Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The limbs develop from permissive and instructive interactions between epithelium and mesenchyme at specified times and places along the lateral body wall. The outgrowths are initiated at defined positions along the embryonic axis where these cell lines continue to proliferate, giving rise to local thickenings that soon develop into limb buds ( ). The early limb bud elongates and, gradually, the different limb regions become apparent. A broad plate forms at the tip and, within it, digital rays develop that mark the position of the forming digits. The digits later separate and become tipped with nails. Fig. 19.1 shows the main stages in the development of a human upper limb; the stages in development of the lower limb are basically the same.
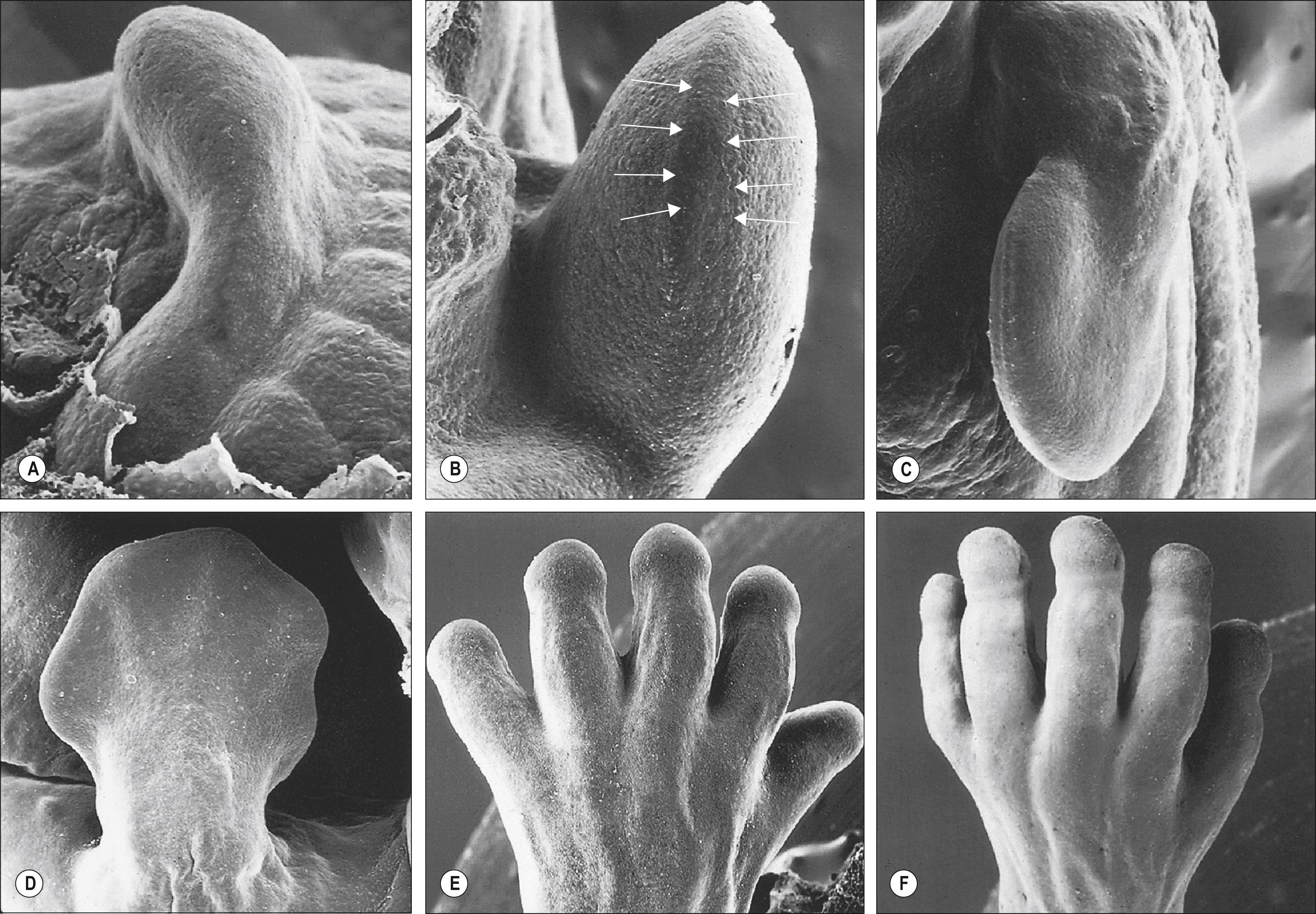
Upper and lower limb buds protrude laterally from the trunk, encased in an ectodermal epithelium rimmed by a longitudinal ridge of columnar epithelial cells: the apical ectodermal ridge. The ectoderm will ultimately form the epidermis of the skin. Beneath the epithelium is a mixed population of mesenchymal cells derived from the proliferating epithelial somatopleuric lateral plate ( Ch. 12, p. 205 ); these cells have already undergone restriction to develop into upper or lower limbs before the limb buds are visible. They give rise to the connective tissues, bone, cartilage, ligament, tendon, muscle attachment and encasing layers, loose connective tissue and dermis. Muscle in the limb originates from paraxial mesenchyme cells that migrate from the somites; this cell population also contributes to some of the endothelial cells that produce an extensive vascular network in the early limb bud. Motor and sensory nerves and their associated Schwann cells, together with melanocytes destined for the skin, migrate into the developing limb somewhat later; motor neurones derive from the neural plate; sensory neurones, Schwann cells and melanocytes are derived from the neural crest.
For descriptive, experimental and conceptual purposes, various ‘axes’, borders, surfaces and lines in relation to the developing limb bud are defined and named ( Fig. 19.2 ). An imaginary line from the centre of the elliptical base of the bud, through the centre of its mesenchymal core to the centre of the apical ectodermal ridge, defines the proximodistal axis of the limb bud (previously known in descriptive embryology simply as the axis). Named in relation to the proximodistal axis, the cranially placed limb border is the preaxial border and the caudally placed limb border is the postaxial border. (In vertebrate embryos used as experimental models, e.g. chick embryos, the pre- and postaxial borders are termed anterior and posterior borders, respectively.) Any line that passes through the limb bud from preaxial to postaxial border, orthogonal to the proximodistal axis, constitutes a craniocaudal axis. The dorsal and ventral ectodermal surfaces clothe their respective aspects from preaxial to postaxial borders, and any line that passes from dorsal to ventral aspect, orthogonal to both proximodistal and craniocaudal axes, constitutes a dorsoventral axis. It should be noted here that the terms dorsal and ventral axial lines are to be used exclusively in relation to developing and definitive patterns of cutaneous innervation of the limbs and their associated levels of the trunk.
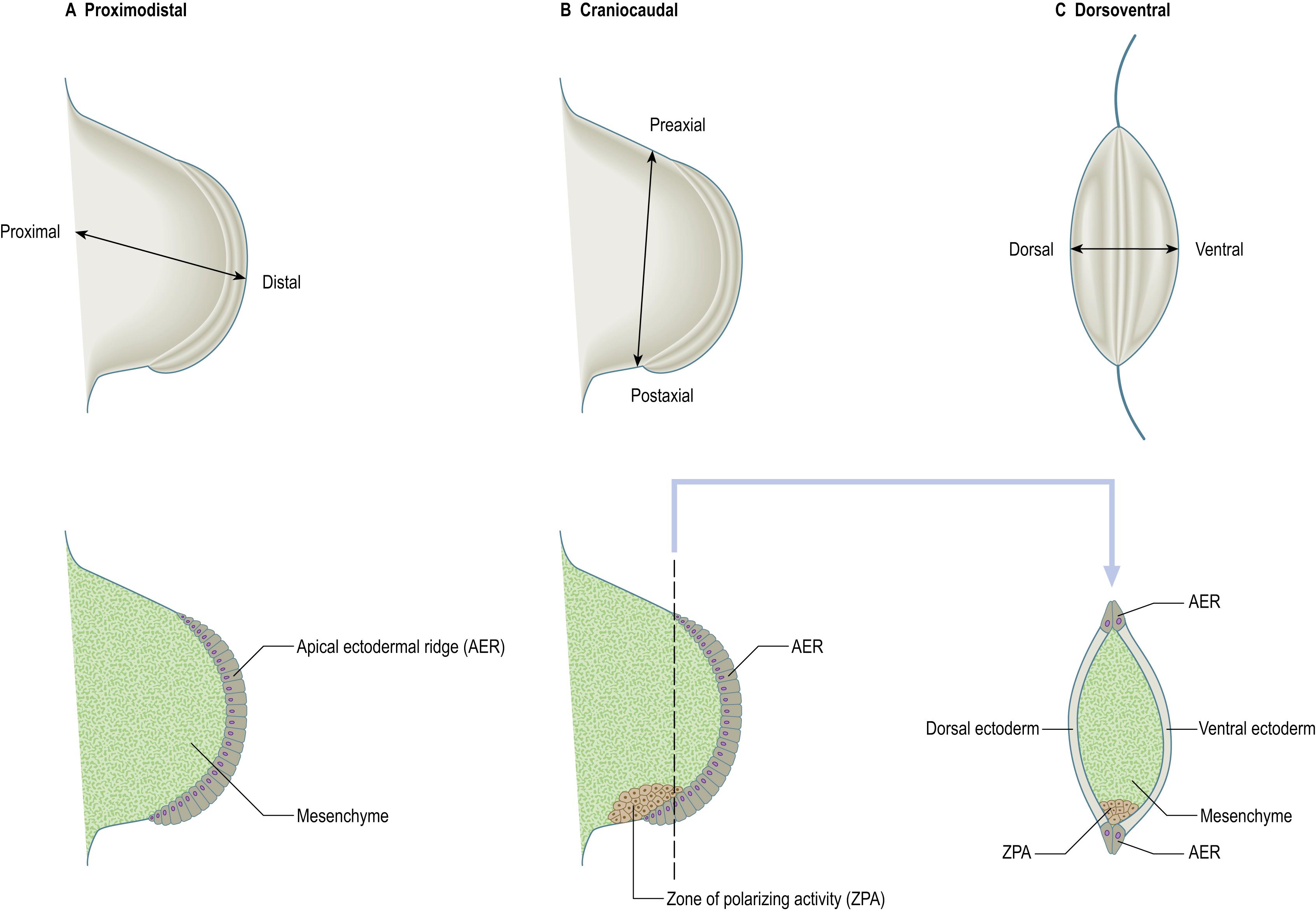
The three developmental axes (proximodistal, craniocaudal and dorsoventral) can be identified in the human developing limb bud by stage 13 (31–33 days postfertilization, see Fig. 23.3 ). Experiments on wing buds of chick embryos have shown that a different set of cell–cell interactions control the development of structures in relation to each of the three principal axes of the limb bud: proximodistal (e.g. humerus to fingers), craniocaudal (e.g. thumb to little finger) and dorsoventral (e.g. extensors to flexors) (see Fig. 19.2 ). The same sets of interactions take place in chick leg limb buds. These experiments are described in detail in .
Outgrowth of the limb bud along its proximodistal axis is controlled by interactions between the apical ectodermal ridge and underlying somatopleuric mesenchyme, and is accompanied by the sequential formation of limb structures along the proximodistal axis. Growth and development along its craniocaudal axis involves an interaction between a small population of mesenchyme cells on the postaxial border of the limb bud, termed the zone of polarizing activity, or polarizing region, and adjacent mesenchyme. The development of the dorsoventral axis of the limb involves an interaction between the surface ectoderm on the sides of the limb bud and the underlying mesenchyme. These sets of cell–cell interactions are coordinated so that the developing limb develops properly with respect to all three axes.
Early differential growth of parts of the limb bud results in two main changes to the originally symmetric axes of the limb. The dorsal aspect of the limb grows faster than the ventral, which causes the limb bud to curve around the body wall; the ventral surface of the limb (closest to the body wall) remains relatively flat, but the dorsal surface bulges into the amniotic cavity. The apical ectodermal ridge, originally facing laterally, becomes increasingly directed caudally (upper limb) and ventrally (lower limb) as the limbs extend around the body.
The apical ectodermal ridge (AER, or apical ridge) is required for limb bud outgrowth. When it is removed from a chick wing bud, outgrowth ceases and a truncated wing results with the level of truncation depending on the stage at which the ridge is removed; severe truncations result when the AER is removed from early limb buds while progressively more distal structures develop when the AER is removed at later stages. The importance of the AER is further demonstrated by grafts of a second AER to a wing bud inducing a new outgrowth that develops digits ( , ) ( Fig. 19.3 ). The molecular basis of the cell–cell interactions between apical ridge and underlying mesenchyme is conserved between different vertebrates; the apical ectodermal ridge of a mouse limb bud can promote outgrowth of a chick wing bud.
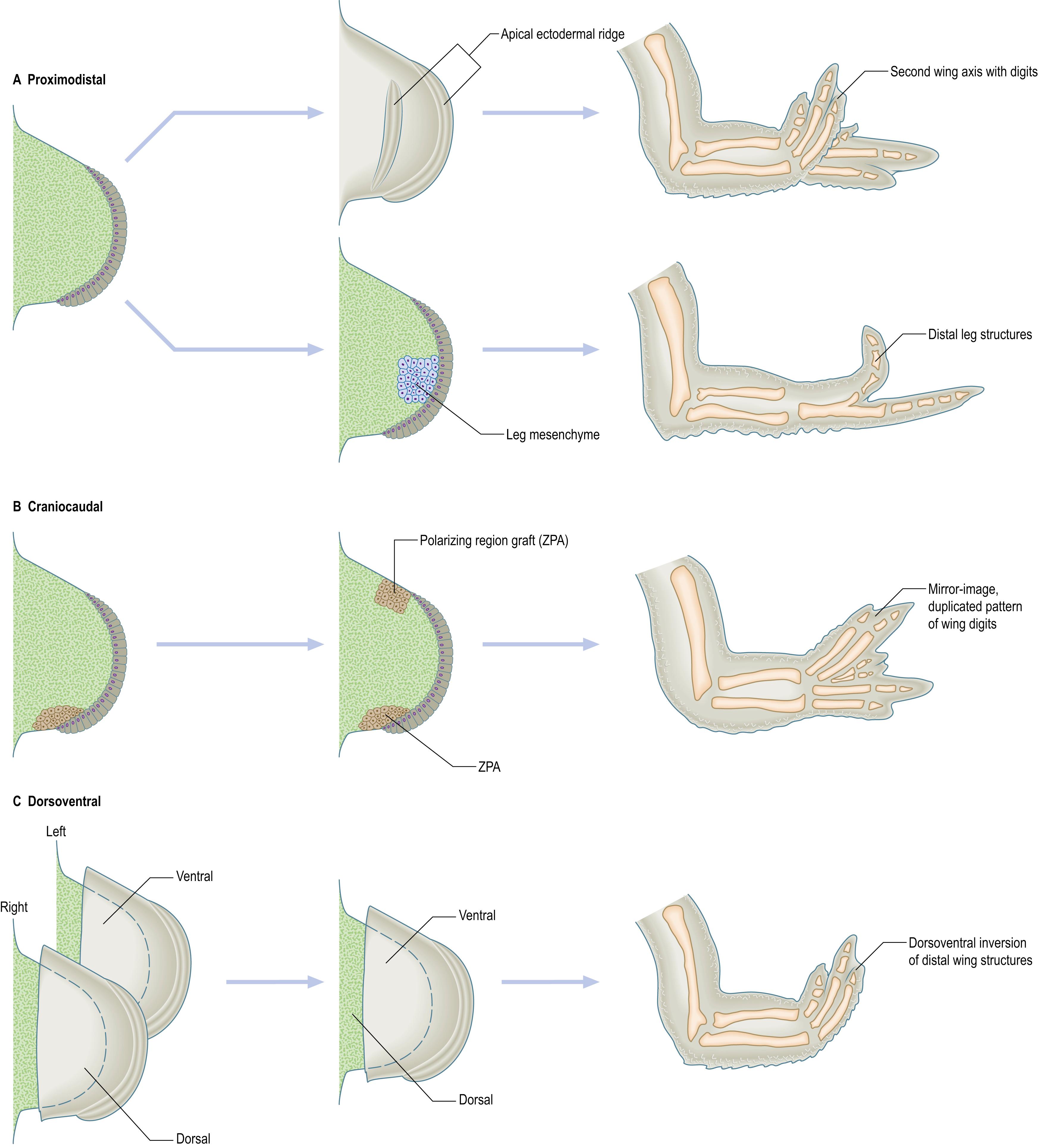
There is evidence that the limb mesenchyme beneath the apical ectodermal ridge provides a factor that is essential for maintaining activity of the ridge (the so-called apical ectodermal ridge maintenance factor). Thus, the craniocaudal extent of production of apical ectodermal ridge maintenance factor determines the length of the apical ectodermal ridge. When cells from the proximal region of a chick leg bud are grafted to the tip of a wing bud beneath the apical ectodermal ridge, the apical ridge is maintained and outgrowth continues. It should be noted that the grafted cells produce the structures according to their new position, but because they are leg cells, they form toes rather than wing digits ( ) (see Fig. 19.3 ). In addition, leg mesenchyme will pass information to local wing ectoderm, eliciting appropriate epidermal development: in this case, formation of scales rather than feathers.
For many years it was thought that a timing mechanism linked to limb bud outgrowth specified the pattern of structures along the proximodistal axis. The timing mechanism was postulated to operate at the tip of a developing limb bud beneath the apical ectodermal ridge in the zone of proliferating undifferentiated mesenchyme cells, which was therefore called the progress zone. It was assumed that, as cells left the progress zone during limb bud outgrowth, their proximodistal fate becomes fixed. Thus, cells spending a short time at the tip of the limb bud would be specified to form proximal structures, while cells spending a longer time would be specified to form distal structures ( ). It was suggested that the proximodistal pattern is specified in the very early limb bud ( ) and that the prespecified structures are progressively elaborated as the bud grows out. It now seems that the proximal part of the limb (humerus/femur) is specified in the very early limb bud by signals from the body wall ( , ) while the more distal structures are specified as the bud grows out by a timing mechanism ( ).
The zone of polarizing activity (or polarizing region) at the postaxial border was discovered by a grafting experiment on chick wing buds ( , ). When a polarizing region is grafted to the preaxial border of an early chick wing bud, so that there are polarizing region cells at both borders, duplication of distal limb structures occurs, with two ulnae (instead of an anterior radius and posterior ulna) and a mirror-image pattern of six digits (the normal chick wing has three digits (see Fig. 19.3 )). The digit closest to the polarizing region is always the most postaxial digit (in the chick wing, traditionally designated as digit 4), while more preaxial digits develop further away. Thus, it was proposed that the polarizing region is the source of a diffusible morphogen which controls both digit pattern and digit number. Digit pattern is specified by concentration of the morphogen although timing is also important, e.g. an additional digit can be specified by a prolonged exposure to a low morphogen concentration or by a short exposure to a high morphogen concentration. Digit number is controlled by regulating the width of the limb bud: both directly, via promoting cell proliferation, and indirectly, by controlling production of the apical ectodermal ridge maintenance factor. The width of the limb bud is also limited by zones of programmed cell death on the preaxial and postaxial borders. (It should be noted that, in the literature relating to work on animal embryos, pre- and postaxial borders are termed anterior and posterior borders, respectively, and therefore the regions in which programmed cell death occurs are termed the anterior and posterior necrotic zones.) When the length of the apical ectodermal ridge is reduced, fewer digits form (oligodactyly), while, when the apical ectodermal ridge becomes longer, more digits form (polydactyly). Other regions of apoptotic cell death occur between the digits and result in separation of the digits, but these appear later than the anterior and posterior necrotic zones; the resultant debris in the necrotic zones is removed by macrophages.
Grafts of the zone of polarizing activity only generate mirror-image digit patterns when placed beneath or adjacent to the apical ectodermal ridge in a chick wing, suggesting that the apical ridge produces a factor that maintains polarizing region activity. A positive feedback loop, in which the polarizing region and the apical ectodermal ridge mutually maintain each other’s activity, ensures that development of craniocaudal and proximodistal axes is coordinated. Like that of the apical ectodermal ridge, the function of the polarizing region is conserved across species and grafts of the postaxial border of limb buds from mammals, including human limb buds ( ), generate additional chick digits. This shows that the polarizing region produces the same molecular signal in both birds and mammals, but that the character of a digit depends on the responding cells.
Signals from the ectoderm control the dorsoventral axis. It is possible to remove the surface ectodermal epithelium (like peeling off a glove) from the mesenchymal core of an early chick limb bud. When the ectodermal epithelium from a left limb bud is recombined with the mesenchymal core from a right limb bud, keeping the craniocaudal axis in register, the distal part of the limb that develops conforms to the polarity of the ectoderm rather than the polarity of the mesenchyme ( ) (see Fig. 19.3 ). This can be seen in the pattern of muscles, orientation of the joints and eventual characteristic differentiation of the epidermis.
The apical ectodermal ridge produces fibroblast growth factors, extracellular cell–cell signalling molecules that act on the underlying mesenchyme cells and are essential for limb bud outgrowth ( ). Fibroblast growth factors also maintain the activity of the polarizing region. The latter expresses Sonic hedgehog ( Shh ), a gene encoding another class of secreted protein that acts as diffusible morphogen ( ). Sonic hedgehog protein (Shh) plays a pivotal role in the specification of the craniocaudal digit pattern: it spreads across the chick wing bud so that cells at different positions across the craniocaudal axis are exposed to different Shh concentrations ( ). In mouse limb buds, the two most postaxial digits (corresponding to the little finger and the ring finger in the hand) come from the polarizing region itself and are specified by the length of exposure to Shh ( ). Responding cells transduce the signal through the Gli family of transcription factors. (The Gli proteins are bifunctional effectors of Shh signalling and can lead to either activation or repression of expression of target genes.) Shh secreted by the polarizing region also acts on adjacent mesenchyme cells, controlling their proliferation and maintaining the expression of the Gremlin gene, encoding an extracellular antagonist of secreted bone morphogenetic proteins (BMP). Gremlin functions as the apical ectodermal ridge maintenance factor by antagonizing BMP signalling which would otherwise inhibit fibroblast growth factor ( Fgf ) expression in the apical ectodermal ridges ( , ). Dorsal limb ectoderm expresses Wnt7a , a gene encoding another extracellular signalling molecule that acts as a dorsalizing signal ( ).
Among the genes expressed in response to cell–cell signalling molecules in the limb bud and which form dynamic and overlapping domains of expression are 5′ members of the Hoxa and Hoxd gene complexes. Hox genes code for transcription factors that act in a cell-autonomous fashion to control expression of many target genes. There are two phases of Hoxd and Hoxa expression in the developing limb. In the first phase in the early limb bud, the expression of 5′ Hoxd genes ( Hoxd9 , d10, d11 d12 and d13 ) is activated sequentially and in progressively more restricted distal and proximal domains, while the expression of 5′ members of the Hoxa gene complex is activated similarly, but slightly later than the Hoxd genes, in overlapping domains along the proximodistal axis. The first phase of Hox gene expression establishes the growth and pattern of proximal limb structures, i.e. upper and lower arm; the second phase, which occurs in the broad digital plate, establishes the growth and pattern of distal structures, i.e. the hand/foot. Regulatory gene sequences in the DNA on either side of the Hoxd complex have been identified that control the switch in expression from the first phase to the second phase as the limb bud develops ( ). The gene encoding the transcription factor Lmxb1 is expressed in the dorsal part of the limb, in response to Wnt7a signalling by dorsal ectoderm, and establishes the pattern of dorsal structures.
The molecular basis of limb development is conserved in humans. Mutations associated with many of the genes described above are responsible for human congenital limb malformations. Details can be found in , and . There are, however, many human congenital limb anomalies for which the genes involved are not known. A global gene expression analysis of developing mouse limbs estimates that several thousand genes, maybe around 35% of the genome, participate in some aspect of limb development ( ), with relatively fewer genes (hundreds) preferentially expressed in either forelimb or hindlimb. The first genome-wide study on developing human limbs has documented patterns of histone modifications marking active regulatory sequences in the DNA that control gene expression, with the aim of identifying regulatory regions of genes active specifically in human limb buds ( ).
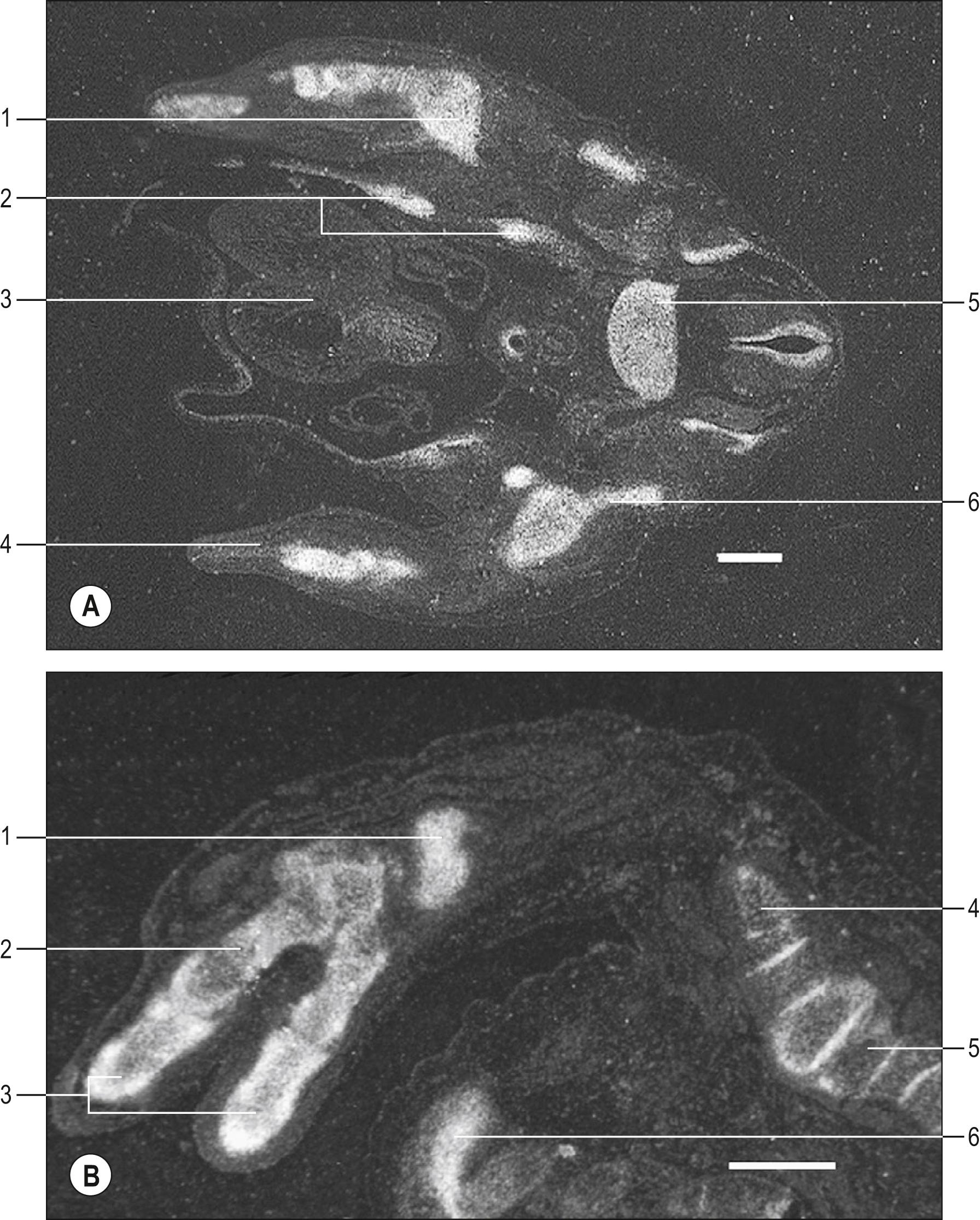
Somatopleuric mesenchyme in the limb buds gives rise to the connective tissues of the limb. Cell lineage analyses in early chick limb buds show that a single mesenchyme cell can give rise to the major types of connective tissue including cartilage, perichondrium, tendon and epi-, peri- and endomysial layers around the muscles. The first signs of the formation of skeletal elements are regions of increased cell density in the core of the limb bud. The cells in these regions go on to differentiate into chondrocytes, which produce large quantities of extracellular matrix, in which they become embedded, giving rise to a cartilage model of the bone that will eventually develop. One centre of chondrogenesis forms in the proximal region of an early human limb bud; two centres of chondrogenesis then form more distally as the limb bud lengthens, followed by five centres in the broadened distal tip. The Sox9 transcription factor is essential for chondrocyte differentiation in mouse embryos; the Sox9 gene is expressed before and during deposition of cartilage ( ). Sox9 gene transcripts are expressed in the humerus and forearm skeletal elements of human embryos at approximately 44 days postfertilization, and in the carpals, metacarpals and phalanges, in addition to the more proximal elements, at approximately 52 days postfertilization ( Fig. 19.4 ). Mutations in human Sox9 are associated with campomelic dysplasia, a skeletal dysmorphology syndrome ( ). Some bony eminences, which are sites of entheses, have been shown to derive from chondrogenic foci which form separately and later than the cartilage model of the long bones ( ). The cells co-express Sox9 and the transcription factor scleraxis ( Scx ) and blockage of Scx arrests eminence development. Scleraxis is also expressed in the early limb subjacent to the ectoderm in tendon progenitors and later in the cells of all tendons. Studies of Scx knockout mice show that Scx is required for the differentiation of force-transmitting tendons but not for muscle-anchoring tendons ( ). A population of Scx + /Sox9 + progenitor cells have been shown to give rise to Scx − /Sox9 + chondrocytes and Scx + /Sox9 − tenocytes/ligamentocytes. The Scx + /Sox9 + cells contribute particularly to establishing the enthesis organ between the cartilage and tendon. The closer the tendon is to the cartilage primordium (anchoring tendon), the more tenocytes arise from the Scx +/ Sox9 + lineage. In general, the number of tenocytes derived from this lineage decreases with increasing distance from the cartilage model, although there is variation between individual force-transmitting tendons ( ). For further details on tendon development see .
Two methods of bone ossification occur ( Ch. 5 ). In intramembranous ossification mesenchymal bone precursors differentiate directly into osteoblasts; in endochondral ossification, osteoprogenitor cells which give rise to osteoblasts enter the cartilage models of the long bones via vascular invasion. Initially a periosteal collar forms, and osteoblasts proliferate and secrete bone matrix within the mid-section of the future diaphysis, forming a primary ossification centre; this extends proximally and distally until it reaches the future epiphysial levels, where growth plates (epiphysial plates) will be established. For further details on the cellular development of cartilage and bone, see Chapter 5 . For further details on the development of specific limb bones, see appropriate regional chapter. It should be noted that maternal nutrition and vitamin status affects fetal bone growth and that despite vitamin supplementation in Western countries, pregnant women in northern latitudes may remain vitamin D deficient in winter pregnancies. Infant femur length was shorter in pregnancies with early serum 25-hydroxyvitamin D below the median value ( ).
The first evidence of bone formation in human embryos is intramembranous ossification of the clavicle (stage 18–19) followed by the mandible. Endochondral ossification of cartilage models of long bones is initially seen in the limbs in stage 21–23 human embryos (50–58 days postfertilization). By postmenstrual week 10, columns of chondrocytes can be seen at the epiphysial level of most bones. However, only the lower end of the femur and upper end of the tibia develop secondary ossification centres before birth.
Regions of developing cartilage are easily recognized histologically in the developing limb because they consist of widely spaced cells surrounded by matrix. These regions are separated by transverse bands of relatively flattened cells – interzones – which mark the sites of future joints. Their subsequent development varies according to the type of joint that is formed.
In developing fibrous joints, the interzone is converted into collagen, which is the definitive medium connecting the bones involved. In developing synchondroses, the interzone becomes growth cartilage of the modified hyaline type; in developing symphyses, it is predominantly fibrocartilage. In developing synovial joints, the interzonal mesenchyme becomes trilaminar when a more tenuous intermediate zone appears, splitting the mesenchyme into two dense strata. As the skeletal elements chondrify, and in part ossify, the dense strata of the interzonal mesenchyme also become cartilaginous; subsequent cavitation of the intermediate zone establishes the cavity of the joint. The loose mesenchyme around the cavity forms the synovial membrane and probably also gives rise to all other intra-articular structures, such as ligaments, discs and menisci. In joints containing discs or menisci, and in compound articulations, more than one cavity may appear initially, sometimes merging later into a complex single cavity. For details of the genes expressed in joint development consult . As development proceeds, thickenings in the fibrous capsule can be recognized as the specializations peculiar to a particular joint. (In some joints, such accessions to the fibrous capsule are derived from neighbouring tendons, muscles or cartilaginous elements.) Although the initial stages in the process of cavitation of joints are independent of movements, a true joint cavity can generally form only in the presence of movements ( ).
It is well established that all limb myogenic precursor cells originate from the somites ( , ). These cells are committed at an early stage and can be identified in the lateral halves of the somites. After the mesenchymal sclerotome cells have migrated from the epithelial somite, the remaining dorsolateral portion is termed the epithelial plate or dermomyotome of the somite (see Fig. 18.2 ). Cells from the dorsomedial edge of the dermomyotomes form the axial musculature, whereas, at limb levels, cells de-epithelialize and migrate from the ventrolateral edges of the dermomyotome into the limb bud. The myogenic precursor cells do not differentiate into muscle before their migration into the limb, probably because of inhibitory signals produced by the somatopleuric mesenchyme. Muscle differentiation depends on the key activity of the MyoD family of transcription factors ( ).
The myogenic precursor cells colonize the limb bud in a proximodistal direction but never reach the most distal portion of the limb. Experiments in chick embryos revealed that myogenic cells are still indifferent regarding their region-specific determination when they first enter the limb. Thus, when brachial-level somites are grafted opposite the leg-forming region, or when pelvic-level somites are grafted opposite the wing-forming region, a normal limb musculature develops, irrespective of the origin of the somites, i.e. putative myoblasts, unlike the somatopleuric (lateral plate) mesenchymal cells, are not specified to be wing or leg ( ). Other experiments in the chick wing show that the muscle pattern developed in the limb reflects the pattern of the skeletal elements: duplication or lack of lower arm elements is accompanied by the duplication or lack of the corresponding muscles ( ).
It is thought that in all classes of tetrapods the first myogenic cells to arrive in the limb bud form principal dorsal and ventral premuscle masses that undergo specific spatiotemporal sequences of divisions and subdivisions as the limb lengthens. In human embryos, this leads to the individualization of about 19 muscles in the upper limb and 14 muscles in the lower limb. In the upper limb, the premuscle masses first divide into three masses, the next division gives rise to the muscles attached to the carpus, and the final division produces the long muscles of the digits. A similar pattern is seen in the lower limb. The mechanisms that divide the muscle masses are not known; one suggestion is that they are created by invading somatopleuric cells which ultimately form the myotendinous junctions, and endo-, peri and epimyseal layers which complete the composite anatomical muscle. For details on the development of the myotendinous junction see and . For further development of skeletal muscle, see Chapter 5 .
The literature suggests that all musculoskeletal elements are in their appropriate positions in human limbs by postmenstrual week 10. For detailed accounts of the process, consult and . For reviews on limb development, including tissue formation, see .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here