Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neuroendocrinology, in its broadest sense, is the study of the coordinated interaction of the nervous, endocrine, and immune systems to maintain the constancy of the internal milieu (homeostasis). This chapter concentrates on the functions of the hypothalamus and its interaction with the pituitary gland.
One of the features of the neuroendocrine system is that it uses neuropeptides as both neurotransmitters and neurohormones. The term neurotransmitter is applied traditionally to a substance that is released by one neuron and acts on an adjacent neuron in a stimulatory or inhibitory fashion. The effect is usually rapid, brief, and confined to a small area of the neuronal surface. In contrast, a hormone is a substance that is released into the bloodstream and usually travels to a distant site to act over seconds, minutes, or hours to produce its effect over a large area of the cell or over many cells. Neuropeptides can act in either fashion. For example, the neuropeptide vasopressin, produced by the neurons of the supraoptic and paraventricular nuclei, is released into the bloodstream and has a hormonal action on the collecting ducts in the kidney. Vasopressin is also released within the central nervous system (CNS), where it acts as a neurotransmitter. Similarly, the neuropeptide substance P acts as a neurotransmitter in primary sensory neurons that convey pain signals and as a neurohormone in the hypothalamus.
The influence of neurohormones and neuropeptides on the brain can be divided into two broad categories: organizational and activational. Organizational effects occur during neuronal differentiation, growth, and development and bring about permanent structural changes in the organization of the brain and therefore brain function. An example of this is the structural and organizational changes brought about in the brain by prenatal exposure to testosterone. Activational effects are those that change preestablished patterns of neuronal activity, such as an increased rate of neuronal firing caused by exposure of a neuron to substance P or vasopressin.
Numerous neuropeptides are found in the brain, where they have a wide variety of effects on neuronal function ( Table 50.1 ). Current understanding of all the actions of neuropeptides in the nervous system is far from complete.
| Neuropeptide | Central Nervous System Function and Selected Function Outside the Central Nervous System |
|---|---|
| Hypothalamic Peptides Modulating Pituitary Function | |
| Corticotropin (ACTH)-releasing hormone (CRH) | Regulation of ACTH secretion |
| Integration of behavioral and biochemical responses to stress | |
| Modulatory effects on learning and memory | |
| Growth hormone-releasing hormone (GHRH) | Regulation of growth hormone secretion |
| Growth hormone release-inhibiting hormone (somatostatin) | Regulation of growth hormone secretion |
| Ghrelin | Regulation of growth hormone secretion |
| Regulation of feeding | |
| Thyrotropin-releasing hormone (TRH) | Regulation of thyroid-stimulating hormone secretion |
| May be involved in depression | |
| Enhances neuromuscular function (in the periphery) | |
| Stimulates prolactin release | |
| Gonadotropin-releasing hormone (luteinizing hormone-releasing hormone; GnRH) | Regulates gonadotropin secretion Influences sexual receptivity |
| Prolactin-releasing peptide | Stimulates prolactin secretion |
| Neurotensin | Endogenous neuroleptic |
| Regulates mesolimbic, mesocortical, and nigrostriatal dopamine neurons | |
| Thermoregulation | |
| Analgesia | |
| Neuropeptide Y | Stimulates hunger, food intake, and drinking |
| Sexual behavior | |
| Locomotion | |
| Memory | |
| Agouti-related protein | Stimulate hunger and food intake |
| Orexins (hypocretins) | Stimulate CRH and antidiuretic hormone (ADH) |
| Inhibit GHRH | |
| Stimulate GnRH | |
| May stimulate preovulatory prolactin release | |
| Inhibit TRH release | |
| Inhibit hunger and food intake | |
| Pituitary Peptides | |
| Prolactin | Maternal behavior |
| Mood | |
| Anxiety | |
| Growth hormone | |
| Thyroid-stimulating hormone | |
| Follicle-stimulating hormone | |
| Luteinizing hormone | Elevated levels may promote neurodegeneration |
| Pro-opiomelanocortin | |
| ACTH | |
| ACTH-like intermediate lobe peptides | |
| β-Endorphin | Analgesic mechanisms |
| Feeding | |
| Thermoregulation | |
| Learning and memory | |
| β-Lipotropic hormone | Skin tanning |
| Melanocyte-stimulating hormone (α- and γ-) | Weight loss |
| Skin tanning | |
| Increased sexual desire | |
| Anti-inflammatory effect | |
| Important mediator of leptin control on energy homeostasis | |
| Oxytocin | Anxiety and mood |
| Active/passive stress coping | |
| Maternal behavior, aggression | |
| Pair bonding | |
| Vasopressin | Active/passive stress coping |
| Anxiety | |
| Spatial memory | |
| Social discrimination, social interaction | |
| Pair bonding | |
| Activation of the hypothalamic-pituitary-adrenal (HPA) axis | |
| Neurophysins | |
| Brain–Gastrointestinal Tract Peptides | |
| Vasoactive intestinal polypeptide | Cerebral blood flow |
| Potent anti-inflammatory factor | |
| Somatostatin | |
| Insulin | Feeding behavior |
| Modulatory effect on learning and memory | |
| Hunger | |
| Glucagon | Inhibition of feeding |
| Pancreatic polypeptide | |
| Gastrin | |
| Cholecystokinin | Feeding behavior |
| Satiety | |
| Modulates dopamine neuron activity | |
| Facilitates memory processing (especially under stress) | |
| Tachykinins (e.g., substance P) | Substance P colocalizes with serotonin and is involved in nociception |
| Secretin | Modulates motor and other functions in brain, facilitating GABA |
| Thyrotropin-releasing hormone | |
| Bombesin | Thermoregulation |
| Inhibition of feeding | |
| Modulatory effect on learning and memory | |
| Orexins (hypocretin) | Gastric and gastrointestinal motility and secretion |
| Pancreatic hormone release | |
| Regulation of energy homeostasis | |
| Feeding behavior | |
| Locomotion and muscle tone | |
| Wakefulness/sleep | |
| Galanin | Modulates release of gonadotropin-releasing hormone, prolactin, insulin, glucagons, growth hormone, and somatostatin |
| Affects feeding, sexual behavior, and anxiety | |
| Potent anticonvulsant effects | |
| Leptin | Satiety factor |
| Growth Factors | |
| Insulin-like growth factors (IGF) 1 and 2 | |
| Nerve growth factors | Axonal plasticity |
| Opioid Family | |
| Endorphins | Analgesia |
| Enkephalins (met-, leu-) | Analgesic mechanisms |
| Feeding | |
| Temperature control | |
| Learning and memory | |
| Cardiovascular control | |
| Dynorphins | |
| Kytorphin | |
| Neuropeptides Modulating Immune Function | |
| ACTH | |
| Endorphins | |
| Interferons | |
| Neuroleukins | |
| Thymosin | |
| Thymopeptin | |
| Other Neuropeptides | |
| Atrial natriuretic factors | Possibly a role in cerebral salt wasting |
| Bradykinins | Cerebral blood flow |
| Calcitonin gene-related peptide (CGRP) | Migraine and other vascular headaches Movement of water into the gastrointestinal lumen Vasodilatation of microvascular beds |
| Other Neuropeptides | |
| Angiotensin | Hypertension |
| Thirst | |
| Synapsins | |
| Calcitonin | |
| Sleep peptides | Regulation of sleep cycles |
| Orexins (hypocretin) | Sleep-wake regulation |
| Narcolepsy | |
| Energy homeostasis | |
| Carnosine | |
| Pituitary adenylate cyclase–activating polypeptide (PACAP) | Migraine and other vascular headaches |
| Precursor Peptides | |
| Pro-opiomelanocortin | |
| Proenkephalins (A and B) | |
| Calcitonin gene product | |
| Vasoactive intestinal polypeptide gene product | |
| Proglucagon | |
| Proinsulin | |
It has been known for many years that stress, through activation of the hypothalamic-pituitary-adrenal (HPA) axis, modulates the function of the immune system ( ). Infections in the periphery convey information to the CNS through humoral (cytokines and bacterial toxins) and neuronal routes to bring about behaviors that enhance survival ( ). Certain peptides and their receptors, once thought to be unique to either the immune or the neuroendocrine system, are actually found in both.
Cytokines—interleukins (IL)-1, -2, -4, and -6 and tumor necrosis factor (TNF)—are synthesized by glial cells in the CNS in response to cell injury. IL-1 and the other cytokines, through their ability to stimulate the synthesis of nerve growth factor, may be important promoters of neuronal damage repair. Circulating cytokines have been thought to play a role in the hypothalamus to activate the HPA axis in response to inflammation elsewhere in the body (see the section titled “Fever,” later in this chapter) and inhibit the pituitary-thyroid and pituitary-gonadal axes in response to systemic disease.
Several other hormones and neuropeptides have modulatory effects on immune function. Similarly, immunocompetent cells contain hormones and neuropeptides that may affect neuroendocrine and brain cells ( Table 50.2 ). There is accumulating evidence that stress and the psyche-brain-immune network plays a role in psychiatric disease ( ). The overall ability of the psyche to influence immunological function and therefore disease outcome is less clear.
| Hormone or Peptide | Immunocompetent Cell in Which Hormone Is Found | Comments |
|---|---|---|
| Inhibitory | ||
| Glucocorticoids | Inhibit lymphokine synthesis, inflammation | |
| Corticotropin (ACTH) | B lymphocytes | Stimulated by corticotropin-releasing hormone; inhibited by cortisol |
| Macrophage activation, synthesis of IgG and γ-interferon | ||
| Chorionic gonadotropin | T cells | Stimulated by thyrotropin-releasing hormone; inhibited by somatostatin |
| Activity of T cells and natural killer cells | ||
| α-Endorphin | IgG synthesis, T-cell proliferation | |
| Somatostatin | Mononuclear leukocytes, mast cells | T-cell proliferation, inflammatory cascade |
| Vasoactive intestinal peptide | Mononuclear leukocytes, mast cells | T-cell proliferation and migration in Peyer patches |
| Potent anti-inflammatory effect | ||
| α-Melanocyte-stimulating hormone | Fever, prostaglandin synthesis, secretion of interleukin 2 | |
| Impairs function of antigen-producing cells and T cells | ||
| Anti-inflammatory effects | ||
| Stimulatory | ||
| Estrogens | Lymphocyte proliferation and secretion | |
| Growth hormone | T lymphocytes | Stimulated by growth hormone |
| Thymic growth, lymphocyte reactivity | ||
| Prolactin | Mononuclear cells | Thymic growth, lymphocyte proliferation |
| Stimulatory | ||
| Thyrotropin (TSH) | T cells | Stimulated by thyrotropin-releasing hormone; inhibited by somatostatin |
| IgG synthesis | ||
| β-Endorphin | Activity of T, B, and natural killer cells | |
| Substance P | Proliferation of T cells and macrophages, inflammatory cascade | |
| Corticotropin (ACTH)-releasing hormone | Lymphocyte and monocyte proliferation and activation | |
| Not Known to Be Stimulatory or Inhibitory | ||
| Enkephalins | B lymphocytes | |
| Vasopressin | Thymus | |
| Oxytocin | Thymus | |
| Neurophysin | Thymus | |
The hypothalamus plays a key role in ensuring that body temperature is maintained within narrow limits by balancing the heat gained from metabolic activity and the environment with the heat lost to the environment ( ). A theoretical schema of the mechanisms of hypothalamic temperature regulation is depicted in Fig. 50.1 . Although numerous neurotransmitters and peptides can alter body temperature, their physiological roles remain unclear.
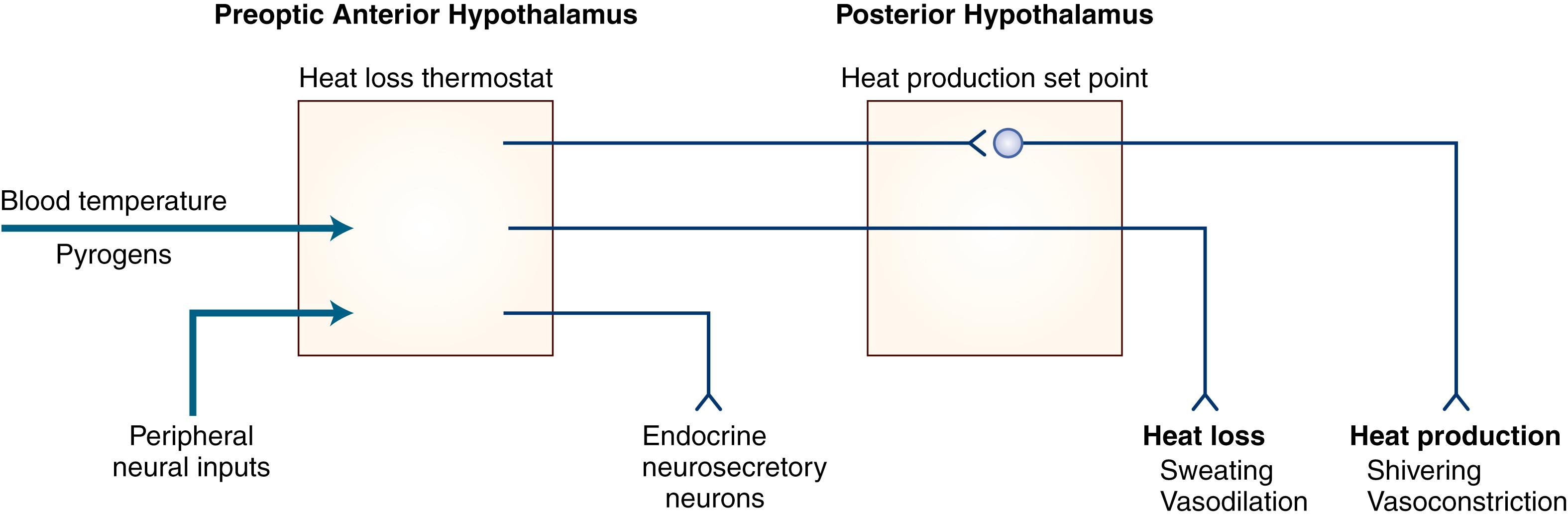
Hypothalamic injury can cause disordered temperature regulation. One potentially serious consequence is the hyperthermia that may occur when the preoptic anterior hypothalamic area is damaged or irritated by ischemia, subarachnoid hemorrhage, trauma, or surgery. In some patients, the marked impairment of heat loss mechanisms and the resulting hyperthermia may be fatal. In those individuals who survive, temperature control usually returns to normal over a period of days to weeks. Chronic hyperthermia of hypothalamic origin is extremely uncommon; it may occur with continued impairment of the ability to dissipate heat adequately or with difficulty sensing temperature elevations. Chronic hypothalamic hyperthermia does not respond to salicylates and other antipyretics because it is not mediated by prostaglandin. Hypothermia, both acute and chronic, can be due to hypothalamic injury, the most common causes being head trauma, infarction, and demyelination. Other entities to be considered in the differential diagnosis are severe hypothyroidism, Wernicke disease, and drug effect. Some patients with no apparent hypothalamic structural abnormalities may have episodes of recurrent hypothermia. The cause of this syndrome is unclear, although the response of some patients to anticonvulsant agents and of others to clonidine or cyproheptadine suggests a possible neurotransmitter abnormality. Agenesis of the corpus callosum in association with episodic hyperhidrosis and hypothermia (Shapiro syndrome) is caused in some individuals by an abnormally low hypothalamic “set point.” These symptoms may respond to clonidine (a centrally acting α 2 -adrenergic agonist). A similar condition associated with hyperthermia (so-called reverse Shapiro syndrome) has been found to respond with normalization of temperature to low-dose l -dopa, higher doses causing hypothermia. Large lesions in the posterior hypothalamus may impair both heat production (by altering the set point) and heat loss (by damaging the outflow from the preoptic anterior hypothalamic area). This results in poikilothermia, a condition in which body temperature varies with the environmental temperature.
Classical teaching has been that inflammatory cells in the periphery (primarily monocytes) release cytokines in response to infection and inflammation. These cytokines were thought to act on the hypothalamus to induce the production of prostaglandin E 2 (PGE 2 ) and cause elevation of the body temperature set point. The body then used its normal physiological mechanisms of vasoconstriction, vasodilation, sweating, and shivering to maintain this new higher set point (i.e., fever). This view of the mechanism by which bacterial infections cause fever is probably incorrect. Bacterial endotoxic lipopolysaccharide (LPS) does appear to work in the periphery to cause macrophages to release a variety of factors; however, the initial signal to the brain probably travels by vagal afferents to the preoptic anterior hypothalamus via norepinephrine, which activates the cyclooxygenase isoenzyme COX-2 to generate and release PGE 2 . The slower or second febrile increase in PGE 2 is due to COX-2 activation by IL-1β produced locally in the brain, not to circulating factors ( ). Acetylsalicylic acid and acetaminophen reduce fever by inhibiting cyclooxygenase, thereby reducing the levels of PGE 2 within the hypothalamus.
In otherwise healthy persons, extreme elevations of body temperature (as high as 41.1°C [106°F]) can sometimes be tolerated without serious effects. However, hyperthermia associated with prolonged exertion, heatstroke, malignant hyperthermia, neuroleptic malignant syndrome (NMS), hyperthyroidism, pheochromocytoma crisis, and some drugs may have serious and even fatal consequences. Exertional hyperthermia occurs with prolonged physical activity, particularly in hot, humid weather. It usually decreases athletic performance initially and can cause muscle cramps or heat exhaustion. When severe, it may result in heatstroke, a syndrome characterized by hyperthermia, hypotension, tachycardia, hyperventilation, hypoglycemia, and a decreased level of consciousness. Seizures are common in heatstroke and can further exacerbate the elevated body temperature. Even in the face of early and vigorous treatment, deaths continue to occur.
Drug-induced hyperthermic syndromes include anticholinergic poisoning, sympathomimetic poisoning, malignant hyperthermia, NMS, and serotonin syndrome. In malignant hyperthermia , a syndrome associated most often with the use of various general anesthetic agents and muscle relaxants, an inherited defect leads to excessive release of calcium from sarcoplasmic reticulum, stimulating severe muscle contraction (see Chapter 99, Chapter 110 ). The neuroleptic malignant syndrome is characterized by diffuse muscular rigidity, akinesia, and fever accompanied by a decreased consciousness level and evidence of autonomic dysfunction—namely, labile blood pressure, tachyarrhythmias, excessive sweating, and incontinence. NMS can be associated with the administration of major tranquilizers (primarily those that work by blocking dopamine receptors); with rapid withdrawal from dopaminergic agents including entacapone; and less commonly with the administration of tricyclic antidepressants. It appears to result from an alteration of temperature control mechanisms in the hypothalamus. As part of treatment, the withdrawal of all neuroleptics is mandatory. In addition to general supportive measures and cooling, the use of bromocriptine—a dopamine agonist (2.5–10 mg four times daily, increasing by 7.5 mg daily in divided doses to a maximum of 60 mg daily) can be helpful. Hypotension, psychosis, and nausea are possible side effects. An alternative is dantrolene (50–200 mg/day orally, or 2 to 3 mg/kg intravenously (IV) per day to a maximum of 10 mg/kg/day).
The serotonin syndrome is characterized by mental status changes, neuromuscular symptoms, autonomic dysfunction, and gastrointestinal (GI) dysfunction. In addition to tremor and rigidity (seen also in NMS), other features may include shivering, myoclonus, and hyperreflexia. Nausea, vomiting, and diarrhea are common in serotonin syndrome but uncommon in NMS. Treatment entails withdrawal from the offending drug and general supportive care.
Given free access to food and water, most animals maintain their body weight within narrow limits. With a change in energy intake/expenditure (a change in the size or number of individual meals that is not balanced by an equal and opposite change in energy use), the animal experiences a change in weight. One possible model of nutrient balance is depicted in Fig. 50.2 . The four components of energy balance are (1) the afferent system, (2) the CNS processing unit, (3) the efferent system, and (4) the absorption of food from the gut and its metabolism in the liver. Defects at any point in these systems may lead to weight loss or weight gain ( ).
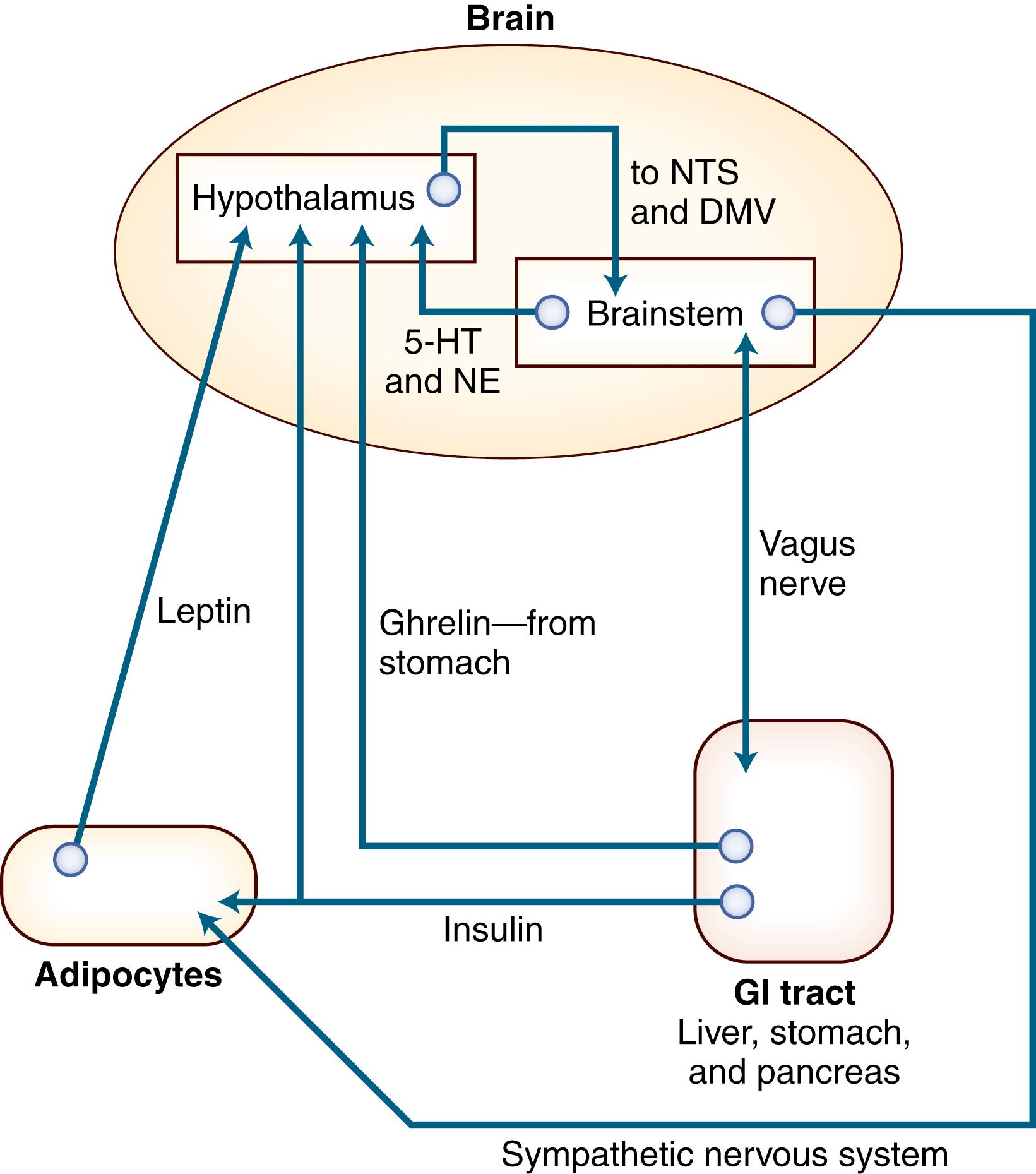
In response to a meal or to starving, hormonal and neural signals are generated in the periphery. Some are of short duration and others of long duration. Some relate to satiety, others relate to feeding behavior, and still others relate to “thinness and fatness.” Leptin is secreted by fat cells in the periphery and is sensed in the brain. Leptin seems to be most important if the levels fall below normal. In that circumstance it stimulates hunger. High levels of leptin tend not to suppress hunger. Neurons in the hypothalamus release the pro-opiomelanocortin (POMC) segment alpha-melanocyte–stimulating hormone (α-MSH), which suppresses appetite. Neurons that release agouti-related peptide (AgRP) also corelease neuropeptide Y (NPY) and γ-aminobutyric acid (GABA), both of which stimulate appetite ( ). Ghrelin, a stimulator of growth hormone (GH) release, is released from the stomach in the fasting state. Ghrelin activates NPY and agouti-related protein in the hypothalamus, leading to increased feeding and the deposition of energy into body fat. Peripheral insulin seems to mediate a satiety signal in the ventromedial hypothalamus. Destruction of the ventromedial hypothalamus, in both animals and humans, leads to obesity. Lesions in the paraventricular nucleus have a similar effect ( ). Overeating (hyperphagia) is only one of the mechanisms that produce hypothalamic obesity; more efficient handling of calories by the eater is probably another important factor. Hypothalamic lesions can also cause weight loss. Lesions in the dorsomedial nucleus lead to a reduction in body weight and fat stores, as do lesions in the lateral hypothalamus. Oral motor and meal-size responses may be dependent on centers in the caudal brainstem modulated by the hypothalamus.
Meal size and food intake are influenced by many different stimuli. Sensory cues such as the sight, aroma, and taste of food are major factors in dietary obesity. A decrease in blood glucose or in the oxidation of fatty acids in the liver stimulates the act of eating. Stomach distention gives rise to neural and hormonal signals that reduce food intake. GI peptides—such as cholecystokinin (CCK), bombesin, and glucagon—inhibit feeding by their actions on the autonomic nervous system, particularly the vagal nucleus ( ). Increased fatty acid oxidation leads to higher levels of 3-hydroxybutyrate, which then acts on the hypothalamus to reduce food intake. Interference with any of these sensing systems in the CNS can lead to obesity. NPY infused into the ventromedial nucleus of the hypothalamus induces obesity, perhaps by inhibiting sympathetic drive and stimulating insulin release. Although this may explain obesity with hypothalamic lesions, obesity related to eating highly palatable food is probably not related to central changes in NPY.
In animals, the ob, db , and fa genes play a role in the ability of adipose tissue to regulate feeding through a circulating factor. When the product of the ob gene, leptin, is administered peripherally to a genetically obese (ob/ob) mouse deficient in leptin, the animal reduces its intake of food, with a resulting decrease in body weight. The role of leptin in appetite regulation is complex. Leptin does not reverse the obesity seen in db/db mice and in obese humans. In these groups, serum leptin concentrations are higher than in subjects of normal weight, suggesting an insensitivity to the effects of endogenously secreted leptin.
When α-MSH binds to its receptor in the hypothalamus, it causes satiety. Up to 5% of obese children have been found to have an abnormality of the α-MSH receptor MC 4 R as a cause of their obesity ( ).
The orexins (hypocretins) are neuropeptides that play a role in energy balance and arousal ( ). Narcolepsy is caused by failure of orexin-mediated signaling. Orexins are found in the hypothalamus, where they regulate sleep/wake cycles ( ), and in the GI tract, where they excite secretomotor neurons and modulate gastric and intestinal motility and secretion ( ).
Anorexia nervosa and bulimia nervosa are clinical eating disorders of unknown etiology seen primarily in young women and girls. Anorexia nervosa is characterized by reduced caloric intake and increased physical activity associated with weight loss, a distorted body image, and a fear of gaining weight. Bulimia nervosa is characterized by episodic gorging followed by self-induced vomiting or laxative and diuretic abuse or dieting and exercise to reduce weight. Initially these syndromes were considered to be neuroendocrine in origin; then, for many years, they were assumed to be purely psychiatric. Although malnutrition produces changes in neuroendocrine function, disturbances in corticotropin-releasing hormone (CRH), opioids, NPY, vasopressin, oxytocin, CCK, ghrelin, and leptin as well as the monoamines—serotonin, dopamine, and norepinephrine—have been found in patients with eating disorders ( ). These neurotransmitters play a role not only in appetite but also in mood and impulse control. The abnormalities have been found to persist, in some instances, long after recovery. Autoantibodies have been implicated in the eating abnormalities seen in this condition ( ). Furthermore, patients with anorexia nervosa seem to have an increased total daily energy expenditure because of their increased physical activity. All of this suggests a complex interaction between the psyche and the endocrine system as a cause of these syndromes.
Experimental and clinical data support the hypothesis that interaction of the frontal and temporal lobes and the limbic system is necessary for normal emotional function. Lesion and stimulation experiments in cats have shown that rage reactions can be provoked from the hypothalamus. In humans, electrical stimulation of the septal region produces feelings of pleasure or sexual gratification, whereas lesions of the caudal hypothalamus or manipulation of this area during surgery may cause attacks of rage. The amygdala (with its rich input from polysensory areas and limbic-associated areas and its output to the hypothalamus) and other subcortical areas are important structures through which the external environment can influence and cause emotional responses. In depression, activation of the HPA axis has been recognized, but the story is more complex than this simple observation. Data have shown that depressed patients with suicidal ideation have less activation of the HPA axis, whereas those who commit suicide have a more active axis. Unfortunately, many of these data are derived from peripheral tests that primarily examine pituitary function rather than assessing true hypothalamic function.
Libido, like other feelings, requires the participation of both hypothalamic and extrahypothalamic sites. In most instances of hypothalamic disease, loss of libido is caused by the impaired release of gonadotropin-releasing hormone (GnRH) and a subsequent decrease in testicular testosterone in men. In women, libido is related more to adrenal androgens and in menstruating women to ovarian androgens. Adrenal androgen levels may be low in women with corticotropin (i.e., adrenocorticotropic hormone [ACTH]) deficiency and secondary adrenal insufficiency. Hypersexuality associated with hypothalamic disease is rare and may occur with or without a subjective increase in libido. The melanocortins (ACTH and α-, β-, and γ-MSH) may play a role in the motivational aspects of sexual behavior as well as having a sildenafil-like effect on penile and vaginal blood flow, albeit through a central rather than a peripheral mechanism. Melanocortinergic agents may be useful in the treatment of sexual dysfunction in both males and females ( ).
Current understanding of the human hypothalamus in relation to normal development, sexual differentiation, and behavior is expanding gradually. Sexual differentiation of the genitals occurs during the first 2 months of pregnancy, whereas sexual differentiation of the brain does not occur until the second half of pregnancy. Gender identity appears to result from the developing brain being exposed to testosterone, resulting in a male direction if exposed or a female direction if not. In addition to brain differences related to gender, there are brain differences related to sexual orientation ( )
It has been known for some time that oxytocin, the classical posterior pituitary hormone that has effects on uterine contraction and milk letdown, works in the brain to promote social affiliation in humans. This brain effect is important for parent-infant bonding and romantic attachments (Feldman, 2012). It also offers hope for the treatment of some of the behavioral aspects of frontotemporal dementia ( ).
Most endocrine rhythms are circadian—that is, a complete cycle takes approximately 24 hours. Although longer and shorter cycles do occur, the circadian rhythms have been studied most extensively. In many animals, light plays an important role in regulating circadian rhythms. Nerve fibers project from the optic chiasm to the suprachiasmatic and arcuate nuclei of the hypothalamus. The hypothalamus is responsible for the hormonal rhythms, such as cortisol and GH secretion, and for the behavioral rhythms, such as sleep/wake cycles and estrous activity. Although patients with hypothalamic disease often have disturbances in their biological rhythms, these are usually of less clinical importance than other problems caused by such lesions, and this is seldom if ever a presenting complaint.
In humans, discernible hypothalamic-pituitary tissue begins to develop during week 5 of embryonic life. The Rathke pouch, a diverticulum of the buccal cavity, forms and expands dorsally to contact and invest the diverticulum that develops from the floor of the third ventricle. By week 11, the buccal tissue has lost its connection with the foregut and has flattened to form the primitive anterior pituitary, whereas the neural tissue from the floor of the third ventricle is forming the posterior pituitary. Residual Rathke pouch tissue is postulated to give rise to the craniopharyngiomas that can occur in this region. Rarely, ectopic functional pituitary tissue in the oropharynx can cause signs and symptoms of hyperpituitarism.
The hypothalamus, despite its small size, is the region of brain with the highest concentrations of neurotransmitters and neuropeptides. Beginning with the pioneering work of Ernst and Berta Scharrer and Geoffrey Harris in the 1940s, the hypothalamus has been assigned a central role in regulating anterior pituitary function. In addition to the identified hypophysiotropic hormones ( Table 50.3 ), other peptides with putative regulatory functions are found in high concentration in the hypothalamus: neurotensin, substance P, CCK, NPY, vasoactive intestinal polypeptide, and the opioid peptides. The hypothalamus also is rich in acetylcholine, norepinephrine, dopamine, serotonin, histamine, and GABA. In many neurons, these neurotransmitters colocalize with peptides, although this colocalization and presumptive co-release have uncertain physiological significance. In patients with nonfunctioning pituitary or parapituitary tumors, symptoms produced by compression of neural structures adjacent to the pituitary gland are a common presentation. Understanding these symptoms requires knowledge of the anatomy of the region ( Fig. 50.3 ).
| Pituitary Hormone | Hypothalamic Factor |
|---|---|
| Growth hormone (somatotropin) | Growth hormone–releasing hormone (GHRH) |
| Growth hormone releasing–inhibiting hormone (somatostatin) | |
| Prolactin | Prolactin-releasing factors (PRFs) |
| Prolactin releasing–inhibiting factor: dopamine and possibly the precursor of gonadotropin-releasing hormone (GnRH) | |
| Thyrotropin | Thyrotropin-releasing hormone (TRH) |
| Thyrotropin releasing–inhibiting factor: somatostatin can do this but has not been confirmed to do so physiologically | |
| Pro-opiomelanocortin is cleaved to form corticotropin (ACTH) | Corticotropin-releasing hormone (CRH) |
| Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) | Gonadotrophin-releasing hormone (GnRH) |
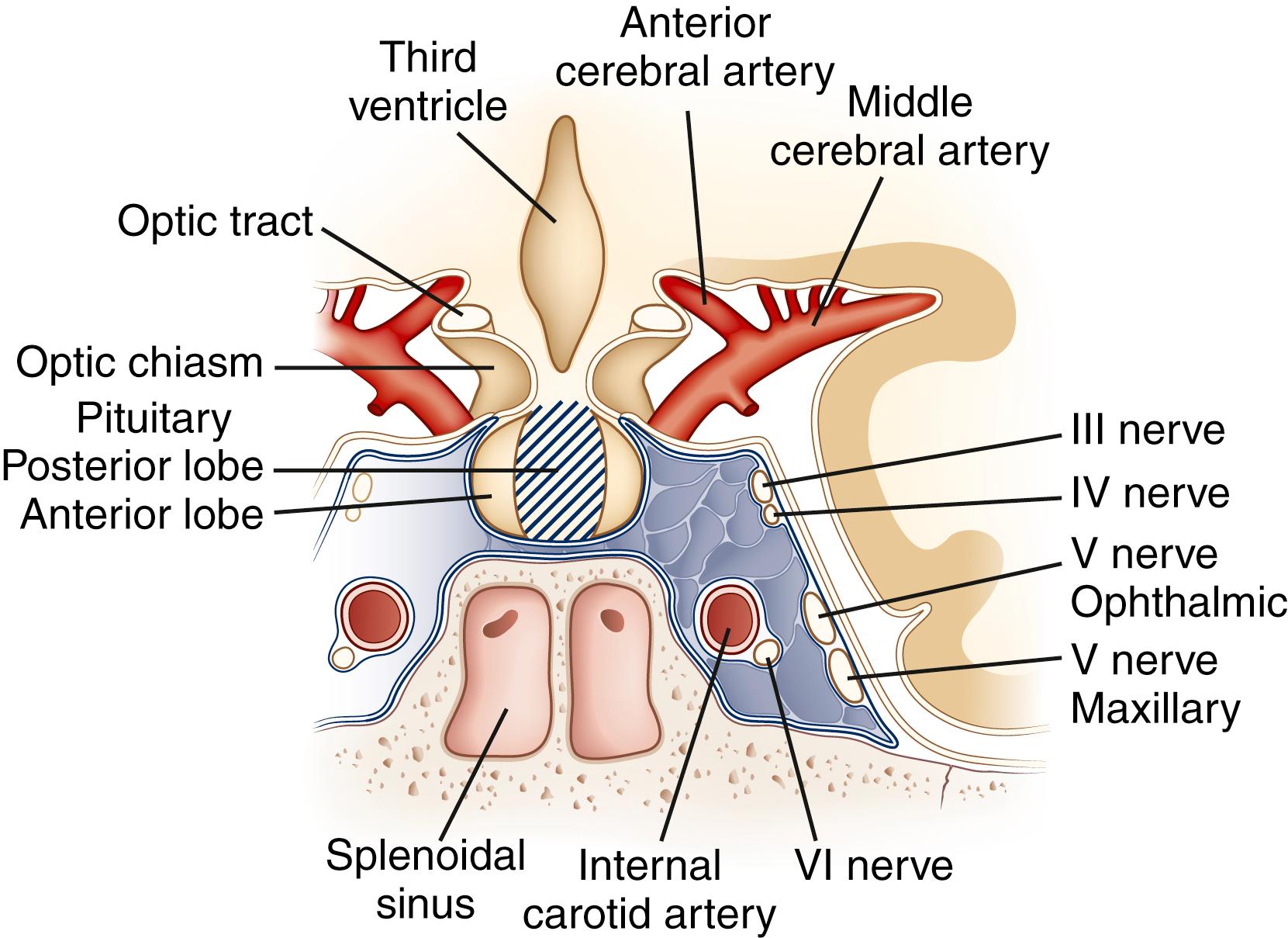
Tumor erosion of the floor of the sella turcica may lead to cerebrospinal fluid (CSF) rhinorrhea. Conversely, sinusitis or sphenoid sinus mucocele can invade the sella, resulting in anterior pituitary dysfunction. Expansion of pituitary tumors into the cavernous sinus can produce palsies of the third, fourth, fifth, and sixth cranial nerves. The development of such deficits is especially common with the sudden expansion of pituitary tumors that occurs in pituitary apoplexy. Carotid aneurysms or ectatic carotid arteries in the cavernous sinus may expand medially and mimic pituitary adenomas by enlarging the sella and causing anterior pituitary hypofunction. Appropriate evaluation with computed tomography (CT) or magnetic resonance imaging (MRI) can identify these conditions.
The dura overlying the sella is sensitive to pain, and stretching of this structure by expanding pituitary tumors gives rise to headache referred to the vertex and retro-orbital area. In some cases, especially if intracranial pressure is elevated, the dura may herniate into the sella, where continued pulsation of the CSF over time leads to remodeling and expansion of the sella. This produces the radiological finding of the empty sella syndrome, another cause of which is lymphocytic hypophysitis. The pituitary gland becomes a thin ribbon of tissue along the walls of the normal-sized or expanded sella, and the sella contains mostly CSF. Only rarely can evidence of impairment of pituitary function be found in such patients. In cases of marked hydrocephalus, accompanying pituitary-hypothalamic dysfunction can be seen, presenting most commonly in females as amenorrhea.
Expansion of pituitary tumors out of the sella tends to lead to compression of the anterior and inferior crossing fibers of the optic chiasm (see Chapter 16, Chapters 45 ). These fibers subserve vision in the superior temporal quadrants. Therefore pituitary adenomas typically cause bitemporal superior quadrantanopias. Lesions such as craniopharyngiomas that impinge on the posterior and superior fibers of the optic chiasm tend to manifest with bitemporal inferior quadrantanopias. Nevertheless, owing to the variability of the positioning of the optic chiasm and the tendency for tumors to be asymmetrical in their growth, parasellar lesions result in a wide variety of field defects. Because pressure on the optic chiasm preferentially affects the fibers subserving color vision, these visual field defects may be missed on bedside confrontation testing with the fingers. A better bedside test is to look for subtle color desaturation in the affected visual field.
The superior and inferior hypophysial arteries are the pituitary’s major source of blood ( Fig. 50.4 ). The posterior pituitary gland is supplied principally by the inferior hypophysial arteries and drained by the inferior hypophysial veins. The superior hypophysial artery forms a primary capillary plexus in the median eminence of the hypothalamus. From here, blood flows into the long hypophysial portal veins, which carry it to the anterior pituitary. Although some blood from the anterior pituitary drains into the cavernous sinus, some drains into the posterior pituitary and some returns to the median eminence by way of the long portal veins, which are capable of bidirectional blood flow. This vascular anatomy provides a potential mechanism for the important feedback loops necessary for the regulation of hypothalamic-pituitary function.
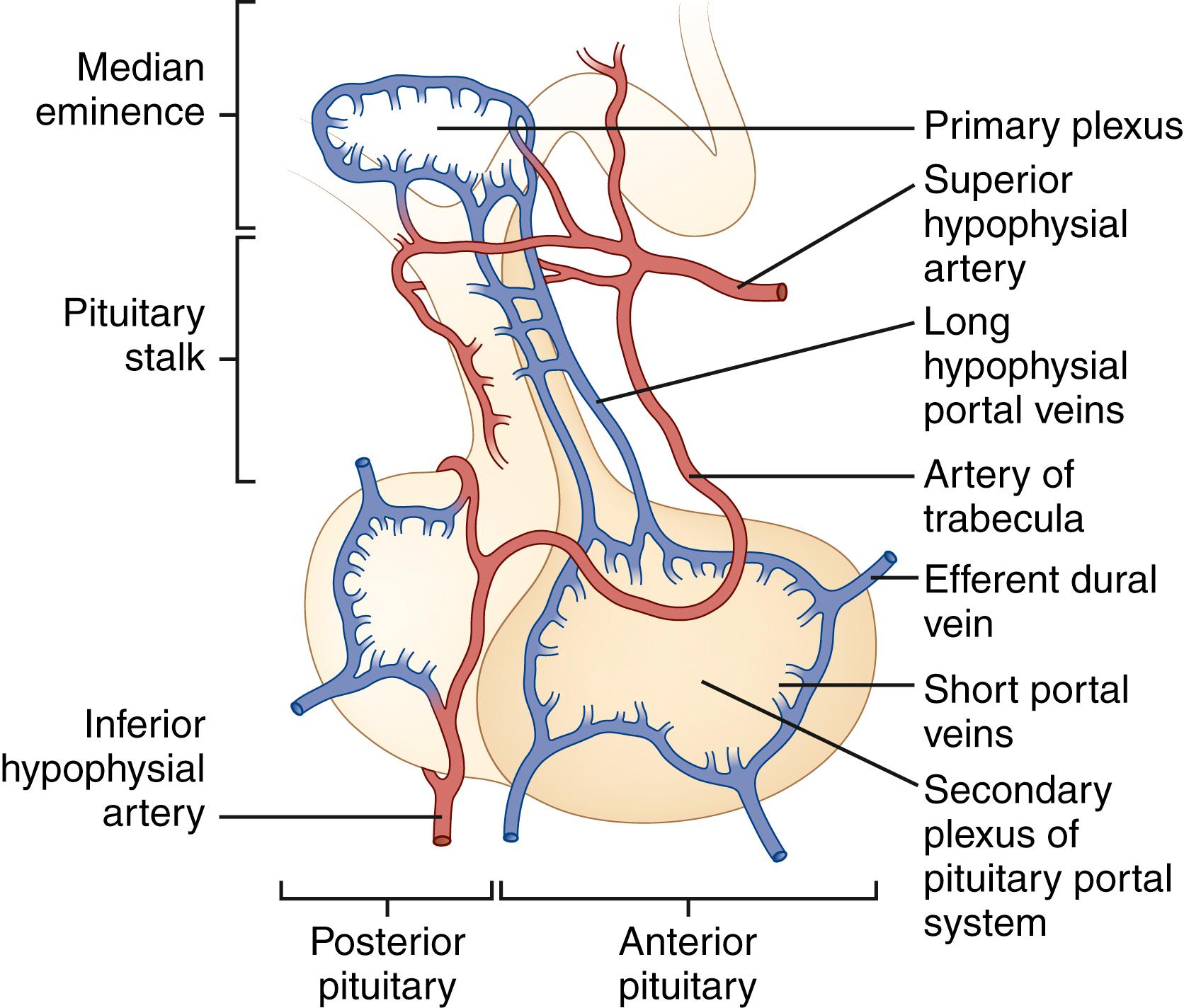
The hypothalamus produces hypophysiotropic substances that control the secretion of anterior pituitary hormones. Five neuropeptides and one neurotransmitter (dopamine) are known to be important physiological regulators of pituitary function (see Table 50.3 ). In addition, several neurotransmitters affect pituitary hormone release, although their physiological role remains uncertain. Since their discovery in 1998, the orexins (hypocretins) have been found to regulate virtually all the hypothalamic-pituitary axes as well as to participate in the coordination of anterior pituitary function with sleep, arousal, and general metabolism. This is well reviewed in an article by .
The causes of pituitary insufficiency are summarized in Box 50.1 . In general the symptoms of hypopituitarism ( Table 50.4 ) are those of the secondary failure of end-organ function. Because the associated changes usually develop slowly and some autonomous end-organ function remains, the symptoms are often less severe than those that occur with primary end-organ disease. The term Simmonds disease is applied to panhypopituitarism of the anterior pituitary gland. When this syndrome develops in the postpartum period after an episode of pituitary infarction, it is called Sheehan syndrome .
Pituitary aplasia
Complete
Monohormonal
Trauma
Head injury
Surgery
Radiotherapy
Compression by cysts or tumors
Pituitary apoplexy
Pituitary infarction
Hypophysitis
Infection
Granulomatous disease
Autoimmune disease
Hypothalamic failure
Drugs
Opioids
Cancer chemotherapy
| Hormone | Excess Secretion | Deficient Secretion |
|---|---|---|
| Growth hormone | In children : Gigantism | In children : Growth failure and tendency to hypoglycemia |
| In adults : Acromegaly | Increased body fat, decreased exercise capacity, and decreased bone density | |
| Prolactin | In children : Delayed puberty | |
| In adults : | In adults: | |
| Women : Amenorrhea, galactorrhea, and infertility | Women: Inability to breastfeed and possible infertility | |
| Men : Impotence, infertility, and (rarely) galactorrhea | ||
| Luteinizing hormone and follicle-stimulating hormone | In children : Precocious puberty In adults : Infertility, hypogonadism, polycystic ovary syndrome |
In children : Delayed puberty In adults: Amenorrhea, infertility, erectile dysfunction |
| Thyrotropin (TSH) | Hyperthyroidism | Hypothyroidism |
| Hyperprolactinemia (due to excessive TRH stimulation) | ||
| Pro-opiomelanocortin | Cushing disease, Nelson syndrome | Hypoadrenalism; glucocorticoids affected more severely than mineralocorticoids |
Intrauterine growth is independent of GH. Therefore, although GH-deficient children are of normal size at birth, they subsequently fail to grow. Insulin-like growth factors (IGFs) 1 and 2 are important mediators in human somatic growth. IGF-1 production in the liver is dependent on GH, whereas IGF-2 is relatively insensitive to GH. True GH deficiency is rare. It may manifest in an isolated fashion or as part of general pituitary failure. Apparent GH deficiency may result from an isolated deficiency of growth hormone–releasing hormone (GHRH) or from a lack of GH receptors in the liver, leading to failure of IGF-1 production (resulting in Laron dwarfism). GH opposes insulin action; in children especially, its deficiency may be associated with episodes of fasting hypoglycemia.
Pituitary insufficiency of GH in children may manifest as delayed or absent puberty. Onset of puberty depends to some extent on the achievement of a critical body mass. Thus anything that delays growth—such as GH deficiency, adrenal insufficiency, or hypothyroidism—delays puberty. If breasts or sexual hair growth have not started to develop in girls by age 14 or if testicular enlargement and sexual hair growth have not occurred in boys by age 15, puberty should be considered delayed. Luteinizing hormone (LH) or follicle-stimulating hormone (FSH) deficiency may occur as part of generalized pituitary failure, from isolated GnRH deficiency, or as a result of high prolactin levels inhibiting their release from the pituitary.
One cause of hypopituitarism is pituitary apoplexy , a term that should be reserved for infarction of or hemorrhage into the normal pituitary gland or into a pituitary adenoma. To be classified as true apoplexy, the hemorrhage should be of sufficient severity to produce signs of compression of parasellar structures or evidence of meningeal irritation. A sudden expansion of the pituitary gland may lead to chiasmal compression or cranial nerve palsies. Rupture of the necrotic gland into the CSF may be clinically indistinguishable from subarachnoid hemorrhage due to rupture of a berry aneurysm or an arteriovenous malformation. Hypotension, aggravated by coexisting ACTH deficiency, may further complicate the picture. The diagnosis can usually be made readily by CT scanning (although apoplexy may be missed on CT slices 1-cm thick) or MRI. Treatment includes admission to hospital; close clinical observation with general supportive measures; corticosteroid replacement; and, in case of worsening neurological symptoms, surgical decompression by an experienced pituitary surgeon ( ).
Development of secondary sexual characteristics before age 8 in girls and age 9 in boys is considered abnormal. In approximately one-fifth of affected girls and one-half of affected boys, the cause of precocious puberty is a neurological lesion. Various types of tumors have been associated with the development of precocious puberty—including hamartoma, teratoma, ependymoma, optic nerve glioma, glioma, and neurofibroma—either alone or as part of von Recklinghausen syndrome. Tumors are most commonly located in the posterior hypothalamus, pineal gland, or median eminence, or they put pressure on the floor of the third ventricle. The cause of precocious puberty in these circumstances has not been clearly delineated. However, some of these tumors may be an ectopic source of GnRH or of human chorionic gonadotropin, a placental peptide with LH- and FSH-like activity.
In the investigation of precocious puberty, LH and FSH levels as well as human chorionic gonadotropin should be measured along with testosterone (in males) and estradiol (in females). An MRI study of the head is mandatory. If LH and FSH levels are in the adult range and the head imaging result is negative, it is most likely that the precocious puberty is idiopathic. Very high human chorionic gonadotropin levels suggest ectopic production. If LH and FSH levels are low, adrenal, ovarian, testicular, or exogenous causes must be sought.
Chronic administration of long-acting analogs of GnRH results in an initial stimulation of LH and FSH secretion, followed by complete inhibition. This effect on LH and FSH release can be used to stop and prevent progression of hypothalamic precocious puberty.
Probably the most common abnormality of pituitary function encountered by the neuroendocrinologist is hyperprolactinemia; its causes are summarized in Box 50.2 . Prolactin levels in excess of 200 ng/mL (normal <25 ng/mL) if not caused by pregnancy are usually due to excessive production of the hormone by a pituitary adenoma. In premenopausal women, the development of amenorrhea secondary to direct inhibition of LH and FSH by prolactin leads to early investigation and diagnosis of prolactin-secreting tumors at the microadenoma (<10 mm in diameter) stage. In men, the insidious onset of reduced libido usually means that these tumors are found late, often only after they have produced signs and symptoms of optic nerve compression. Galactorrhea, a rare accompaniment of elevated prolactin in men, is seen frequently in women with hyperprolactinemia.
Drugs
Dopamine receptor blockers
Phenothiazines such as chlorpromazine
Butyrophenones such as haloperidol
Metoclopramide
Reserpine
α-Methyldopa
Monoamine oxidase inhibitors
Tricyclic antidepressants (unusual, probably idiosyncratic)
Benzodiazepines (unusual, probably idiosyncratic)
Verapamil
Cocaine
Fluoxetine
Amoxapine
Hormones
Estrogens
Thyrotropin-releasing hormone (as can occur in primary hypothyroidism)
Pituitary tumor
Prolactin-secreting adenoma
Interference of flow of dopamine down the pituitary stalk by a large pituitary or parapituitary tumor
Chest wall stimulation
Chronic skin disease (e.g., severe acne) chronic skin disease and tumours of the chest wall should be indented slightly—they are subforms of chest wall stimulation
Tumors of chest wall
Chronic renal failure
Cirrhosis
Ectopic production
Hypothalamic disease
Pseudocyesis
Idiopathic
Serum prolactin levels increase after generalized tonic-clonic seizures and complex partial seizures due to temporary lack of hypothalamic dopaminergic inhibition of prolactin release, but they show no change after virtually all cases of psychogenic, absence, or simple or complex partial seizures of frontal lobe origin. After a seizure, prolactin levels peak at 15–20 minutes and then decrease to baseline levels within 60 minutes. The increase should be at least two times baseline. Caution should be exercised in interpreting early-morning prolactin levels because a 50%–100% increase in prolactin is normal just before waking. Furthermore, prolactin elevations are far from specific for epilepsy, and some tendency for the elevation to attenuate in patients with frequent seizures has been observed.
Because prolactin secretion is under strong inhibitory control by the hypothalamus (owing to the secretion of dopamine), anything that interferes with the free flow of blood down the pituitary portal veins can reduce the exposure of the pituitary to the dopamine released by the hypothalamus. This results in raised peripheral blood prolactin levels. In patients with this condition, prolactin levels commonly range from 50 to 150 ng/mL (usually <100 ng/mL; normal is <25 ng/mL); such elevations can be seen, for example, in patients with granulomatous disease involving the pituitary stalk. However, probably the most common situation in which this occurs is in patients in whom the pituitary stalk is “kinked” by a nonsecretory pituitary adenoma. In such circumstances, this may lead to the erroneous assumption that the pituitary adenoma is secreting prolactin, and long-term therapy with bromocriptine might be undertaken. We have seen such patients whose tumors continued to grow despite normalization of prolactin levels. The mistake with these patients is to assume that a prolactin-secreting macroadenoma would result in a moderately elevated prolactin level. Patients with macroadenomas that secrete prolactin usually have prolactin levels above 200 ng/mL, and not infrequently in excess of 1000 ng/mL.
Patients taking neuroleptic medications may also have elevated prolactin levels; occasionally the elevation is enough to cause galactorrhea or amenorrhea. In such patients it may be uncertain whether symptoms are secondary to drug-induced hyperprolactinemia or to a microadenoma. In the ideal situation, serum prolactin levels are measured before starting the patient on these medications. Our practice is to perform an MRI study of the pituitary to look for a tumor. Occasionally we will perform dynamic pituitary testing with thyrotropin-releasing hormone and metoclopramide. In most patients, drug-induced hyperprolactinemia responds normally to stimulation with these agents. The treatment of drug-induced hyperprolactinemia is difficult if the causative drug cannot be stopped. Some patients may benefit from the use of atypical antipsychotics with reduced or no action at dopamine receptors (at normal therapeutic doses).
The presence of excessive amounts of circulating GH before closure of the epiphyses leads to gigantism. If the epiphyses have closed, the only tissue still capable of responding to GH will grow, leading to the clinical syndrome of acromegaly; its clinical features are summarized in Box 50.3 . Of particular note for the neurologist and neurosurgeon is the frequent complaint of headache and symptoms related to carpal tunnel syndrome. It is not uncommon to find patients with acromegaly in whom surgery for carpal tunnel release was performed bilaterally 3–5 years before diagnosis of their disease.
Headache—nonspecific, tension-type, felt at the vertex and behind the eyes
Impaired glucose tolerance or diabetes mellitus
Enlargement of hands and feet
Enlargement of the jaw, with increased spacing between the teeth and malocclusion
Hypertension
Menstrual irregularities
Soft-tissue growth
Thick skin
Dough-like feel to palm (e.g., during handshake)
Carpal tunnel syndrome
Arthralgia and osteoarthritis
Proximal muscle weakness
Hyperhidrosis
Most cases of gigantism and acromegaly are due to excess GH production by a pituitary adenoma. Rare cases of ectopic GH production have been described. Excessive GHRH production by pancreatic tumors can cause acromegaly. Excess production of GHRH by the hypothalamus could theoretically cause an identical clinical syndrome.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here