Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The lymphatic system includes primary and secondary lymphoid organs .


The primary lymphoid organs produce the cell components of the immune system (see 10-1 ). They are:
The bone marrow .
The thymus .
The secondary lymphoid organs are the sites where immune responses occur. They include:
The lymph nodes .
The spleen .
The tonsils .
Aggregates of lymphocytes and antigen-presenting cells in the lung (bronchial-associated lymphoid tissue, BALT) and the mucosa of the digestive tract (gut-associated lymphoid tissue, GALT), including Peyer's patches .
The lymphatic system is widely distributed because pathogens can enter the body at any point.
The main function of the lymphoid organs , as components of the immune system, is to protect the body against invading pathogens or antigens (bacteria, viruses and parasites). The basis for this defense mechanism, or immune response , is the ability to distinguish self from non-self substances .
The two key cell components of the immune system are lymphocytes and accessory cells (see 10-2 ). Lymphocytes include two major cell groups:
B cells , responding to cell-free and cell-bound antigens.
T cells , subdivided into two categories: helper T cells and cytolytic or cytotoxic T cells . T cells respond to cell-bound antigens presented by specific molecules.
After leaving the two primary organs (bone marrow and thymus), mature B and T cells circulate in the blood until they reach one of the various secondary lymphoid organs (lymph nodes, spleen and tonsils).
B and T cells can leave the bloodstream through specialized venules called high endothelial venules , so called because they are lined by tall endothelial cells instead of the typical squamous endothelial cell type.
The accessory cells include two monocyte-derived cell types: macrophages and dendritic cells . An example of a dendritic cell is the Langerhans cell found in the epidermis of the skin. A third type, the follicular dendritic cell , is present in lymphatic nodules of the lymph nodes. Follicular dendritic cells differ from ordinary dendritic cells in that they do not derive from a bone marrow precursor.
Before we start our discussion of the origin, differentiation and interaction of lymphocytes and accessory cells, we need to define the characteristics of the immune system. Then, we will be able to integrate the structural aspects of each major lymphatic organ with the specific characteristics of the immune responses.
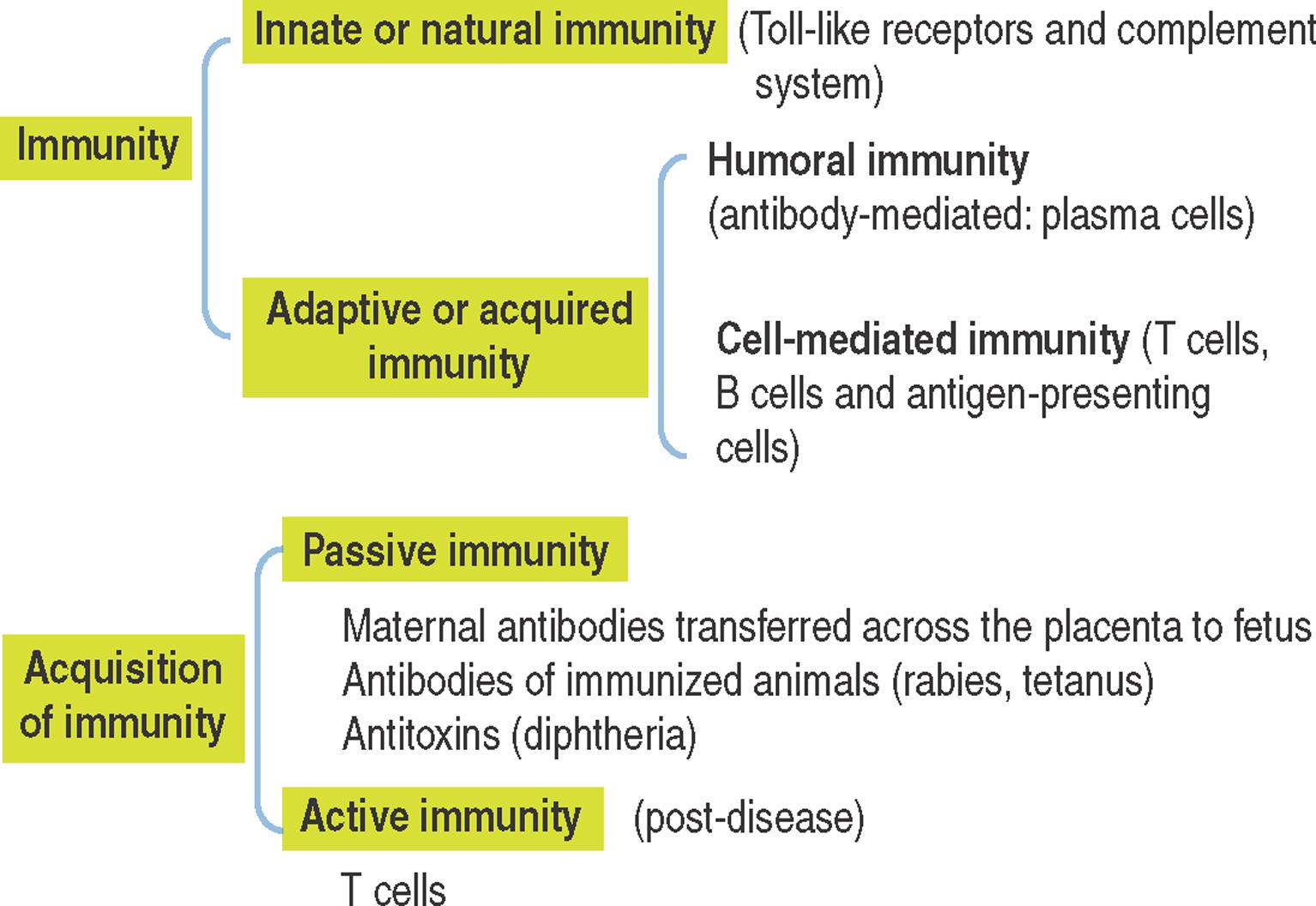
Immunity in general is the reaction of cells and tissues to foreign (non-self) substances or pathogens , including bacterial, viral and parasite antigens. Two types of immunity are distinguished:
Innate or natural immunity.
Adaptive or acquired immunity.
Innate or natural immunity of the newborn is the simplest mechanism of protection. It does not require previous exposure to a pathogen and elicits rapid responses by macrophages and dendritic cells.
Toll-like receptors (TLRs; see Box 10-A ) initiate innate immunity against components of invading pathogens (such as nucleic acids, proteins, lipids and polysaccharides). Different TLRs recognize distinct types of conserved microbial structures, a condition that provides specificity to the innate response.
Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns (PAMPs) . The term PAMPs designates proteins associated with a wide spectrum of pathogens recognized by cells of the innate or natural immune system.
Activated TLR activate in turn the NF-κB transcription factor pathway (see Necroptosis in Chapter 3 , Cell Signaling | Cell Biology | Pathology), which regulates cytokine expression. Activation of the NF-κB pathway links innate and adaptive immune responses by stimulating the production of proinflammatory cytokines, such as interleukines and tumor necrosis factor ligand and chemokines, as well as triggering the expression of costimulatory molecules (CD40, CD80 and CD86).
The intracellular domain of the TLR has structural homology with the cytoplasmic region of interleukin-1 receptors. It is known as Toll-interleukin-1 receptor domain , or TIR domain , and participates in signaling by recruiting downstream proteins.
The extracellular region of the TLR contains leucine-rich repeat (LRR) motifs, whereas the extracellular domain of interleukin receptors contains three immunoglobulin-like domains. LRR is involved in the recognition of PAMPs, facilitated by accessory proteins (for example, lipopolysaccharides).
We discuss later in this chapter that the complement system , consisting of serum proteins, membrane-bound regulators and receptors, is also a key mechanism of innate defense, triggered rapidly upon infection. Stimulation of macrophages and dendritic cells by activated TLRs and the complement system leads to the production and secretion of proinflammatory cytokines, thereby initiating an inflammatory response.
Adaptive or acquired immunity develops when an individual is exposed to a pathogen with the aims of eliminating the pathogen as well as the generation of immunologic memory.
To achieve adaptive or acquired immunity, it is necessary to select lymphocytes (clonal selection) from a vast repertoire of cells bearing antigen-specific receptors generated by a mechanism known as gene rearrangement . You can regard adaptive immunity as the perfection of innate or natural immunity because it recognizes vital components of the microorganism utilizing a limited number of pattern-recognition receptors , expressed on all cells of a given type (non-clonal) and independent of immunologic memory.
Adaptive or acquired immunity involves two types of responses to an antigen (or pathogen):
The first response is mediated by antibodies produced by plasma cells, the final differentiation product of B cells as we have seen in Chapter 4 , Connective Tissue. This response is known as humoral immunity and operates against antigens located outside a cell or bound to its surface. When antibodies bind to an antigen or toxins produced by a pathogen, they can facilitate the phagocytic action of macrophages or recruit leukocytes and mast cells to take advantage of their cytokines and mediators, respectively, and strengthen a response. Humoral immunity results in persistent antibody production and production of memory cells.
The second response requires the uptake of a pathogen by a phagocyte . An intracellular pathogen is not accessible to antibodies and requires a cell-mediated response, or cell-mediated immunity . T cells, B cells and antigen-presenting cells are the key players in cell-mediated immunity.
A consequence of adaptive or acquired immunity is the protection of the individual when a second encounter with the pathogen occurs. This protection is specific against the same pathogen and, therefore, adaptive or acquired immunity is also called specific immunity .
Passive immunity is a temporary form of immunity conferred by serum or lymphocytes transferred from an immunized individual to another individual who has not been exposed or cannot respond to a pathogen. The transfer of maternal antibodies to the fetus is a form of passive immunity, which protects newborns from infections until they can develop active immunity. Active immunity is the form of immunity resulting from exposure to a pathogen.
Properties of adaptive or acquired immunity
Both humoral and cell-mediated immunity developed against foreign pathogens have the following characteristics:
Specificity: Specific domains of an antigen are recognized by individual lymphocytes. We will see later how cell membrane receptors on lymphocytes can distinguish and respond to subtle variations in the structure of antigens offered by an antigen-presenting cell. This molecular interaction between cells is known as immunologic synapse .
Diversity: Lymphocytes utilize a gene rearrangement mechanism to modify their antigen receptors in such a way that they can recognize and respond to a large number and types of antigenic domains.
Memory: The exposure of lymphocytes to an antigen results in two events: their antigen-specific clonal expansion by mitosis, as well as the generation of reserve memory cells . Memory cells can react more rapidly and efficiently when exposed again to the same antigen.
Self-limitation: An immune response is stimulated by a specific antigen. When the antigen is neutralized or disappears, the response ceases.
Tolerance: An immune response pursues the removal of a non-self antigen while being „tolerant” to self-antigens. Tolerance is achieved by a selection mechanism, which eliminates lymphocytes expressing receptors specific for self-antigens. A failure of self-tolerance (and specificity) leads to a group of disorders called autoimmune diseases .
The bone marrow is the site of origin of B and T cells from a lymphoid stem cell. The same hematopoietic stem cell gives rise to a lymphoid stem cell , which generates precursors for B cells and T cells (see 10-1 ) . B cells mature in the bone marrow , whereas the thymus is the site of maturation of T cells .
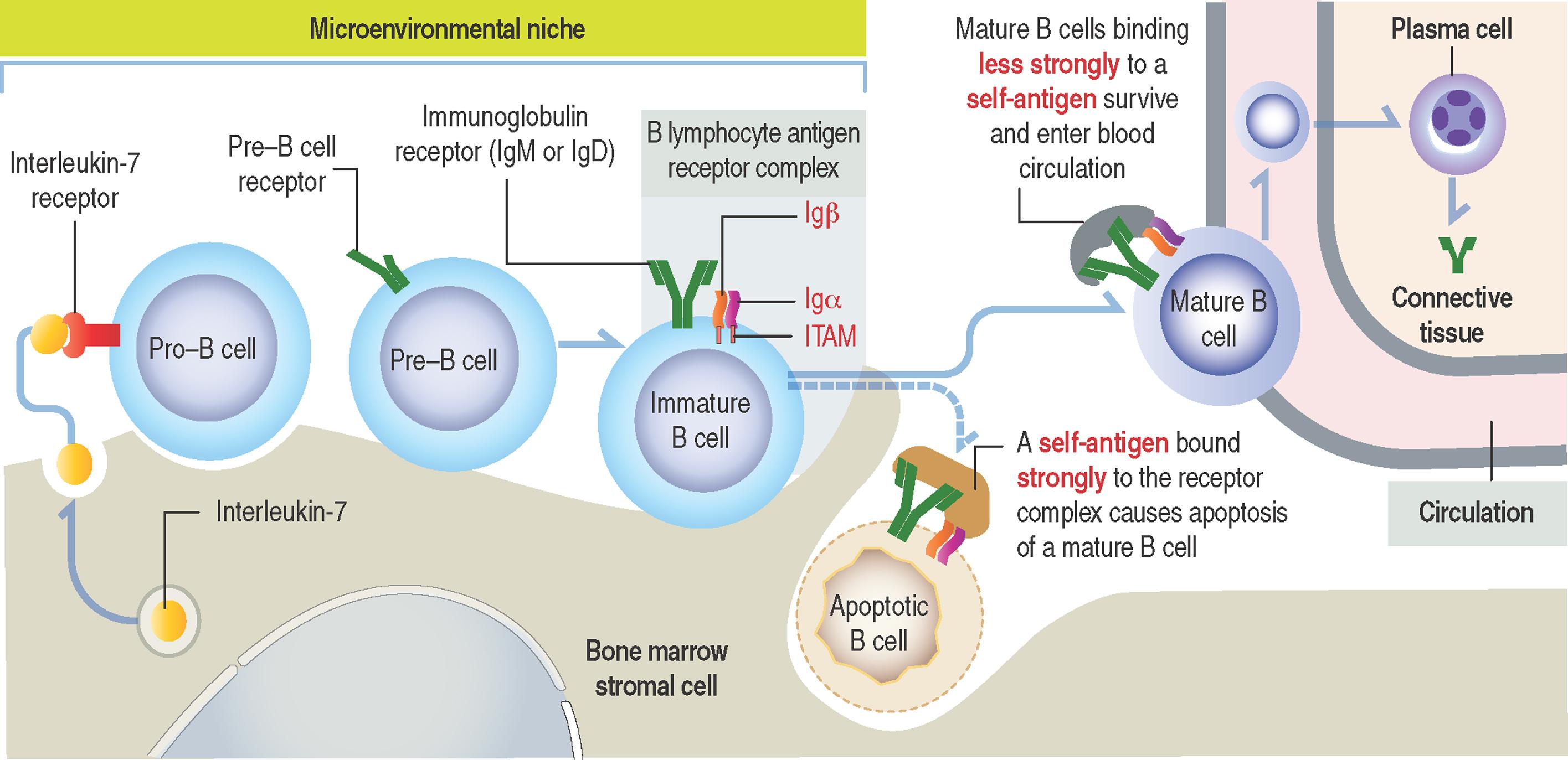
Stem B cells in the bone marrow proliferate and mature in a microenvironmental niche provided by bone marrow stromal cells producing interleukin-7 (IL-7) (see 10-4 ).
During maturation, B cells express on their surface immunoglobulins M (IgM) or D (IgD) interacting with two additional proteins linked to each other, immunoglobulins α(Igα) and β (Igβ) . The cell surface IgM or IgD, together with the conjoined Igα and Igβ , form the B cell antigen receptor complex . The intracellular domains of Igα and Igβ contain a tyrosine-rich domain called immunoreceptor tyrosine-based activation motif (ITAM) .
Binding of an antigen to the B lymphocyte antigen receptor complex induces the phosphorylation of tyrosine in the ITAM, which, in turn, activates transcription factors driving the expression of genes required for further development of B cells.
Self-antigens present in the bone marrow test the antigen-binding specificity of IgM or IgD on B-cell surfaces. This is a required testing step before B cells can continue their maturation, enter peripheral lymphoid tissues and interact with foreign (non-self) antigens.
Self-antigens binding strongly to two or more IgM or IgD receptor molecules on B cells induce apoptosis. Self-antigens with a weaker binding affinity for the B-cell antigen receptor complex enable the survival and maturation of these B cells when ITAMs of IgM- or IgD-associated Igα and Igβ transduce signaling events, resulting in further differentiation of B cells and the entrance of mature B cells into the circulation.
Major histocompatibility complex (MHC) and the human–equivalent leukocyte antigens (HLA) ( 10-5 ) T-cell recognition of an antigen and the resulting signaling that follows are key steps in the initiation of the adaptive immune response.
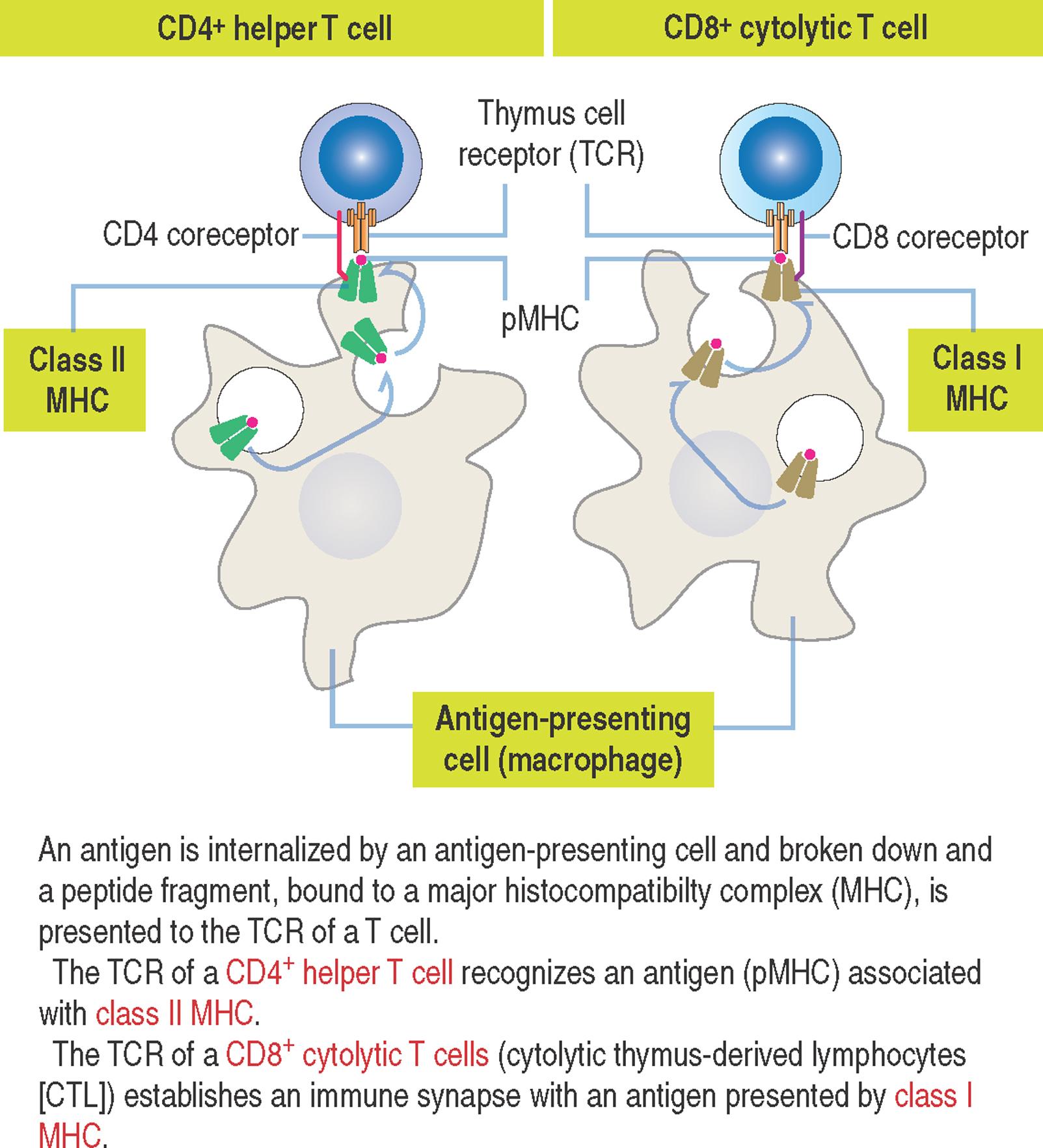
The presentation of antigens to T cells is carried out by specialized proteins encoded by genes in the major histocompatibility locus and present on the surface of antigen-presenting cells, the macrophages.
Antigen-presenting cells survey the body, find and internalize antigens by phagocytosis, break them down into antigenic peptide fragments and bind them to major histocompatibility complex (MHC) . The antigen peptide fragment–MHC complex (designated pMHC) can be exposed later on the surface of the cells and recognized by T cells.
There are two types of mouse MHC gene products: class I MHC and class II MHC .
Class I MHC consists of two polypeptide chains: an α chain , consisting of three domains (α 1 , α 2 and α 3 ) encoded by the MHC gene locus, and β 2 -microglobulin , not encoded by the MHC gene locus.
Antigens are housed in a cleft formed by the α 1 and α 2 domains. CD8 , a coreceptor on the surface of cytolytic T cells , binds to the α 3 domain of class I MHC.
Class II MHC consists of two polypeptide chains, an αchain and a βchain. Both chains are encoded by the MHC gene locus. The α 1 and α 1 domains form an antigen-binding cleft. CD4 , a coreceptor on the surface of helper T cells , binds to the β 2 domain of class II MHC.
CD4 and CD8 are cell surface identifiers, members of the cluster of differentiation or designation (abbreviated as CD; see Box 10-B ).
Cell surface molecules recognized by monoclonal antibodies are called antigens . These antigens are markers that enable the identification and characterization of cell populations. A surface marker that identifies a member of a group of cells, has a defined structure and is also recognized in other members of the group by a monoclonal antibody is called a cluster of differentiation or designation (CD) .
A helper T cell , which expresses the CD4 marker, can be differentiated from a cytolytic T cell , which does not contain CD4 but expresses the CD8 marker.
CD markers permit the classification of T cells that participate in inflammatory and immune reactions. CD antigens promote cell-cell interaction and adhesion, as well as signaling leading to T-cell activation .
All nucleated cells express class I MHC molecules. Class II MHC molecules are restricted mainly to antigen-presenting cells (macrophages, dendritic cells and B cells), thymic epithelial cells of the thymus and endothelial cells.
The MHC-equivalent molecules in the human are designated human leukocyte antigens (HLAs) . HLA molecules are structurally and functionally homologous to mouse MHC molecules and the gene locus is present on human chromosome 5 (β 2 -microglobulin is encoded by a gene on chromosome 15).
The class I MHC locus encodes three major proteins in the human: HLA-A, HLA-B and HLA-C . The class II MHC locus encodes HLA-DR (R for antigenically related), HLA-DQ and HLA-DP .
T cells have cell surface receptors for the recognition of antigenic pMHC presented by MHC. This is the fundamental initiating event of T-cell activation and induction of an effector function.

Antigen recognition involves an immunologic synapse mechanism consisting of the formation of stable pMHC–T cell adhesiveness, followed by an activating signaling cascade by T cells (see Box 10-C ).
The initiation and regulation of a specific immune response depends on the communication between T cells and antigen-presenting cells (APCs) . The immune response results from molecular interactions at the site of T-cell and APC contact also known as the immune synapse . The immune synapse is a combined cell-cell adhesion and cell signaling device.
The diversity of cell surface molecules of APCs (class I MHC and class II MHC) and T cells (T cell receptors and coreceptors) provides a framework for the molecular regulation and activity of the immune synapse. The immune synapse has a significant role in T-cell maturation, activation and differentiation taking place in the cortex of the thymus. The immune synapse concept also applies to B-cell maturation in bone marrow.
How a p-MCH, presented by an antigen-presenting cell, can be read and then translated by a T cell into a functional response represents a key step to discriminate between self and foreign antigens, to recognize tumor antigens or to avoid autoimmune responses.
The receptor which recognizes specific pMHC presented by class I and class II MHC molecules, is the T-cell receptor (TCR) .
TCR consists of two disulfide-linked transmembrane polypeptide chains: the α chain and the β chain . The α-β heterodimer confers ligand binding specificity to the TCR but lacks the capability to transduce signaling activity.
To acquire signaling activity, TCR associates with multiple transducing proteins, including CD3γ, CD3δ, CD3ε and CD3ζ , each containing immunoreceptor tyrosine-based activation motifs (ITAMs). Phosphorylated ITAMs recruit proteins with an SH2 domain, in particular the tyrosine protein kinase ZAP70 , to the TCR. The ITAM cytoplasmic domain was previously mentioned as a component of the B-cell antigen receptor complex involved in signaling functions.
The initiation of signaling by TCR is integrated and propagated by tyrosine phosphorylation of three associated proteins: ZAP70, LCK (for lymphocyte cell kinase) and LAT (for linker for activation of T cells). LCK is an important participant in TCR signal propagation.
How does TCR signaling start? It appears that binding of the pMHC ligand determines a conformation change in the TCR complex, which enables the CD3 chain to become susceptible to phosphorylation. The CD3 ζ -CD3 ζ pair, which remains apart in a native state, approach each other upon pMHC binding to the TCR, activate tyrosine phosphorylation of ITAM, increase the concentration of LCK and signal initiation takes place.
Does activation require the triggering of a number of TCRs? A single pMHC complex can engage multiple TCRs. In other words, low concentrations of antigen peptides or a short time interval of pMHC-TCR engagement can trigger the initiation of TCR signaling.
In addition to activating proteins associated with the TCR, T cells have coreceptors connected to MHC proteins. CD4 and CD8 are T-cell surface proteins interacting selectively with MHC proteins (see Primer 10-A ).
C D4 and CD8 are members of the immunoglobulin (Ig) superfamily . Members of the Ig superfamily have a variable number of extracellular Ig-like domains. The intracellular domain of CD4 and CD8, in turn, binds to LCK .
LCK, a member of the Src family of protein kinases, binds to the cytoplasmic domains of the CD4 and CD8 coreceptors. LCK is recruited when CD8 or CD4 binds to class I MHC or class II MHC complexes, respectively.
Two terminal Ig-like domains of CD4 bind to the β 2 domain of the class II MHC. A single Ig-like domain of CD8 binds to the α 3 domain of the class I MHC.
Following pMHC ligand binding, LCK phosphorylates tyrosine residues in the CD3 chains, enabling the docking and activation of the protein tyrosine kinase ZAP70. Then, ZAP70 phosphorylates the transmembrane adapter protein LAT, leading to the recruitment of multiple adapters.
Remember: CD4 + helper T cells recognize antigens associated with class II MHC and CD8 + cytolytic T cells (cytolytic thymus-derived lymphocytes [CTL]) respond to antigens presented by class I MHC (see 10-5 ).
Thymocyte maturation in the thymus: Positive and negative selection ( 10-6 )

The recruitment and entry of bone marrow–derived T lymphoid cell progenitors, called thymocytes, to the thymus depends on multiple chemokine receptors, including CC-chemokine receptor 7 (CCR7), CCR9 and CXC-chemokine receptor 4 (CXCR4).
Two initial events take place in the thymus during the maturation of thymocytes:
A sequence rearrangement of the gene encoding the protein components of the TCR .
The transient coexistence of TCR-associated coreceptors CD4 and CD8 . Thymocytes progress through three major developmental stages defined by expression of the CD4 and CD8 coreceptors.
When precursor cells, derived from the bone marrow, enter the cortex of the thymus, they lack surface molecules typical of mature T cells. Because they still do not express CD4 and CD8, they are called „double-negative” (DN) thymocytes .
After interacting with thymic epithelial cells , the stroma components of the thymus, DN thymocytes proliferate, differentiate and express the first T cell–specific molecules: TCR and coreceptors CD4 and CD8.
As we have already seen, TCR consists of two pairs of subunits: αβ chains . Each chain can vary in sequence from one T cell to another. This variation is determined by the random combination of gene segments and has a bearing on which foreign antigen thymocytes can recognize by a screening process called thymocyte selection . The outcome of the selection process is dictated by the affinity of their expressed TCRs for self-peptides bound to MCH molecules (pMHC) in the thymus.
Maturation of thymocytes proceeds through a stage where both CD4 and CD8 coreceptors and low levels of TCR are expressed by the same cell . These cells are known as „double-positive„(DP) thymocytes .
Three possibilities can take place:
TCR–self-pMHC interactions result in the transduction of TCR signals, which are sufficient to promote the survival of DP thymocytes and their transition to the SP stage. This outcomer is called positive selection .
If the expressed TCR on a DP thymocyte fails to engage self-pMHCs, the required TCR signals for positive selection are not generated and the cell undergoes death by apoptosis. This outcome is called non-selection .
Thymocytes expressing TCRs, which bind with high affinity to self-pMHCs, generate strong signaling, which also induces death by apoptosis. This outcome is called negative selection .
Selected thymocytes must be self-MHC–restricted and self-tolerant . Cells able to recognize self-MHC eventually mature, express one of the two coreceptor molecules (CD4 or CD8) and become „single-positive” (SP) thymocytes . This process is called clonal selection .
Keep in mind that MHC restriction and the two coreceptor molecules CD4 or CD8 play essential roles in pMHC recognition and the ability to generate productive signaling. Remember that LCK, largely associated with CD4 and CD8 in thymocytes, establishes an interaction with the TCR-CD3 complex, which is dependent on binding CD4 or CD8 and TCR to the MHC molecule.
There is an additional test for the selected self-MCH–restricted thymocytes: only those thymocytes that can recognize foreign peptides and self-MHC will survive . If thymocytes bind to body's tissue-specific antigens (self-molecules) , they are eliminated by apoptosis and cleared by macrophages.
So, which is the source of the foreign and self-peptides for testing thymocytes?
Branching and interconnected thymic cortical epithelial cells in the cortex of the thymus synthesize and present self and non-self peptides to the previously selected thymocytes shown to be self-MHC-restricted and self-tolerant.
After completing the positive selection tests in the cortex of the thymus, thymocytes are induced to express chemokine receptor CCR7 to fulfill an additional requirement in the medulla of the thymus. The medulla of the thymus houses thymic medullary epithelial cells , which produce CCR7 cytokines involved in optimizing the negative selection of potentially autoreactive thymocytes.
When thymocytes complete their development in the thymus, they enter the bloodstream and migrate to the peripheral lymphoid organs in search of an antigen on the surface of an antigen-presenting cell. We come back to additional details of the thymocyte's maturation sequence in our discussion of the thymus.
We have seen that B cells can differentiate into immunoglobulin-secreting plasma cells under the influence of cytokines produced by CD4 + helper T cells.

B cells can present antigens, thus allowing direct interaction with T cells, which produce and secrete cytokines for plasma cell development. Plasma cells are effector cells; they use antibodies to neutralize extracellular pathogens. In contrast, T cells are primary effector cells for controlling or killing intracellular pathogens.
There are four CD4 + T-cell subsets: T h 1, T h 2, T h 17 AND T fh (for T follicular helper) cells. They are defined by a repertoire of produced specific cytokines in response to specific pathogens and immunological functions.
T h 1 cells produce interferon-γ (IFN-γ) and their function (defined as type 1 cell-mediated immunity) is associated with intracellular pathogens (such as Mycobacterium tuberculosis, Leishmania major, Toxoplasma gondii and others). Interferon-γ (IF-γ), produced by T h 1 cells, stimulates the differentiation of T h 1 cells but suppresses the proliferation of T h 2 cells.
T h 2 cells are involved in immune responses (defined as type 2 cell-mediated immunity) , which are critical for protecting the host from infections with helminthic (Greek helmins , worm) intestinal parasites and for stimulating repair of damaged tissue.
T h 2 cells produce specific interleukins (ILs): IL-4, IL-5, IL-9 and IL-13. T h 2 cells are unable to secrete IF-γ. T h 2 cells produce several ILs, which promote effects such as eosinophilia (IL-5), intestinal mastocytosis to induce parasite expulsion (IL-4 and IL-9) and macrophage activation (IL-4 and IL-13). IL-4 stimulates the production of immunoglobulin E (IgE) by B cells to activate the responses of mast cells, basophils and eosinophils. T h 2-derived IL-4 suppresses the activation of T h 1 cells. As you can see, T h 1 and T h 2 produce cytokines (IFN-γ and IL-4, respectively), which reciprocally inhibit the development of the other subset.
T h 17 cells secrete IL-17 and are associated with bacterial and fungal infections (defined as type 3 cell-mediated immunity) . IL-17 recruits and activates neutrophils and macrophages.
T fh cells promote the survival, proliferation and differentiation of B cells in the light zone of the germinal centers of lymph nodes. Within the germinal centers, B cells, interacting with T fh cells, proliferate by entering the dark zone of the germinal center. B cell–derived progenies re-enter the light zone and establish contact with antigens and T fh cells. B cells, selected by T fh , will produce long-lived plasma cells and memory B cells persisting for life. T fh cells are also associated with B-cell antibody responses, consisting of driving Ig class switching from IgM to IgG.
CD4+ helper T cells are activated when they recognize the pMHC–class II MHC complex.

In the presence of cells with pMHC bound to class II MHC, CD4+ helper T cells proliferate by mitosis and secrete cytokines , also called ILs . These chemical signals, in turn, attract B cells, which also have receptor molecules of single specificity on their surface (Ig receptor). Unlike helper T cells, B cells can recognize free antigen peptides without MHC molecules .
When activated by ILs produced by the proliferating helper T cells, B cells also divide and differentiate into plasma cells secreting Igs .
Secreted Igs diffuse freely, bind to antigen peptides to neutralize them, or trigger their destruction by enzymes or macrophages.
Plasma cells synthesize only one class of Ig (several thousand Ig molecules per second; the lifetime of a plasma cell is from 10 to 20 days).
Five classes of Igs are recognized in humans: IgG, IgA, IgM, IgE and IgD (see Box 10-D ). Abnormal plasma cells may accumulate in bones and bone marrow, causing bone destruction and affecting the production of normal blood cells. This pathologic condition is called multiple myeloma (see Box 10-E ).
An immunoglobulin (Ig) molecule or antibody is composed of four polypeptide chains: two identical light chains and two identical heavy chains. One light chain is attached to one heavy chain by a disulfide bond. The two heavy chains are attached to each other by disulfide bonds.
Heavy and light chains consists of amino terminal variable regions that participate in antigen recognition (Fab region) and carboxyl terminal constant regions. The constant region (Fc region) of the heavy chains mediates effector functions.
Immunoglobulins can be membrane-bound or secreted.
Types of immunogloblins: IgA forms dimers linked by a J chain and participates in mucosal immunity. IgD is a receptor for antigens of inmature B cells. IgE participates in mast cell and basophil activation (degranulation). IgG is the most abundant immunogloblin and the only one to cross the placental barrier. It participates in opsonization, a mechanism that enhances phagocytosis of pathogens. IgM molecules normally exist as pentamers.
Multiple myeloma is caused by the abnormal proliferation of plasma cells in bone marrow and bone . An excessive growth of malignant plasma cells in bone and marrow causes bone fractures and prevents the production of normal blood cells in the marrow. Anemia, abnormal bleeding and high risk of infections may develop. Compression of the spinal cord by myeloma cells growing in vertebrae can cause back pain, numbness or paralysis.
Myeloma cells produce an excessive amount of an abnormal immunoglobulin, called Bence-Jones protein , present in serum and urine. Renal failure may occur because of the accumulation of immunoglobulins in the kidneys.
Bone marrow transplantation (autologous , from the same patient, or allogenic , from a healthy and compatible donor) is a form of treatment in patients resistant or non-responsive to chemotherapy. First, the bone marrow of the recipient is depleted with very high doses of chemotherapy and low doses of radiation therapy and then followed by the administration of donor's marrow cells into the blood of the patient. Hematopoietic stem cells will then localize in bone marrow and repopulate it.
Some T and B cells become memory cells , ready to eliminate the same antigen if it recurs in the future. The secondary immune response (re-encounter with the same antigen that, triggered their production) is more rapid and of greater magnitude. Memory cells recirculate for many years and provide a surveillance system directed against foreign antigens.
Another function of CD4 + helper T cells is to secrete cytokines to stimulate the proliferation of CD8 + cytolytic T cells , which recognize the pMHC–class I MHC complex on the surface of antigen-presenting cells. Cytolytic, or cytotoxic, T cells display TCR and CD8 coreceptors .

CD8 + cytolytic T cells initiates a target cell destruction process by:
Attaching firmly to the antigen-presenting cell with the help of integrins and cell adhesion molecules on the cell surface of the target cell.
Inducing cell membrane damage by the release of pore-forming proteins (called perforins) .
These pores facilitate the unregulated entry of the pro-apoptotic protease granzyme , water and salts. The cytolytic T cell protects itself by a membrane protein, protectin , which inactivates perforin, blocking its insertion into the cytolytic T cell membrane.
CD8 + cytolytic T cells can also destroy target cells by the Fas-Fas ligand mechanism seen during apoptosis (see Chapter 3 , Cell Signaling | Cell Biology | Pathology).
When the cytolytic T cell receptor recognizes an antigen on the surface of a target cell, Fas ligand is produced in the cytolytic T cell. The interaction of Fas ligand with the trimerized Fas receptor on the target cell surface triggers the apoptotic cascade by activation of procaspases into caspases, which determine cell death.
Natural killer (NK) cells are lymphocytes, which are activated on encounter with virus-infected cells and tumor cells. The role of NK cells in immune surveillance against tumor cells highlights the development of therapeutic approaches targeting NK cells in cancer treatment.

The rapid cytolytic activity of NK cells does not depend on antigen activation . Instead, NK cells need to be primed by cytokines (INF-γ and ILs) or dendritic cells to accomplish an optimal effector response. Dendritic cells are specialized antigen-presenting cells monitoring their environment for non-self antigens, which they internalize, process and present to antigen-specific T cells and NK cells. NK cells do not belong to the T or B cell types; they do not express TCR.
NK cells are present in most human tissues and in peripheral blood but are not abundant in lymph nodes and tonsils. Bone marrow and secondary lymphoid organs are believed to be the development site of NK cells.
Tissue-resident human NK cells have CD56 receptors as well as inhibitory and activating receptors interacting respectively with class I MHC and an activating ligand of normal cells. Target cells lacking class I MHC activate the destructive function of NK cells mediated by perforin or tumor necrosis factor ligand derived from activated macrophages.
Activating and inhibitory receptors deliver a balance of signals that regulate the recognition of normal cells by NK cells.
For example, when class I MHC is downregulated in tumor cells, NK cells destroy the MHC-deficient tumor cell. Alternatively, tumor cells can overexpress ligands, which are recognized by the activating receptor in NK cells. Signaling from the activated receptor overrides the inhibitory receptor and tumor cells are lysed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here