Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acquired and congenital bone marrow failure syndromes are characterized by a reduction in the effective production of mature erythrocytes, granulocytes, and platelets by the bone marrow. Bone marrow failure leads to various peripheral blood cytopenias. In some conditions, only one or two cell lines may be affected. In others such as aplastic anemia the result is pancytopenia. In this chapter, we deal only with the acquired bone marrow failure syndromes, though we use the term rather loosely because the acquired marrow failure syndromes may have a genetic basis. The known inherited/congenital bone marrow failure syndromes are described in detail in Chapter 7 . The acquired marrow failure syndromes that affect only platelets or granulocytes are described in Chapters 22 and 34 respectively. The acquired single lineage deficiency syndromes that involve red cell production (pure red cell aplasia) are also described in this chapter, except that the 5Q-syndrome is discussed in Chapter 7 and in more detail Chapter 51 .
Decreased production of mature blood cells may result from a reduction in the number or function of their progenitors . Aplastic anemia is a descriptive term referring to a clinical state in which peripheral blood pancytopenia results from reduced or absent production of blood cells in the bone marrow. Aplastic anemia may arise in the setting of inherited/congenital syndromes associated with a predisposition to marrow failure (discussed in Chapter 7 ), may develop secondary to marrow-toxic stressors in an otherwise seemingly normal host, or may lack any apparent underlying cause. Classifications of the aplastic anemias are presented in Box 6-1 . A careful medical history, physical examination, and laboratory evaluation are critical to discern inherited versus acquired causes of aplastic anemia. The distinction between inherited and acquired causes of aplastic anemia carries profound implications for medical management and treatment, so a careful search for possible underlying inherited syndromes should be undertaken prior to initiation of therapy.
Secondary
Radiation
Drugs and chemicals
Direct toxicity: chemotherapy; benzene
Idiosyncratic: chloramphenicol; antiinflammatory drugs; antiepileptics; carbonic anhydrase inhibitors
Viruses
Epstein-Barr virus
Hepatitis (non-A, B, C, E, or G)
Human immunodeficiency virus
Immune diseases
Eosinophilic fasciitis
Hypoimmunoglobulinemia
Systemic lupus erythematosis (uncommon)
Thymoma
Graft-versus-host disease in immunodeficiency
Pregnancy
Paroxysmal nocturnal hemoglobinuria
Myelodysplasia
Idiopathic
Fanconi anemia
Dyskeratosis congenita
Shwachman-Diamond syndrome
Amegakaryocytic thrombocytopenia
Diamond-Blackfan anemia
Reticular dysgenesis
GATA-2 syndromes
Familial aplastic anemias
Nonhematologic syndromes (e.g., Down, Dubowitz, and Seckel syndromes)
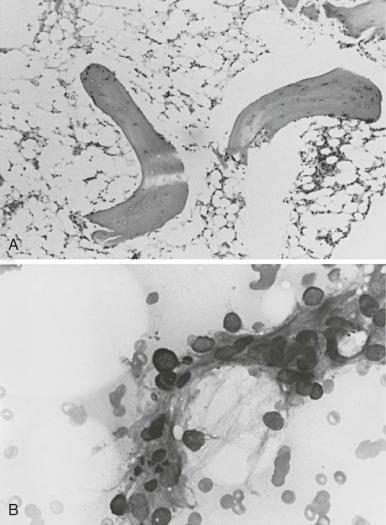
Camitta and co-workers classified the severity of aplastic anemia in an effort to make possible the comparison of diverse groups of patients and different therapeutic approaches. Diagnosis of severe aplastic anemia requires that the patient have at least two of the following anomalies: a granulocyte count below 500/µL, a platelet count below 20,000/µL, and an absolute reticulocyte count less than or equal to 40 × 10 9 /L . In addition the bone marrow biopsy must contain less than 25% of the normal cellularity or less than 30% hematopoietic elements ( Fig. 6-1 ). Very severe aplastic anemia is further defined by a granulocyte count of less than 200/µL. Mild or moderate aplastic anemia, sometimes called hypoplastic anemia, is distinguished from the severe form by the presence of mild or moderate cytopenias and more variable, but still deficient, bone marrow cellularity. These distinctions are more than semantic; they are critical for the prediction of outcome and the choice of therapy.
Epidemiologic studies performed in Europe estimate that the annual incidence of aplastic anemia is 2 per million per year. By comparison, the incidence of acute leukemia is about 50 per million per year. Higher figures for the incidence of aplastic anemia have been obtained from smaller studies in the United States and earlier surveys in Europe, but these figures may have been inflated by the inclusion of cases of myelodysplasia, a much more common syndrome. Aplastic anemia is more common in Asia (4 to 7 per million per year) than in the West. Chloramphenicol, a known cause of aplastic anemia, has been widely used in much of Asia because of its efficacy and low cost, but reductions in its use have not been accompanied by reductions in aplastic anemia incidence in Japan or elsewhere, and no association was observed in the case control studies in Thailand. A recent case control study in Thailand spanning 1989-2002 described an association between aplastic anemia and exposure to benzene or pesticides. Although an increased risk of aplastic anemia was also associated with animal exposures and ingestion of nonbottled or nondistilled water, no significant associations with known infections or hepatitis were observed.
The peak age of presentation of aplastic anemia is at 15 to 25 years or over 60 years. The male-to-female ratio in acquired aplastic anemia is approximately 1 : 1.
When no causative factors are ascertained, the cases are classified as idiopathic. A search for possible causative agents is warranted because some patients may improve following the removal of the offending agent. However, most cases of aplastic anemia remain idiopathic. The distinction between acquired versus inherited causes of aplastic anemia is crucial to guide clinical management and treatment (see Chapter 8 ).
The incidence of drug- and chemical-related aplastic anemia varies over time and from place to place. Many drugs and toxins have been implicated by inferential and circumstantial evidence; the magnitude of the risk is usually unknown ( Box 6-2 ). Presence of an agent on this list suggests caution regarding its use, but no drug on this list should be proscribed if there are strong clinical indications for its use. From a public health perspective, even drugs associated with an increased risk of marrow failure do not cause large numbers of cases of aplastic anemia.
* Agents are listed because they have been cited in the literature; inclusion in this list does not imply acceptance by the author of a causal relationship.
Antibiotics: daunorubicin, doxorubicin hydrochloride (Adriamycin), chloramphenicol
Antimetabolites: antifolic compounds, nucleotide analogues
Antimitotics: vinblastine, colchicine
Benzene and chemicals containing benzene: carbon tetrachloride, chlorophenols, kerosene, Stoddard solvent
Cytotoxic cancer chemotherapy with alkylating drugs: busulfan, melphalan, cyclophosphamide
Chloramphenicol
Insecticides: chlordane, chlorophenothane (DDT), γ-benzene hexachloride (lindane), parathion
Anticonvulsants: carbamazepine, hydantoins, phenacemide
Nonsteroidal antiinflammatory agents: indomethacin, ibuprofen, oxyphenylbutazone, phenylbutazone, sulindac
Antihistamines: cimetidine, chlorpheniramine, ranitidine
Antiprotozoal drugs: quinacrine, chloroquine
Sulfonamides: some antibiotics, antidiabetics (chlorpropamide, tolbutamide), antithyroid drugs (methimazole, methylthiouracil, propylthiouracil), carbonic anhydrase inhibitors (acetazolamide, methazolamide)
Penicillamine
Metals: gold, arsenic, bismuth, mercury
Allopurinol (may potentiate marrow suppression by cytotoxic drugs)
Antibiotics: flucytosine, mebendazole, methicillin, sulfonamides, streptomycin, tetracycline, trimethoprim/sulfamethoxazole
Carbimazole
Guanidine
Lithium
Methyldopa
Potassium perchlorate
Quinidine
Sedatives and tranquilizers: chlordiazepoxide, chlorpromazine, meprobamate, methyprylon, piperacetazine, prochlorperazine
Thiocyanate
Note that even confirmed associations do not substantiate causality. Antibiotics felt to be causative may have been administered for the viral infection that led to the aplastic anemia or for symptoms from already established neutropenia in an undiagnosed case of aplastic anemia. Bleeding may be precipitated in undiagnosed thrombocytopenic patients who receive nonsteroidal antiinflammatory drugs. As an example of known errors in associations, among six patients reported to have sniffed glue and become aplastic, five had sickle cell anemia and aplastic crises now known to be due to parvovirus infection.
The incidence of drug-related aplasia in pediatric cases is low, mainly because many of the drugs felt to be related to aplasia are not used in childhood, with the exception of antiepileptic drugs, carbonic anhydrase inhibitors, nonsteroidal antiinflammatory medications, and some antibiotics.
Drug-related aplasia may occur in several ways. Drugs may exert direct cytotoxic or suppressive effects on the bone marrow. Myelosuppressive drugs, such as those used in cancer chemotherapy, lead to predictable and dose-related marrow suppression. Benzene, too, can be demonstrated regularly to suppress the bone marrow in animals in a dose-linked manner, and most individuals exposed to sufficient amounts of benzene would probably suffer some type of marrow damage. In practice, most drug-related aplastic anemia is idiosyncratic and occurs unpredictably in only rare individuals who are prescribed the medication, sometimes weeks to months after its administration is discontinued. This last category of patients may possess a genetic propensity for this phenomenon or be due to a direct effect of the drug on antigen presentation of abnormal peptides (see below).
Chloramphenicol was considered to be the commonest cause of aplastic anemia at the peak of its use, which began in 1949. A genetic predisposition may exist. The mechanism of the idiosyncratic aplasia remains unknown despite extensive investigation.
Chloramphenicol contains a nitrobenzene ring and thus resembles amidopyrine, a drug known to cause agranulocytosis. Chloramphenicol is the prime example of a drug that causes both dose-related marrow suppression through mechanisms that include mitochondrial inhibition, and idiosyncratic aplastic anemia.
The signs of dose-related toxicity appear more rapidly in patients with hepatic or renal disease because the drug must be inactivated by conjugation with glucuronide in the liver and excreted in the urine. High doses and high plasma levels correlate with the characteristic reversible erythroid depression. In vitro, chloramphenicol inhibits the growth of both colony-forming units granulocyte/macrophage (CFU-GMs) and colony-forming units erythroid (CFU-Es) and also may inhibit the hematopoietic microenvironment (HM).
Nonsteroidal antiinflammatory drugs, which are used more extensively in adults than in children, are associated with aplasia. Nonsteroidal antiinflammatory drugs associated by occasional case reports with aplastic anemia include aspirin, indomethacin, and ibuprofen. Several large studies reported an increased risk of aplastic anemia with phenylbutazone and identified even higher probabilities with some of the other nonsteroidal antiinflammatory drugs. Cimetidine, another commonly used drug, is associated with a 2 per 100,000 user risk of cytopenias. Sulfa-containing compounds, which appear as risk factors in most case-control studies of drugs and aplastic anemia, are used in a wide variety of clinical circumstances. Other drugs implicated in aplastic anemia that are commonly used in the pediatric population include anticonvulsants (hydantoins, carbamazepine ) and carbonic anhydrase inhibitors (acetazolamide and methazolamide ). Many of the drugs listed in Figure 6-1 also have been associated with agranulocytosis. In general only a minority of cases of aplastic anemia can be assigned a drug association. The distinction between aplasia secondary to the medication versus aplasia arising from the underlying disorder (or occult viral infection) requiring treatment can be difficult.
Benzene is a particularly dangerous environmental contaminant. It is found in organic solvents, coal tar derivatives, and petroleum products. Fatal aplastic anemia, leukemia, or both, have been reported years later in factory workers who had benzene exposure. Benzene is concentrated in bone marrow fat, forms water-soluble intermediates, and damages DNA. It decreases the numbers of progenitors and damages stroma. The risk of cytopenias is likely related to cumulative exposure.
Toxicity thought to be due to other organic solvents may in some instances be caused by benzene contaminants. Neither pure toluene nor xylene is a marrow toxin. Aplastic anemia has been linked by many case reports to insecticides, particularly γ-hexachlorobenzene (lindane) in children. Aromatic hydrocarbons are present in insecticides and herbicides and may comprise the solvents for these agents. Some organophosphate insecticides have been shown to inhibit in vitro hematopoietic colony formation, as has lindane.
Marrow aplasia may occur as an acute toxic sequela of irradiation due to nuclear bomb explosion, radioactive fallout, reactor accidents, and accidental exposure in medicine and industry. Bone marrow cells may be affected by high-energy γ-rays as well as by ingested or absorbed lower-energy α particles. The radiation injury is to the actively replicating pool of precursor and progenitor cells and also to stem cells, in which DNA damage may have a more severe effect. Nonetheless, radiation-related marrow aplasia is infrequent. Even in a restricted episode, such as the Chernobyl reactor accident in 1986, most immediate deaths were due to skin burns and damage to gastrointestinal and pulmonary systems.
Chronic radiation-induced aplasia is dose related. Patients who were irradiated for ankylosing spondylitis had an increased risk of aplastic anemia, and American radiologists have been reported to have an increased death rate from aplasia (for both groups, the pathologic distinction of aplasia and myelodysplasia was not made). The incidence of late aplasia in atomic bomb victims was not increased, nor was it increased in patients receiving radiation therapy for malignancies. Knospe and co-workers suggested that irradiation with an exposure greater than 4.4 Gy was required for the development of aplasia; they also postulated that low doses might damage only stem cells, whereas high doses would also damage the supportive hematopoietic stromal microenvironment.
Patients with bacterial or viral illnesses frequently develop mild pancytopenia during or following the infection (see Chapter 37 ). Patients with bacterial or viral infections often receive antibiotics and other medications, and it is frequently not clear whether an ensuing aplastic anemia was caused by the infection, by the drug, or by the combination of the two, or even whether the infectious illness was the result and not the cause of the pancytopenia.
Although hepatitis is frequently associated with mild depression of blood cell counts, aplasia is a rare sequela, estimated to occur in fewer than 0.07% of the total number of pediatric hepatitis cases and in fewer than 2% of those with non-A, non-B hepatitis. Nonetheless as an identifiable clinical event, a prior episode of hepatitis is recognized in 2% to 5% of aplastic anemia patients in a Western series. The prevalence of prior hepatitis is about twofold this proportion in the Far East. Among children with aplastic anemia in Taiwan, 24% had a history of recent acute hepatitis. Antecedent hepatitis may be subclinical, as about 50% of patients with aplastic anemia may have elevated hepatic transaminases before their first transfusion. In a report of 32 patients with liver transplantation for hepatic failure following non-A, non-B hepatitis, 28% developed aplastic anemia. While aplasia has been reported following both hepatitis A and B virus infections, the majority of cases of the hepatitis/aplasia syndrome are not associated with serotypable hepatitis virus.
A study of aplastic anemia patients reported to the European Registry from 1990-2007 found that 5% of patients with aplastic anemia had an antecedent seronegative hepatitis. Hepatitis-associated aplastic anemia was slightly more common in males and tended to present at a younger age. Actuarial survival after treatment with either immunosuppression or hematopoietic stem cell transplant was similar between patients with hepatitis-associated aplastic anemia versus other acquired aplastic anemia.
Flaviviruses cause arbovirus hemorrhagic fevers, dengue, and other hematodepressive syndromes. Dengue can propagate in bone marrow cultures without direct cytotoxicity, and dengue antigens induce lymphocyte activation and the release of marrow suppressive cytokines.
Herpesviruses such as Epstein-Barr virus (EBV) are large, complex DNA viruses. EBV causes infectious mononucleosis, which has pancytopenic complications in less than 1% of cases. More than 12 such cases have been reported, and one half of these cases had a fatal outcome. In one study, EBV was demonstrated by immunologic and molecular methods in the bone marrow of six patients with aplastic anemia. Only two had a history of typical mononucleosis, although all six had serologic evidence, suggesting that EBV may be an unrecognized cause of aplastic anemia. The EBV's target is B cells, although T cells also may be infected. Because EBV is a common infection, issues of ascertainment can render the causative determination of aplasia difficult.
Infection with cytomegalovirus (CMV) may lead to graft failure in immunosuppressed bone marrow transplant (BMT) recipients. CMV can infect marrow stromal cells in vitro and can inhibit their ability to produce growth factors; direct progenitor cell infection by some CMV strains also has been documented. Herpesvirus 6 is the cause of exanthem subitum and, like CMV, may be found in the marrow of patients with graft failure after transplantation, as well as in hematopoietic progenitors infected in vitro. As with other viruses, both of these are ubiquitous infections making causality difficult to ascertain.
Patients with acquired immunodeficiency syndrome (AIDS) often have cytopenias, but their marrow is much more commonly cellular and dysplastic than empty. Colony formation by marrow from patients may be diminished. The action of human immunodeficiency virus-1 (HIV-1), a lentivirus, on hematopoietic cells remains a subject of controversy. HIV-1 infection of CD34+ cells has been difficult to detect in vivo from patient material or after tissue culture infection of normal cells. The virus apparently directly infects megakaryocytes, which bear the CD4 receptor present on T cells. The virus also may affect stroma functions, at least in vitro. HIV-1 can act indirectly on hematopoiesis through inhibitory lymphokine production: The envelope glycoprotein can stimulate macrophages to produce tumor necrosis factor (TNF), which in turn inhibits hematopoietic colony formation. Hematologic suppression can also be due to opportunistic infections, neoplasms, or marrow suppression from the drugs used to treat AIDS and its complications.
Blood count abnormalities, which are rarely severe, may be observed in the course of rubella, measles, mumps, varicella, and influenza A.
Paroxysmal nocturnal hemoglobinuria (PNH) is a disease characterized by variable combinations of mild to severe intravascular hemolysis, large venous thromboses, and aplastic anemia. It is uncommon in adults and even rarer in children (see Chapter 13 ). There is a clear association of PNH with aplastic anemia: Many patients with PNH develop pancytopenia and marrow hypoplasia, and PNH clones are detectable in up to 50% to 70% of patients with acquired aplastic anemia.
PNH is characterized by an inability to inactivate complement on the erythrocyte cell surface, resulting in increased sensitivity to complement. Deficits were subsequently identified in a family of membrane proteins, all of which were anchored to the cell membrane via glycosylphosphatidylinositol (GPI). GPI binds covalently to specific carboxyl terminal protein sequences and attaches them to cell membrane phosphatidylinositol residues. The genetic defect in PNH was localized to the X-linked phosphatidylinositol glycan class A (PIG-A) gene, whose product functions in the transfer of N-acetylglucosamine to phosphatidylinositol as an early step in GPI anchor formation. Early tests for PNH, such as the Ham test or sucrose hemolysis test, relied on the demonstration of increased sensitivity to complement-mediated red cell lysis. Increased sensitivity and specificity are achieved using flow cytometry assays for the absence of GPI-anchored proteins such as CD59 and CD55. More sensitive tests utilize aerolysin, a channel-forming toxin that binds GPI-anchored proteins to result in cell lysis but leaves the GPI-deficient PNH cells intact. The use of a fluorescently labeled aerolysin variant that binds to GPI but fails to lyse the cells offers increased sensitivity and specificity for the detection of the PNH clone.
The clinical significance of small populations of PNH clones is unclear, and most of the published data are derived from adult patients. Small numbers of PNH cells are detectable in healthy controls, although the PNH cells in this context are typically polyclonal. Despite the frequent finding of PNH clones in the bone marrows of aplastic anemia patients, only 10% to 15% of patients subsequently develop the clinical syndrome of PNH. In the majority of patients, such clones may persist or disappear; however, some patients may develop large symptomatic PNH clones requiring therapeutic intervention. Patients with small asymptomatic PNH clones may respond to the same treatments as other patients with aplastic anemia. Patients who develop large PNH clones are at risk for developing symptoms of hemolysis or thrombosis. One recent retrospective study included 47 pediatric patients with aplastic anemia of whom 14 had a PNH clone greater than 1% at baseline (median clone size 16%). The median clone size remained small for all patients, and none of these patients developed clinical symptoms of PNH.
The etiology of marrow aplasia in PNH remains an area of active investigation. Although severe combined immunodeficiency (SCID) mice infused with bone marrow from PNH patients show preferential engraftment with the PNH clones, studies of hematopoiesis in PNH patients have not detected any selective proliferative advantage of the PIG-A − hematopoietic clones. A subsequent study comparing in vitro proliferation of PIG-A − and PIG-A + CD34 cells from PNH patients found a selective growth deficiency in the PIG-A + cell population rather than an advantage for the PIG-A mutant cells: Fas expression was elevated on the wild type compared with the GPI-deficient cells, suggesting increased resistance to apoptosis as one potential mechanism to explain their findings. No proliferative advantage of PNH hematopoietic clones was observed in mice mosaic for the PIG-A gene. To evaluate the hypothesis that autoreactive T cells might preferentially eliminate PIG-A + hematopoietic stem cells while sparing the PNH clones, the sensitivity of normal versus PNH EBV-transformed B-cell lines to autologous EBV-specific T-cell lines was examined. The PNH cells were no less sensitive to T-cell-mediated cytotoxicity than the non-PNH cells; thus the GPI-linked cell surface molecules are not required for killing by T cells. An abnormal distribution of expanded T-cell clones detected by size analysis of the complementarity-determining region 3 (CDR3) in the β-variable region (BV) mRNA of the T-cell receptor (TCR) has been noted in PNH patients, although the targets of such T-cell populations remain to be ascertained. The mechanisms underlying the clonal expansion of the GPI-negative cells in PNH or aplastic anemia is currently unclear. Despite the presence of clonal populations of PNH cells, patients with PNH do not exhibit an increased incidence of leukemias, and the GPI-negative clones do not behave in a malignant fashion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here