Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Access video content for this chapter online at Elsevier eBooks+ ![]()
The deep inferior epigastric perforator (DIEP) flap was first introduced by Koshima and Soeda in 1989 and popularized for postmastectomy reconstruction by Treece and Allen in 1994. It has become the most recognized perforator flap today, and although it has been described for reconstruction of a wide variety of defects, including those of the head and neck and lower limb, it has assumed its rightful primary role as a gold standard flap for breast reconstruction.
Abdominal tissue is an ideal source of tissue for autologous breast reconstruction. It is commonly present in excess in patients undergoing breast reconstruction. The flap consists of soft, pliable fat and a large skin island that provides tissue with similar texture to the native breast. The flap can be optimally positioned on the chest and shaped to form a new breast mound for either immediate or delayed reconstruction. The resulting breast aesthetics are largely dependent on the volume of available abdominal donor tissue that can be transferred with adequate vascular perfusion and transformed into a 3D construct.
The DIEP flap represents the final stage in the evolution of lower abdominal flaps for breast reconstruction, and maximizes vascular perfusion and aesthetics while simultaneously minimizing functional donor site morbidity. Prior to the DIEP flap, other lower abdominal flap options such as the pedicled VRAM, pedicled transverse rectus abdominis (TRAM), and free TRAM flaps required harvest of the muscle as a passive muscular carrier to ensure adequate flap perfusion. While this produced excellent breast cosmesis, it was at the expense of donor site morbidity, particularly abdominal wall bulges and hernias. The superficial inferior epigastric artery (SIEA) flap became an attractive alternative, as harvest of this flap does not injure the anterior rectus fascia or underlying rectus abdominis muscle, thus potentially eliminating donor-site complications such as bulge or hernia formation. However, the disadvantages are a smaller pedicle diameter, shorter pedicle length, and inadequacy of an arterial pedicle in most patients. Attempts to minimize donor site morbidity, and improve aesthetics with a more reliable flap, led to muscle preservation techniques, and the emergence of the muscle-sparing TRAM flap, and ultimately to full preservation of the rectus abdominis muscle with the introduction of the DIEP flap ( Table 30.1 ).
| MS-0 Full width, partial length |
| MS-1 Preservation of medial or lateral segment |
| MS-2 Preservation of medial and lateral segments |
| MS-3 (DIEP) Preservation of entire muscle |
As a perforator flap, the skin and subcutaneous tissue of the DIEP flap may be supplied by a single perforator vessel that passes from its source vessel, the deep inferior epigastric artery and its accompanying venae comitantes, through the rectus abdominis muscle. The main clinical benefit of a perforator flap is that innervated muscle is left in situ . This means that motor nerves to the rectus abdominis muscle, in addition to the majority of the muscle, remain intact in order to ultimately reduce functional donor site morbidity ( Fig. 30.1 ).

The abdominal donor has the added bonus of usually producing an improved abdominal contour after flap harvest, which approximates that of an abdominoplasty. Donor site aesthetics are related to the creation of an aesthetically-pleasing, low scar on the abdominal wall, adequate closure of the abdominal soft tissues without undue tension, continuity and innervation of the rectus abdominis muscles, and maximal preservation and meticulous closure of the anterior rectus sheath.
Abdominal wall flaps have been used for many years in breast reconstruction. The first use of the musculocutaneous rectus abdominis flap for breast reconstruction was by Robbins in 1979, who used a pedicled vertical rectus abdominis musculocutaneous (VRAM) flap. Holström, in 1979, described the equivalent of a free transverse rectus abdominis musculocutaneous (TRAM) flap with his description of a “free abdominoplasty flap” for breast reconstruction. This was followed by Hartrampf’s description of the pedicled TRAM flap in 1982.
As a greater understanding of the abdominal wall vasculature emerged, it became apparent that the sacrifice of rectus abdominis muscle was not required to adequately perfuse a lower abdominal wall flap. Onishi and Maruyama in 1986 provided the anatomic basis for a perforator-based fasciocutaneous flap of the lower abdominal wall. In addition, attempts were made to reduce the muscle bulk that was removed with the flap to limit donor site morbidity.
In 1989, Koshima and Soeda described using DIEP flaps to reconstruct a contralateral groin defect, and an oral floor defect. Independently, Allen and Treece, in 1994, successfully described the first DIEP flap for breast reconstruction.
Patient selection is key for achieving successful surgical outcomes in autologous breast reconstruction. It is imperative to fully understand the patient’s goals for reconstruction, as not all patients are candidates for autologous breast reconstruction. A donor scar may be unacceptable to some patients, whereas others may have a preference for implant-based reconstruction, or sometimes decline reconstruction.
It is important that patients are in good health, and that medical comorbidities are optimized prior to embarking upon an autologous free flap breast reconstruction. Comorbidities such as poorly-controlled diabetes mellitus or debilitating cardiovascular disease may represent contraindications to autologous reconstruction. Obesity increases the risk of overall complications, and risks become prohibitively high when BMI is greater than 40. Smoking is also associated with higher risks of complications, particularly mastectomy skin flap necrosis, partial flap loss and poor wound healing. Although studies suggest that successful free tissue transfer is possible in smokers, smoking cessation should be encouraged at least 4 weeks prior to surgery to minimize complications.
A prerequisite for using the abdomen as a donor site is the presence of a sufficient quantity of fat in the lower abdomen that will provide adequate volume for breast reconstruction. It is important to note scars on the abdomen that may suggest previous injury to the vascular pedicle of the DIEP flap that may potentially compromise flow within the flap, or increase the risk of abdominal skin necrosis on closure.
A previous abdominoplasty is a contraindication to a DIEP flap reconstruction because the perforators supplying the subcutaneous tissue have been divided, whereas previous abdominal liposuction does not preclude DIEP flap elevation as those connections should theoretically still exist. However, caution should be exercised to ensure proper preoperative and intraoperative perforator evaluation in these patients. Subcostal scars, typically from an open cholecystectomy, might also compromise the vascularity of the remaining abdominal wall, particularly inferomedial to the scar. However, operative strategies can be used to lower the risk of abdominal wall necrosis in these instances such as an eccentric skin paddle design, and minimal abdominoplasty flap elevation. A Pfannenstiel scar, on the other hand, may even augment the calibre of the remaining perforator vessels, which would usually be higher up on the abdomen.
It is important to identify scars which will likely be incorporated into the flap skin paddle as they may result in compromised blood flow to portions of the flap. For example, in the presence of a midline abdominal scar within the flap, skin contralateral to the midline is unlikely to perfuse well after elevation and transfer to the breast. However, such a scar will still allow each hemi-flap to be viable if elevated on its own vascular pedicle.
The abdominal examination should also evaluate the patient for existing hernias or diastasis of the rectus muscles – the reasons are two-fold. First, this may signal pre-existing weakness of the abdominal wall fascia and predict postoperative abdominal morbidity. Second, the location of the hernia may provide information as to location of disrupted perforators, and will predict need for caution in flap elevation and direct intraoperative repair. These findings would be seen on preoperative imaging of the abdominal wall if that is incorporated into DIEP flap planning.
Furthermore, consideration needs to be given to the tumor characteristics. Of particular importance for the reconstructive surgeon is the likelihood of adjuvant radiation as this can impact on the final reconstruction; thus, delayed reconstruction may be recommended in this situation. Coordination with the oncologic plan for type of mastectomy (non-skin-sparing, skin-sparing, or nipple-sparing) should occur to plan access incisions on the breast and requirements for size of skin paddles on the abdomen. Several factors should be considered relating specifically to characteristics of the breast – including the shape, volume, and ptosis of the breast.
The internal mammary artery and its accompanying veins are the preferred vessels for microvascular anastomosis in DIEP breast reconstruction given their calibre, medial positioning, and relative sparing from the effects of radiation ( Fig. 30.2 ). Anastomosis is most commonly performed anterograde to the internal mammary vessels. Alternative options include retrograde anastomosis to the internal mammary vessels, anastomosis to internal mammary perforator vessels, or the thoracodorsal vessels ( Fig. 30.3 ).


The internal mammary vessels are typically 2.5–3 mm in diameter. The artery is usually of sufficient calibre for microvascular anastomosis. The mean distances of the artery from the costochondral junction is approximately 1 cm, 2 cm and 3 cm at the costal levels 2, 3 and 4, respectively. The internal mammary venous anatomy demonstrates variation, with veins typically being larger on the right side (up to 3 mm) than the left (<2 mm). The level of bifurcation of the veins also differs, bifurcating most frequently in the third intercostal space on the left, compared to the fourth intercostal space on the right. For these reasons, dissection of the vessels is commonly performed at the level of the left third or fourth rib, and the fourth rib on the right side.
The deep inferior epigastric artery (DIEA) emerges as a single trunk from the external iliac artery, and courses superomedially posterior to the rectus abdominis muscle, before entering the muscle substance. Within the muscle, the DIEA travels cephalad giving perforator branches which supply the skin and muscle ( Fig. 30.4 ).

The traditional classification of the DIEA vessels is described in anatomical studies by Moon and Taylor. They describe three vessel branching types: Type I occurred in 29% of patients and included a single vessel, Type II occurred in 57% of patients and included a bifurcating vessel, and Type III occurred in 14% of patients and included a trifurcating vessel.
Rozen and colleagues modified this classification based on the results obtained using CTA in 498 abdominal walls. This classification system describes five groups, with Type 0 (<1%) in which the DIEA was absent, Type I (43%) in which there was one DIEA trunk, Type II (48%) in which there were two DIEA trunks, Type III (9%) in which there were three DIEA trunks, and Type 4 (<1%) where four DIEA trunks were present ( Fig. 30.5 ).

The superficial inferior epigastric artery (SIEA) is the second important blood supply provider to the skin of the lower abdomen. It arises as a direct cutaneous vessel, from either the common femoral artery 2–3 cm below the inguinal ligament or from the common trunk of the superficial circumflex iliac artery. It can be absent or hypoplastic in 35%. The SIEA may be sufficient to adequately supply enough abdominal tissue for a breast reconstruction. However, the SIEA is smaller in calibre than the DIEA, and is more prone to spasm, and has an overall higher flap failure rate. The territory of perfusion is typically lower on the abdomen than that of the DIEP flap, and is usually less likely to cross the midline.
Perfusion of the lower abdominal tissue has been classically described by Hartrampf’s perfusion zones, which describe a centrally-perfused skin ellipse with declining perfusion at the peripheral ends. In this model, zone I lies directly over the muscle pedicle, and zone II is across the midline. This model was developed to describe perfusion of unipedicled TRAM flaps, and is not accurate for use in DIEP flaps. Unlike pedicled TRAM flaps, DIEP flaps depend on the inferior epigastric pedicle and are nourished by a lower number of perforators.
For DIEP flaps, several anatomical and clinical studies have demonstrated a reliable, axial pattern of perfusion of the lower abdominal skin and fat on the hemiabdomen ipsilateral to the pedicle. There is significant variability in the branching pattern of the venous plexus across the midline. Ultimately, a less reliable, random pattern blood supply across the midline is present in DIEP flaps.
The Holm-Ninkovic Classification is based on in vivo indocyanine green perfusion studies, and suggests that Hartrampf zones II and III should be reversed for DIEP flaps ( Fig. 30.6 ). In this classification, zone I constitutes the area directly over the ipsilateral rectus abdominis muscle from which the pedicle will arise, and zone II lies lateral to the ipsilateral rectus abdominis muscle. This suggests that the blood flow from the pedicle travels to the ipsilateral side before crossing the midline. The contralateral hemiabdomen is composed of zone III (the area adjacent to zone I which lies across the midline, overlying the contralateral rectus abdominis muscle) and zone IV is the remaining portion lateral to the contralateral rectus abdominis muscle which is furthest from the blood supply of the flap.

Early reports of higher rates of fat necrosis and partial flap loss in DIEP flaps compared with TRAM flaps was likely due to an incomplete understanding of the vascular perfusion of the DIEP flap. The perforasome theory explained how perfusion of the flap differed based on which perforator was chosen for the flap. The perforasome is the distinct vascular territory that is perfused by a perforator ( Fig. 30.7 ). The perforasome for a lateral row perforator tends to be more lateralized, and more closely follows the Holm-Ninkovic pattern of zonal perfusion – with supply to the ipsilateral portion of the flap first, before crossing the midline. By contrast, a single medial row perforator perfuses a portion of the flap which is more centralized and follows the Hartrampf’s classification system more closely. Based on the perforasome concept, a single medial row perforator perfuses a larger vascular territory and makes it ideal for large volume reconstruction, compared to a lateral row perforator, which may be better suited to a smaller volume reconstruction, particularly in the bilateral setting.

Perforator mapping can provide useful information on the location and course of perforators and can reduce operating times. While not required for routine DIEP flap harvesting, it can be useful for delineating anatomy if modifications are planned, or there is concern that the perforators may have been damaged during a previous operation. Similarly, if a robotic harvest is planned, perforator mapping is necessary. Commonly used techniques include ultrasonography, angiography, and thermal imaging.
Ultrasonography is a well-established method for preoperative perforator mapping. It is commonly used because it is a relatively cheap, non-invasive modality, which poses no risks to the patient. A unidirectional handheld doppler probe is most commonly used, particularly to confirm the site of perforators on the surface of the skin, or intraoperatively to assess flow characteristics within vessels. However, it is unable to differentiate axial or intramuscular vessels from perforating vessels, which can result in false positive localization of perforators, and low-specificity for this technique.
Colour Doppler ultrasonography (CDU), on the other hand, provides real-time imaging that allows for delineation of anatomic detail, and colour Doppler, which provides haemodynamic information such as the direction of blood flow, differentiation between venous and arterial flow, and flow velocity measurement. It is an excellent modality to map perforators, give anatomical information about the course of the vessels, and it is very safe. However, its use has fallen out of favour as it is time-consuming, operator-dependent, and the image quality is inferior to that of angiography.
Computed tomography angiography (CTA) ( Fig. 33.8 ) and magnetic resonance angiography (MRA) ( Fig. 33.9 ) are considered the gold-standard in perforator imaging modalities. They allow for precise anatomic localization of the perforators ( Fig. 33.10 ) and assessment of their course through the muscle.

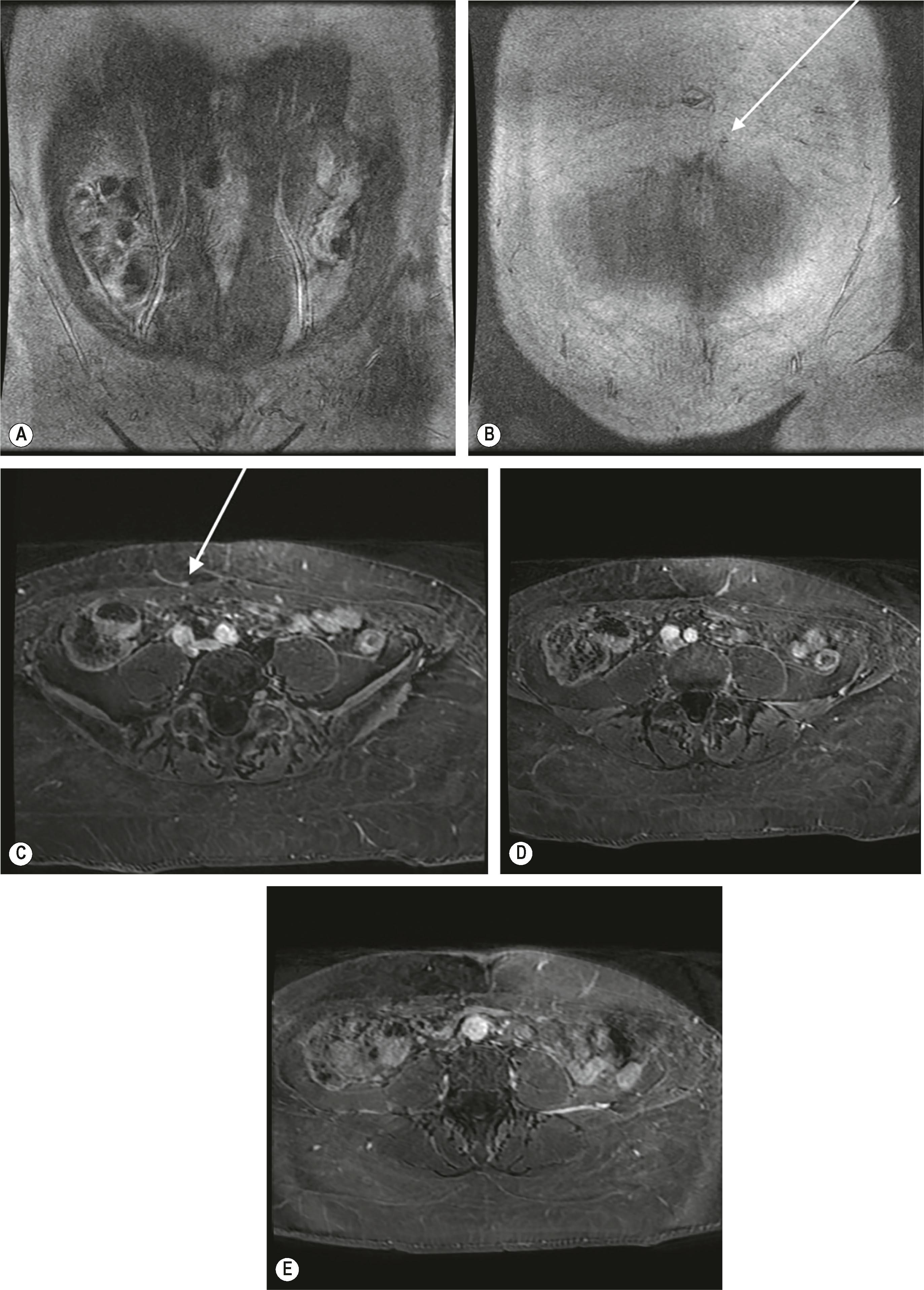

CTA with intravenous iodinated contrast enables the precise demonstration of the size, location, and course of lower abdominal perforators with a high degree of sensitivity and specificity. Scanning time is approximately 10 min, and is often well-tolerated. However, it delivers an ionizing radiation dose of 6–10 mSv, which poses a small but statistically significant risk including the possibility of developing de novo cancer.
Magnetic resonance imaging (MRI), on the other hand, does not rely on ionizing radiation to provide images with similar detail to CTA. The main drawbacks are a longer scanning time for image acquisition and a risk of anaphylaxis with the gadolinium contrast agent. It may also be contraindicated in patients with incompatible metallic implants, and is poorly tolerated by those with claustrophobia.
Fluorescent angiography allows for direct visualization of cutaneous perfusion following intravenous injection of indocyanine green (ICG). It requires a specialized image-capturing device to generate real-time images. Intraoperatively it can allow for dynamic assessment of physiologic perfusion of a selected perforator. This is a useful adjunct in identifying hypoperfused areas within the flap, thereby potentially reducing the risk of fat necrosis within the flap. It also plays a valuable role in the assessment of mastectomy skin flap viability to reduce the risk of skin necrosis. This imaging strategy is reviewed in detail in another chapter in this volume.
Thermal imaging, or dynamic infrared thermography (DIRT), is a straightforward technique that identifies hot spots on the skin that correlate to the location of the perforator vessels, which are usually validated with Doppler for accuracy. It involves the administration of a cold challenge, which causes a relative hypoperfusion of the cutaneous surface, and once the cold challenge is terminated the skin rewarms. As the tissue rewarms, an infrared camera identifies the hot spots.
The highest concentration of perforators from the deep inferior epigastric system is found in an area between 2 cm cranially and 6 cm caudally of the umbilicus, and between 2 cm and 6 cm lateral to the umbilicus. However, to keep the donor site scar as low as possible, usually it only the perforators caudal to the umbilicus that are incorporated in the flap design. Ultimately, surgical judgement is necessary in perforator selection and there is a steep learning curve to intramuscular vessel interpretation. Significant perforators should not be sacrificed until the anticipated perforator(s) have been exposed and evaluated in terms of calibre and physiologic zone of perfusion. This assessment can be done either clinically following temporary occlusion of non-candidate perforators, or with intraoperative ICG angiography.
For a unilateral DIEP flap reconstruction, our preference is to incorporate the central portion of the abdomen in the flap for the best breast contour, and reliable perfusion of zones I to III can be obtained from a single adequately-sized medial row perforator. Using a medial row perforator can also help to avoid denervation of the rectus abdominis muscle. Ideally the selected perforator should have a vein greater than 1 mm in diameter where it enters the flap, and a palpable or visibly pulsating artery. The flap should be centered on the dominant perforator to optimise flap perfusion and minimize fat necrosis; essentially this is the perforasome.
For a bilateral reconstruction that uses a hemi-abdominal flap distribution, use of lateral row perforators that arborize both medially and laterally may result in a lower incidence of fat necrosis.
Occasionally a paucity of donor abdominal tissue is available for transfer, particularly in women with large ptotic breasts, those with midline scars, or thin patients with scant abdominal tissue. To overcome this issue, certain strategies may be used at the time of the reconstruction to maximize the volume of flap tissue available for transfer.
If a single perforator will not adequately perfuse the flap, more perforators can be included with the flap during harvest. One technique is to include multiple perforators along the same anatomic row. Occasionally, it is necessary to take perforators in close proximity to each other, with minimal transection of muscle fibers, or through division and reanastomosis of a secondary perforator back to the main perforator trunk to avoid muscle transection, as has been described in the “abdominal perforator exchange” (APEX) breast reconstruction technique.
Augmentation of the vascular supply through turbocharging or supercharging may allow for perfusion of the entire lower abdomen when a larger flap is required. Turbocharging involves an anastomosis of one vascular pedicle to another vascular pedicle, within the flap. For example, one can anastomose the superficial epigastric system from one hemi-abdomen to the deep inferior epigastric system of the other side and transfer the entire flap on its deep inferior epigastric pedicle.
Alternatively, if bilateral deep inferior epigastric systems are dissected, they can be anastomosed to two separate donor vessels – as is the case with bilateral bipedicled flaps. These supercharged constructs can be anastomosed both anterograde and retrograde to the internal mammary systems, or alternatively one hemi-abdomen to the thoracodorsal system and the other to the internal mammary system. This technique provides an adequate skin envelope as well as additional volume and projection. The complication rate of this approach is comparable to single-pedicled free flap reconstruction, although the operative times are longer.
Utilization of two separate unipedicled hemi-abdominal flaps allows for the creation of stacked flap. Unlike bipedicled flaps, there is greater freedom to inset the flaps independently allowing greater control in determining fill and projection within the breast pocket. Other free flaps may be stacked with a DIEP flap for additional volume.
Another way to increase the volume of tissue that can be safely harvested on a chosen perforator is to perform a delay procedure on the flap. The delay phenomenon involves a staged surgical procedure, which attempts to increase flap vascularity through ischaemic preconditioning. During the first stage, the flap is partially elevated, maintaining some cutaneous connections (such as laterally), on the planned chosen perforator and all other perforators are divided. During the next 2–3 weeks, relative tissue ischaemia is present, and dilation of the selected perforator occurs and adjacent perforasomes are recruited. This technique can play an important role in DIEP flap reconstruction by providing flexibility to choose a “non-ideal perforator” to improve aesthetics, and also to improve perfusion and reduce the risk of fat necrosis in the flap.
Augmentation of the DIEP flap volume has also been described using a breast implant both at the time of the reconstruction, and also during revision procedure. However, early complications include infection, hematoma, and partial flap necrosis.
Local tissue rearrangement has been described to increase projection beneath the flap in the central lower pole in a delayed reconstruction. Termed the “hug flap”, it involves de-epithelialization of the caudal mastectomy skin flap, followed by medial and lateral wings which are undermined, allowing for the wings to be mobilized and folded over the central portion to increase volume in this area.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here