Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Function of the heart valves was discussed in Chapter 9 , where we pointed out that closing of the valves causes audible sounds. Ordinarily, no audible sounds occur when the valves open. In this chapter, we first discuss the factors that cause the sounds in the heart under normal and abnormal conditions. Then we discuss the overall circulatory changes that occur when valvular or congenital heart defects are present.
When listening to a normal heart with a stethoscope, one hears a sound usually described as “lub, dub, lub, dub.” The “lub” is associated with closure of the atrioventricular (A-V) valves at the beginning of systole, and the “dub” is associated with closure of the semilunar (aortic and pulmonary) valves at the end of systole. The “lub” sound is called the first heart sound , and the “dub” is called the second heart sound , because the normal pumping cycle of the heart is considered to start when the A-V valves close at the onset of ventricular systole ( ).
The main cause of the first heart sound is vibration of the taut valves immediately after closure , along with vibration of the adjacent walls of the heart and major vessels around the heart . That is, in generating the first heart sound, contraction of the ventricles first causes sudden backflow of blood against the A-V valves (tricuspid and mitral valves), causing them to close and bulge toward the atria until the chordae tendineae abruptly stop the back bulging. The elastic tautness of the chordae tendineae and valves then causes the back-surging blood to bounce forward again into each respective ventricle. This mechanism causes the blood and the ventricular walls, as well as the taut valves, to vibrate and causes vibrating turbulence in the blood. The vibrations travel through the adjacent tissues to the chest wall, where they can be heard as sound by using the stethoscope.
The second heart sound results from sudden closure of the semilunar valves (aortic and pulmonary valves) at the end of systole. When the semilunar valves close, they bulge backward toward the ventricles, and their elastic stretch recoils the blood back into the arteries, which causes a short period of reverberation of blood back and forth between the walls of the arteries and the semilunar valves, as well as between these valves and the ventricular walls. The vibrations occurring in the arterial walls are then transmitted mainly along the arteries. When the vibrations of the vessels or ventricles come into contact with a sounding board, such as the chest wall, they create sound that can be heard using a stethoscope.
The duration of each of the heart sounds is slightly more than 0.10 second, with the first sound about 0.14 second and the second about 0.11 second. The reason for the shorter second sound is that the semilunar valves are tauter than the A-V valves, so they vibrate for a shorter time than do the A-V valves.
The audible range of frequency (pitch) in the first and second heart sounds, as shown in Figure 23-1 , begins at the lowest frequency the ear can detect, about 40 cycles/sec, and goes up above 500 cycles/sec. When a special electronic apparatus is used to record these sounds, a larger proportion of the recorded sound is at frequencies and sound levels below the audible range, going down to 3 to 4 cycles/sec and peaking at about 20 cycles/sec, as illustrated by the lower shaded area in Figure 23-1 . For this reason, major portions of the heart sounds can be recorded electronically by phonocardiography, even though they cannot be heard with a stethoscope.
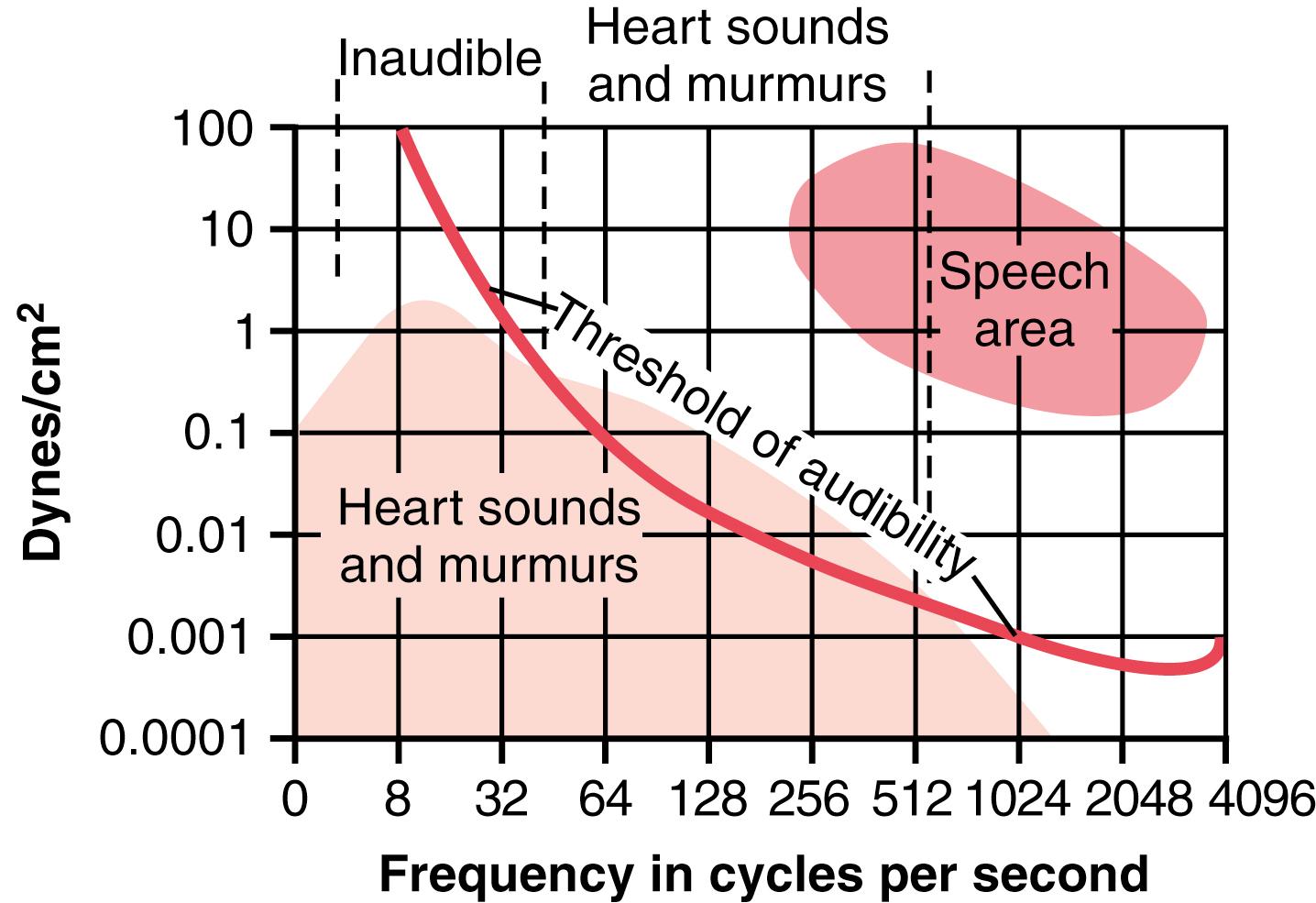
The second heart sound normally has a higher frequency than the first heart sound for two reasons: (1) the tautness of the semilunar valves in comparison with the much less taut A-V valves; and (2) the greater elastic coefficient of the taut arterial walls, which provides the principal vibrating chambers for the second sound, in comparison with the much looser, less elastic ventricular chambers, which provides the vibrating system for the first heart sound. The clinician uses these differences to distinguish special characteristics of the two respective sounds.
Occasionally, a weak, rumbling third heart sound is heard at the beginning of the middle third of diastole . A logical but unproved explanation of this sound is oscillation of blood back and forth between the walls of the ventricles initiated by inrushing blood from the atria. This is analogous to running water from a faucet into a paper sack, with the inrushing water reverberating back and forth between the walls of the sack to cause vibrations in its walls. The reason the third heart sound does not occur until the middle third of diastole is believed to be that in the early part of diastole, the ventricles are not filled sufficiently to create even the small amount of elastic tension necessary for reverberation. The frequency of this sound is usually so low that the ear cannot hear it, yet it can often be recorded in the phonocardiogram. The third heart sound may be normally present in children, adolescents, and young adults but generally indicates systolic heart failure in older adults.
An atrial heart sound can sometimes be recorded in the phonocardiogram, but it can almost never be heard with a stethoscope because of its weakness and very low frequency—usually 20 cycles/sec or less. This sound occurs when the atria contract, and presumably, it is caused by the inrush of blood into the ventricles, which initiates vibrations similar to those of the third heart sound. A fourth heart sound is common in persons who derive benefit from atrial contraction for ventricular filling as a result of decreased ventricular wall compliance and increased resistance to ventricular filling. For example, a fourth heart sound is often heard in older patients with left ventricular hypertrophy.
Listening to the sounds of the body, usually with the aid of a stethoscope, is called auscultation . Figure 23-2 shows the areas of the chest wall from which the different heart valvular sounds can best be distinguished. Although the sounds from all the valves can be heard from all these areas, the cardiologist distinguishes the sounds from the different valves by a process of elimination. That is, he or she moves the stethoscope from one area to another, noting the loudness of the sounds in different areas and gradually picking out the sound components from each valve.
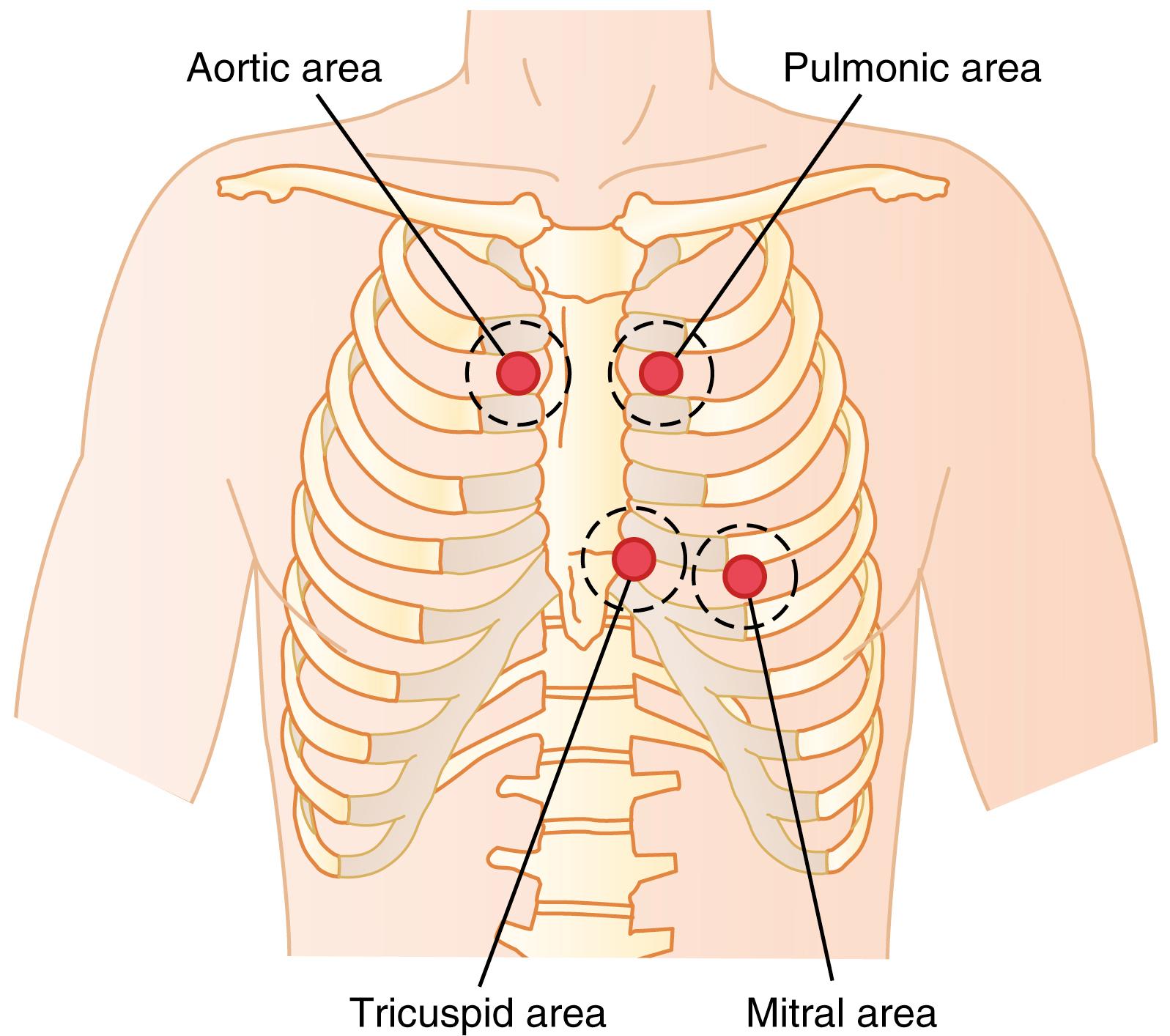
The areas for listening to the different heart sounds are not directly over the valves themselves. The aortic area is upward along the aorta because of sound transmission up the aorta, and the pulmonic area is upward along the pulmonary artery. The tricuspid area is over the right ventricle, and the mitral area is over the apex of the left ventricle, which is the portion of the heart nearest the surface of the chest; the heart is rotated so that the remainder of the left ventricle lies more posteriorly.
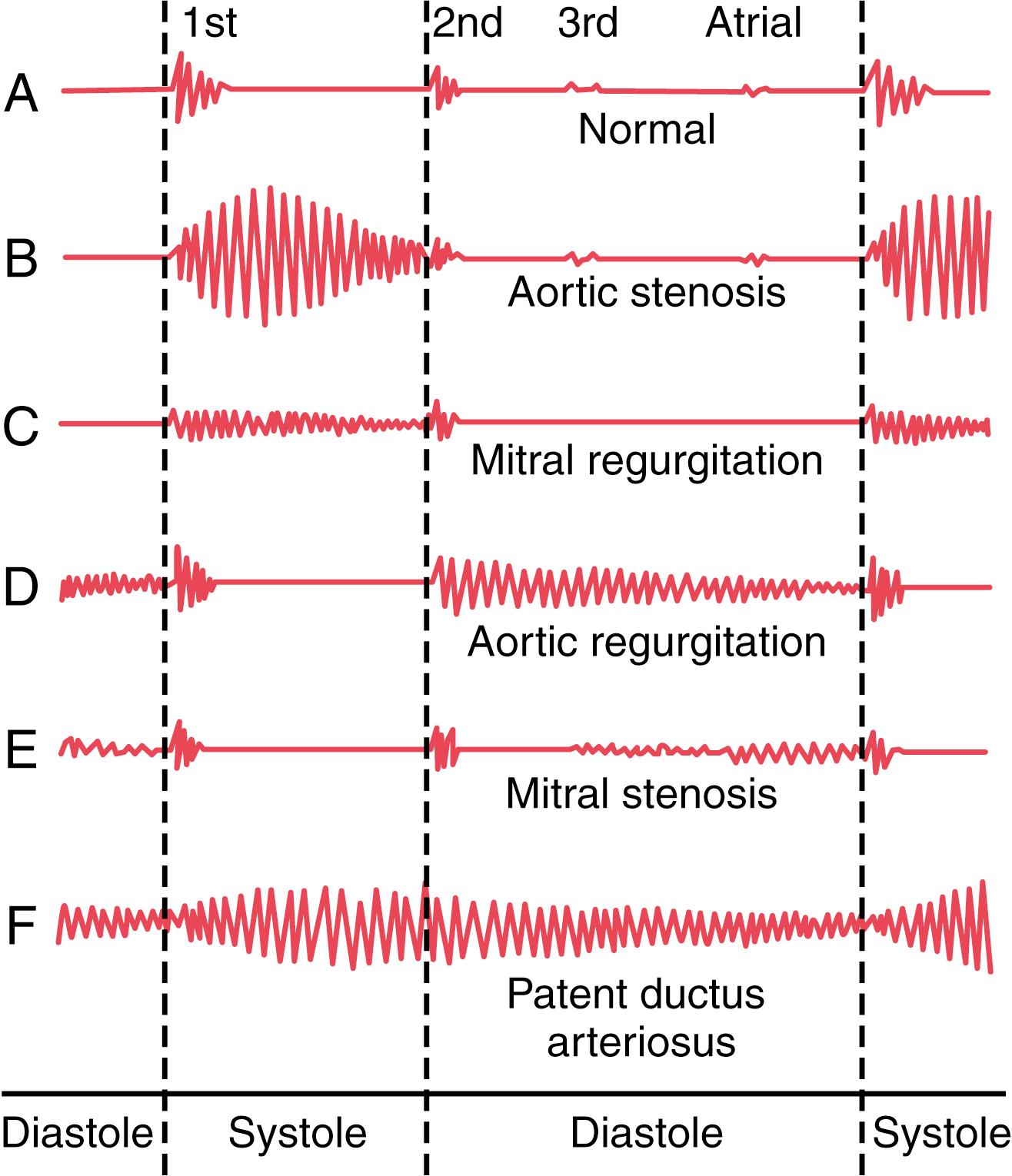
If a microphone specially designed to detect low-frequency sound is placed on the chest, the heart sounds can be amplified and recorded by a high-speed recording apparatus. The recording is called a phonocardiogram , and the heart sounds appear as waves, as shown schematically in Figure 23-3 . Recording A is an example of normal heart sounds, showing the vibrations of the first, second, and third heart sounds and even the very weak atrial sound. Note specifically that the third and atrial heart sounds are each a very low rumble. The third heart sound can be recorded in only one-third to one-half of people, and the atrial heart sound can be recorded in perhaps one-fourth of people.
Rheumatic fever is an autoimmune disease in which the heart valves are likely to be damaged or destroyed. The disease is usually initiated by a streptococcal toxin.
The sequence of events almost always begins with a preliminary streptococcal infection caused specifically by group A hemolytic streptococci. These bacteria initially cause a sore throat, scarlet fever, or middle ear infection. However, the streptococci also release several different proteins against which the person’s reticuloendothelial system produces antibodies . The antibodies react not only with the streptococcal protein but also with other protein tissues of the body, often causing severe immunologic damage. These reactions continue to take place as long as the antibodies persist in the blood—1 year or more.
Rheumatic fever particularly causes damage in certain susceptible areas, such as the heart valves. The degree of heart valve damage is directly correlated with the concentration and persistence of the antibodies. The principles of immunity that relate to this type of reaction are discussed in Chapter 35 , and it is noted in Chapter 32 that acute glomerular nephritis of the kidneys has a similar immunologic basis.
In persons with rheumatic fever, large hemorrhagic, fibrinous, bulbous lesions grow along the inflamed edges of the heart valves. Because the mitral valve undergoes more trauma during valvular action than any of the other valves, it is the one most often seriously damaged, and the aortic valve is the second most frequently damaged. The right heart valves—that is, the tricuspid and pulmonary valves—are usually affected much less severely, probably because the low-pressure stresses that act on these valves are slight compared with the high-pressure stresses that act on the left heart valves.
The lesions of acute rheumatic fever frequently occur on adjacent valve leaflets simultaneously, so the edges of the leaflets become stuck together. Then, weeks, months, or years later, the lesions become scar tissue, permanently fusing portions of adjacent valve leaflets. Also, the free edges of the leaflets, which are normally filmy and free-flapping, often become solid scarred masses.
A valve in which the leaflets adhere to one another so extensively that blood cannot flow through it normally is said to be stenosed . Conversely, when the valve edges are so destroyed by scar tissue that they cannot close as the ventricles contract, regurgitation (backflow) of blood occurs when the valve should be closed. Stenosis usually does not occur without the coexistence of at least some degree of regurgitation, and vice versa.
With aging, the aortic valve often thickens, becomes calcified and stiffer, and may partially obstruct outflow from the left ventricle. With increased life expectancy and aging of the population, aortic valve stenosis has become the most common heart valve disease.
Stenosis of a previously normal aortic valve, often called senile calcific aortic valve stenosis , is characterized by valve calcium deposition and ossification, which lead to narrowing of the aortic valve orifice. As a compensatory response to the increased workload imposed on the heart by the stenotic aortic valve, the left ventricle undergoes concentric hypertrophy. This type of hypertrophy is associated with increased left ventricular wall thickness, which permits the heart to pump with greater vigor against the partially obstructed outflow. An increasing pressure gradient then develops across the calcified valve, reaching 75 to 100 mm Hg in severe cases of aortic valve stenosis.
The hypertrophied left ventricle also becomes more fibrotic and tends to be ischemic because of impaired microcirculatory perfusion, although some patients may also have atherosclerosis of the coronary arteries. The ejection fraction may be normal, and the patient may be able to maintain adequate cardiac output under resting conditions, but with even moderate exercise, symptoms of heart failure may appear. As the stenosis progressively worsens, there are reductions in systolic heart function and inability of the left ventricle to develop enough pressure to pump effectively against the load imposed by the partially obstructed aortic valve. Consequently, symptoms of congestive heart failure appear, with reductions in stroke volume and cardiac output.
Calcific aortic valve stenosis usually does not become severe enough to draw clinical attention until after age 70. Important symptoms of aortic valve stenosis are exertion-related angina, reduced exercise tolerance, and congestive heart failure. Shortness of breath ( dyspnea ) is due to increased left ventricular filling pressure or inability to increase cardiac output adequately with exercise. Early recognition and management of aortic stenosis are important because untreated symptomatic aortic valve stenosis is progressive and will ultimately be fatal.
The development of transcatheter aortic valve replacement technologies has provided new therapeutic opportunities, especially for older patients, in whom traditional surgical procedures cannot be performed or are associated with high risk.
As shown by the phonocardiograms in Figure 23-3 , many abnormal heart sounds, known as heart murmurs , occur when abnormalities of the valves are present, as discussed here.
In persons with aortic stenosis, blood is ejected from the left ventricle through only a small fibrous opening of the aortic valve. Because of the resistance to ejection, the blood pressure in the left ventricle sometimes rises as high as 300 mm Hg; the pressure in the aorta is still normal. Thus, a nozzle effect is created during systole , with blood jetting at tremendous velocity through the small opening of the valve. This phenomenon causes severe turbulence of the blood in the root of the aorta. The turbulent blood impinging against the aortic walls causes intense vibration, and a loud murmur occurs during systole (see recording B, Figure 23-3 ; ) and is transmitted throughout the superior thoracic aorta and even into the large arteries of the neck. This sound is harsh, and in persons with severe stenosis it may be so loud that it can be heard several feet away from the patient. Also, the sound vibrations can often be felt with the hand on the upper chest and lower neck, a phenomenon known as a thrill .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here