Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter reviews the history of patient-specific instrumentation (PSI) and then outlines the design rationale, surgical techniques, and results seen in kinematically aligned (KA) total knee arthroplasty (TKA). The goals of KA TKA are to (1) restore the native femoral and tibial articular surfaces, (2) restore the native knee and limb alignment, and (3) reestablish the native tibial compartment forces and laxities of the knee. Achieving KA with PSI is made possible using techniques and technologies developed over the past decade. Using an accurate and reliable PSI system, coupled with secondary caliper checks, enables successful kinematic balancing in every patient. PSI offers advantages in surgical precision, potentially improved operative time, and advanced preoperative planning. It also obviates the need for additional pinholes or intraoperative registration otherwise seen in navigated knee replacements.
PSI for TKA has been available for over 10 years with often mixed results. Many early versions of this technology relied on magnetic resonance imaging (MRI) modeling of the distal femur and proximal tibia. This yielded an inexact delineation between subchondral bone and cartilage. It also led to intraoperative uncertainty on the part of the surgeon during its application, because of a less reproducible positioning of the guide on cartilage. Furthermore, most early systems did not come with three-dimensional (3D) models of the distal femur and proximal tibia, etchings on the models, or the ability to attach extramedullary alignment rods to verify registration of the guide to the articular surface. In one instance, a manufacturer uses axial imaging of the knee in conjunction with standing full-length X-rays to properly align the hip-knee-ankle (HKA) axis. Flexion contractures of the knee and rotation of the limb make this type of system prone to error. Newer designs often use computed tomography (CT) scans for computer modeling, which has yielded greater predictability in its application once remaining cartilage is removed. , Studies have shown CT-scan modeling to be dimensionally more accurate than MRI. Nonetheless, the ultimate goals of any PSI system remain unchanged: allow preoperative planning, enhance surgical workflow, and increase precision and reproducibility.
In designing the ideal PSI system, we must consider limitations of former versions. As many have failed to achieve the goals of accuracy and reliability, there have been numerous reports from authors who discourage the routine use of PSI during TKA. , Many of these unfavorable accounts relate to the inaccuracy of the underlying PSI system itself. It should be stressed that not all PSI systems are created equal. Unique limitations exist for each system that may not translate to other manufacturers. These inaccuracies can stem from a number of potential issues. However, based on our extensive experience, we have concluded that several factors can adversely affect the quality of the PSI product.
First, as alluded to previously, MRI-based PSI cutting blocks tend to be less accurate than CT-based ones. MRI scans are less accurate for determining joint line definition because cartilage margins can only be estimated, leading to potential reconstruction errors, cutting block mismatch, and lack of accuracy. MRI scans are also more expensive, require longer scan times, are more susceptible to patient motion artifact, and are often adversely affected by periarticular hardware. The MRI-based PSI blocks themselves rest on cartilaginous surfaces, which are inherently softer and less precise. Conversely, CT-based cutting blocks facilitate more reproducible positioning and bone resections once cartilage and synovium are surgically removed with Bovie cautery. In addition, large osteophytes and prominent bony landmarks can serve as contact points for accurate CT-based PSI block placement with minimal intersurgeon variability.
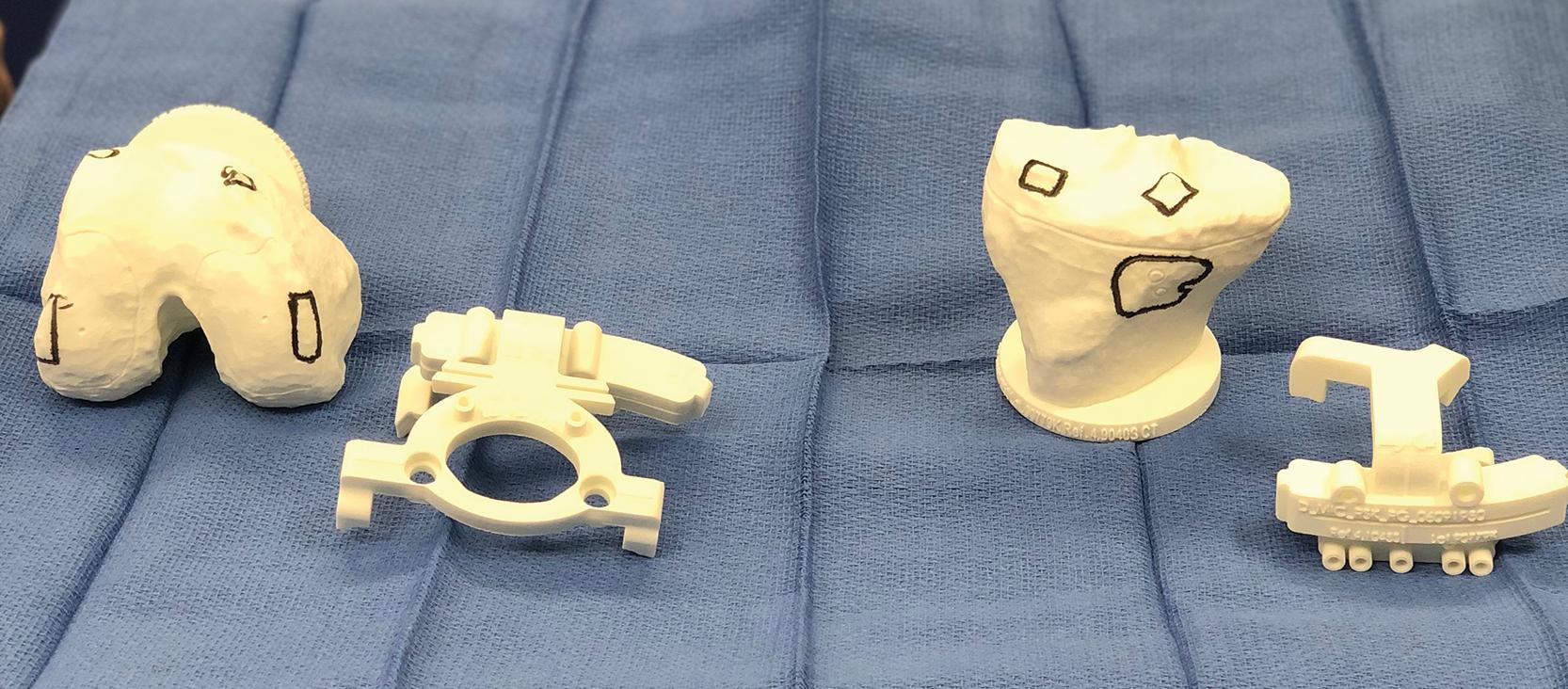
Second, the modern design advocated by the lead authors uses 3D-printed bone models. Exact replicas of the distal femur and proximal tibia are provided sterile to the surgeon for intraoperative use ( Fig. 6.1 ). These yield direct visualization of intended resections, angles, and component positions before making the actual bone cuts. The 3D models with etchings define contact points for cutting blocks. The intended resections can be confirmed visually on the bone and cross-referenced with the model. In addition, alignment slots exist for secondary check of accurate cutting block placement by adding extramedullary alignment rods. Therefore, in addition to the unambiguous registration of these guides to the bone, these resection etchings and alignment slots serve as secondary checks of planned resections, before actually completing the bone cuts.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here