Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Four regions of the cornea are: (1) epithelium, (2) subepithelial zone (epithelial basement membrane, Bowman layer, superficial stroma), (3) stroma, and (4) endothelium and Descemet membrane.
Six corneal responses are: (1) defects and their repair, (2) fibrosis and vascularization, (3) edema and cysts, (4) inflammation and immune responses, (5) deposits, and (6) proliferation.
There are two distinct types of immune response to antigens: (1) innate and (2) adaptive.
The major types of immunocompetent cells include cells of the lymphoid system, mononuclear phagocytic system, myeloid system, and auxiliary cells.
Although the immune response is usually protective, tissue injury may occur from an exuberant immune reaction (hypersensitivity reactions).
Regulation of the immune response is complex, and several immunoregulatory phenomena occur in the anterior segment.
The eye has its own mucosa-associated lymphoid tissue (MALT), the eye-associated lymphoid tissue (EALT).
The cornea is bound by two cellular layers, the epithelium and the endothelium. Each layer rests on a basement membrane: the epithelial basement membrane and the Descemet membrane, respectively. Sandwiched between these cellular layers is the Bowman layer, a thin layer of acellular connective tissue, and the stroma, a thicker, cellular layer of connective tissue. For the purpose of discussing pathologic responses in the cornea, we can divide the cornea into four regions ( Fig. 5.1 ):
Epithelium
Subepithelial zone
Epithelial basement membrane
Bowman layer
Superficial stroma
Stroma
Endothelium and Descemet membrane.
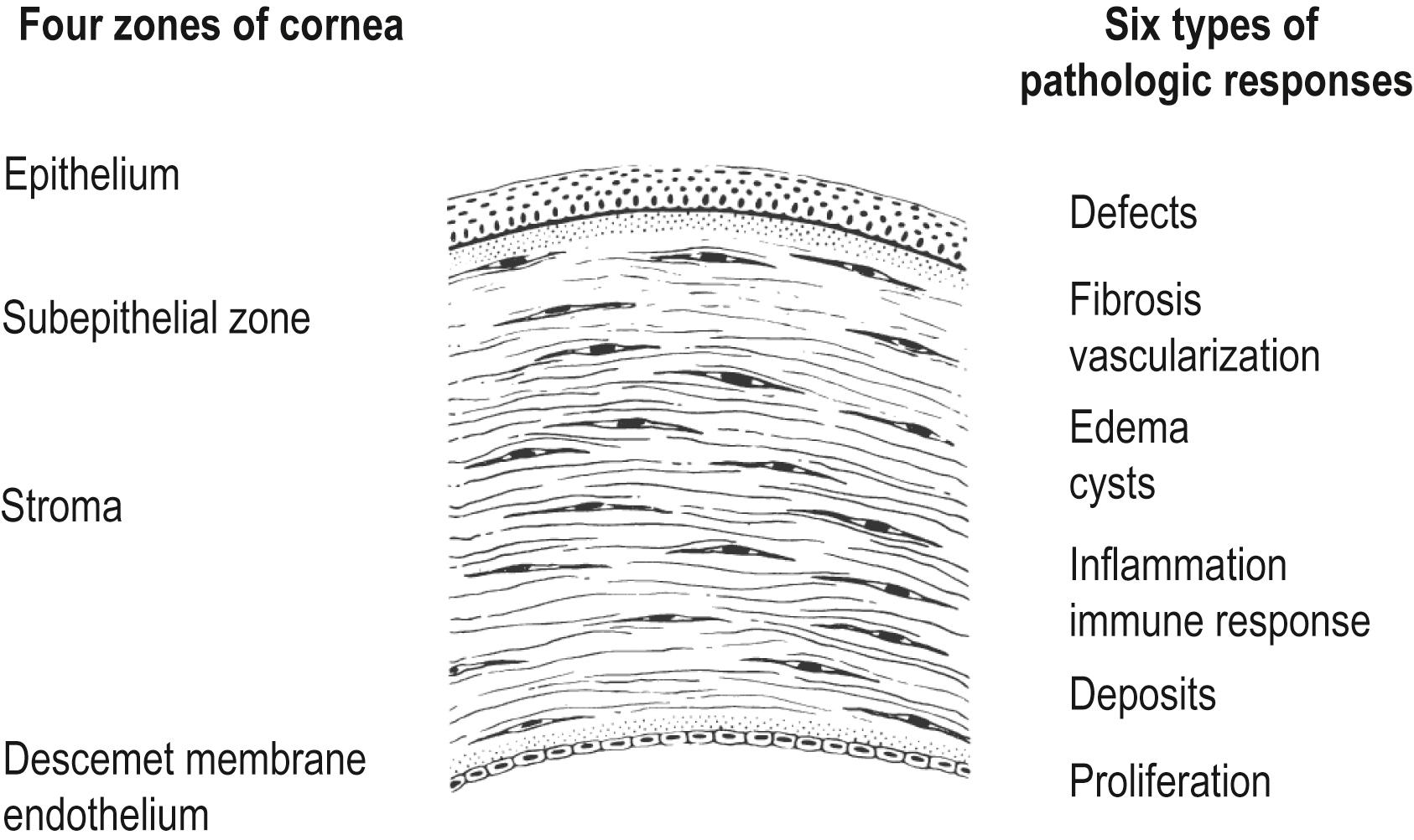
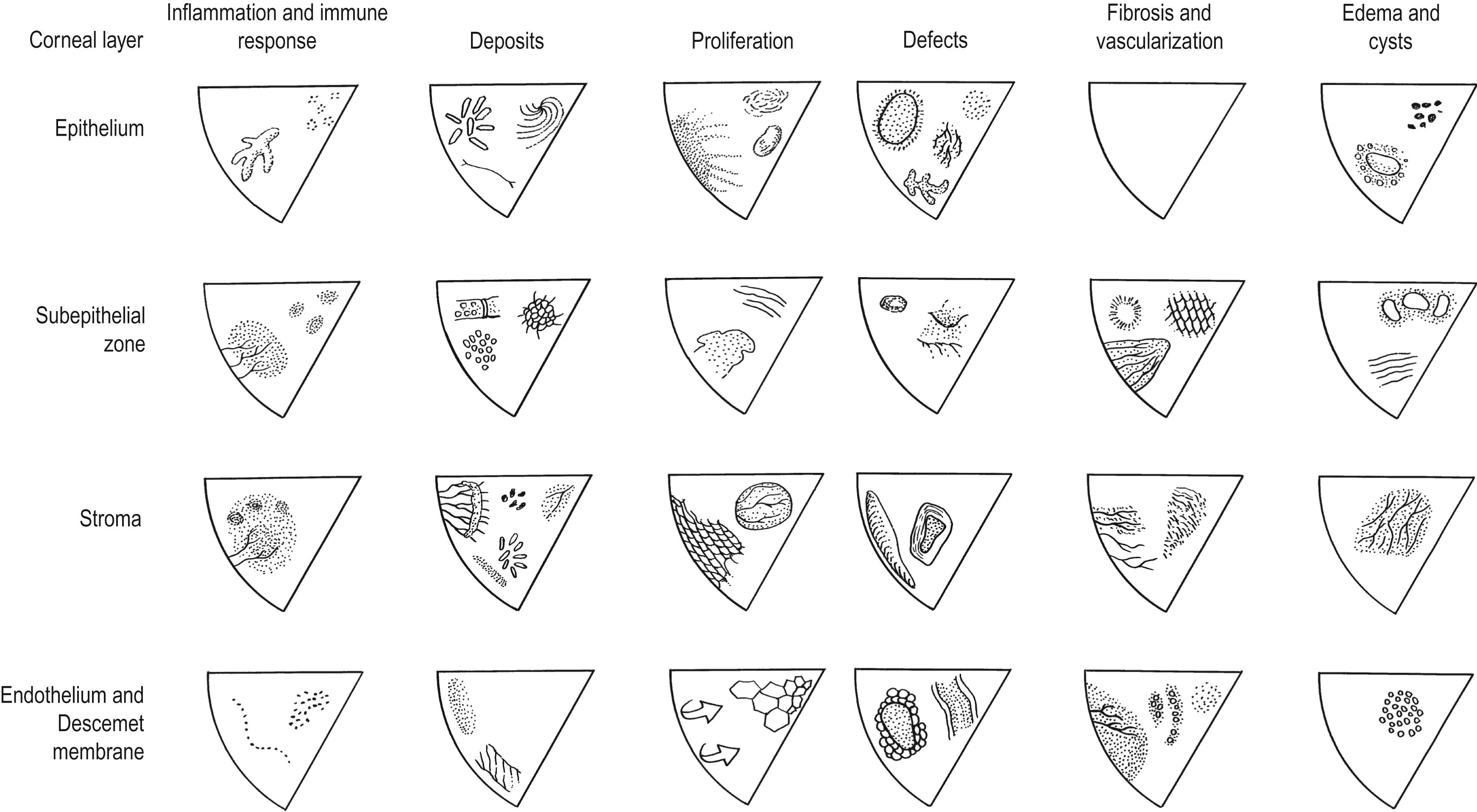
A spectrum of pathologic processes can disrupt the structure of these four zones and interfere with corneal function. However, the cornea can generate only a limited number of responses to these insults. These responses can be grouped into six distinct categories, although there is some overlap among them (see Fig. 5.1 ):
Defects and their repair
Fibrosis and vascularization
Edema and cysts
Inflammation and immune responses
Deposits
Proliferation.
In this section, the authors describe the patterns of tissue response that characterize each zone and provide representative clinicopathologic examples, as originally presented by Waring and Rodrigues and elaborated by Leibowitz and Waring ( Fig. 5.2 ).
Defects are a partial or complete absence of a portion of corneal tissue. A defect is acute if it appears suddenly, recurrent if it appears repeatedly, and chronic if it persists.
Fibrosis and vascularization are part of the normal repair process in connective tissues. In most tissues, these processes are beneficial; however, in the cornea, they lead to stromal scarring with opacification and disruption of optical function. Because the normal cornea is avascular, the appearance of blood vessels in the cornea is always abnormal.
Edema and cysts are grouped together for simplicity and because they often resemble each other clinically. When edema (i.e., excess fluid in or between cells) occurs, the normal architecture is disrupted and may lead to opacification. The edema can be diffuse (stromal edema) or focal (epithelial bullae). Corneal cystic areas are focal collections of fluid or solid material without an epithelial lining.
Inflammation and immune reactions result from a variety of insults that can lead to reversible or irreversible changes. In general, three basic steps are involved: (1) an inciting pathologic event; (2) a host cellular and/or humoral inflammatory and/or immune response; and (3) a repair process. These three steps are beneficial when they contain and control the pathologic process but harmful if they lead to corneal damage.
Abnormal types or amounts of material can be deposited in the cornea from exogenous or endogenous sources, including corneal dystrophies and degenerations and autologous breakdown products.
There are three basic types of abnormal proliferative responses: (1) abnormal growth and maturation; (2) ectopic migration; and (3) corneal limbal stem cell deficiency, leading to conjunctivalization of the corneal surface.
A summary of the six pathologic responses in the four corneal zones is presented in Fig. 5.2 , with representative disorders occupying each box of this matrix. The amount of functional deficit inflicted by a disease process depends on the type, duration, severity, and location of the insult, as well as the cornea’s ability to repair and restore normal structure and function.
The normal corneal epithelium is continuously replaced every 4–7 days, a process that involves: (1) differentiation of the basal cells toward the surface; pathologic example: epidermalization and keratinization in vitamin A deficiency; (2) centripetal movement of limbal and peripheral cells; pathologic example: chemical damage of the limbal epithelium; and (3) desquamation of epithelial cells from the surface; pathologic example: extended-wear soft contact lenses interfering with normal desquamation. Among the causes of epithelial defects are corneal abrasions, focal foreign bodies, neurotrophic keratopathy, and sloughing of cells in recurrent erosion.
Repair of damage to the corneal epithelium involves four major stages: sliding of cells to cover the defect, mitosis of cells to restore normal thickness, attachment of cells to the basement membrane, and remodeling to establish normal architecture. , Four factors are required to reestablish normal epithelial integrity: a normal basement membrane, vitamin A, normal tear film, and an intact sensory innervation.
Because the corneal epithelium lacks connective tissue, it is not subject to fibrosis or vascularization. However, either process can occur beneath the epithelium and may affect epithelial repair.
The epithelium takes on a cystic appearance when fluid accumulates within or between the cells (e.g., endothelial dysfunction) or when abnormal epithelial maturation creates small, debris-filled cystic spaces (e.g., Cogan microcysts in epithelial basement membrane degeneration [EBMD]). Even mild changes can reduce visual acuity by creating an irregular surface that diffracts and scatters light.
There are two common causes of epithelial edema: endothelial dysfunction and epithelial hypoxia and trauma. When intercellular fluid lifts cells from the basement membrane, bullae appear. The epithelial sheet is held together by desmosomal connections ( Fig. 5.3 ). Contact lens–induced edema can be caused by epithelial hypoxia, hypercapnia, trauma due to improper fitting or overwear, or a combination of these. Intracellular edema results when the compensatory mechanism of the epithelial cells is exceeded.
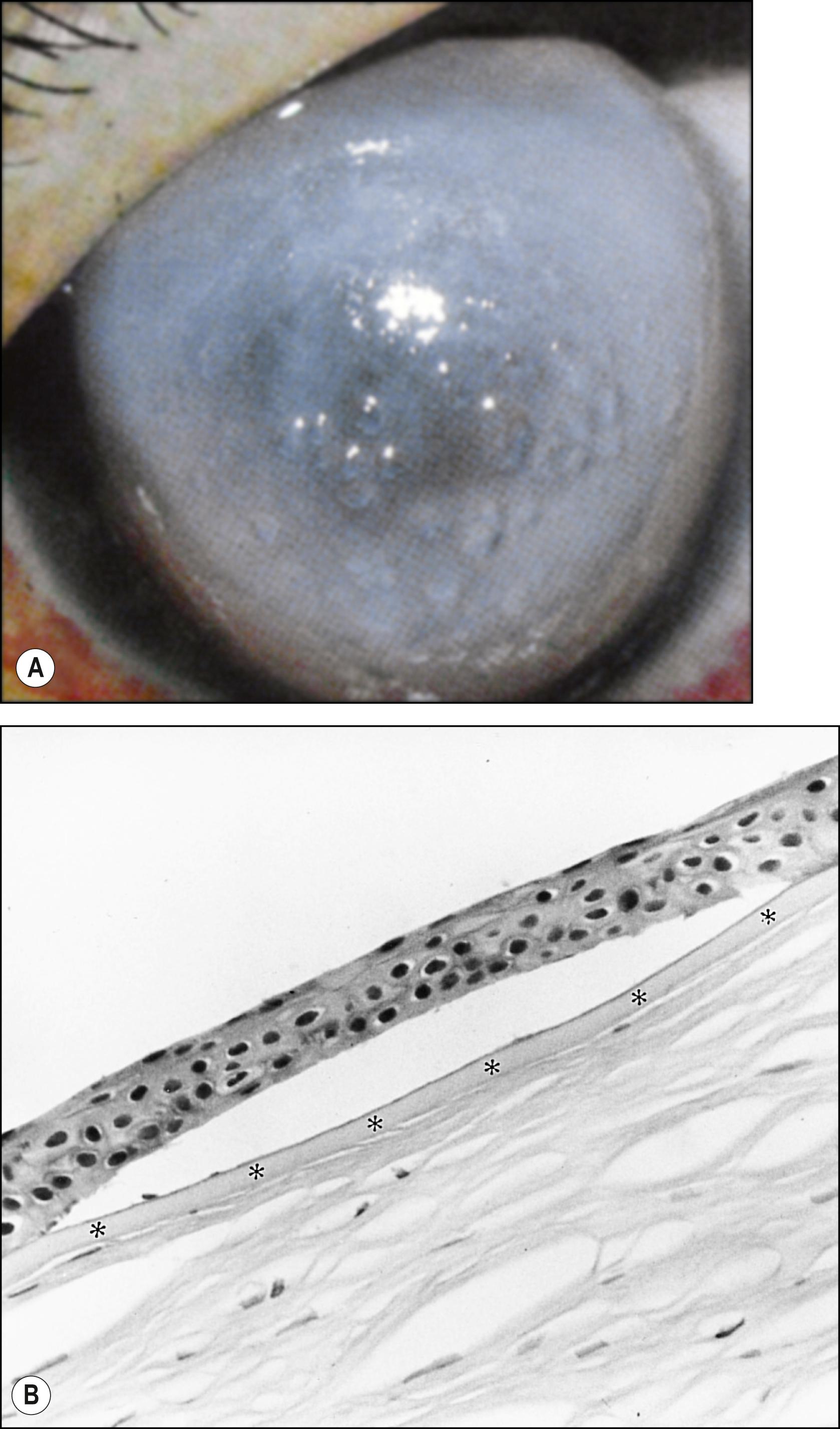
Cysts result from accumulation of rapidly multiplying (e.g., Meesmann dystrophy) or degenerating (e.g., EBMD) epithelial cells. In recurrent epithelial erosions, chronically regenerating epithelium results in clusters of clear, pinpoint microcysts in the area of a previous erosion.
In corneal allograft rejection, the donor epithelium may be attacked by sensitized cytotoxic T (Tc) lymphocytes. This is a specific response to foreign antigens (e.g., the human leukocyte antigens [HLAs] in epithelial cells) and appears clinically as a serpentine line that spreads from the graft-host margin toward the center of the transplant. Epithelial healing often keeps pace with cell death and may make epithelial rejection a passing, asymptomatic phenomenon.
Epithelial deposits can be divided into four categories based on their origin: (1) elements, (2) drugs, (3) systemic diseases, and (4) corneal dystrophies and degenerations.
The most common intraepithelial deposit is iron; hemosiderin pigment is deposited in lysosomes of the basal epithelial cells in a linear pattern ( Fig. 5.4 ).
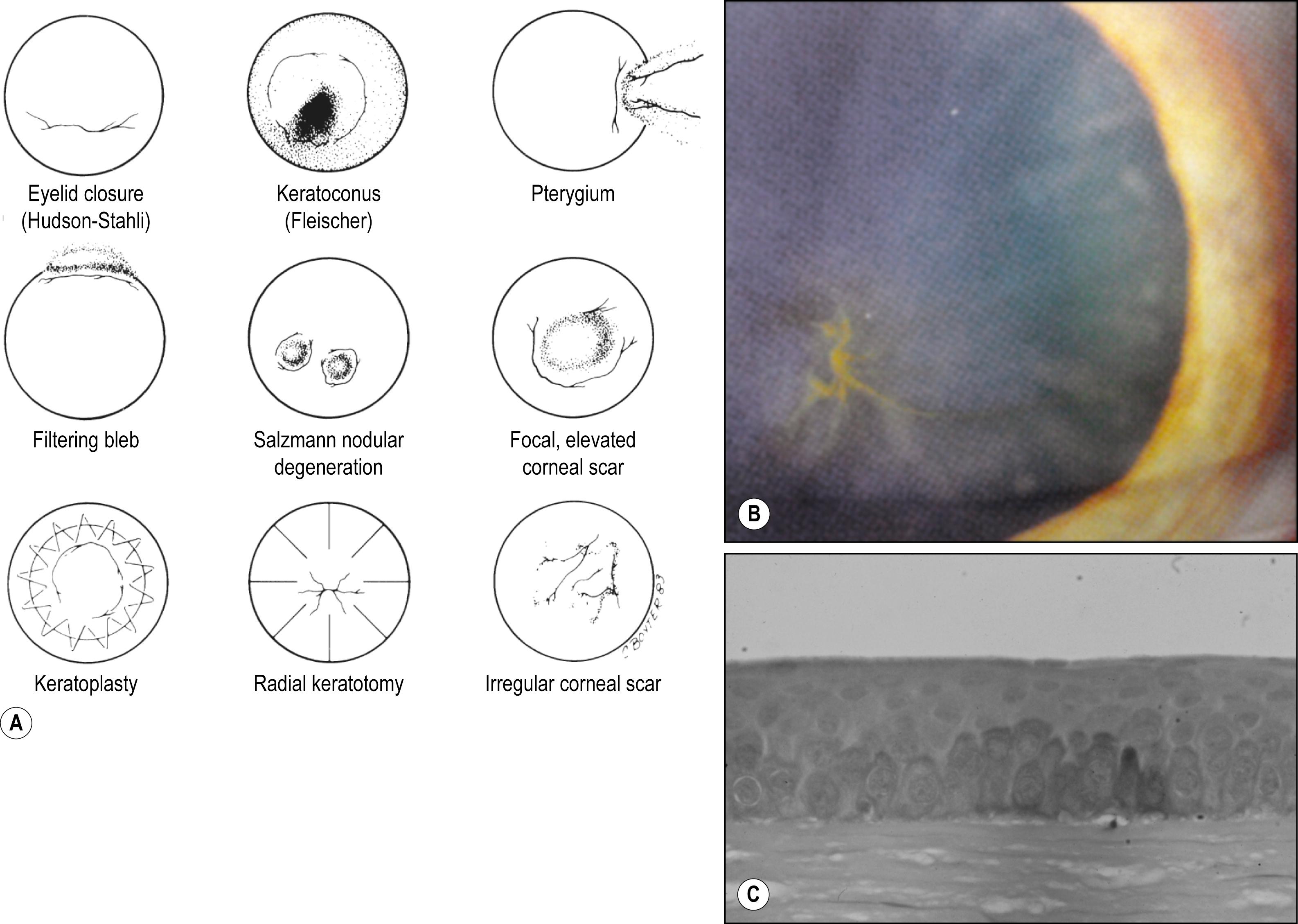
Numerous systemically administered drugs can accumulate in the epithelium; the most common being the antiarrhythmic drug amiodarone, which presents in a characteristic whorl-like pattern. The severity of the deposits is directly proportional to the total drug dose. In general, most of the corneal deposits disappear slowly over time when the drug is withdrawn.
Epithelial deposits from systemic diseases seldom reduce visual acuity. Exceptions include certain inherited metabolic disorders (e.g., mucopolysaccharidosis type VI-A, Maroteaux-Lamy, and the sphingolipidosis of Fabry disease), multiple myeloma, and cystinosis.
Corneal dystrophies rarely produce deposits within the epithelium. Meesmann epithelial dystrophy is the exception.
The epithelium manifests a full spectrum of growth and maturation disorders, including hyperplasia, metaplasia, and dysplasia-neoplasia. Because the epithelium conforms to the contour of the underlying basement membrane and stroma, its thickness may vary. Areas of thinning occur over elevations (e.g., over Salzmann nodules or keratoconic cones), whereas areas of thickening occur when the epithelium fills in defects (e.g., a facet). These adjustments in epithelial thickness preserve a smooth corneal surface to help maintain optimal optical function, but the exact mechanisms involved are unknown.
Corneal metaplasia from a normal to an abnormal keratin-forming epithelium can occur in severe ocular inflammation (e.g., Stevens-Johnson syndrome) and is often associated with conjunctival metaplasia.
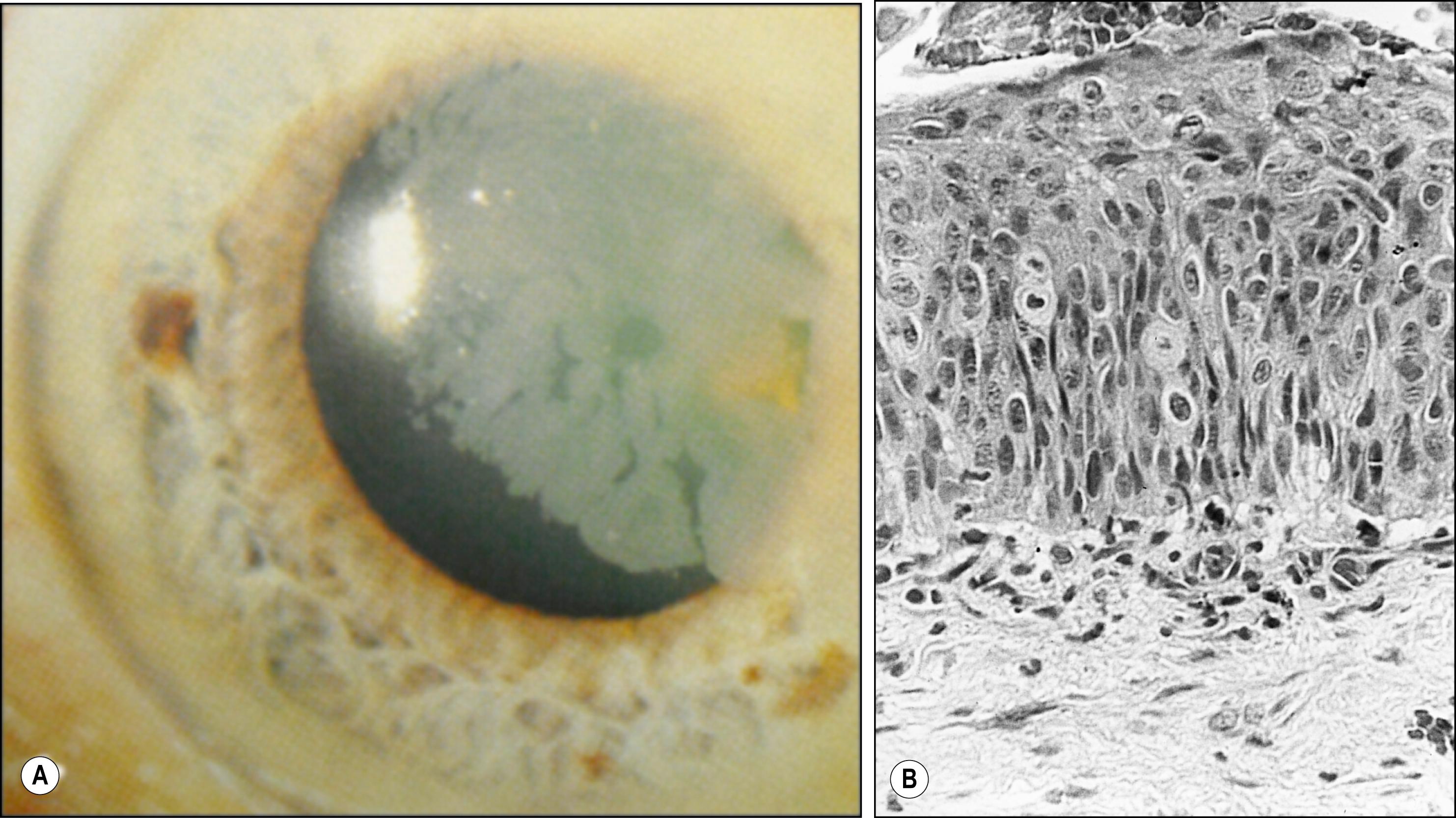
The epithelium is the only layer of the cornea that can become neoplastic, giving rise to squamous cell carcinoma. This process often begins at the limbus where the stem cells are located. The full spectrum of changes, from mild dysplasia to carcinoma in situ, is termed “corneal intraepithelial neoplasia (CIN).” It commonly appears as a gray intraepithelial sheet advancing onto clear cornea but can also appear as a raised limbal mass ( Fig. 5.5 ).
Ectopic migration can be seen after perforating, accidental, or surgical trauma. In these cases, proliferating corneal epithelium can invade the anterior chamber through a fistula and form a cyst or a downgrowth sheet. Epithelium can also proliferate under a laser in situ keratomileusis (LASIK) flap (ingrowth).
The epithelial basement membrane and Bowman layer are both acellular tissues and have different healing responses. The basement membrane is secreted by the basal epithelial cells and can be regenerated or produced in an excess or altered form. However, the Bowman layer does not regenerate. A defect in the Bowman layer is filled in with fibroblasts and connective tissue, creating a permanent scar.
Neither the epithelial basement membrane nor Bowman layer can become fibrotic or vascularized. Fibrous or vascular tissues can spread between the two layers as either an avascular or vascular pannus.
Patches of avascular fibrous tissue can appear beneath the epithelium as a nonspecific response (e.g., advanced Fuchs endothelial dystrophy). The subepithelial haze that can occur 4–8 weeks after photorefractive keratectomy (PRK) is another example. This haze corresponds to a layer of subepithelial collagen and proteoglycans that remodels over time.
Salzmann nodular degeneration is a distinctive type of avascular subepithelial fibrosis. The grayish “stuck-on” lesions consist of hyaline and basement membrane material and accumulate between the Bowman layer and the thinned but continuous epithelium.
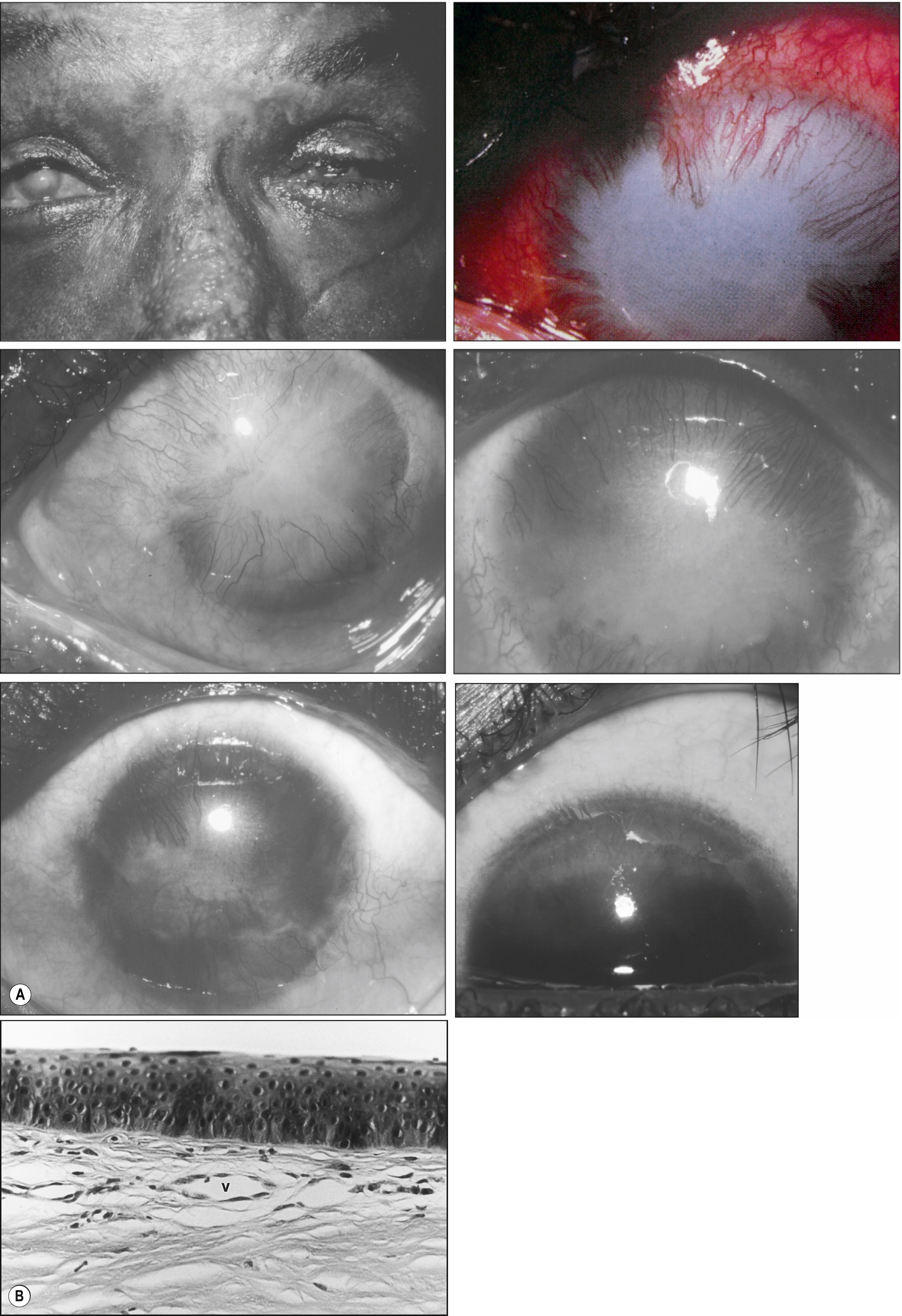
Subepithelial vascular fibrosis involves three basic cells: leukocytes, proliferating vascular endothelial cells, and active fibroblasts that secrete an extracellular connective tissue matrix. This process can occur after the following: (1) mild acute inflammation (e.g., hypoxia beneath an extended-wear soft contact lens) in which a very fine sheet of fibrovascular tissue slowly migrates from the limbus; (2) mild chronic inflammation (e.g., trachomatous eyelid scarring) where a progressive dense pannus spreads centrally; and (3) severe acute inflammation (e.g., alkali burns) in which a thick layer of exuberant fibrovascular tissue can progress across the entire cornea ( Fig. 5.6 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here