Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The field of pulmonary mechanics —the physics of the lungs, airways, and chest wall—deals with how the body moves air in and out of the lungs, producing a change in lung volume (V L ). When we examine these mechanical properties while no air is flowing, we are studying static properties. The situation becomes more complicated under dynamic conditions, when the lungs are changing volume and air is flowing either in or out.
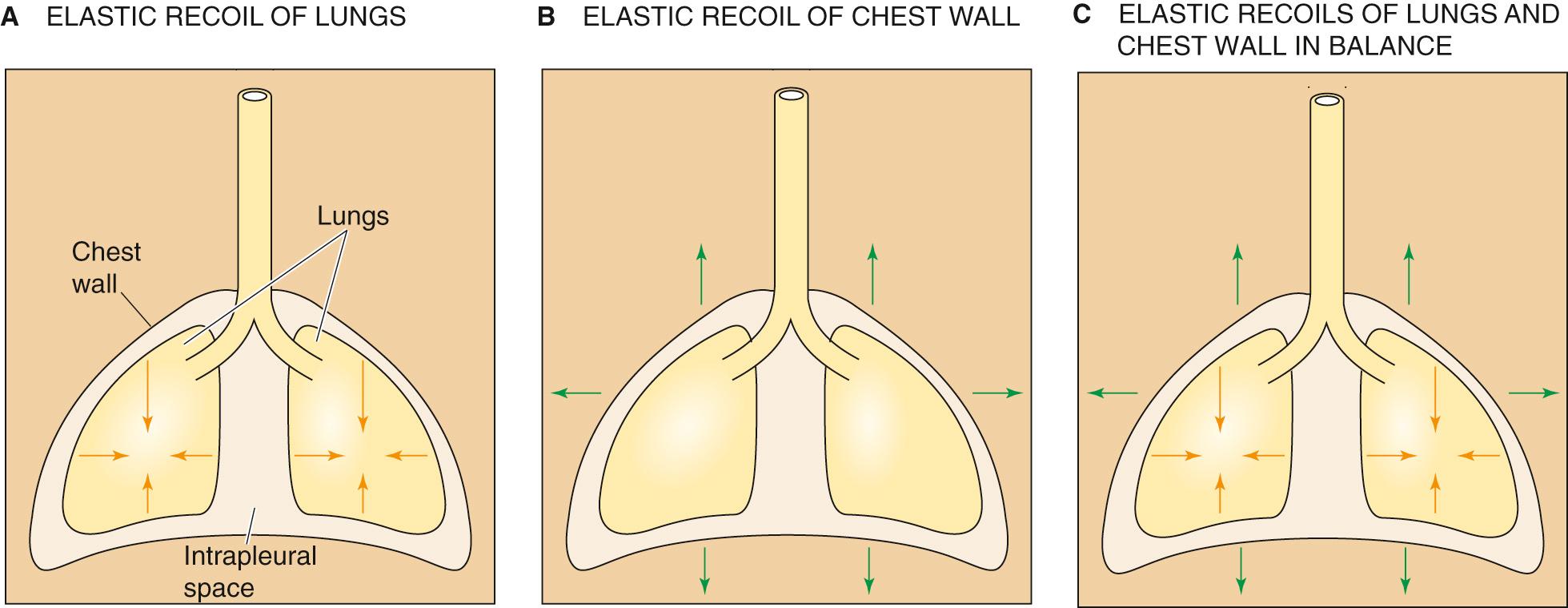
The interaction between the lungs and the thoracic cage determines V L . The lungs have a tendency to collapse because of their elastic recoil, a static property represented by the inwardly directed arrows in Figure 27-1 A . The chest wall also has an elastic recoil. However, this elastic recoil tends to pull the thoracic cage outward (see Fig. 27-1 B ). The stage is thus set for an interaction between the lungs and the chest wall: at equilibrium, the inward elastic recoil of the lungs exactly balances the outward elastic recoil of the chest wall (see Fig. 27-1 C ). This interaction between lungs and chest wall does not occur by direct attachment but via the intrapleural space between the visceral and parietal pleurae (see p. 597 ). This space is filled with a small amount of pleural fluid and is extremely thin (5 to 35 µm). Because the lungs and chest wall pull away from each other on opposite sides of the intrapleural space, the intrapleural pressure (P IP ) is less than barometric pressure (P b ); that is, the intrapleural space is a relative vacuum. Although the designation P IP implies that we are referring exclusively to the intrapleural space, this description is not entirely accurate. Indeed, P IP is probably similar to the pressure in several other regions of the chest cavity in addition to the intrapleural space:
The virtual space between the chest wall or diaphragm and the parietal pleura
The virtual space between the lung and the visceral pleura
The interstitial space that surrounds all pulmonary airways
Around the heart and vessels
Around and—to the extent that smooth-muscle tone can be neglected—inside the esophagus
It is helpful to think of P IP as the intrathoracic pressure—the pressure everywhere in the thorax except in the lumens of blood vessels, lymphatics, or airways. ![]() N27-1
N27-1
Measuring P IP is intrinsically difficult because the space between the visceral and parietal pleurae is very thin (5 to 35 µm). The approaches include use of a pleural needle, pleural catheter, esophageal balloon, pleural balloon, or rib capsule (embedded in a rib) in direct contact with pleural fluid.
Each of these methods reports a vertical gradient in pleural pressure, on the order of 0.5 cm H 2 O/cm in head-up dogs. This pressure gradient drives a downward viscous flow of pleural fluid, presumably along the flat surfaces of the ribs. According to a model, recirculation of pleural fluid would be achieved by an upward flow of fluid along the margins of adjacent lobes of the lungs (here the fluid-filled space is larger, which leads to a reduced resistance), energized by movements of the beating heart and ventilating lungs. Finally, a transverse flow of pleural fluid from these margins back to the flat portions of the ribs would complete the circuit.
The vacuum is not uniform throughout the intrapleural space. When the subject is upright, the vacuum is greatest (i.e., P IP is least) near the apex of the lungs and progressively falls along the longitudinal axis to its lowest value near the bases of the lungs ( Fig. 27-2 ). If a subject whose lungs are ~30 cm tall has finished a quiet expiration, and if P b is 760 mm Hg, P IP is ~753 mm Hg near the apices of the lungs and ~758 mm Hg near the bases. The P IP gradient is about what one would expect, given the density of the lungs. Note that P IP is subatmospheric throughout the chest cavity. Because respiratory physiologists historically measured these small pressures with water manometers rather than with less sensitive mercury manometers, it has become customary to express P IP in centimeters of H 2 O relative to a P b of 0 cm H 2 O. Thus, P IP is about −10 cm H 2 O at the apex and −2.5 cm H 2 O at the base of the lungs.
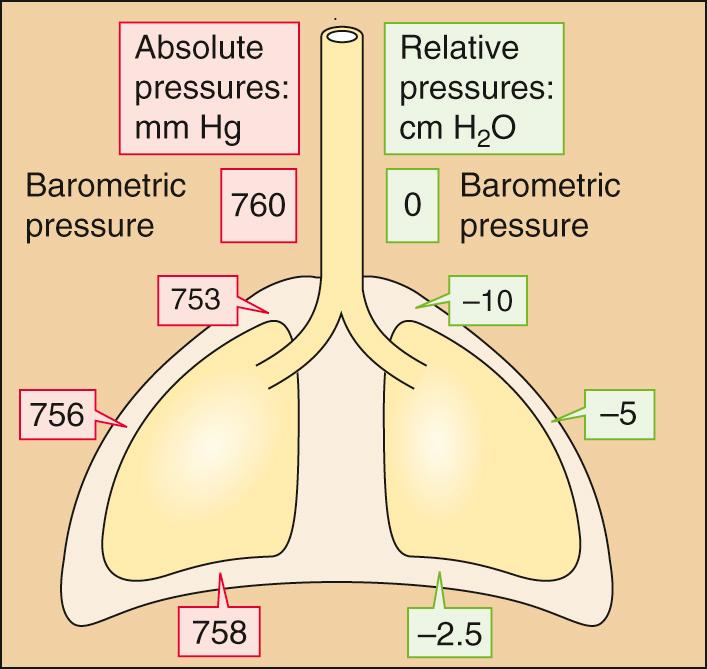
The reasons for the apex-to-base P IP gradient are gravity and posture. When an individual stands vertically on the surface of the earth, gravity pulls the lungs downward and away from the apex of the thoracic cage. This force creates a greater vacuum (i.e., a lower P IP ) at the apex. Gravity also pushes the bases of the lungs into the thoracic cavity, reducing the vacuum there. Standing on one's head would invert these relationships. Lying on one's side would create a P IP gradient along a frontal-horizontal axis (i.e., from side to side), although the P IP gradient would be much smaller because the side-to-side dimension of the thorax (and therefore the gradient created by the weight of the lungs) is less than the longitudinal dimension. In outer space, the P IP gradient would vanish. Thus, the local P IP depends on the position within the gravitational field.
For most of the remainder of this book, we ignore the P IP gradient and refer to an average P IP of about −5 cm H 2 O after a quiet expiration (see Fig. 27-2 ).
We have seen that the opposing elastic recoils of the lungs and chest wall create a negative P IP that keeps the lungs expanded. Any change in the balance between these elastic recoils will cause V L to change as well. For example, imagine a healthy person with a functional residual capacity (FRC) of 3 L and a P IP of −5 cm H 2 O. If that person now develops pulmonary fibrosis, which increases the elastic recoil of the lungs, FRC would decrease because a P IP of −5 cm H 2 O would no longer be adequate to keep the resting V L at 3 L. Moreover, as the lungs shrink, P IP would become more negative, causing chest volume to decrease as well. Under normal circumstances, the key elastic recoil is the one we control: the elastic recoil of the chest wall, which we change moment to moment by modulating the tension of the muscles of respiration.
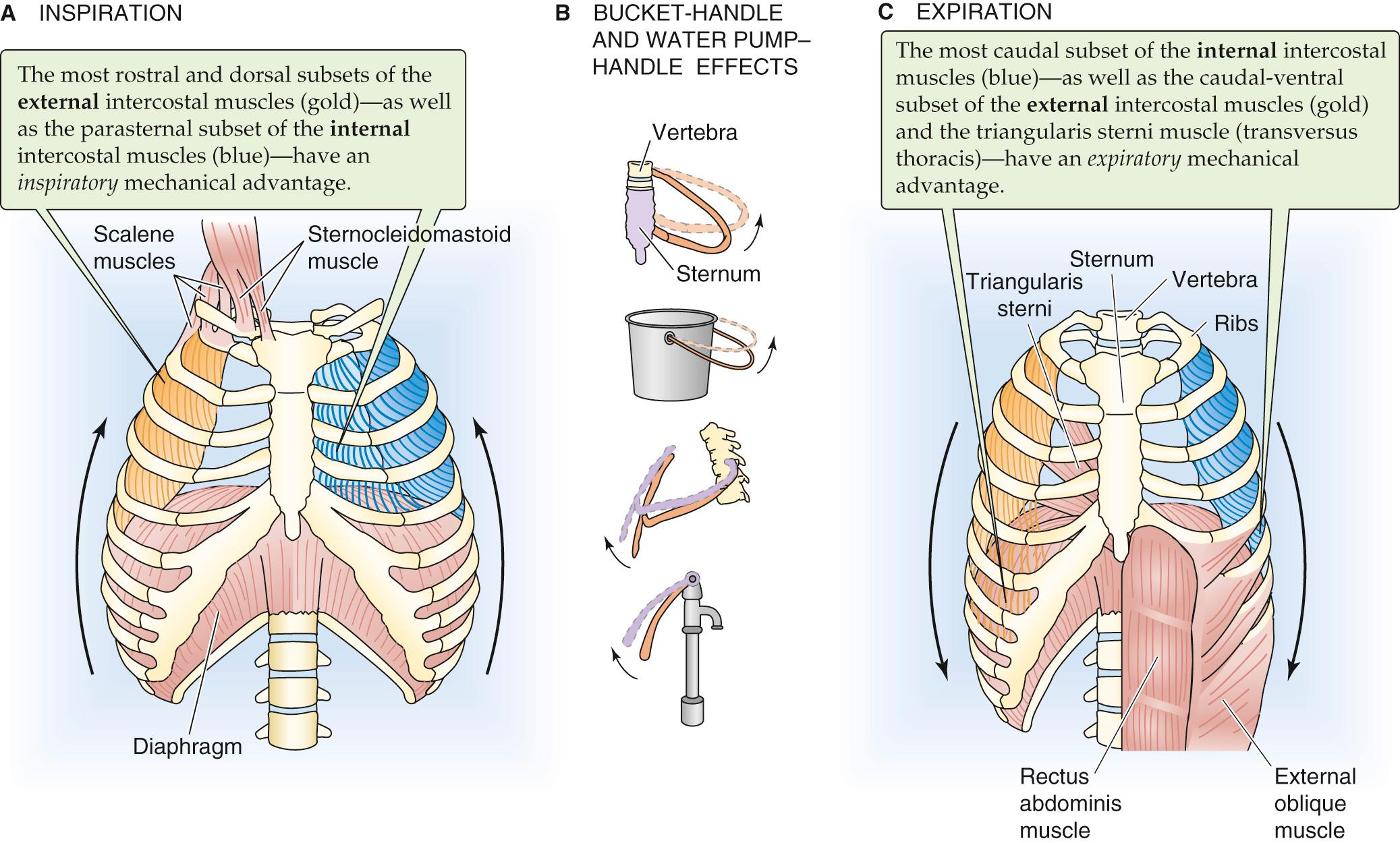
The muscles of inspiration expand the chest, increasing the elastic recoil of the chest wall and making P IP more negative. Despite the P IP gradient from the apex to the base of the lungs when no air is flowing at FRC (see Fig. 27-2 ), the ΔP IP during inspiration is similar throughout the thoracic cavity. Responding to this enhanced intrathoracic vacuum, the lungs expand passively. The increase in V L is virtually the same as the increase in thoracic volume. The muscles that produce a quiet inspiration are called the primary muscles of inspiration and include the diaphragm and many intercostal muscles.
The most important component of the increase in chest volume is the rise in the chest cavity's rostral-caudal diameter, a result of the action of the diaphragm. Stimulated by the phrenic nerves (derived from cervical roots C3 to C5), the diaphragm contracts and moves downward into the abdomen ~1 cm during quiet ventilation.
The external and internal intercostal muscles, innervated by segmental spinal nerves, span the space between adjacent ribs. The action of each such muscle depends partly on its orientation but especially—because of the shape of the rib cage—on its position along the rostral-caudal axis and around the dorsal-ventral circumference of the rib cage. Thus, not all external intercostals are inspiratory, and not all internal intercostals are expiratory. Inspiratory neurons preferentially stimulate the most rostral and dorsal external intercostals and the parasternal internal intercostals, both of which have inspiratory mechanical advantages. ![]() N27-2 The contraction of these muscles has two consequences ( Fig. 27-3 A ). First, the rib cage and the tissues between the ribs stiffen and are therefore better able to withstand the increasingly negative P IP . Second, thoracic volume increases as (a) ribs 2 through 10 rotate upward and outward, increasing the transverse diameter (bucket-handle effect; see Fig. 27-3 B ) and (b) the upper ribs rotate the sternum upward and outward, increasing the anterior-posterior diameter (water pump–handle effect).
N27-2 The contraction of these muscles has two consequences ( Fig. 27-3 A ). First, the rib cage and the tissues between the ribs stiffen and are therefore better able to withstand the increasingly negative P IP . Second, thoracic volume increases as (a) ribs 2 through 10 rotate upward and outward, increasing the transverse diameter (bucket-handle effect; see Fig. 27-3 B ) and (b) the upper ribs rotate the sternum upward and outward, increasing the anterior-posterior diameter (water pump–handle effect).
Contrary to conventional wisdom, recent work shows that not all external intercostal muscles are inspiratory and that not all internal intercostal muscles are expiratory. For an exhaustive analysis of this subject, consult the review below.
During a forced inspiration, the accessory (or secondary ) muscles of inspiration also come into play:
Scalenes. These muscles lift the first two ribs.
Sternocleidomastoids. These muscles lift the sternum outward, contributing to the water pump–handle effect.
Neck and back muscles. These elevate the pectoral girdle (increasing the cross-sectional area of the thorax) and extend the back (increasing the rostral-caudal length).
Upper respiratory tract muscles. The actions of these muscles decrease airway resistance.
During a quiet inspiration, normal lungs store enough energy in their elastic recoil to fuel a quiet expiration, just as stretching of a rubber band stores enough energy to fuel the return to initial length. Thus, a quiet expiration is normally passive, accomplished simply by relaxation of the muscles of inspiration. Thus, there are no primary muscles of expiration.
Expiration is not always entirely passive. One example is a forced expiration in an individual with normal airway resistance. Another is even a quiet expiration of a person with a disease that increases airway resistance (e.g., asthma, chronic bronchitis, emphysema). In either case, the accessory muscles of expiration help make P IP more positive:
Abdominal muscles (internal and external oblique, rectoabdominal, and transverse abdominal muscles). Contraction of these muscles (see Fig. 27-3 C ) increases intra-abdominal pressure and forces the diaphragm upward into the chest cavity, decreasing the rostral-caudal diameter of the thorax and increasing P IP .
Intercostals. The most ventral and caudal external intercostals, the most dorsal and lateral of the caudal internal intercostals, and an intercostal-like muscle called the triangularis sterni all have an expiratory mechanical advantage. Expiratory neurons selectively stimulate these muscles so as to reduce both the anterior-posterior and the transverse diameters of the thorax. These actions are particularly important for coughing.
Neck and back muscles. Lowering of the pectoral girdle reduces the cross-sectional area of the thorax, whereas flexion of the trunk reduces the rostral-caudal diameter.
During a forced inspiration, the accessory muscles of inspiration use their energy mainly to increase V L (rather than to overcome resistance to airflow); the lungs store this extra energy in their elastic recoil. During a forced expiration, the accessory muscles of expiration use their energy mainly to overcome the resistance to airflow, as discussed below.
Imagine that a person experiences a puncture wound to the chest cavity, so that air enters the thorax from the atmosphere, raising P IP to the same level as P b . This condition is called a pneumothorax (from the Greek pneuma [air]). With no vacuum to counter their elastic recoil, alveoli will collapse—a condition known as atelectasis. The upper part of Figure 27-4 A illustrates an extreme hypothetical case in which pressure is atmospheric throughout the thorax. Even though the lungs are collapsed, V L is not zero because proximal airways collapse before smaller ones farther downstream, trapping air. The resulting minimal air volume is ~10% of total lung capacity (TLC), typically ~500 mL.
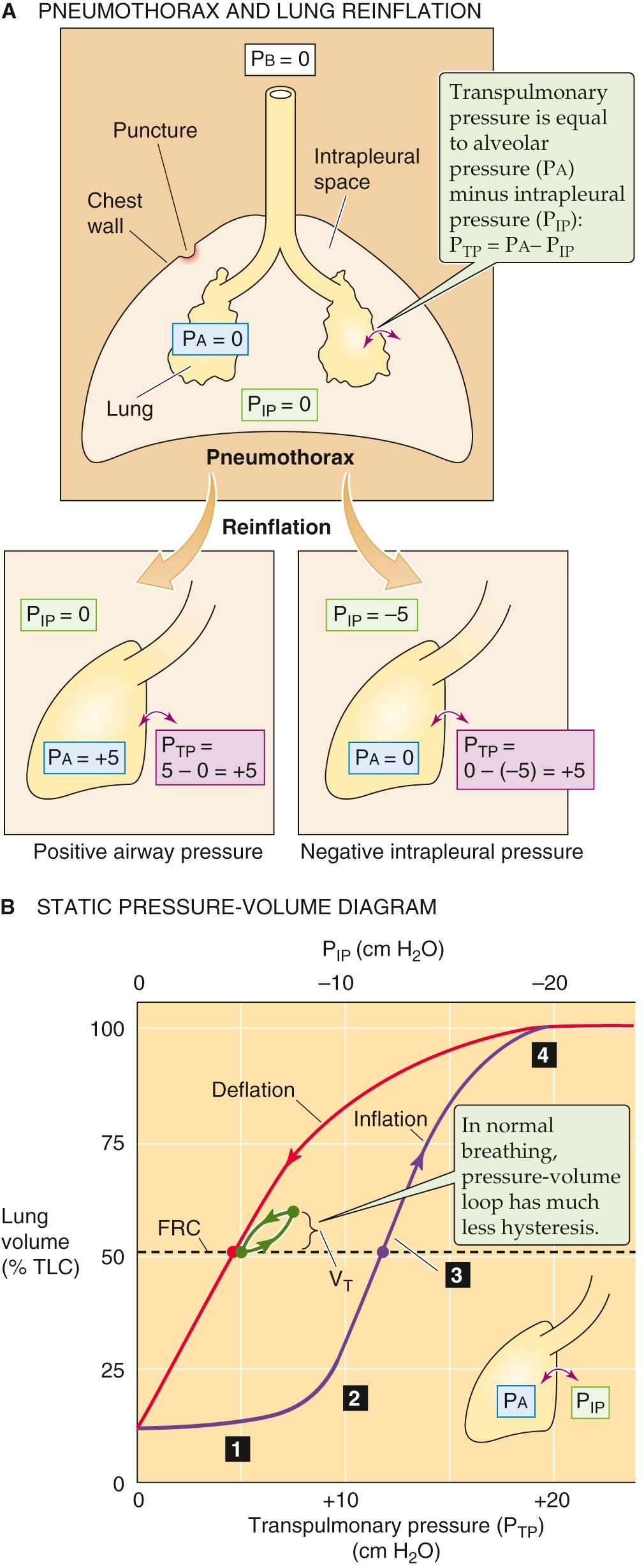
We now wish to re-expand the collapsed lungs to their original volume (i.e., FRC). What are the forces at work during such a re-expansion? The force responsible for distending an airway is the transmural pressure (P TM ) —the radial pressure difference across an airway wall at any point along the tracheobronchial tree:
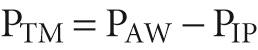
P AW is the pressure inside the airway, and P IP is the pressure in the interstitial space surrounding the airway. A special case of P TM is the transmural pressure across the alveolar wall— trans pulmonary pressure (P TP ):
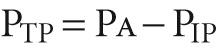
P a is alveolar pressure. When the glottis is open and no air is flowing, the lungs are under static conditions, and P a must be 0 cm H 2 O:
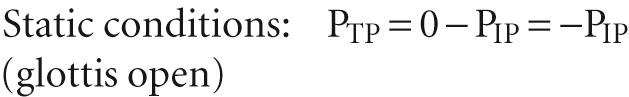
Thus, with the glottis open under static conditions, the pressure that inflates the alveoli (i.e., P TP ) is simply the negative of P IP . We can re-expand the lungs to FRC by any combination of an increase in P a and a decrease in P IP , as long as P TP ends up at 5 cm H 2 O (see Fig. 27-4 A , lower panels). Thus, it makes no difference whether we increase P a from 0 to +5 cm H 2 O with P IP fixed at zero (the principle behind positive-pressure ventilation in an intensive care unit) or whether we decrease P IP from 0 to –5 cm H 2 O with P a fixed at zero (the principle behind physiological ventilation). In both cases, V L increases by the same amount.
A clinician would treat the pneumothorax by inserting a chest tube through the wound into the thoracic cavity and gradually pumping out the intrathoracic air. The clinician might also insert a tube through the mouth and into the upper trachea (to ensure a patent airway), use a mechanical ventilator (to ensure gas exchange), and sedate the patient (to prevent the patient from fighting the ventilator). Between the inspiratory cycles of the ventilator, the lungs are under static conditions and V L depends only on P TP —that is, the difference between P a (which is set by the ventilator) and P IP . As we remove air from the thorax, P IP becomes more negative and the alveoli re-expand. We can characterize the elastic (or static) properties of the lungs by plotting V L versus P TP as V L increases (see Fig. 27-4 B , purple curve). How do we obtain the necessary data? In principle, we could determine V L by using Equation 26-4 . We could read off P a (needed to compute P TP ) directly from the ventilator. Finally, we could in principle measure P IP by using a pressure transducer at the tip of the chest tube. Most important, we must take our readings between inspiratory cycles of the ventilator—under static conditions.
During the reinflation, measured under static conditions, we can divide the effect on V L into four stages, starting at the left end of the purple curve in Figure 27-4 B :
Step 1: Stable V L . In the lowest range of P IP values, making P IP more negative has little or no effect on V L . For example, decreasing P IP from 0 to –1 cm H 2 O (i.e., increasing P TP from 0 to +1 cm H 2 O), we record no change in V L . Why? As discussed below, it is very difficult—because of the surface tension created by the air-water interface—to pop open an airway that is completely collapsed. Until P TP is large enough to overcome the collapsing effects of surface tension, a decrease in P IP has no effect on V L .
Step 2: Opening of airways. Decreasing P IP beyond about –8 cm H 2 O produces V L increases that are at first small, reflecting the popping open of proximal airways with the greatest compliance. Further decreasing P IP produces larger increases in V L , reflecting the expansion of already-open airways as well as recruitment of others.
Step 3: Linear expansion of open airways. After all the airways are already open, making P IP increasingly more negative inflates all airways further, causing V L to increase in a roughly linear fashion.
Step 4: Limit of airway inflation. As V L approaches TLC, decreases in P IP produce ever smaller increases in V L , which reflects decreased airway and chest-wall compliance and the limits of muscle strength.
What would happen if, having inflated the lungs to TLC, we allowed P IP to increase to 0 cm H 2 O once again? Obviously, the V L would decrease. However, the lungs follow a different path during deflation (see Fig. 27-4 B , red curve), creating a P IP -V L loop. The difference between the inflation and the deflation paths— hysteresis —exists because a greater P TP is required to open a previously closed airway, owing to a deficit of surfactant at the air-water interface, than to keep an open airway from closing, due to the abundance of surfactant. We will discuss surfactant in the next section. The horizontal dashed line in Figure 27-4 B shows that inflating previously collapsed lungs to FRC requires a P IP of –12 cm H 2 O (purple point), whereas maintaining previously inflated lungs at FRC requires a P IP slightly less negative than –5 cm H 2 O (red point). During normal ventilation, the lungs exhibit much less hysteresis, and the green P IP -V L loop in Figure 27-4 B lies close to the red deflation limb of our original loop. The changes in V L in Figure 27-4 B reflect mainly changes in the volume of alveoli, with a small contribution from conducting airways.
We will now focus on just the red curve in Figure 27-4 B , a portion of which is the middle curve in Figure 27-5 . Here, P TP is +5 cm H 2 O when V L is at FRC. As the subject makes a normal inspiration with a tidal volume (V T ) of 500 mL, P TP increases (i.e., P IP decreases) by 2.5 cm H 2 O. The ratio of ΔV L to ΔP TP (i.e., the slope of the P TP -V L curve) is the compliance, a measure of the distensibility of the lungs. In our example,
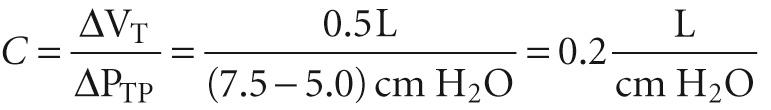
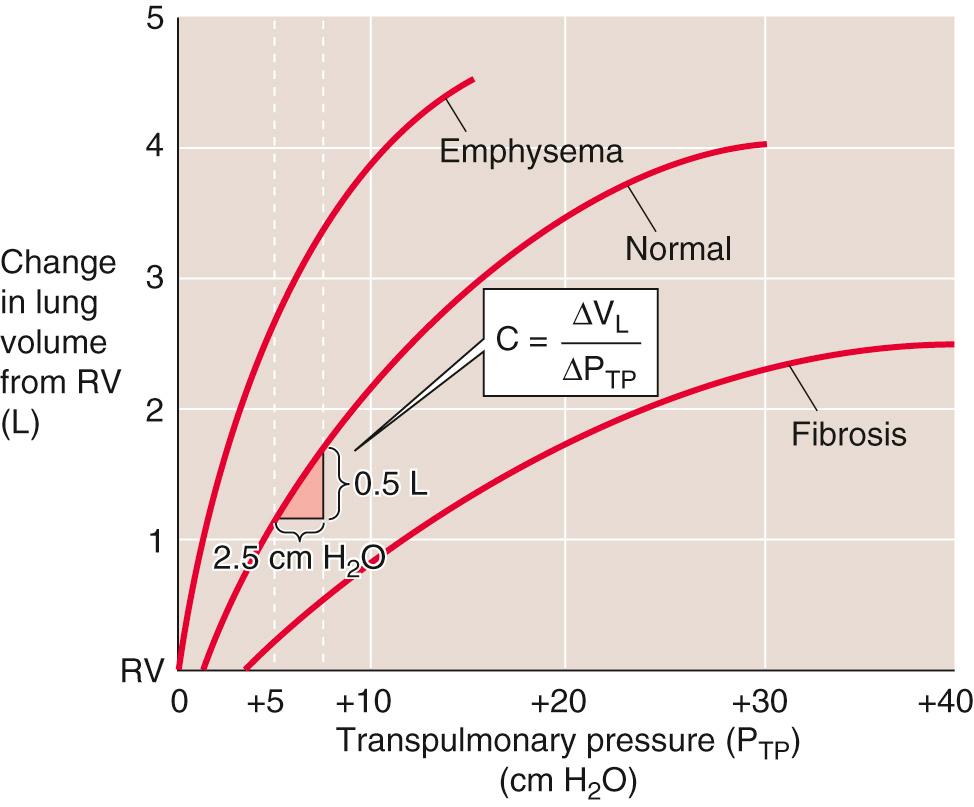
Because we made this measurement under conditions of zero airflow, C is the static compliance. Static compliance, like V L , is mainly a property of the alveoli. The elastance of the lungs, which is a measure of their elastic recoil, is the reciprocal of the compliance ( E = 1 /C ). Lungs with a high compliance have a low elastic recoil, and vice versa.
Figure 27-5 also shows representative P TP -V L relationships for lungs of patients with pulmonary fibrosis (bottom curve) and emphysema (top curve). In pulmonary fibrosis, the disease process causes deposition of fibrous tissue, so that the lung is stiff and difficult to inflate. Patients with restrictive lung disease, by definition, have a decreased C (i.e., a decreased slope of the V L -P TP relationship in Fig. 27-5 ) at a given V L . The same ΔP TP that produces a 500-mL V L increase in normal lungs produces a substantially smaller V L increase in fibrotic lungs. In other words, static compliance (ΔV L /ΔP TP ) is much less, or elastic recoil is much greater.
In emphysema, the situation is reversed. The disease process, a common consequence of cigarette smoking, destroys pulmonary tissue and makes the lungs floppy. An important part of the disease process is the destruction of the extracellular matrix, including elastin, by elastase released from macrophages. Normal mice that are exposed to cigarette smoke develop emphysema rapidly, whereas the disease does not develop in “smoker” mice lacking the macrophage elastase gene. The same increase in P TP that produces a 500-mL V L increase in normal lungs produces a substantially larger V L increase in lungs with emphysema. In other words, static compliance is much greater (i.e., much less elastic recoil).
Because it requires work to inflate the lungs against their elastic recoil, one might think that a little emphysema might be a good thing. Although it is true that patients with emphysema exert less effort to inflate their lungs, the cigarette smoker pays a terrible price for this small advantage. The destruction of pulmonary architecture also makes emphysematous airways more prone to collapse during expiration, which drastically increases airway resistance.
Two additional points are worth noting. First, compliance (i.e., slope of the P TP -V L curve) decreases as V L increases from FRC to TLC (see Fig. 27-5 ). Second, the P TP -V L curve is the amalgam of pressure-volume relationships of all alveoli. Different alveoli have different P TP -V L curves and may experience different intrapleural pressures, depending on their position within a gravitational field (see Fig. 27-2 ). This inhomogeneity of static parameters contributes to regional differences in ventilation (see pp. 687–689 ; Box 27-1 ).
Two major categories of pulmonary disease—restrictive and obstructive—can severely reduce total ventilation; that is, the amount of air entering and leaving the lungs per unit of time. We discuss obstructive diseases (which affect the resistance of the conducting airways) in Box 27-2 .
Pulmonologists use the term restrictive lung disease in an inclusive sense to refer to any disorder that reduces FRC, vital capacity, or TLC (see Fig. 26-8 B ) and thereby makes the lungs difficult to inflate. Pure restrictive disease does not affect airway resistance. Restrictive disease can target the lung parenchyma or three extrapulmonary structures, as outlined in the next four paragraphs.
Restrictive diseases of the lung parenchyma decrease the static compliance of the lung—mainly a property of the alveoli. To overcome increased elastic recoil, the patient must make extra effort to inhale. The patient compensates by making rapid but shallow inspirations. In newborns, an example is infant respiratory distress syndrome, caused by a deficiency in surfactant. Pulmonary edema is a buildup of fluid in the interstitial space between the alveolar and capillary walls and, eventually, the alveolar space. Interstitial inflammation of a variety of etiologies (e.g., infection, drugs, environmental exposure) can lead to the deposition of fibrous tissue and a group of diseases called diffuse interstitial pulmonary fibrosis.
A buildup in the intrapleural space of either air (pneumothorax) or fluid (pleural effusion) can restrict the expansion of a vast number of alveoli.
Rigidity of the chest wall makes it difficult to increase thoracic volume even if the neuromuscular system (see next) can generate normal forces. Ankylosing spondylitis is an inflammatory disorder of the axial skeleton that may reduce the bucket-handle rotation of the ribs during quiet inspirations and the flexion and extension of the trunk during forced inspirations and expirations. In kyphoscoliosis (angulation and rotation of the spine), deformation of the vertebrae and ribs may reduce ventilation. In both conditions, impairment of coughing predisposes to lung infections.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here