional area of 2 to 2.5 cm 2 (mild MS), resistance to flow leads to increased LA blood volume “pooling.” Increased LA pressure generated by Frank Starling mechanics maintains adequate diastolic flow across the resistive valve orifice.
When progressive MS leads to a transvalvular gradient of greater than 5 to 10 mm Hg, typically corresponding to a valve orifice of less than 1.5 cm 2 , MS is classified as “severe”. The resultant increase in LA pressure is transmitted upstream to the pulmonary veins, capillaries, and arteries. At an LA pressure of 25 mm Hg, pulmonary edema typically develops, with prolonged arterial vasoconstriction and vascular remodeling eventually leading to fixed pulmonary hypertension with chronically increased pulmonary pressure. Elevated pulmonary arterial systolic pressure greater than 60 mm Hg imparts significant RV afterload may also ultimately lead to RV dilatation, TR, and RV failure. MS spares left ventricle function in two-thirds of cases, with normal or less than normal LV chamber hemodynamics in 85% of cases and impaired output primarily due to restricted LV inflow.
Any increase in cardiac output, as with exercise, will lead to an increase in mitral transvalvular pressure gradients according to the law of Poiseuille: flow α Δp/resistance, where Δp signifies pressure gradient. At a given cardiac output, decreased diastolic filling time caused by increased heart rates, such as with exercise or the onset of atrial fibrillation, will also cause increased transvalvular gradient as more flow must occur per unit time.
Chronically increased transmitral pressure gradients caused by MS typically lead to atrial hypertrophy and dilatation. Associated LA fibrosis and disorganization of the atrial muscle fibers cause abnormal atrial conduction velocities and refractory times. Increased automaticity, ectopic foci, and reentry circuits eventually lead to supraventricular tachyarrhythmias in nearly 40% of patients. Loss of the “kick” generated by normal atrial contraction, responsible for 30% of ventricular filling, results in a 20% decrease in cardiac output and necessitates increased atrial pressure to allow ventricular loading. Atrial fibrillation consequently causes increased diastolic pressures and volume overload, potentially leading to worsening congestion.
MS patients may remain asymptomatic for many years. As valvular stenosis gradually worsens, however, symptoms characteristic of low cardiac output and pulmonary venous congestion eventually develop, including fatigue, dyspnea, and orthopnea. Ultimately, peripheral edema and other congestive symptoms caused by volume overload and right heart failure ensue. Increased heart rate caused by atrial fibrillation or supraventricular tachycardia, exercise or other factors may exacerbate symptomatology.
Patients with MS who develop atrial fibrillation may complain of palpitations and symptomatic tachycardia. More ominously, thromboembolization will occur in 20% of patients with MS and may be its first symptom in 10% of cases, presenting as stroke, myocardial ischemia or infarction, renal infarction, and gut or limb ischemia. Half of all thromboembolic events will involve the cerebral circulation. Rarely, a large, pedunculated atrial thrombus may form and obstruct the valve inlet, resulting in hemodynamic collapse and sudden death. With advanced disease, LA enlargement may cause hoarseness from left recurrent laryngeal nerve compression onto the pulmonary artery, dysphagia from esophageal compression, or persistent cough from bronchial compression. Pulmonary venous congestion may induce hemoptysis from sudden rupture of a dilated bronchial vein. RV failure and TR can cause abdominal pain and swelling from hepatomegaly and ascites, or even florid peripheral edema.
The characteristic physical finding of MS is a low-pitched, rumbling diastolic murmur which is best heard at the apex with the patient in the left lateral decubitus position. In patients who are in sinus rhythm, the murmur increases in intensity during late diastole (known as presystolic accentuation) due to the increased flow across the stenotic valve with atrial contraction. A high-pitched “opening snap” or an accentuated first heart sound caused by forceful opening or closing, respectively, of an inflexible but still mobile mitral leaflet may be heard with early MS. The Graham Steell diastolic murmur of MS can result from pulmonary regurgitation caused by pulmonary hypertension and right-sided overload.
Advanced MS is typically associated with rales developing with the onset of pulmonary edema. As RV failure develops, an RV heave, jugular venous distension, hepatomegaly, ascites, and lower extremity edema may be found.
The earliest appreciable changes of MS discernable by routine chest radiography include evidence of an enlarged LA seen as a straightening of the left cardiac border, a double shadow in the cardiac silhouette, or an elevated left main stem bronchus. Prominent pulmonary vessels may also be appreciated. If stenosis is severe, congested pulmonary lymphatics in the lower lung fields may be present as horizontal linear opacities, known Kerley B lines. Mitral valve calcification may also be visible. Stigmata of heart failure, such as opacification of the lung fields and pleural effusions, may follow.
The electrocardiogram of MS patients is often grossly normal, although 90% of patients will demonstrate evidence of LA enlargement as a widened, notched P wave (p mitrale). Atrial arrhythmias may also be appreciated if present. With advanced MS, RV hypertrophy may be associated with right-axis deviation.
Echocardiography, as with other valve lesions, is the primary diagnostic method to determine the presence and severity of MS and associated abnormalities. Commissural fusion, leaflet immobility and leaflet as well as annular/subvalvular thickening and calcification can be well assessed by transthoracic and especially by transesophageal echo. Three-dimensional (3D) echocardiography provides further definition of valve morphology and function.
Doppler echocardiography has largely replaced cardiac catheterization in accurately assessing the hemodynamic parameters of MS as well as other valve lesions. Doppler blood velocity measurement allows mean and peak transvalvular mitral pressure gradient determination as a function of the simplified Bernoulli equation: p = 4v 2 , where v equals the velocity of blood crossing the valve orifice. Orifice area can be measured by planimetry (tracing the valve-opening orifice on a still echocardiographic image) or as a derivative of velocity measurements, based on continuity equations. Mitral valve area can also be determined based upon the pressure half-time, the time in which the transvalvular velocity decreases by half. Pressure half-time will become prolonged with increasing severity of stenosis.
Ten-year patient survival is greater than 80% in the asymptomatic patient with MS, and interventional treatment is consequently not recommended in this setting. In comparison, 10-year survival for symptomatic patients with severe MS who forgo intervention is less than 15%, and mean survival is less than three years in patients with MS and severe pulmonary hypertension.
Medical management of patients with symptomatic MS includes the use of diuretics to reduce LA pressure and vascular congestion. Beta-blockers and calcium channel-blocking agents are recommended to provide heart rate control and help maintain sinus rhythm. Anticoagulation therapy is recommended via CHADS 2 and the updated CHADS 2 -VASc (modification to better assess low-risk patients) scoring systems in patients developing atrial fibrillation ( Table 61.3 ), and in patients without atrial fibrillation but who have suffered a prior embolic event or have a documented LA thrombus. Use of anticoagulation therapy must be tempered against the risk of major bleeding, which can now be calculated using the Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly [>65 years], Drugs/alcohol concomitantly (HAS-BLED) scoring system developed in 2010 from data in the Euro Heart Survey to assess 1-year risk of major bleeding in patients taking anticoagulants with atrial fibrillation.
| CHADS 2 Score | Points ∗ | CHADS 2 - VASc Score | Points ∗ | |
|---|---|---|---|---|
| C | Congestive heart failure | 1 | Congestive heart failure | 1 |
| H | Hypertension | 1 | Hypertension | 1 |
| A | Age ≥ 75 years | 1 | Age ≥ 75 years | 2 |
| D | Diabetes mellitus | 1 | Diabetes mellitus | 1 |
| S | Prior stroke, TIA or thromboembolism | 2 | Prior stroke, TIA, or thromboembolism | 2 |
| V | Vascular disease | 1 | ||
| A | Age 65–74 years | 1 | ||
| Sc | Sex category (female) | 1 |
Early intervention is associated with improved long-term survival for patients with MS compared to patients in whom intervention is delayed until the development of symptomatology. Five-year survival is 62% for NHYA Class III patients, and 15% for Class IV patients. The current American Heart Association/ American College of Cardiology (AHA/ACC) guidelines accordingly recommend intervention for MS in patients with symptomatic severe or asymptomatic very severe MS (valve orifice area <1.5 cm 2 or <1 cm 2 , respectively). , Additionally, surgery is recommended for patients with moderate or severe MS undergoing cardiac surgery for other reasons, and for patients with severe MS who have recurrent embolic events while on anticoagulation (with LA excision recommended). Options for mechanical intervention include percutaneous mitral balloon commissurotomy, open mitral commissurotomy and surgical repair, or replacement of the mitral valve.
This endovascular procedure, first reported by Inoue and colleagues in 1984, uses a balloon catheter advanced into the left atrium via transseptal puncture or retrograde transaortic delivery to dilate the stenotic mitral valve. Based upon compelling safety and efficacy data, percutaneous balloon mitral commissurotomy (PBMC) has largely replaced surgical interventions in appropriately selected patients based upon (Wilkins) echocardiographic criteria ( Table 61.4 ). In general, PBMC is indicated by the presence of mobile, noncalcified, thin valves with minimal fusion, scarring or calcification of the subvalvular apparatus, and the absence of moderate-to-severe MR or a LA thrombus. ,
| Grade | Mobility | Thickening | Calcification | Subvalvular Thickening |
|---|---|---|---|---|
| 1 | Highly mobile valve with only leaflet tips restricted | Leaflets near normal in thickness (4–5 mm) | A single area of increased echocardiographic brightness | Minimal thickening just below the mitral leaflets |
| 2 | Leaflet mid and base portions have normal mobility | Midleaflets normal, considerable thickening of margins (5–8 mm) | Scattered areas of brightness confined to leaflet margins | Thickening of chordal structures extending to one-third of the chordal length |
| 3 | Valve continues to move forward in diastole, mainly from the base | Thickening extending through the entire leaflet (5–8 mm) | Brightness extending into the midportions of the leaflets | Thickening extended to distal third of the chords |
| 4 | No or minimal forward movement of the leaflets in diastole | Considerable thickening of all leaflet tissue (>8–10 mm) | Extensive brightness throughout much of the leaflet tissue | Extensive thickening and shortening of all chordal structures extending down to the papillary muscles |
PBMC is associated with a mortality risk of 0.5%, and a less than 10% risk of cardiac or vascular complications, embolization, or creation of severe MR. Successful PBMC, defined as a postdilation valve area of more than 1.5 cm 2 with MR less than 2/4, is achieved in over 80% of patients. Although reintervention is often required, over half of patients can expect to remain free from surgery at 20 years.
Open mitral commissurotomy is the primary form of surgical repair of MS, and is limited to use for patients in whom intervention is needed but PBMC is contraindicated, or have failed previous percutaneous intervention. Open mitral commissurotomy requires use of cardiopulmonary bypass, and involves division of fused commissures, mobilization of scarred chordae, and ligation of the LA appendage. The mortality rate for open commissurotomy is less than 2%, and the 10-year freedom from reoperation rate after open mitral commissurotomy is approximately 90%. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here