Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Disorders of sex development (DSD) is an umbrella term referring to the large collection of conditions in which establishment of chromosomal, gonadal, or anatomic sex development is atypical.
Estimates of the prevalence of DSD vary widely, but it is likely 1 in 5000 to 6000 in the general population.
The genetic regulation of sex determination and differentiation involves a complex cascade of transcription factors beginning with the SRY gene and its major target, SOX9.
Current nomenclature for DSD is mechanistically based and includes categories of 46,XX DSD, 46,XY DSD, and sex chromosome DSD. Within these categories, individual diagnoses are specified based on their genetic, enzymatic, or receptor defect.
The evaluation and management of an infant with DSD should be overseen by a multidisciplinary DSD team.
Using panels of targeted genes permits a genetic diagnosis in about 20% to 40% of infants with DSD, with higher diagnostic rates in cases of 46,XY DSD.
The most common identifiable cause of DSD is congenital adrenal hyperplasia due to 21-hydroxylase deficiency.
When assigning a gender to an infant with DSD, the overarching goal is to make an assignment that will match the individual's gender identity.
Although there is a paucity of data to inform the gender assignment decision, there is sufficient information for some of the more common or well-defined conditions.
Disorders of sex development (DSD) was coined in 2005 as an umbrella term referring to the large collection of conditions in which establishment of chromosomal, gonadal, or anatomic sex development is atypical. Many, but not all, individuals included in the DSD rubric have genital ambiguity at birth. The term is controversial among those affected, and some have called for it to be changed to differences of sex development or abolished entirely. Nevertheless, it remains in widespread use as an overarching term encompassing conditions leading to over- or undermasculinization of a fetus.
Congenital malformations of the genitalia, including isolated cryptorchidism and mild hypospadias, occur in approximately 1 in 200 to 300 live born infants, but these conditions usually do not constitute DSD. Estimates of the prevalence of DSD range widely, depending on the definition and which specific disorders are included. A reasonable estimate is 1 in 5000 to 6000, although some have placed the number as high as 2%. , Although chromosomal disorders such as Turner and Klinefelter syndromes may be included in the spectrum of DSD, the most common cause of genital ambiguity is congenital adrenal hyperplasia (CAH), the vast majority of which is caused by deficiency of the 21-hydroxylase enzyme. CAH is estimated to occur in 1 in 14,000 to 15,000 live births.
In order to understand the pathophysiology and the medical evaluation of the infant with a DSD, a firm grasp of normal embryology is critical.
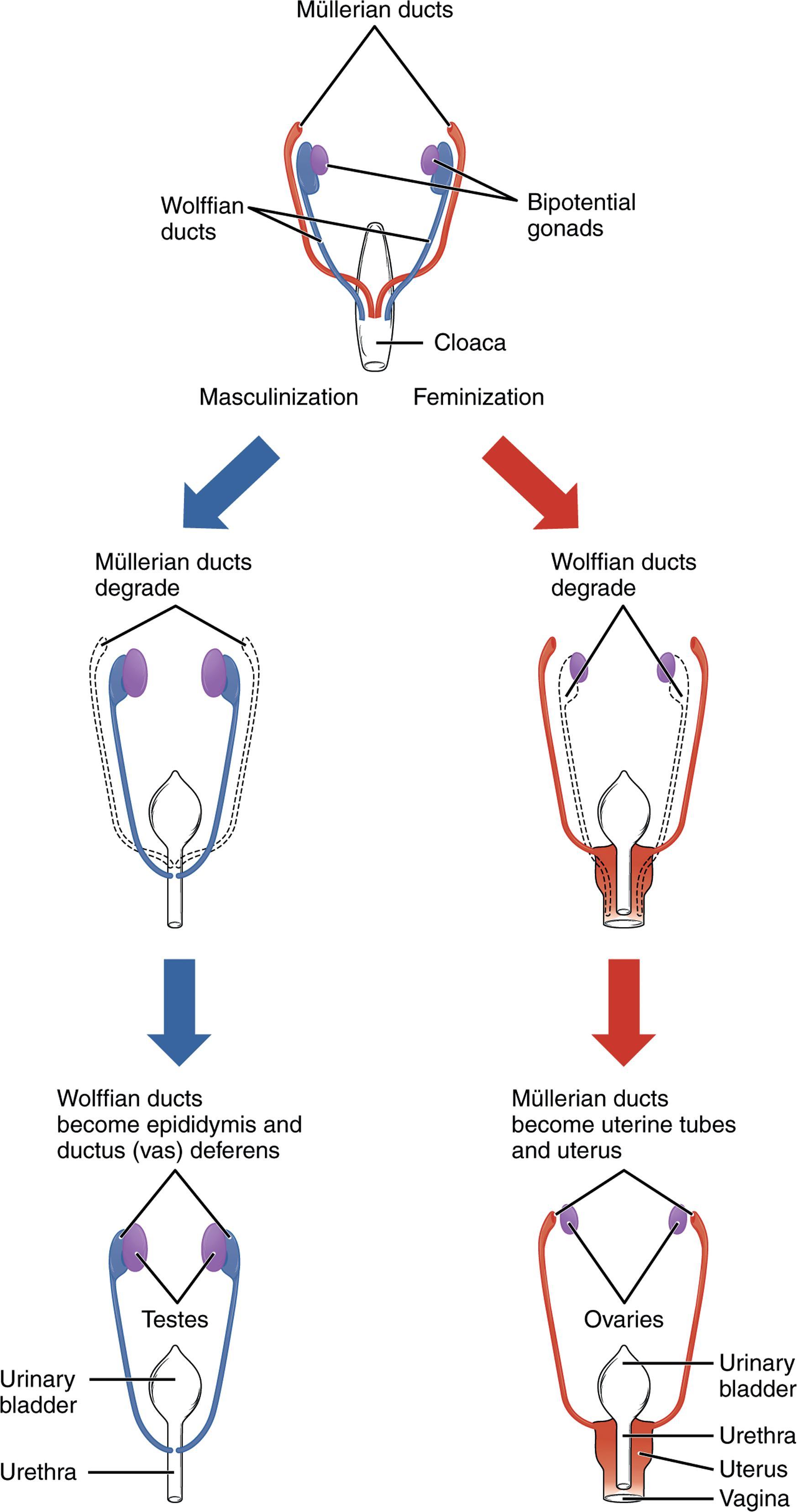
At approximately 5 weeks of gestation in the human embryo, the gonadal ridge appears on the surface of each mesonephros (primitive kidney). The gonadal ridge is morphologically undifferentiated in both 46,XX and 46,XY embryos, hence it is frequently termed the bipotential gonad ( Fig. 29.1 ). Germ cells arise from the wall of the yolk sac and migrate toward the undifferentiated gonad, arriving at 5 to 6 weeks’ gestation.
The earliest morphologic changes specific to the testis occur at about 6 weeks of gestation. At this time, Sertoli cells begin to differentiate within the primitive sex cords. The Sertoli cells cluster around the germ cells as the primitive sex cords develop into the seminiferous tubules. The appearance of Sertoli cells is generally recognized to be the first sign that the gonad will become a testis. Approximately 1 week after Sertoli cells appear, Leydig cells differentiate from the mesenchyme between the primitive sex cords and become functional soon after their appearance.
In the absence of testis determination, ovarian determination occurs. The earliest sign that the bipotential gonad will become an ovary occurs at approximately 7 weeks’ gestation, when the cortical cords form. Germ cells become incorporated into these cords and enter prophase I of meiosis at approximately 10 weeks’ gestation. The cortical cords subsequently break up into clusters of cells surrounding each germ cell, forming ovarian follicles. The precursors of the follicular cells are of the same lineage as the Sertoli cells of the testis.
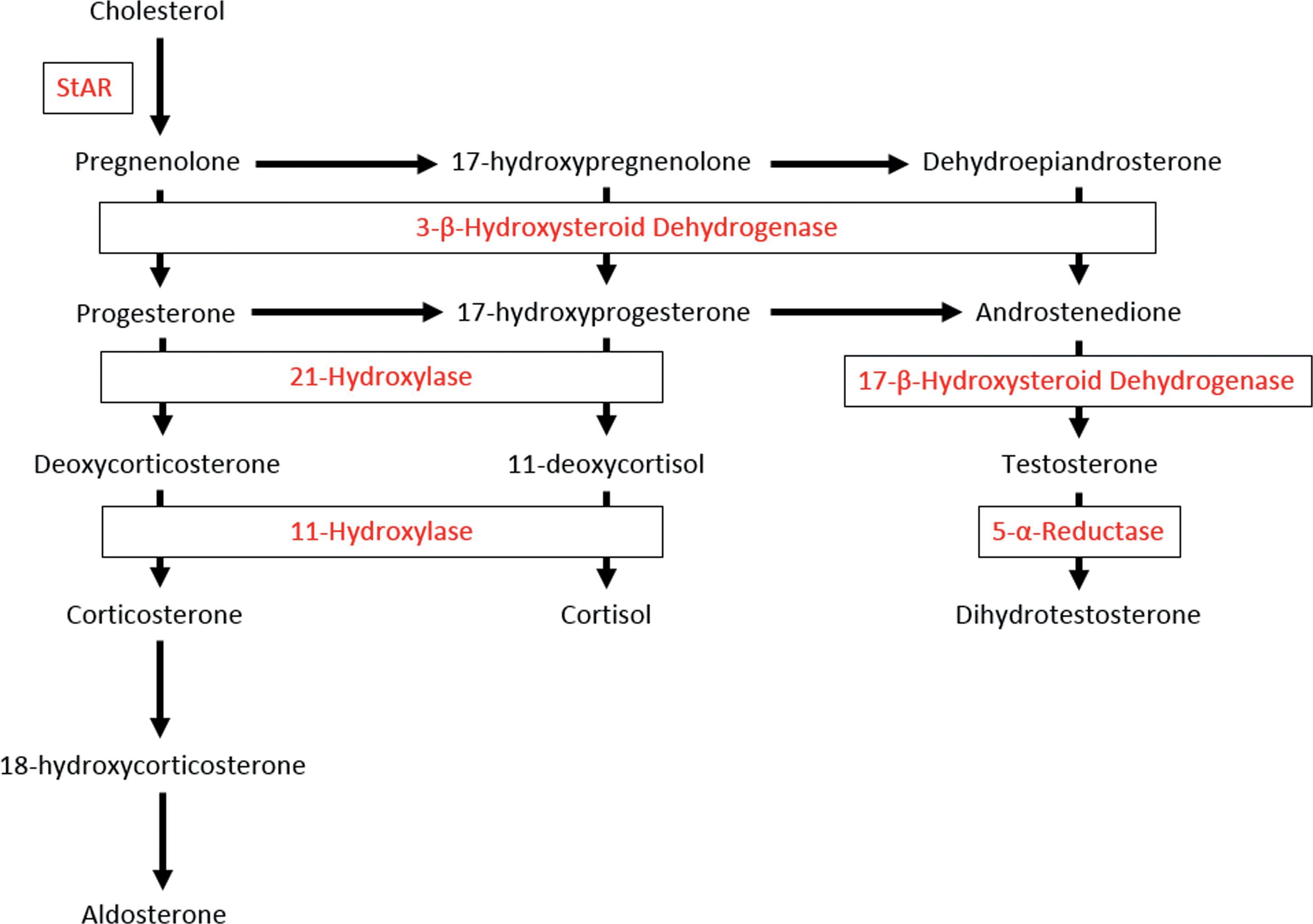
Sertoli cells produce anti-Müllerian hormone (AMH), beginning at 7 weeks’ gestation. AMH acts to disrupt development of the Müllerian ducts (see below). At 8 weeks’ gestation, the fetal testis starts to produce testosterone. Leydig cells require stimulation by human chorionic gonadotropin (hCG) to produce testosterone in appropriate amounts. The effects of hCG are mediated via the luteinizing hormone (LH)/CG receptor on the Leydig cell. Pituitary LH is not initially required for testosterone production, and indeed, it is not secreted from the pituitary until 11.5 weeks’ gestation. In response to hCG, the first of a series of enzymatic steps resulting in testosterone synthesis occurs ( Fig. 29.2 ). Secreted testosterone subsequently diffuses into target cells, where it may act directly or be enzymatically converted by 5α-reductase to the more potent androgen, dihydrotestosterone (DHT). Placental aromatase serves the important role of protecting the mother from virilization due to placental transfer of fetal androgens. Conversely, aromatase also protects XX fetuses from the masculinizing effects of placentally transferred maternal androgens.
By 6 weeks of gestation, all embryos possess two sets of ducts (see Fig. 29.1 ). The Wolffian ducts, precursors of the male system, originate as the excretory ducts of the mesonephros. The Müllerian ducts, precursors of the female system, form lateral to the Wolffian ducts at 6 weeks of gestation. The Müllerian ducts meet in the midline to form the uterine canal.
In the XY fetus, testis determination occurs, and AMH secretion begins. In the presence of AMH, the Müllerian ducts involute. The timing of this is critical, because the ducts become insensitive to the effects of AMH after 8 weeks of gestation. Testosterone leads the Wolffian ducts to differentiate into the epididymis, vas deferens, and seminal vesicles.
In the XX fetus, no testosterone or AMH is produced early in gestation. The Wolffian ducts regress if they are not exposed to androgen by 10 weeks of gestation. In the absence of AMH exposure early in gestation, the Müllerian ducts proliferate. The superior portion of the Müllerian ducts eventually differentiates into the fallopian tube, whereas the uterine canal differentiates into the uterus, cervix, and upper third of the vagina.
The external genitalia of a 46,XY embryo prior to 8 weeks of gestation cannot be distinguished from those of an XX embryo. Bilateral ridges of tissue known as the urethral folds meet anteriorly to form the genital tubercle. The labioscrotal folds develop lateral to the urethral folds. Under the influence of androgen, the genital tubercle elongates to form the phallus. The urethral folds begin to fuse, starting posteriorly and moving anteriorly along the ventral surface of the phallus to form the penile urethra. The labioscrotal folds increase in size and fuse in the midline to form the scrotum. The differentiation of the external genitalia in the male is complete by 12 to 13 weeks gestation. If androgen secretion is delayed past this time, development of the external genitalia cannot be completed, even in the face of normal androgen levels. Masculinization of the external genitalia in XY fetuses occurs under the influence of DHT.
In the XX embryo, few changes occur in the appearance of the external genitalia after 6 weeks’ gestation. The genital tubercle enlarges slightly to form the clitoris, whereas the urethral folds form the labia minora and the labioscrotal folds develop into the labia majora.
Sex determination refers to the path by which the bipotential gonad forms either a testis or ovary, whereas sex development includes the subsequent formation of internal and external genital structures. These processes are mediated by a complex network of genes required for formation and maintenance of the gonadal ridge/bipotential gonad and testis or ovarian determination, which require a fine balance between genetic factors. This has been described as “a story of opposing forces and crucial alliances but, although the winning team takes all, its rule can be surprisingly tenuous.” , Although in many cases the specific genes and their interactions in humans are known, much of our understanding comes from mouse models.
Normal formation of the gonadal ridge is critical for both testis and ovarian determination. Although much of the genetic regulation remains unknown, the WT1 gene is a critical component of this mechanism. WT1 activates the NR5A1 gene, which produces a protein known as steroidogenic factor 1 (SF-1). Both WT1 and SF-1 proteins are critical for activation of NR0B1, producing the DAX-1 protein. Maintenance of the gonadal ridge prior to testis determination requires a balance of this genetic interaction, likely involving additional genetic inputs.
Beginning at 6 weeks of gestation, SRY expression commences in the XY fetus. Initiation of SRY expression likely involves input from GATA4 and its cofactor FOG2, which upregulate SRY expression. WT1 appears to stabilize SRY mRNA. The MAPK pathway also appears to have a role in SRY regulation.
The major target of SRY appears to be SOX9. In turn, SOX9 activates a cascade of genetic events comprising dozens of genes leading to formation of a functioning testis. This activation of SOX9 is enhanced by SF-1, which also serves to activate AMH expression. Interestingly, SF-1 is also critical for expression of a number of genes encoding steroidogenic enzymes and for genetic control of gonadotropin releasing hormone, LH, and FSH. While SOX9 is the mediator of SRY's role in sex determination, abnormal overexpression of other SOX genes may mimic SOX9 action, leading to testis development in an XX individual.
An alternative genetic cascade exists for ovarian determination, although it is less well understood than that for the testis. WNT4 gene expression promotes ovarian differentiation, and abnormalities of WNT4 and altered WNT4 activity in response to RSPO1 mutation may interrupt ovarian development. Genetic variants in NR5A1 encoding SF-1 may interfere with the WNT pathway and normal ovarian development. FOXL2 is another gene that plays a role in ovarian function by suppressing SOX9 activity, thus maintaining ovarian differentiation.
Historical terminology for DSD were fraught with controversy, as many people considered the terms to be pejorative. The nomenclature was revised at a consensus conference held in Chicago in 2005. Although this nomenclature has also proved to be controversial in some quarters, it remains the standard. The new terminology has replaced categories such as “pseudohermaphroditism” and “hermaphroditism” with more mechanistically oriented language such as “sex chromosome DSD,” “46,XY DSD,” and “46,XX DSD.” Within these categories, individual diagnoses are specified based on their genetic, enzymatic, or receptor defect.
This category includes conditions in which a 46,XX fetus is exposed to inappropriately high concentrations of androgens ( Table 29.1 ). The source of these androgens may include aberrantly formed testicular tissue, excessive adrenal or placental androgen production, or exogenous exposure. A subcategory includes isolated abnormal ovarian development.
| I. 46,XX DSD |
| A. Virilizing congenital adrenal hyperplasia a |
| 1. 21-hydroxylase deficiency |
| 2. 11β-hydroxylase deficiency |
| 3. 3β-hydroxysteroid dehydrogenase deficiency |
| B. 46,XX testicular DSD |
| C. 46,XX ovotesticular DSD |
| D. Maternal androgen exposure |
| E. Fetal aromatase deficiency |
| F. 46,XX gonadal dysgenesis |
| II. 46,XY DSD |
| A. 46,XY gonadal dysgenesis |
| 1. Complete gonadal dysgenesis (Swyer syndrome) |
| 2. Partial gonadal dysgenesis |
| B. 46,XY ovotesticular DSD |
| C. Leydig cell hypoplasia |
| D. Defective testosterone biosynthesis |
| 1. Feminizing congenital adrenal hyperplasia |
| a. StAR deficiency (lipoid adrenal hyperplasia) |
| b. 3β-hydroxysteroid dehydrogenase deficiency |
| c. 17α-hydroxylase/17,20 lyase deficiency |
| 2. 17β-hydroxysteroid dehydrogenase deficiency |
| E. 5α-reductase deficiency |
| F. Androgen insensitivity syndrome |
| 1. Complete androgen insensitivity syndrome |
| 2. Partial androgen insensitivity syndrome |
| G. Persistent Müllerian duct syndrome |
| III. Sex chromosome DSD |
| A. 45,X/46,XY mosaicism |
| B. Turner syndrome a |
| C. Klinefelter syndrome a |
| IV. Other abnormalities of sexual differentiation |
| A. Syndromes of multiple congenital anomalies |
a Not considered as a disorder of sex development by some groups.
CAH accounts for the majority of cases of masculinization of female infants. CAH is caused by a group of autosomal recessive disorders of adrenal steroidogenesis in which there is deficient activity of one of the enzymes necessary for the production of cortisol (see Fig. 29.2 ). Cortisol deficiency increases production of ACTH, leading to adrenal hyperplasia and overproduction of adrenal androgens. Virilizing CAH may be caused by 21-hydroxylase deficiency, 11β-hydroxylase deficiency, or 3β-hydroxysteroid dehydrogenase deficiency (3β HSD).
21-Hydroxylase deficiency accounts for more than 90% of CAH and is the most important diagnosis to consider in an infant with genital ambiguity. Based on newborn screening studies, the classical form of the disease occurs in 1 in 10,000 to 15,000 live births. In the classical form there is masculinization, with or without salt-wasting. The salt-wasting form accounts for 75%, whereas the simple-virilizing form accounts for approximately 25% of cases. Diagnosis is supported by increased baseline and adrenocorticotrophic hormone (ACTH)-stimulated 17-hydroxyprogesterone and androstenedione and increased serum androgens and may be confirmed by genetic testing. Prenatal diagnosis is available via molecular analysis of the CYP21A2 gene.
11β-Hydroxylase deficiency accounts for about 5% of cases of CAH. Although salt-wasting may occur in the newborn period, it is less common than in 21-hydroxylase deficiency. In later infancy and childhood, hypertension may occur due to accumulation of 11-deoxycorticosterone and its metabolites.
3β-Hydroxysteroid dehydrogenase deficiency is the least common form of CAH. Clinical presentation is usually that of mild clitoromegaly due to accumulation of dehydroepiandrosterone (DHEA) and its peripheral conversion to testosterone via the type I 3βHSD enzyme, with salt-wasting.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here