Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The middle cerebral artery (MCA) is the largest cerebral artery, and it is the most commonly affected intracranial vessel in cerebrovascular accident. It supplies most of the outer convex brain surface, nearly all the basal ganglia, and the anterior and posterior limbs of internal capsule.
Infarcts that occur within the distribution of MCA territory lead to diverse neurologic sequelae. In general, the clinical features depend on the site of MCA occlusion. More distal blockages tend to produce milder deficits while the proximal occlusions result in widespread effects that could even be fatal.
Contralateral hemiparesis and hemisensory loss are the most common presentations of MCA syndrome. Lower extremity function is more spared than that of the faciobrachial region.
Aphasia is a common feature when the cortical areas of the dominant hemisphere are involved. In comparison, involvement of homologous regions in the nondominant hemisphere often results in impaired awareness of the deficit.
Advances in modern parenchymal, vascular, molecular, and functional imaging techniques have enhanced our understanding of the pathophysiology of various neurologic deficits observed in MCA disease and carry potential to predict the extent of expected recovery as well as influence the results of various therapeutic strategies to improve outcome.
The middle cerebral artery (MCA), the largest of the branches of the internal carotid artery (ICA), is also the most commonly affected artery in stroke syndromes. The MCA supplies most of the convex surface of the brain, leaving to other cerebral arteries only the frontal pole and its immediately adjacent lateral gyro, the extremes of the high parietal and very posterolateral aspects of the occipital gyri, and occipital pole. In the brain parenchyma, its branches irrigate almost all of the basal ganglia and capsules (internal, external, and extreme capsule), claustrum, putamen, the upper parts of the globus pallidus, parts of the substantia innominata of Reichert, the posterior portion of the head and all of the body of the caudate nucleus, and all but the very lowest portions of the anterior and posterior limbs of the internal capsule. Supply to any portion of the thalamus has also been demonstrated.
The internal capsule, classically the MCA territory, has a complex arterial supply. Although its anterior limb has some supply from a large branch of the anterior cerebral artery known as Heubner artery, the MCA supplies the entire anterior limb in at least one-third of cases. Most of the posterior limb of the internal capsule and corona radiata are fed by the deep, lenticulostriate branches of the MCA, whereas the lowest portion of the posterior limb is supplied by the anterior choroidal artery, which usually arises from the ICA just proximal to the circle of Willis.
The anatomy of the MCA tree has been described by named branches and by the relationship between the vessel and the anatomic landmarks of the cerebral surface.
The traditional terminology analogizes the vessel as a tree with a trunk and branches ( Fig. 24.1 ), a clinically useful descriptive method that we employ throughout this chapter. The MCA regularly begins as a single trunk or stem. Its length varies from 18 to 26 mm. The diameter at its origin is about 3 mm (range 2.5–4.9 mm). , The stem gives rise to most of the lenticulostriate branches, so named because they supply the lentiform nucleus (putamen and pallidum), body of the caudate nucleus, and the internal capsule. The claustrum and external capsule are supplied by vessels from the surface, penetrating through the insula. The lenticulostriate arteries are 5–17 branches, each of which is an end artery, which supply cones of varying size of adjacent tissue in their curvilinear (on coronal view) course to end in the corona radiata near the lateral ventricular wall. Some smaller lenticulostriate arteries may arise from the distal ICA.
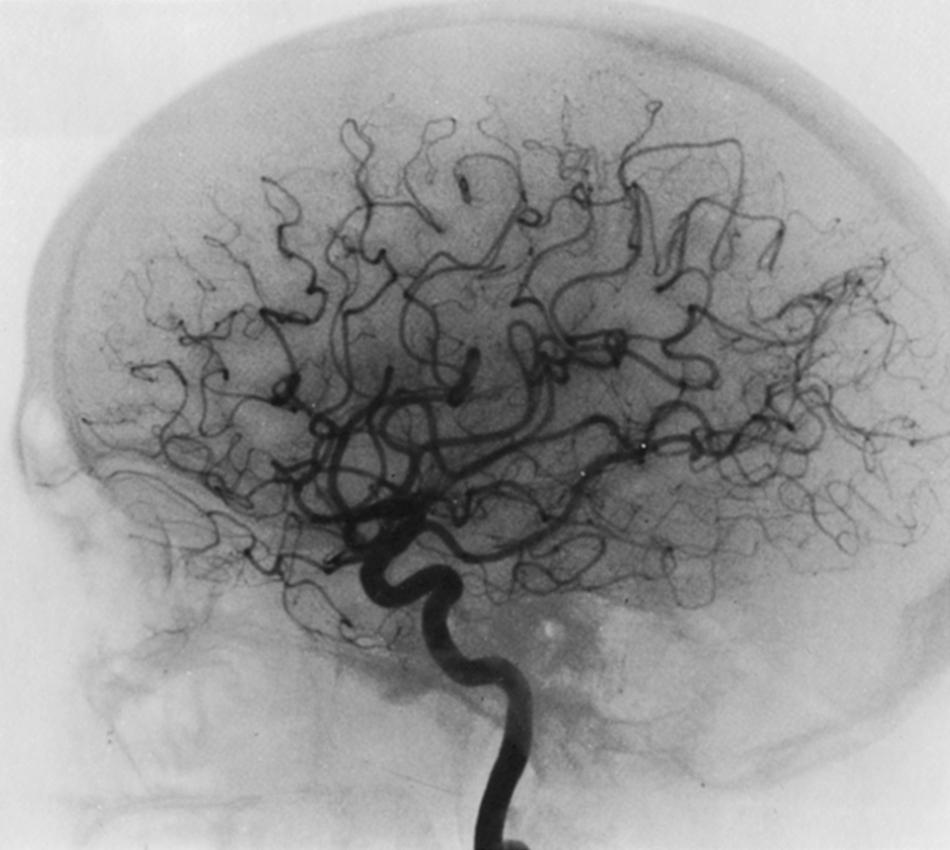
No clear correlation between the length of the MCA stem and the pattern or number of the lenticulostriate arteries has been shown, nor does the pattern on one side predict that on the other. The lenticulostriate arteries arising more medially on the MCA stem are the smaller vessels (50–150 μm), whereas those arising more laterally are larger (some as large as 500 μm). Three patterns of origin of the lenticulostriate arteries from the MCA have been described. In the most common variant (49%), one or more of the larger lenticulostriate branches arise just beyond the major bifurcation (in the territory of the upper division). The next most common permutation (39%) features all the larger lenticulostriate arteries arising from the stem just proximal to its bifurcation. In the least commonly encountered pattern, some of the larger penetrators arise from the medial portion of the stem. These variations may explain differing patterns of infarction from occlusion of the MCA stem alone or its main divisions. One important anatomic feature of these arteries is the lack of anastomoses among themselves.
The cerebral surface, centrum semiovale, claustrum, external capsule, hemispheral cortex, and white matter are supplied by those MCA branches that originate beyond the lenticulostriate arteries. These cortical surface branches usually number 12–15. They arise from the MCA stem in a variety of patterns, and by far the most common (78%) is two large branches (bifurcation pattern). Less often (12%), the 12 branches arise from three major trunks (trifurcation pattern). The least differentiated and least common (10% of cases) is the continuation of the stem with no major divisions, when each of the surface branch arises from the common trunk until the primary vessel has given off 11 of the usual 12 branches, after which it terminates as the angular artery.
In the bifurcation pattern, the two main branches are called the superior and inferior division. The superior division supplies insula, frontal lobe, and rolandic regions and always contains the orbitofrontal and prefrontal branches. The inferior division supplies the temporal polar, anterior temporal, and middle temporal branches. Although the central (rolandic) branch is almost always in the upper division, in a few cases it is included in the lower. Likewise, the posterior temporal branch is almost always in the lower division. The upper division is usually the source of the anterior parietal, posterior parietal, and angular branches. No branch arising from the upper division irrigates brain regions that would be expected to be supplied from a branch of the lower division or vice versa.
In the trifurcation pattern, the orbitofrontal, prefrontal, and precentral branches supplying the frontal lobe are regularly represented in the upper division. The middle division is made up of the central (rolandic), the anterior parietal, and the angular branches. Less often, the precentral branch is a member of this trunk on the frontal side, and in a few other instances, the temporo-occipital and superior temporal branches are added on the inferior side. The inferior division regularly contains the temporal polar, anterior, and middle temporal branches, to which the posterior temporal and temporo-occipital branches are less often added.
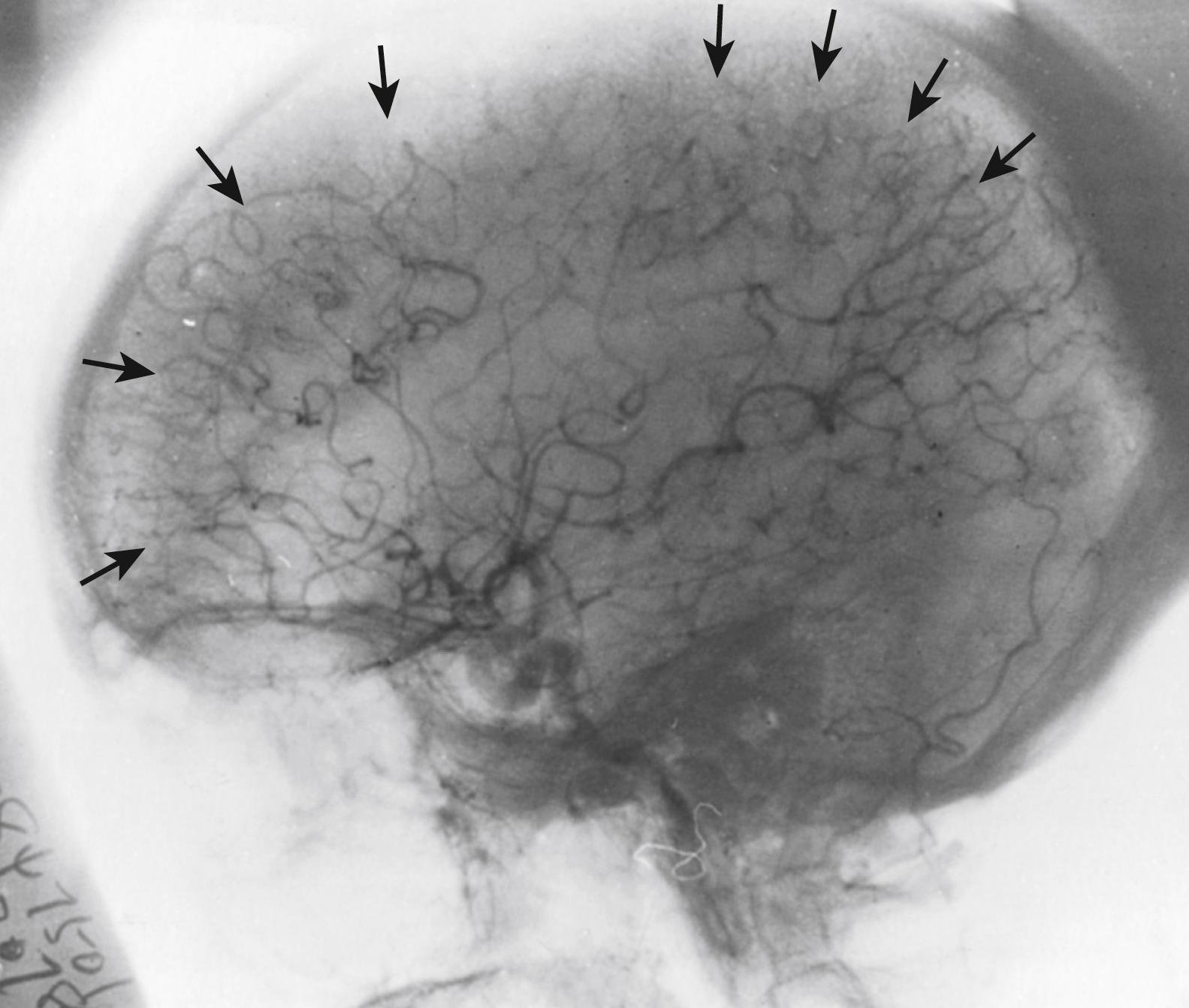
Regardless of the brain regions supplied by each branch, remarkable variations have been found in the position over gyri and sulci of individual branches within their section of the convexity. Variation in length is orderly: the smallest branches supply the frontal lobe while the longest branches supply the high parietal and lateral occipital areas. Only 27% of the orbital frontal branches are as large as 1 mm in diameter. The largest artery is usually the artery of the central (rolandic) sulcus. The more posterior regions of the brain are supplied by fewer arteries, which are larger in diameter, give off fewer major branches, and have the longest course from the circle of Willis to their termination in a borderzone ( Fig. 24.2 ). The temporo-occipital artery is 1 mm in diameter in 90% of cases and more than 1.5 mm in length in up to 63% of cases. This large diameter and the ease with which it can be followed on the surface for long distances helps surgeons to use this branch for the extracranial–intracranial anastomosis operation. The three vessels with the longest course on the cortical surface are the angular, posterior parietal, and temporo-occipital arteries. Intraluminal diameters greater than 1 mm have been encountered in up to 86% of angular arteries, in 68% of temporo-occipital arteries, and in 52% of posterior parietal arteries but in only 14% of central sulcus arteries.
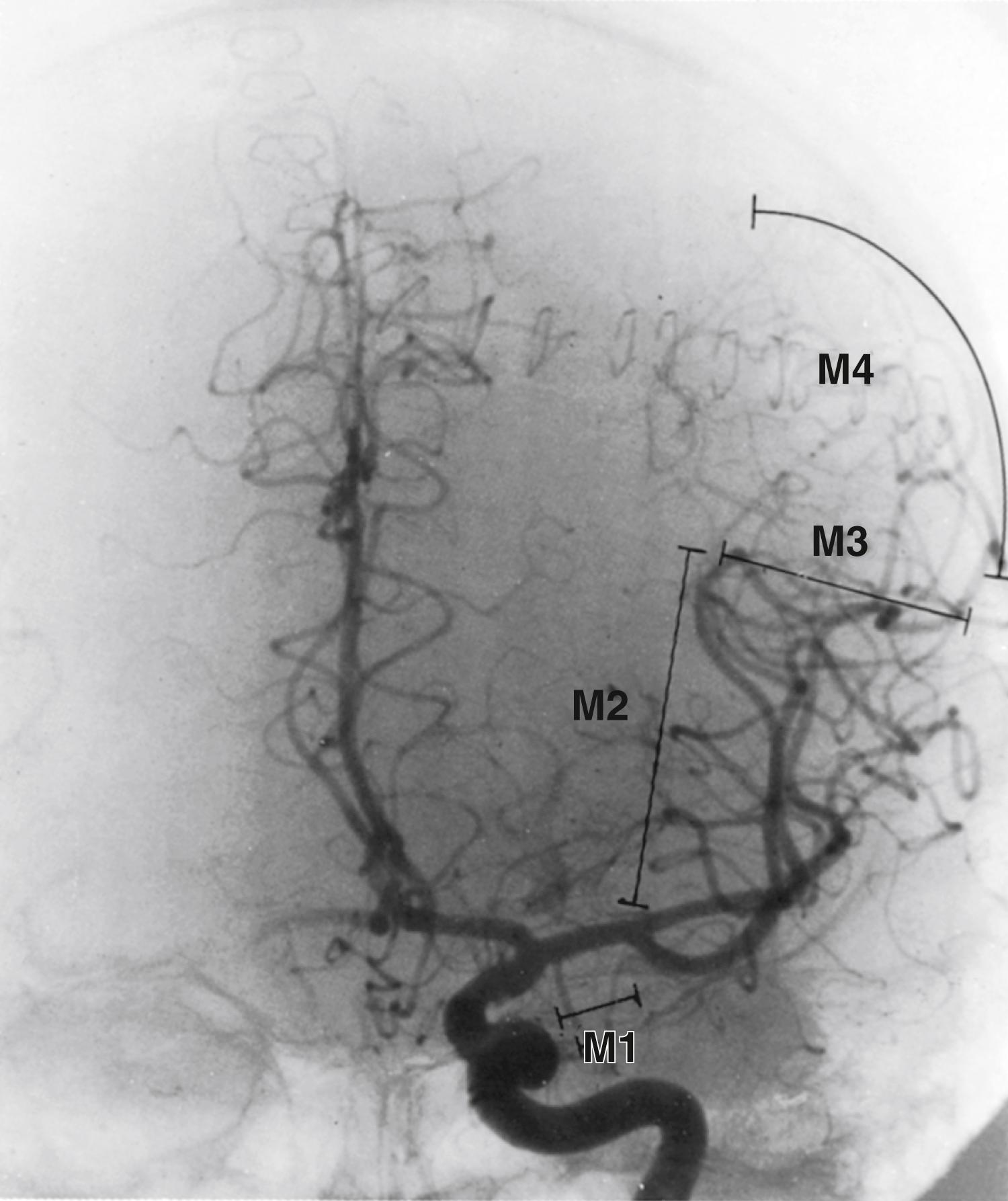
Another method of classifying the MCA branches is based on the relationship of the artery with the major landmarks on the brain, especially the sylvian fissure, the operculum, and the convex surface. This scheme, which has found its greatest use in angiographic descriptions, divides the MCA into four major segments ( Fig. 24.3 ). , The first sphenoidal segment (M1) occupies the space from the origin of the MCA to the limen insulae. The second segment (M2) encompasses the branches of the upper and lower divisions that overlie the insula. The M3 segments are the continuation of these branches as they curve caudally along the undersurface of the operculum, most of which are upper division branches. The M4 segments describe the portions of branches of the MCA over the convex surface of the brain.
The MCA branches that constitute the M3 or opercular segment follow the curve of the operculum back over the surface of the insula. Some of these branches reverse course over as much as 180 degrees. The branches passing over the parietal and temporal operculum make less striking reversals of direction, some turning only a few degrees before reaching the convex surface of the temporal and parietal regions. M4 branches of the MCA emerge from the sylvian fissure beyond the operculum and course along the sulci and gyri of the cerebral convexity. Considerable variation is noted in their path. Upper division M4 branches typically follow a path mainly along the depths of a given sulcus and a few of them pass long distances over the surface of a gyrus, making them also suitable for extracranial–intracranial bypass surgery.
Anomalies of the MCA occur in no more than 3% of cases. , , Some writers even dispute their occurrence. Duplication of the MCA is more common, usually arising from the ICA and supplying the same regions that would otherwise have been supplied by the original MCA. An accessory MCA has also been described that arises from the anterior cerebral artery and runs through the sylvian fissure along with the normal MCA, usually supplying frontal polar areas. It has been reported in 0.3%–4.0% of the general population. , Previous reports mainly focused on the association of the accessory MCA with cerebral aneurysms and the role of the accessory MCA as a collateral blood supply in the event of MCA occlusion. , Rarely, an accessory MCA may develop atherosclerosis and present with ischemic symptoms.
For each cerebral surface branch of the major cerebral arteries, the terminal twigs end in a narrow network of vessels that form the borderzone (see Fig. 24.2 ) between the major arterial territories.
The MCA contains the same intima, media, and adventitia as other arteries, but the relative thicknesses of these component parts differ from those in peripheral arteries of comparable size. Compared with extracranial vessels of similar size, the MCA has a narrower adventitia with little elastic tissue and few perivascular supporting structures; the media is also thinner, with some 20 circular muscle layers. , The internal elastic lamina is thicker and finely fenestrated. The intima, although somewhat thin, seems essentially the same as that of comparably sized vessels elsewhere. No vasa vasorum has been demonstrated in MCA.
The thinner adventitia of intracranial vessels may be a sign of their lower exposure to stretching and trauma. Elastic tissue is concentrated in the internal elastic lamina instead of being scattered through the vessel as in other arterial beds, perhaps making intracranial arteries more prone to dampen pulse waves.
Embolism exceeds atheroma and accounts for 15%–30% of strokes, most of which occur in the territory of the MCA.
Occlusion by embolism has been appreciated since the time of Chiari. These large ones have been traced to “paradoxical” embolus from a leg vein source, , atrial fibrillation, mitral valve prolapse, , marantic embolus, fragmented thrombus complexes from a non-obstructing ICA plaque, , shotgun pellet, metal fragment from a penetrating neck wound, traumatic dissection of the ICA, ICA of various causes, and automobile accident with angiographically normal ipsilateral ICA. , Recent striking images support aortic arch embolism, even in a setting of clinically important carotid stenosis.
Embolism to one or more branches of the MCA may occur from almost any of the above as well as other less well documented sources. , Sources include calcific material from the ipsilateral ICA (although Chiari’s famous case also had a patent cardiac foramen ovale), spontaneous dissection of the ICA from fibromuscular hyperplasia, traumatic ICA dissection, mucin and emulsified fat from breast metastasis, endocarditis due to candida, mitral valve prolapse, cardiac myxoma, marantic embolus, arterial wall fragments after resuscitation, giant fusiform MCA aneurysm, ICA occlusion from various causes, and various types of transcardiac emboli via a patent foramen ovale.
An embolus of merely a few millimeters may occlude the MCA stem. Some calcific plaques may also embolize to the MCA but are usually too small to block the stem. Carotid plaque rupture as a cause of major MCA stem occlusion has been difficult to demonstrate. ,
Accumulating evidence indicates that inflammation is of key importance in atherosclerotic plaque destabilization, thromboembolism, and stroke. Inflammatory cells within plaque, particularly macrophages, are increasingly recognized as key mediators of lipid oxidation, plaque remodeling, and fibrous cap erosion and rupture, leading to acute thrombo-embolic events. Imaging with 18F-fluorodeoxyglucose (18-FDG) positron emission tomography (PET) has significant potential for non-invasive imaging of atherosclerotic inflammation, providing information about plaque biology associated with clinical events independently of the effect of the plaque on narrowing of the arterial lumen. In a recent study, carotid plaque PET 18-FDG uptake predicted early recurrent stroke. This study found that the predictive utility of 18-FDG plaque uptake was independent of age and degree of lumen stenosis (categorized as 50%–69% and 70%–99%, adjusted hazard ratio [HR], 6.1; confidence interval [CI], 1.3–28.8; P = .02).
Although molecular imaging (FDG-PET) can help in imaging plaque inflammation, it is difficult to apply in intracranial stenosis due to high uptake of FDG by surrounding brain parenchyma. Recently sodium fluoride (18F-NaF) PET/computed tomography (CT) imaging was applied in coronary artery diseases to facilitate understanding of plaque calcification. The same radiotracer might help to determine calcification in intracranial arterial stenosis. Furthermore, combining 18F-NaF with Gd-enhanced vessel wall imaging could further elucidate the link between local hemorrhage, micro-calcification, progression to plaque rupture, and potential cerebrovascular event.
Most of the embolic material is a small fragment of a vessel or cardiac wall thrombus or vegetation. Being compressible, such embolic fragments may alter their length and width as they pass through the arterial tree. Rarely, large-particle embolism may occur from fibrocartilaginous material.
Emboli follow preferential paths through the MCA system. , The lower division receives the larger share of the emboli. In the upper division, the serial arrangement of the major branches allows the entire division to be occluded by a large embolus blocking the first branch point. Small emboli often pass by the anterior branches to lodge in the more posterior branches. The sharply angulated orbitofrontal branch is rarely occluded. , The lower division remains a single vessel until it reaches the superior temporal plane, where it gives off its three main branches within the space of 1 cm or less. As a result, even small emboli may result in simultaneous occlusion of more than one or even all the branches.
Autopsies commonly show no occlusion, despite stem, division, or branch occlusion typical of embolism. Many emboli must be poorly organized and may undergo spontaneous dissolution. For vessels with persisting occlusion, the functional prognosis for the infarct is worse. , By imaging alone, such persistent occlusions may have all the features of in situ thrombi. Transcranial Doppler (TCD) studies are often useful in demonstrating rapid recanalization in real time during intravenous thrombolysis as well as persistent occlusion. ,
Unless adequate collateral flow is present, embolic occlusion of the MCA yields a gigantic infarct. On the contrary, in patients with good collateral flow, the brain parenchyma may be remarkably spared.
Intracranial collateral pathways are important for the perfusion of brain regions affected by an intracranial occlusion. These channels may be pre-existing or develop de novo. The collateral status correlates well with final infarct volume and functional outcome. Collaterals can also predict the effectiveness of intravenous thrombolysis as well as various endovascular intervention. Furthermore, good collaterals are believed to reduce hemorrhagic complications of various therapeutic strategies in acute ischemic stroke.
A variety of clinical profiles occur in embolism to MCA branches ( Fig. 24.4 ). In some instances, the deficits are only transient, even with angiographic persisting occlusion, which raises the possibility that the nature of the embolic material may play a role in the severity of the infarct. , In the days of polymeric silicone (Silastic) pellet therapy for arteriovenous malformations (AVMs), aberrant embolism was a well-recognized risk, usually occurring near the end of the embolization procedure. ,
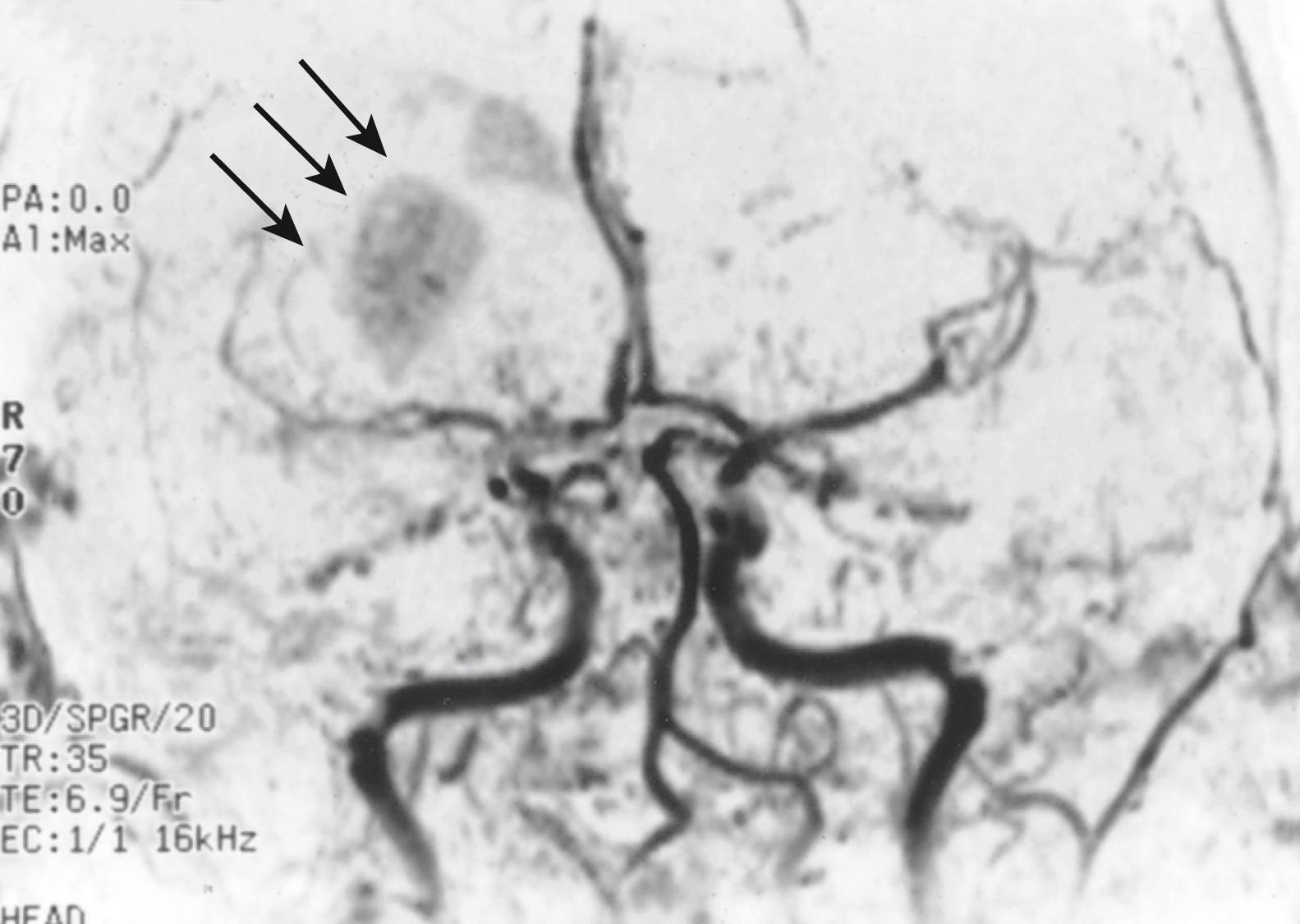
Emboli initially occluding the MCA stem and then migrating to the distal branches may lead to discontinuous multifocal infarction. The lack of collateral branches to the lenticulostriates makes these deep territories especially vulnerable to infarction. The initial major hemispheral clinical syndrome may ameliorate to that of deep infarction affecting the penetrating vessels of the MCA stem. This clinical picture, dubbed spectacular shrinking deficit (SSD), appears to be the consequence of thrombolysis or distal migration of the fragmented clot and its residual particles. Such cases often leave two separate foci of infarction, assumed to be from the same embolic event ( Fig. 24.5 ). Syndromes of nonsudden or fluctuating onset may also occur in 5%–6% of documented embolic strokes. In some instances, the syndrome may take up to 36 hours to evolve. ,
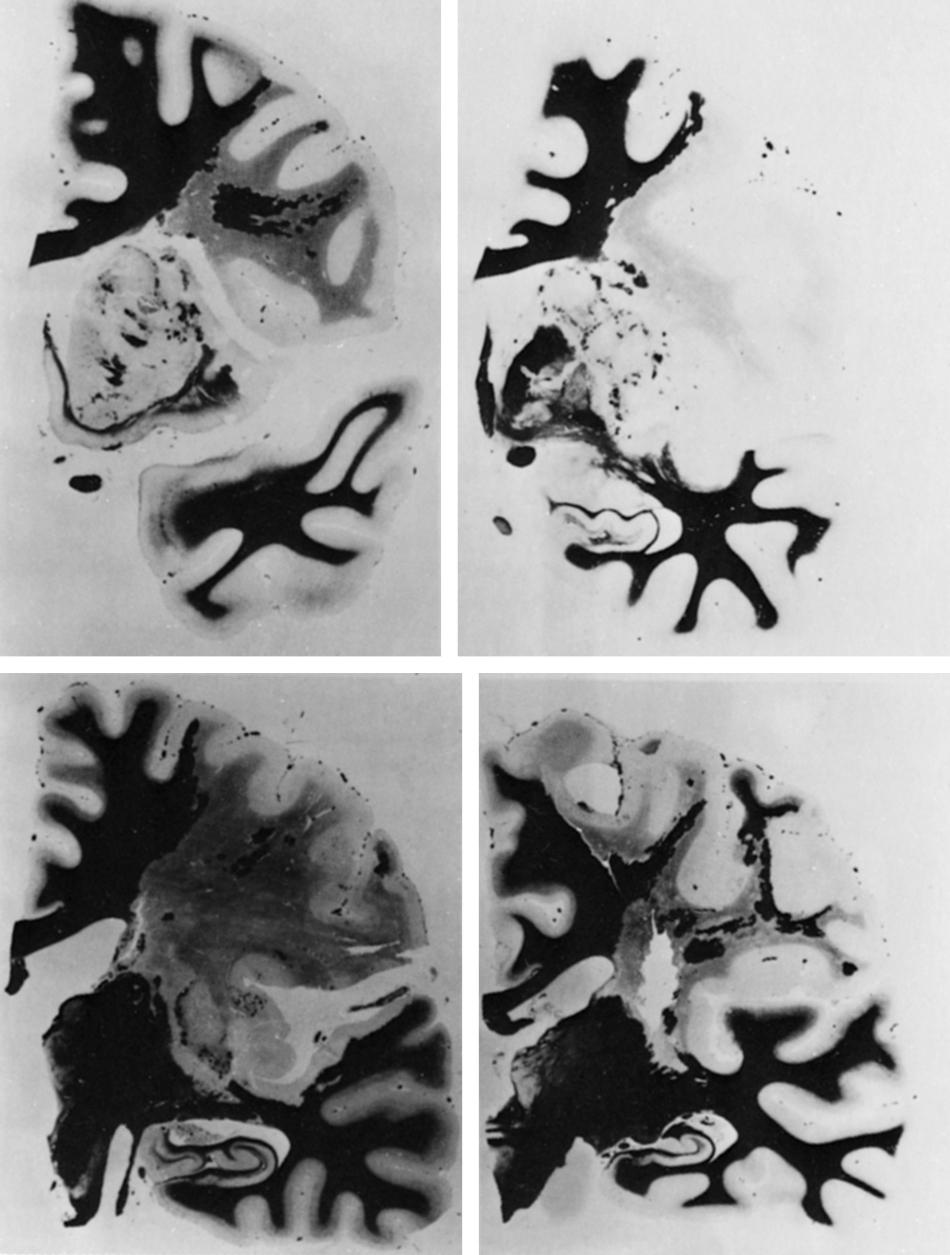
Autopsy studies indicate that thrombotic occlusion accounts for only 2% of cases of ischemic events in the MCA territory. Asymptomatic occlusion of the MCA stem is rare. ,
Primary arteriosclerotic occlusive thrombosis is an uncommon cause of symptomatic MCA disease. , However, it is more common in Asians, Hispanics, and Africans. In acute ischemic stroke, angiographically demonstrated MCA stenosis found above a normal ICA is also compatible with recanalizing embolism. , ,
Although small and deep infarcts, known as lacunes, are usually explained by local microatheroma, some infarcts affecting the subinsula, external, and internal capsule and the upper corona radiata, which lie in the territory of several lateral lenticulostriates, create a curvilinear appearance on coronal imaging. They often spare the putamen, globus pallidus, capsule, and even the entry zone for the corona radiata ( Fig. 24.6 ). Local atheromatous stenosis of the upper division is the usual cause but is not always easily demonstrated.
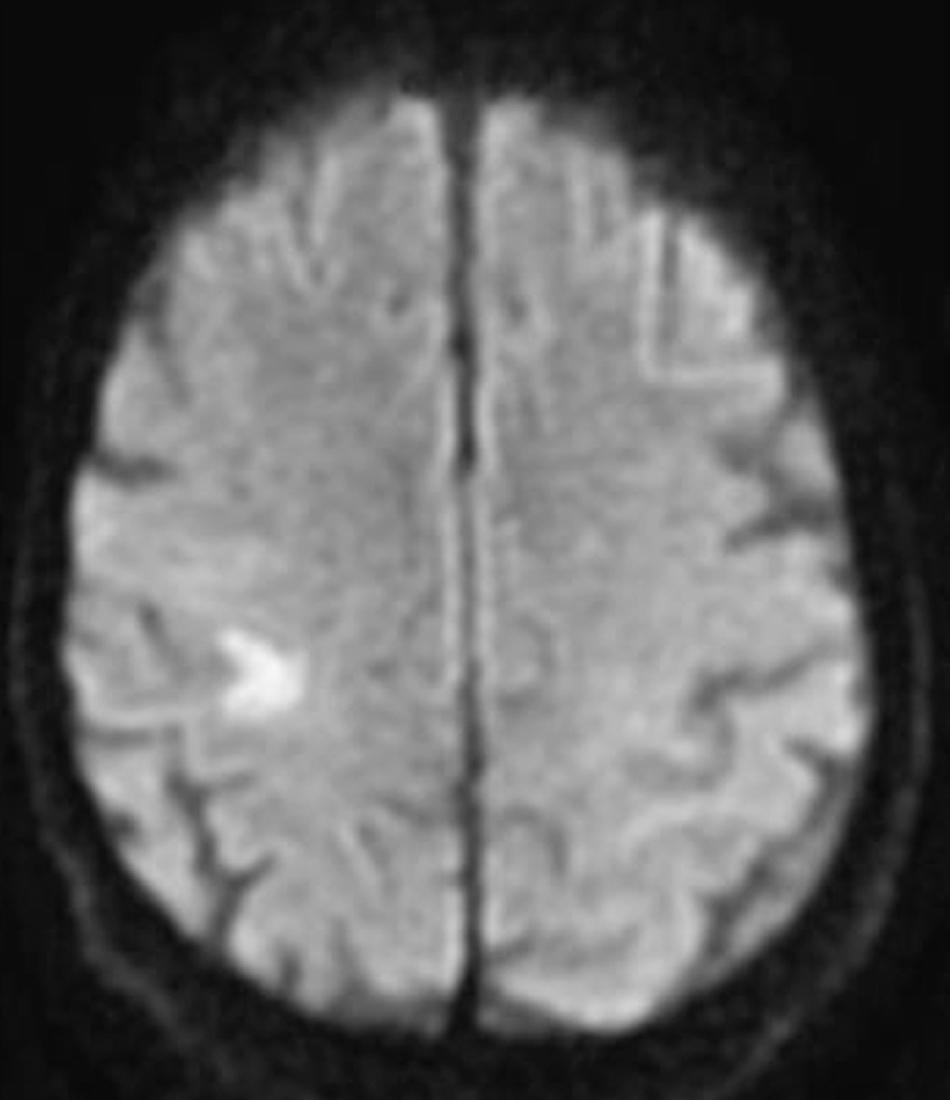
Recent advancements in magnetic resonance imaging (MRI) technology enable direct visualization of vessel lumen and steno-occlusive plaque. A recent study reported good correlation of MRI measurements of MCA stenosis with digital subtraction angiography. High-resolution MRI provides crucial information about plaque characteristics. An example is shown in Fig. 24.7 .
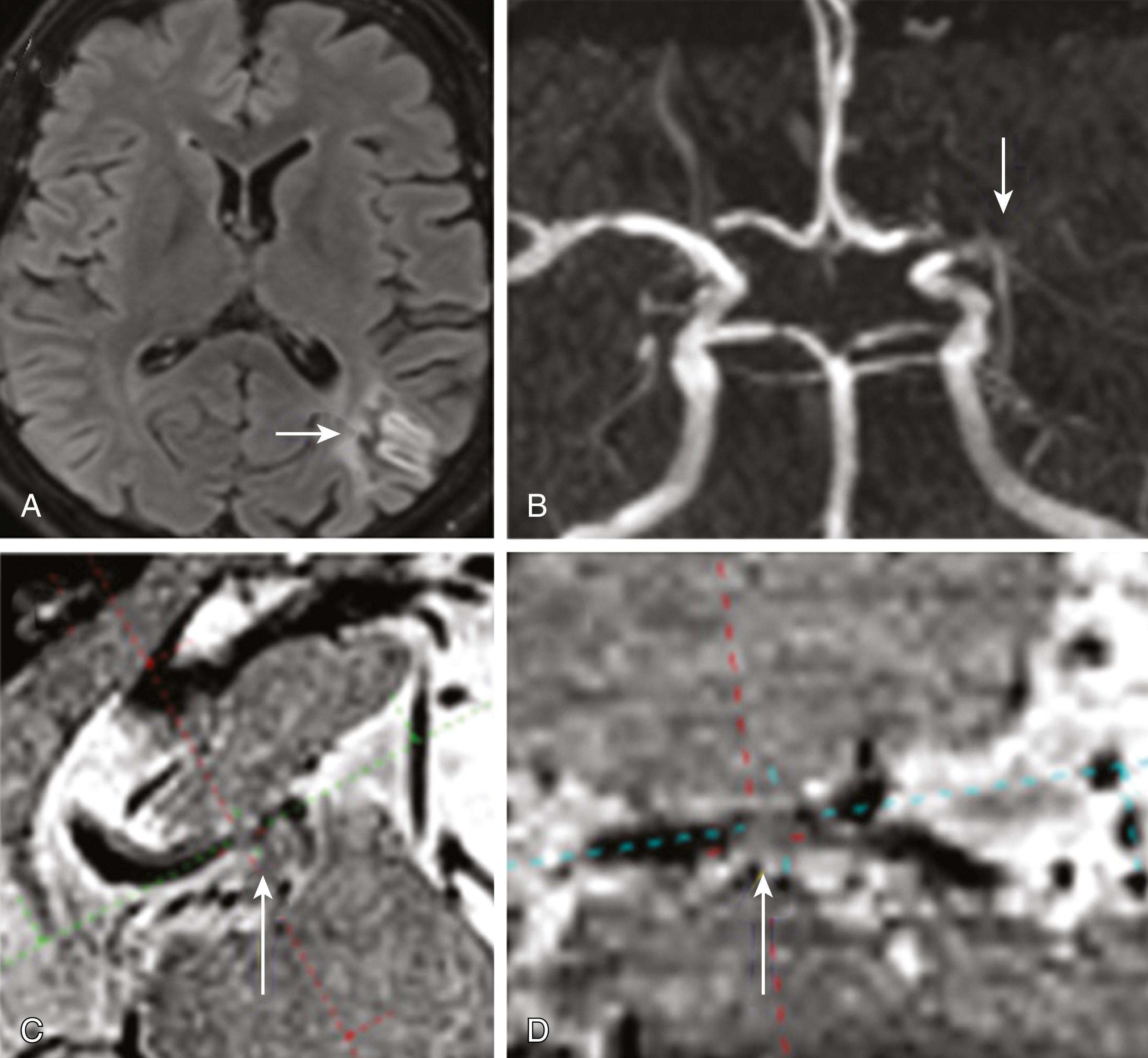
Lumen stenosis of the MCA is commonly found in the stem. In Asians, Hispanics, and Africans, atherosclerosis is a common cause of MCA stenosis. However, other causes such as moyamoya disease, dissection, post-radiation effects, metastatic tumors (including atrial myxoma), and infection may also cause stenotic lesions. The clinical syndromes range from minor pure motor stroke, , minor focal hemispheral syndrome, and transient ischemic attach (TIA) , , , , to severe disability. ,
Autopsy diagnosis of MCA dissection has been rare. A wide variety of conditions that may result in dissection include trauma, strenuous physical exertion, surgery, fibromuscular hyperplasia, atherosclerosis, mucoid degeneration of the media, moyamoya disease, split or frayed internal elastic lamina, congenital defect of the media, syphilis, and even migraine. The disorder has been most often reported in younger patients. The usual site is a short section of the stem, although adjacent branches may also be affected. In some, symptoms have been delayed for minutes, hours, or many days.
Capsular warning syndrome was first described in 1993 in patients who presented with stereotypic recurrent episodes of TIA. This syndrome is associated with a high risk of developing a completed stroke. Although the exact pathogenesis of this syndrome remains debatable, small perforator artery disease is believed to be the most common mechanism. Recent developments in high-resolution MRI enable evaluation of arterial wall characteristics. Accordingly, recent studies suggest eccentric atherosclerosis of a non-stenosing segment of MCA as the underlying pathophysiology.
MCA may be affected by arteritis, fibromuscular hyperplasia, altered coagulation states, delayed effects of radiation, and other conditions.
An important condition that has been described recently is the reversible cerebral vasoconstriction syndrome (RCVS). It is characterized by recurrent thunderclap headache, seizures, stroke, and non-aneurysmal subarachnoid hemorrhage. Reversibility of vasoconstriction within 3 months is the hallmark of RCVS. Common conditions associated with RCVS include postpartum state, and use of vasoactive agents and immunosuppressive agents. The clinico-radiologic features are often dynamic in nature. Nimodipine, a calcium channel blocker, seems to reduce thunderclap headaches. However, it has no proven effect on hemorrhagic and ischemic complications. Corticosteroids are avoided since they may worsen the clinical course.
The textbook accounts, briefly reviewed here, assume total infarction in the territory at risk. Uncollateralized occlusion of the main trunk of the MCA causes softening of the basal ganglia and internal capsule within the substance of the hemisphere as well as a large portion of the cerebral surface and subcortical white matter. , The large infarct produces contralateral hemiplegia, deviation of the head and eyes toward the side of the infarct, hemianesthesia, and hemianopia. Foix and Levy detailed the clinical elements almost a century ago. Major disturbances also occur in behavior. Global aphasia occurs when the hemisphere dominant for speech and language is involved, whereas impaired awareness of the stroke is expected when the non-dominant hemisphere is affected. When the infarct is large, the hemianopia may be due to involvement of the visual radiations. More often, hemianopia is part of a syndrome of hemineglect for the opposite side of the space and is accompanied by failure to turn toward the side of the hemiplegia in response to sounds from that side.
A variant of the syndrome of MCA stem occlusion, colorfully named malignant infarction, applies mainly to those experiencing subsequent herniation. The time to severe decline is brief (2–5 days). Advances in treatment have allowed survival for some; however, most of the syndrome elements persist ( Fig. 24.8 ). Results of a pooled analysis of the three European randomized controlled trials demonstrated better clinical outcome in patients who underwent decompressive surgery within 48 hours of stroke onset. Compared to the best medical therapy, more patients in the decompressive-surgery group achieved modified Rankin Scale (mRS) 0–3 (43% vs. 21%) and mRS 0–4 (75% vs. 24%).
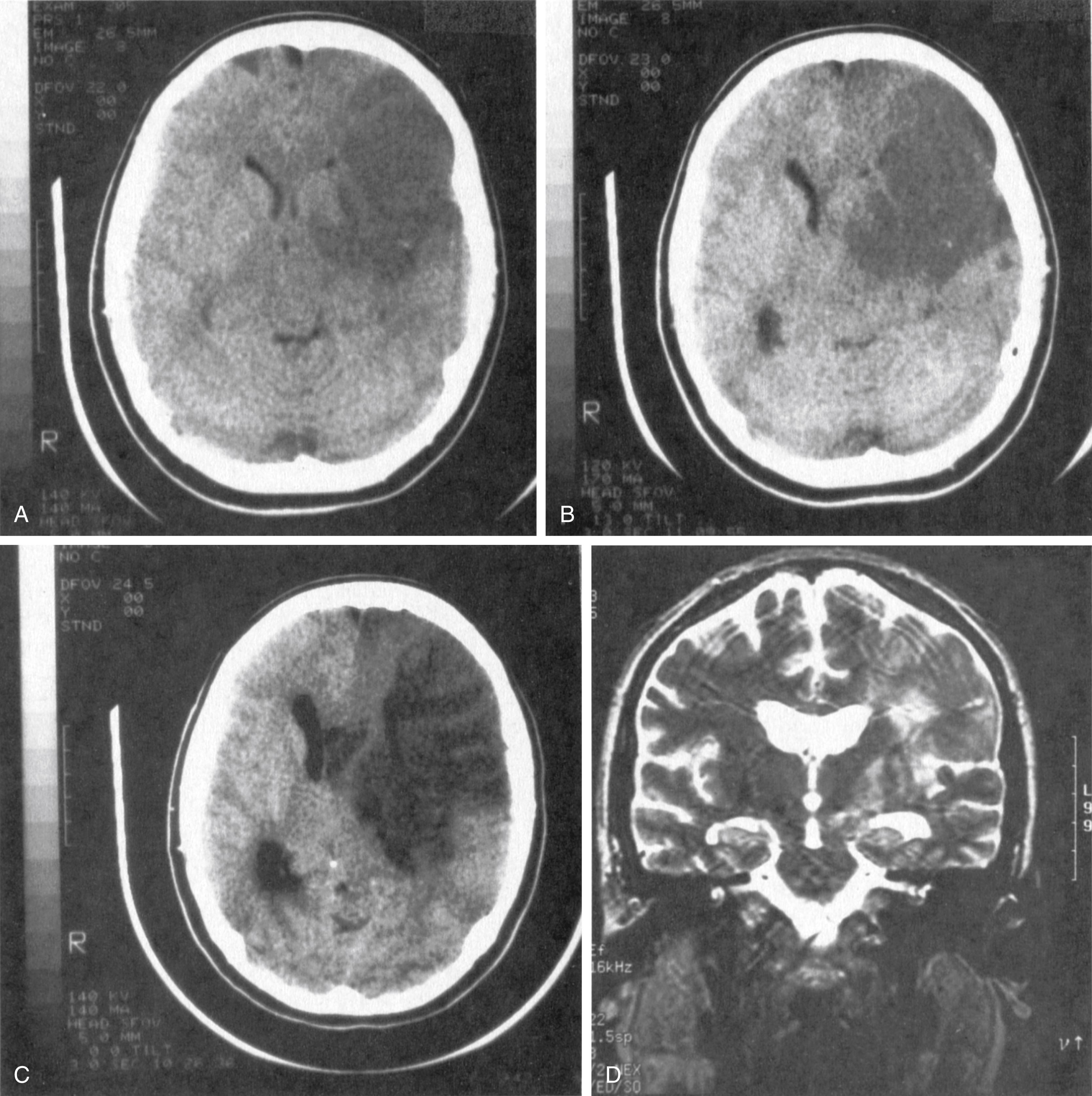
When the occlusion is restricted to the upper division, the sensorimotor syndrome mimics that from occlusion of the main trunk. Added to it is aphasia when the dominant hemisphere is involved or impaired awareness of the deficit when the other hemisphere is affected. However, the hemiparesis usually affects the face and arm more heavily than the leg. Because the occlusions usually affect the upper division, aphasia from dominant hemisphere infarction is usually of the motor (Broca) type ( Figs. 24.9 and 24.10 ), whereas the disturbance in behavior from non-dominant hemisphere infarction may be mild.
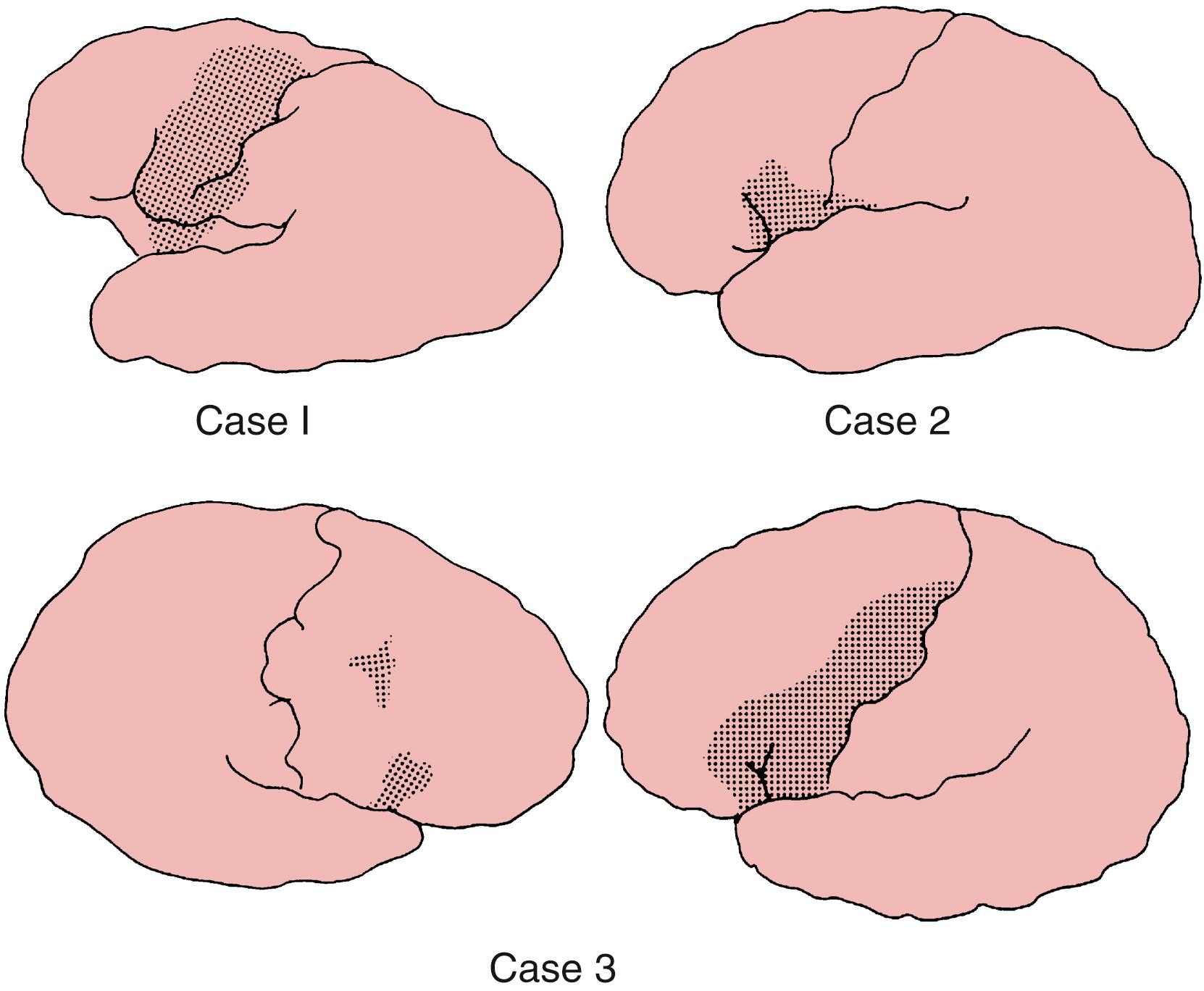
In the lower division syndromes, infarction typically spares the rolandic region, hemiparesis is mild, head and eye deviations are rarely encountered, and even disorders of sensation are infrequent. When the infarct affects the dominant hemisphere, pure aphasia (Wernicke type) is the rule, whereas in non-dominant hemisphere infarction, the behavior disturbances may appear in relative isolation. Hemianopia may be a prominent sign.
When the involvement is limited to the territory of a small penetrating artery branch of the main stem, a small, deep infarct (lacune) occurs, affecting part or the entire internal capsule and producing a syndrome of pure hemiparesis unaccompanied by sensory, visual, language, or behavior disturbances.
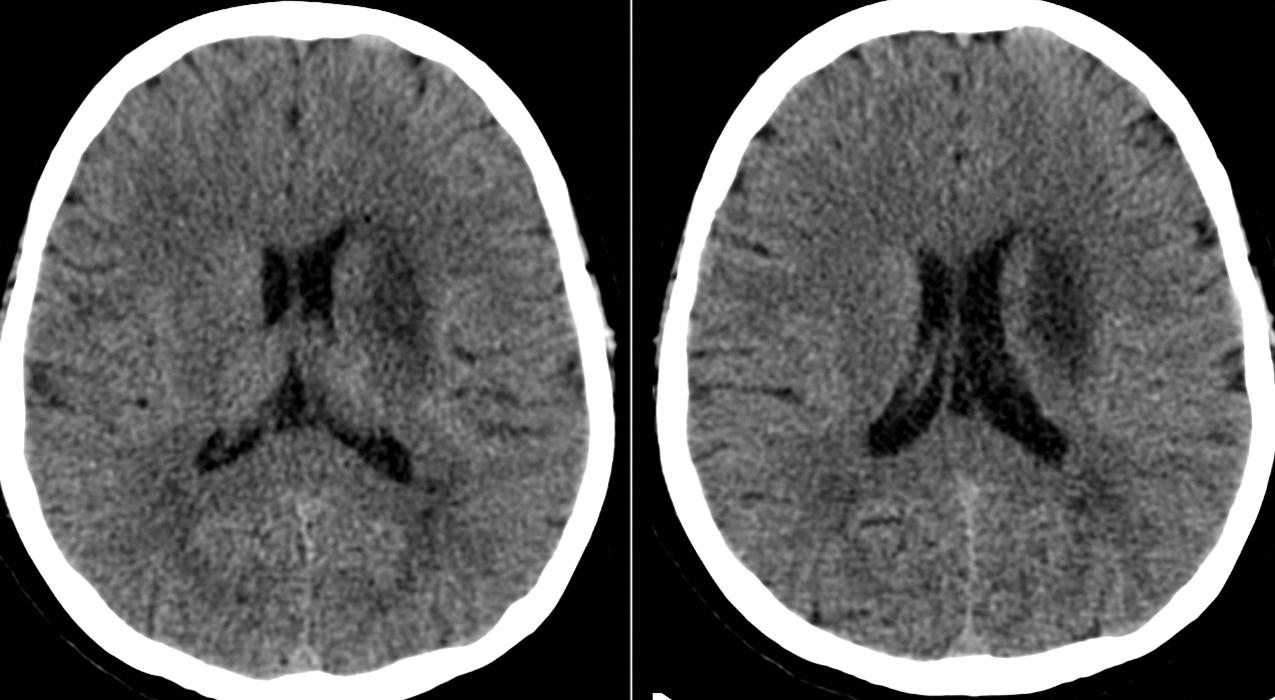
Transient loss of consciousness is rare in MCA territory infarction. It occurs at onset in only 8.4% of carotid ischemic strokes. Delayed loss of consciousness is more common, often occurring 36 hours to 4 days after hemispheral infarcts ranging in size from the entire MCA territory to only the frontotemporal region. The decline in consciousness is usually part of a larger clinical picture of impending cerebral herniation and seems not to be due to an injury to a specific brain region in the MCA territory controlling consciousness ( Fig. 24.11 ).
The terms hemiplegia and hemiparesis have been used rather loosely in the literature, which makes a clear correlation between the severity of weakness and a given site of infarction difficult. The number of cases that correlate the hemiparesis formula and imaging or autopsy findings remains disappointingly small, and some of them, despite an autopsy study, lack credibility. Henschen’s massive review of the published autopsy literature on higher cerebral function before 1920 was typical of most writers. The literature remains frustrating because of many surprising instances in which the motor deficit showed considerable improvement. MCA stem occlusions affecting either side of the brain appear to produce the same basic motor deficit and can be described under the same heading. Such were the findings in the 488 cases of MCA territory infarction published in the pilot phase of the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) Stroke Data Bank project.
The most reliable occurrence of hemiplegia follows complete occlusion of the MCA at its stem (see Figs. 24.10 and 24.11 ). The typical picture consists of dense contralateral hemiplegia, hemianesthesia, homonymous hemianopia, and conjugate gaze deviation to the contralateral side. The syndrome is more severe when the stem is affected. , , , Among the patients who die within days, contralateral hemiplegia is usually accompanied by hemianesthesia and hemianopia.
The syndrome among survivors without hemicraniectomy seems similar. While some have had only mild facial paresis, distal functions of the limbs are much impaired, and hand and finger movements and the foot are often paralyzed. In some patients, movement of the shoulder and elbow may allow the arm to lift, and movement of the hip and knee may suffice for walking.
Hemiplegia from deep infarction alone features several different syndromes. Foix and Levy described two types. In the first, massive hemiplegia occurred, and the appearance was the same as that observed when the infarct involved both the superficial and deep territories. Initial hemiplegia gave way to marked contractures. No involuntary movements, choreoathetosis, parkinsonism or disturbances in balance were described. The second type involved a more marked hemiplegia in the leg than in the arm, rendering the patient unable to walk. Contracture in this syndrome was more common in the leg and was often associated with a permanently flaccid hemiplegia. Later studies and CT scans have reported a range of weakness, from profound hemiplegia to mild weakness, which underwent striking improvement despite persistence of the deep infarct. ,
Hemiplegia from surface infarction is the third type. Infarction of the entire surface territory produces a syndrome essentially identical to a deep territory infarction. Surface infarcts confined to the cortical surface of insula and operculum cause hemiplegia with faciobrachial predominance that soon fades to a facial plegia with mild, predominantly distal paresis of the arm.
Individual branch occlusions uncommonly produce hemiplegia. In most cases, either hemiparesis occurs or the syndrome of paralysis is incomplete and confined to one or more body parts. The most reliable deficit is encountered among patients with occlusion of the ascending frontal branch. Dramatic improvements within weeks are reported. A large number of variables affect the outcome. Efforts are under way to study a variety of treatment options, among them constraint therapy, transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS).
The most commonly encountered pattern of hemiparesis is weakness of the hand, shoulder, foot, and hip. This type was described in 71.2% of the 488 unilateral hemisphere strokes studied during the pilot phase of the NINCDS Stroke Data Bank project. A few other types of hemiparesis are also well known. Among them are the classic syndromes of distal predominance of the hemiparesis (often attributed to Broadbent, although we have found no source among his writings), a faciobrachial paresis, and monoplegia. The main phase of the NINCDS Stroke Data Bank study provided data for 183 of 1276 patients with convexity infarction in the MCA territory and is still the largest reported cohort. Infarct size did not differ according to side, but the location of the main site of the infarct did. On the left side, the infarct was centered in the inferior parietal region, but on the right, it was midfrontal. There was a good correlation between infarct size and extent of weakness as estimated by overall motor function. There was poor correlation for lesion location (lower third, middle third, or upper third on either side of the rolandic fissure) and any of the specific syndromes of focal weakness; no two cases shared the same lesion for the same syndrome, and several cases shared the same lesion with a different syndrome. The findings indicated a difference in weakness syndromes between the two hemispheres and great individual variation of the acute syndrome caused by a given site of focal infarction along the rolandic convexity.
When hemiparesis features distal predominance, it affects the lower face, fingers, forearm, toes, and lower leg, with relative sparing of the forehead, shoulder, upper arm, hip, thigh, neck, and trunk. This lower facial and distal predominance of hemiparesis is believed to represent the density of the homuncular representation over the hemispheral surface, , but occurred in only 23.5% of the 488 patients with unilateral weakness affecting the cerebrum in the Stroke Data Bank. In addition, it occurred with approximately the same frequency regardless of whether the infarct was confined to a single lobe or was as large as several lobes and whether the area involved was frontal, parietal, temporal, or opercular.
The syndrome of faciobrachial paresis also has a widely varying frequency. , , Obvious weakness is present in the jaw muscles. Movements of the tongue and oropharynx show impairment of swallowing and occasionally impairment of vocalization. These lower face and oral pharyngeal disturbances may persist long after forehead movement has been restored. The initial appearance is sometimes similar to Bell palsy, but the upper deviation of the eyes characteristic of peripheral facial palsy (Bell phenomenon) is typically not present, even in the earliest stages. The involvement of the upper extremity is usually more obvious in the form of impaired movement of the fingers and hand.
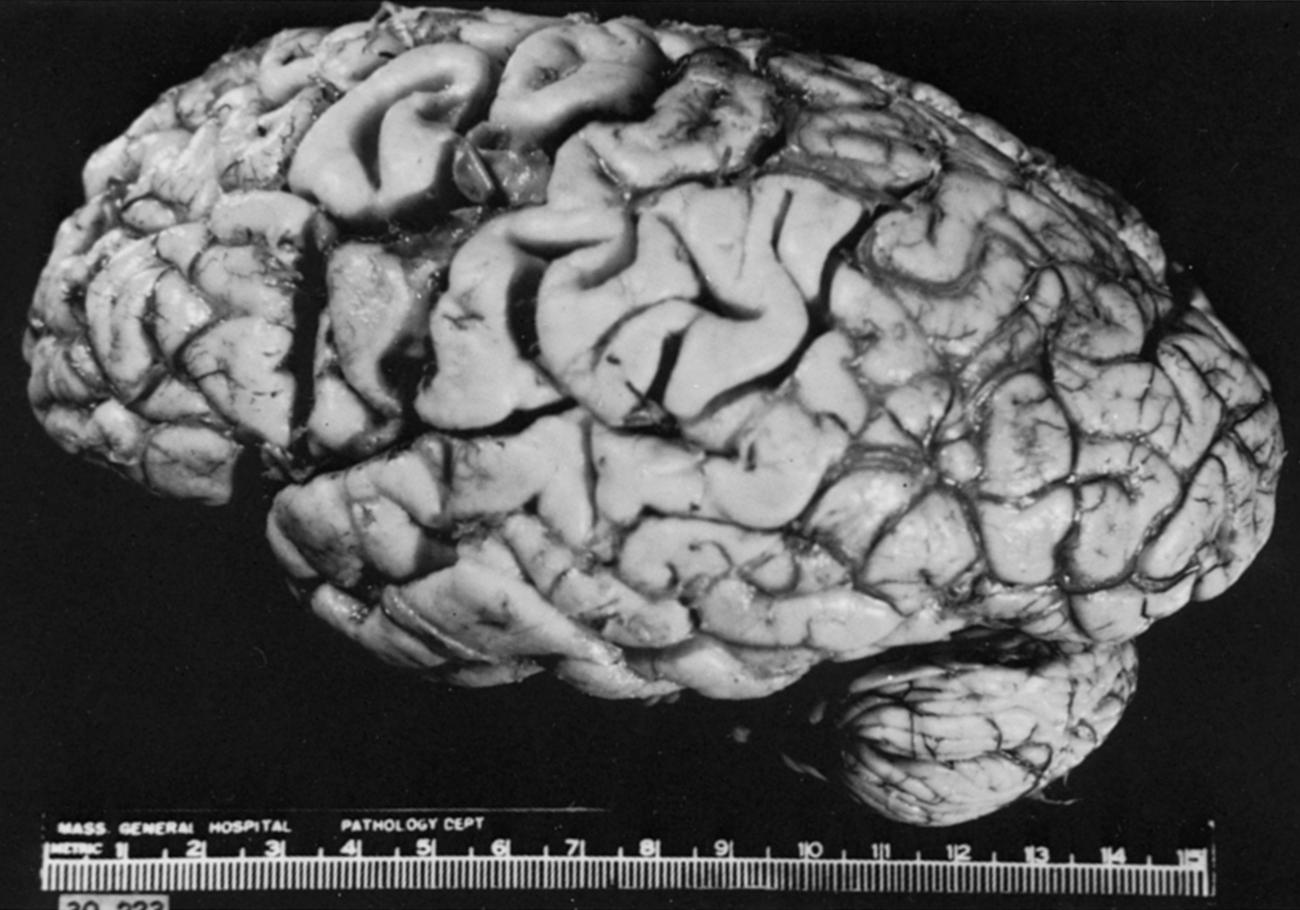
Although described in standard texts, detailed case reports of monoplegia are not easily found in the literature. von Monakow made reference to the possibility of an isolated brachial plegia arising from a lesion confined to the middle of the second frontal gyrus, provided that the lesion is acute and does not extend too deeply into the white matter (“wenn sie akut einsetzt und nicht zu tief in das subcorticale Mark übergrieft”). Dejerine and Regnard found a case with weakness limited to the muscles of the thenar, hypothenar, and interosseous muscles; they did not mention confirmation of the presumed vascular nature of the lesion. Garcin described a monoparesis with weakness predominating in the flexor movements, mimicking median nerve palsy; the locus of the lesion was inferred in the absence of autopsy data. The only case of focal upper rolandic infarction with autopsy documentation ( Fig. 24.12 ) was of an elderly woman, whose examination within hours of onset revealed normal power in the upper extremity. The only clinical signs were slight right facial weakness with initial mutism. She was monitored for months, during which time the initial deficit improved, but no disturbance of limb power occurred at any time. Among recent publications was a well-documented example of “hand knob” infarction.
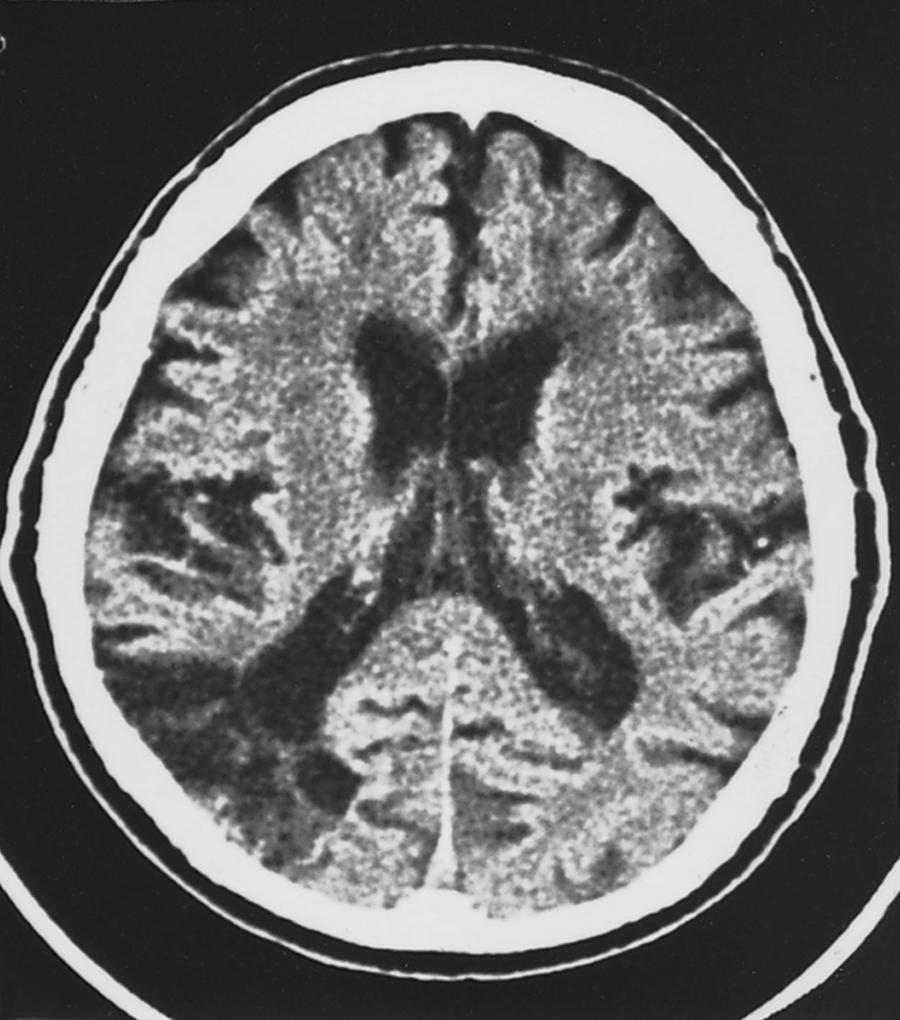
Isolated brachial monoplegia has often been described as a clinical sign in carotid territory TIAs. It has also been encountered as a transient syndrome in aberrant emboli during pellet embolization in the treatment of AVMs. These findings are of great interest but must be interpreted with caution because the setting (an angiogram suite with the patient under a drape) does not lend itself to detailed evaluation of the leg and axial structures during the frantic period when the physicians are striving to reverse the acute deficit. Schneider and Gautier, in an extensive review of 1575 patients with acute stroke and predominance of leg weakness, found that only 63 had predominance of leg weakness. Although 41 patients had hemispheric convexity lesions, the MCA territory was affected in only one. The NINCDS Pilot Stroke Data Bank project contained a mere 31 cases of monoplegia involving the arm among the 488 patients with cerebral stroke, yet even this small number showed a significant correlation with infarct of a single lobe rather than multiple lobes ( P < .002).
The means by which improvement occurs in hemiparesis remains unclear but is persistently described as recovery, a term suggesting a restoration of the original function. Such may not be the case, but the term is too well embedded in usage for us to constantly point out the ambiguities and force readers to face alternative terms throughout this chapter.
“Recovery” after stroke-induced hemiparesis is the rule rather than the exception. Clinical and imaging factors have been put forward to predict who is likely to recover and to what extent. The degree and nature of recovery depends critically on the outcome measure chosen. Several commonly cited observational studies have demonstrated, for example, that the initial severity of hemiparesis and lesion volume are reasonably good at predicting scores on disability scales at 3 or 6 months. Neurologic impairment can dissociate from disability assessments, however, allowing a patient to be deemed recovered by a disability score while still harboring a substantial, unaddressed neurologic deficit. Because the approach of most stroke rehabilitation programs is to teach compensation for deficits, such as the nonparetic hand accomplishing a motor task, rather than to reverse the neurologic impairment, recovery may be reported after a course of rehabilitation; however, at the level of impairment, recovery may not have occurred. Other studies have used impairment of motor function as the primary outcome measure and have shown varying correlations with their independent variables. Duncan et al. ran a widely cited study that serially evaluated the course of motor recovery among 104 hemiparetic stroke patients. Stratifying by severity of the motor impairment as measured by the standardized Fugl-Meyer (FM) stroke scale at day 1, the investigators followed the course of motor recovery at 5 days and at 1, 3, and 6 months. The investigators showed that the FM scores at day 1 accounted for only half of the variance in 6-month motor function ( r = 0.53, P < .001).
Alternatively, if one defines recovery as the change in impairment score from the initial time point to 90 days later, prediction of recovery is more consistent. Newer evidence suggests that for patients who have mild-to-moderate deficits, there is a remarkably consistent course of recovery, and all patients achieve a specific proportion (≈70%) of the potential total remaining recovery. For patients with severe deficits at onset, prediction of recovery is much more difficult. To distinguish those likely to recover from those who are not likely to recover, investigators recently showed that expression of a “recovery pattern” on functional magnetic resonance imaging (fMRI), obtained in the first few days after stroke, correlated well with the degree of subsequent recovery that then occurred.
Functional imaging has also begun to reveal the dynamic process of brain reorganization after stroke. Neuronal activity in regions outside primary motor cortex after stroke-induced hemiparesis may appear both in the opposite hemisphere and in secondary motor areas in the ipsilesional hemisphere as early as 24 hours after stroke onset. , The appearance of this task-related activity suggests that alternative brain regions participate in the performance of motor function when a deficit is present, then revert to a more typical, contralateral pattern as recovery proceeds. Physiologic measures of white matter tracts such as TMS also support the idea that a changing balance of cross-callosal inhibition between the two hemispheres plays a role in recovery. Loss of inhibition from the stroke hemisphere to the contralesional hemisphere may interfere with recovery of motor function through the increased inhibition back on the hemisphere with the stroke. This competitive inhibition between hemispheres after stroke has led to the proposal of rehabilitation measures that restore physiologic balance between hemispheres through the use of TMS , and tDCS. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here