Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radiation therapy (RT) uses ionizing radiation to locally treat cancers, while systemic therapy uses cytotoxic chemotherapy or molecular targeted biologics to systemically treat cancers.
Compared to surgical treatment of head and neck (HN) cancers, radiation potentially offers a curable alternative or adjunct, sometimes with the added benefit of organ preservation.
Prompt dental evaluation with necessary tooth extractions and optimal lifetime dental hygiene are critical for patients with HN cancer who may receive radiation to the mandible to minimize the risk of long-term osteoradionecrosis.
In the combined setting, chemotherapy potentiates the effects of RT, exerts cytotoxic effects on cancer cells, and may modulate the antitumor immune response to improve both local and systemic disease control.
Bolus cisplatin is generally the standard of care for systemic therapy for HN cancer with concurrent radiation, although the final choice of systemic therapy is at the discretion of the medical oncologist.
Postoperative chemoradiation is indicated for positive margins or nodal extracapsular extension.
Definitive HN cancer radiation doses generally range from 66 to 70 Gy.
Definitive radiation is an effective treatment alternative for nonmelanomatous skin lesions.
Risk for long-term complications is a function of the total dose of radiation delivered, the dose per fraction, the time frame over which it was given, the anatomic sites included within the radiation fields, and potential added effects from concurrent therapies.
Induction chemotherapy has been shown to improve disease control and overall survival in nasopharyngeal cancers and improve larynx preservation in some patients, but the value in improving overall survival in HN cancers remains unclear.
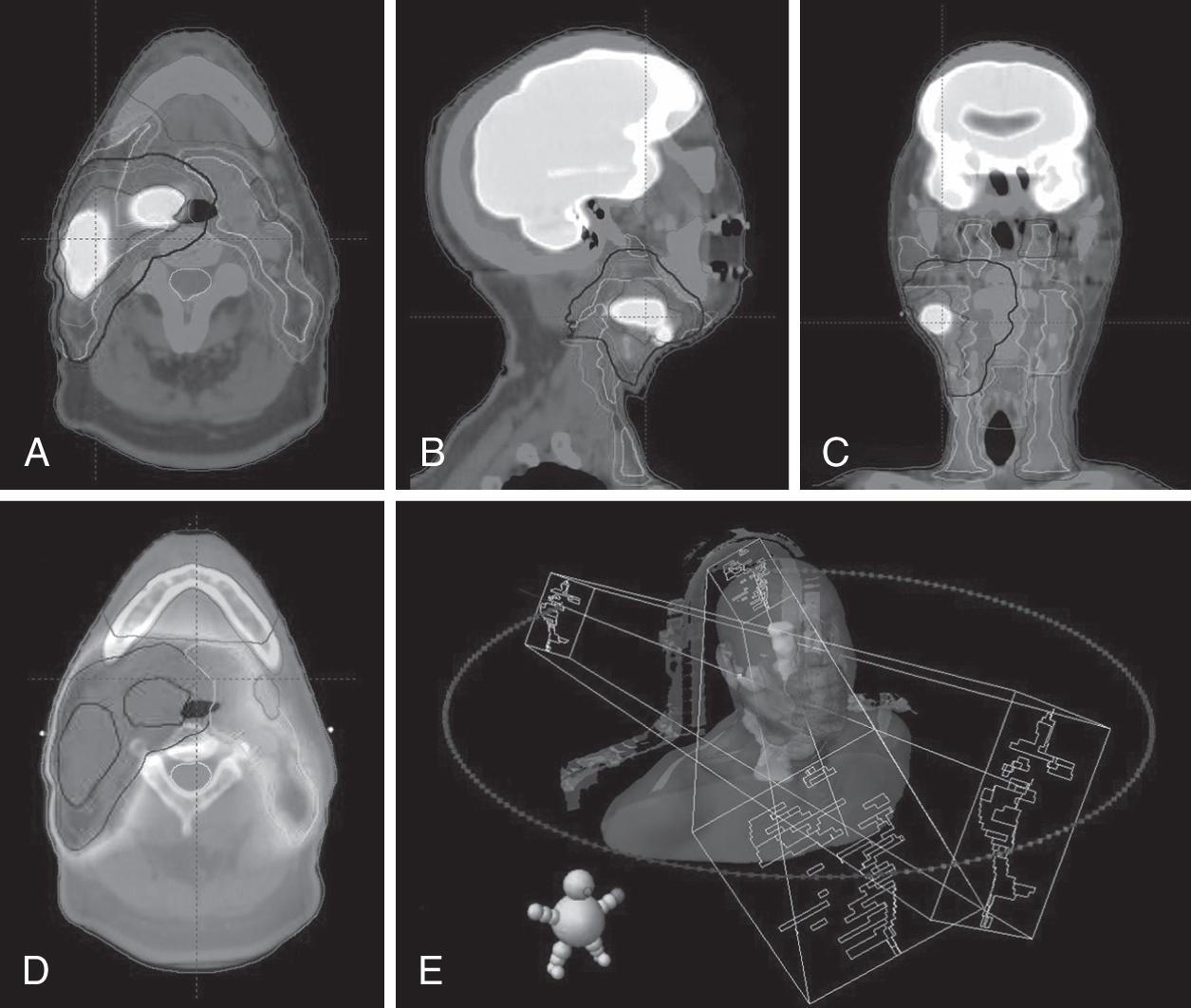
Radiotherapy, also called radiation therapy (RT), is the localized treatment of cancer and other diseases with ionizing radiation. Ionizing radiation induces mitotic cell death via damage to DNA through a variety of atomic interactions, including free radical generation and direct DNA strand breaks. The primary goal of all RT is to maximize cancer cell death while minimizing damage to healthy normal tissues. Radiation is most often delivered by an external source (i.e., external beam RT [EBRT]) using photons, protons, electrons, and other heavy particles but may also be administered by temporarily or permanently placing radioactive sources into a patient’s body (i.e., brachytherapy). The most frequently used modern EBRT techniques for treatment of head and neck (HN) cancers are a complex method of volumetric-modulated arc therapy (VMAT) and occasionally three-dimensional conformal RT (3DCRT). Varying dose rates/energies, multiple beam angles/arcs, and dynamic multileaf collimator shapes combine to optimize delivery of the radiation. Image-guided RT (IGRT) uses imaging capabilities on the treatment machine (e.g., cone beam computed tomography [CT] and x-ray) to verify patient setup. Stereotactic body RT (SBRT) is an additional technique often used in the palliative or re-irradiation setting that delivers high doses of radiation in five or fewer treatments ( Fig. 22.1 ).
Patients undergoing RT for HN cancers require timely coordination and support from a multitude of care providers. The following specialists are often involved:
Radiation oncologist: A formal history and physical evaluation and review of all available imaging are necessary to determine the appropriate radiation targets, dosing, and schedule.
Medical oncologist: A formal medical oncology evaluation is recommended for any patient who may be a candidate for systemic therapies.
Dentist or oral surgeon: Any patient who may receive radiation in the region of the mandible, maxilla, or teeth should receive a formal evaluation and any necessary dental work (e.g., extraction of unhealthy teeth, fillings, fluoride trays) prior to initiating RT, if possible. RT should not start until 2 weeks or more after a dental procedure to ensure proper healing. A delay in the dental evaluation is one of the most common yet significant causes of delay in initiating RT. For this reason, patients with clearly unhealthy teeth who undergo initial surgical resection may have concurrent dental extractions performed in anticipation of adjuvant RT.
Nutritionist: Regular assessments of nutritional status for patients with HN cancer before, during, and after RT and systemic therapy are critical. Nutritionists can also provide teaching and support for patients with and without feeding tubes.
Speech and swallow therapy: A baseline evaluation is recommended for patients with current or anticipated speech or swallowing problems.
Pathology, radiology, and otolaryngology: Involvement is recommended to help with RT planning.
Additional consultants: May include interventional radiology, audiology, ophthalmology, neurosurgery, plastic surgery, physical medicine and rehabilitation, social work, addiction services, smoking cessation, psychology, and palliative care.
CT simulation: Planning, or simulation, CT scans are performed in the radiation oncology department, at which time immobilization devices customized to the patient are made, including a thermoplastic molded mask, a mouthpiece, and a head rest. Magnetic resonance imaging (MRI), positron emission tomography (PET)/CT, and additional scans may be performed to assist with both staging and treatment planning. Whenever possible, patients should receive these additional scans in the radiation treatment position using their mask, mouthpiece, and head rest to assist with immobilization. Having similar positions between all imaging modalities optimizes the accuracy of image fusions used for target delineation planning software ( Fig. 22.1 ).
Drawing volumes: The radiation oncologist will then use planning software to outline gross tumor, areas of potential microscopic disease, and normal tissues. This process is known as “drawing volumes,” or contouring, and often takes several hours and may involve more than 20 distinct volumes or structures.
Planning: A dosimetrist, physicist, and the radiation oncologist then work together to create the optimal plan for delivering the radiation to the target while restricting the dose to normal tissues. These plans are often reviewed by multiple people and run through quality assurance checks on the treatment machine.
Treatment: RT for patients with HN cancer may consist of a schedule such as daily single treatments for 6 to 7 weeks, with weekly check-up appointments with the radiation oncologist to evaluate and treat toxicities.
Both RT and surgery are local therapies. Definitive RT strives for organ preservation to maintain critical functions such as swallowing, normal speech, and breathing while attempting to maximize the quality of life in patients who might otherwise undergo morbid surgical resection. RT is typically delivered daily (Monday through Friday) over 6 to 7 weeks on an outpatient basis without need of anesthesia, making it a more ideal treatment modality for poor surgical candidates. The protracted time commitment can be problematic for noncompliant or elderly patients and those living great distances from a radiation oncology facility. Surgery could be advantageous in providing a one-time procedure and optimal pathologic assessment of primary tumors and nodal disease. While acute side effects of surgery occur primarily in the immediate postoperative time frame, acute RT toxicities typically build up gradually throughout the course of treatment. Extent of disease and involvement of critical structures limit surgeons in their ability to achieve complete resection of disease and radiation oncologists in their ability to treat these disease sites to full definitive doses. Both specialties must balance aggressive treatment with acute and long-term local toxicities.
A gray (Gy) represents one joule per kilogram and is the standard unit of absorbed dose used in clinical radiation oncology. A variety of doses and fractionation schedules are used in treating HN cancers. In the typical daily radiation setting, daily doses of 180 to 225 cGy (1.8 to 2.25 Gy) are used. General, non-site-specific total doses vary based on the setting (definitive RT to gross disease ≈ 66 to 70 Gy; high-risk elective neck coverage ≈ 60 Gy; low-risk elective neck ≈ 54 Gy; and postoperative RT ≈ 54 to 66 Gy).
Patients who should not receive RT are those with collagen vascular diseases, other hypersensitivity conditions (e.g., ataxia-telangiectasia), pregnant women particularly in the first two trimesters, and those who would exceed maximum safe cumulative doses of RT to critical structures. Some patients can be retreated, usually with a lower dose, after time has elapsed since the previous treatment. Additionally, disorders of DNA repair and conditions (e.g., Li-Fraumeni syndrome and xeroderma pigmentosum) put patients at high risk for radiation-induced secondary cancers.
Chemotherapy, biologics, and immunotherapies are the three main groups of systemic therapies. Cytotoxic chemotherapy drugs interfere with DNA replication, microtubule formation, and other cellular processes to impair mitosis and/or induce apoptosis. While these agents tend to primarily damage cells with high mitotic rates (e.g., cancer, mucosal tissues, and bone marrow), they are fairly indiscriminate. Biologics, in turn, typically employ antibodies to specifically target proteins ubiquitous among a clonal cancer cell population, such as surface antigens and/or components critical to a cancer signaling pathway. Immunotherapies help activate the immune system to attack cancer cells through various mechanisms. Systemic therapies and RT may provide a synergistic effect, allowing for improved tumor control. Systemic therapy can put cancer cells into a radiosensitive state by manipulation of the proliferative pathways. At present, immunotherapies are approved as first-line therapy for recurrent or metastatic HN cancers, and trials are underway combining them with RT.
Cisplatin is the most used systemic therapy in concurrent setting. For concurrent chemoradiation, cisplatin bolus is preferred over weekly cisplatin. Systemic agents most often used for treatment of nonmetastatic HN cancers, along with their main adverse effects, are reported in Table 22.1 .
| AGENT | NOTABLE TOXICITIES |
|---|---|
| Cisplatin | Hearing loss, renal failure, peripheral neuropathy, electrolyte abnormalities, gastrointestinal toxicity |
| Carboplatin | Electrolyte abnormalities, myelosuppression |
| 5-Fluorouracil | Mucositis, hand-foot syndrome, photosensitivity, maculopapular rash |
| Paclitaxel | Peripheral neuropathy, arthralgia, myalgia |
| Docetaxel | Peripheral neuropathy, edema, asthenia |
| Cetuximab | Acneiform rash, dermatitis, hypomagnesemia, neutropenia |
| Pembrolizumab | Arthralgia, myalgia, dermatitis, edema, electrolyte abnormalities, gastrointestinal toxicity, pancytopenia, hepatitis, elevated serum creatinine |
| Nivolumab | Arthralgia, peripheral neuropathy, myalgia, dermatitis, edema, asthenia, electrolyte abnormalities, gastrointestinal toxicity, pancytopenia, hepatitis, fever, elevated serum creatinine |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here