Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although anesthesia-related complications have decreased in the last two decades, perioperative mortality has not. The leading precursor to death after surgery is acute organ injury progressing to single-organ or multiorgan failure. The failure of two or more vital organs has been termed “multiorgan dysfunction syndrome” (MODS). The common trigger for development of organ dysfunction or acute organ injury is the systemic inflammatory response. The pathophysiology of MODS involves an indiscriminate activation of the inflammation response by hostile stimuli, including sepsis, hypovolemia, trauma, or other circumstances involving significant tissue damage. Several organ systems are more at risk for perioperative organ injury. The acute stress response related to surgery has been shown to create a hypercoagulable state and to impair neuroprotective mechanisms, forming a potential for cerebral ischemia or stroke. Postoperative pulmonary and renal complications are similarly seen as an exacerbation of inflammatory pathways unless related to direct surgical trauma.
Recent advances in the understanding of the pathogenesis of organ dysfunction in sepsis indicate that coagulation abnormalities contribute strongly to its evolution. With a less organ-based but more histological approach, the systemic endothelial injury appears to be central for the development of organ failure. Shock-induced sympathoadrenal activation with excessive catecholamine levels induces systemic injury to endothelium and microcirculation of all organs. The natural anticoagulation protection of the endothelium is provided primarily by the heparinoid-rich glycocalyx, the tissue factor pathway inhibitor (TFPI), and the protein C/thrombomodulin system. In the case of tissue ischemia or inflammatory stress, the degradation/shedding of glycocalyx combined with coagulation activation and profibrinolytic factors from the injured endothelium may result in profound coagulation imbalance. Major surgery has been shown to induce a patient-specific inflammatory acute stress response contributing to the imbalance of the coagulation system, and the inflammatory environment may contribute to hypo- and hypercoagulability.
The coagulation factor activation, and indeed the entire coagulation cascade, extend beyond the clotting function. Relevant to perioperative stress, the coagulation proteins are connected to numerous proinflammatory signaling pathways. The overall state of coagulation in the circulation depends on the balance between pro- and anticoagulant mechanisms. When the balance is disturbed in favor of a procoagulant state, the proinflammatory signals of the coagulation proteins may be more dominant. Alternatively, disruption of coagulation ability may lead to bleeding, which has major clinical importance in the operative setting. Impaired coagulation ability and the consequences of bleeding in the operative setting are well recognized.
Several mechanisms contribute to the organ injury related to the activation of coagulation system, including extravascular fibrin deposition, small-vessel obstruction by thrombosis, and amplification of cellular inflammatory pathways. The procoagulant state has been shown to be especially important in the pathogenesis of sepsis-associated MODS. Similar mechanisms are probably pertinent to organ dysfunction in the perioperative period, when multiple proteins intermingle to alter the balance between procoagulant and anticoagulant. Attributing organ dysfunction solely to a specific type of surgical procedure is difficult, because the true incidence of perioperative organ dysfunction is unknown, and perioperative organ dysfunction severity appears to vary between surgical sites and procedures. Perioperative complications include postoperative sepsis and shock, which may produce coagulation abnormalities and organ dysfunction that are difficult to disassociate from the surgical event. The following paragraphs cover the basic coagulation and fibrinolytic functions and discuss alterations of these functions in the perioperative period.
The traditional understanding of the coagulation cascade is that the coagulation is initiated by a sequential activation of several serine proteases, leading to a polymerization of the fibrin clot. The extrinsic pathway of the coagulation cascade is initiated by cell surface expression of tissue factor (TF), which binds to activated factor VII (FVIIa) to form a complex. The intrinsic pathway is initiated by contact activation. The TF–FVIIa complex converts factor X to factor Xa, which converts prothrombin to thrombin. Thrombin cleaves fibrinogen to fibrin. The cross-linking of fibrin generates the mechanical durable fibrin clot.
Focusing on a more in vivo understanding of coagulation, a cell-based model of the coagulation cascade was introduced by Hoffman and colleagues in 2003. The cell-based coagulation model operates on the notion that the “intrinsic” and “extrinsic” pathways are operating in parallel on different cell surfaces. The model describes four overlapping stages of coagulation: initiation, amplification, propagation, and stabilization. In the initiation phase, TF is expressed, and the extravascular TF binds to FVII, leading to FVII activation and forming the activated TF–FVIIa complex, which leads to the thrombin generation as described before. In the amplification phase, the small amount of thrombin produced by the TF–FVIIa complex allows platelet adhesion and activation. Thrombin is a very potent in vivo platelet activator. The activated platelets bind von Willebrand factor, cleave FVIII and activate it to FVIIIa. The propagation phase begins when FVa and FVIIIa are bound on the platelet surface. Large numbers of platelets are engaged to the site of injury, and surface FXa binds to FVa to produce a burst of thrombin generation for clot formation. During the stabilization phase, the formation of fibrin monomer and sufficient thrombin amount activate FXIII, which cross-links the fibrin into a stable fibrin clot ( Fig. 3.1 ).
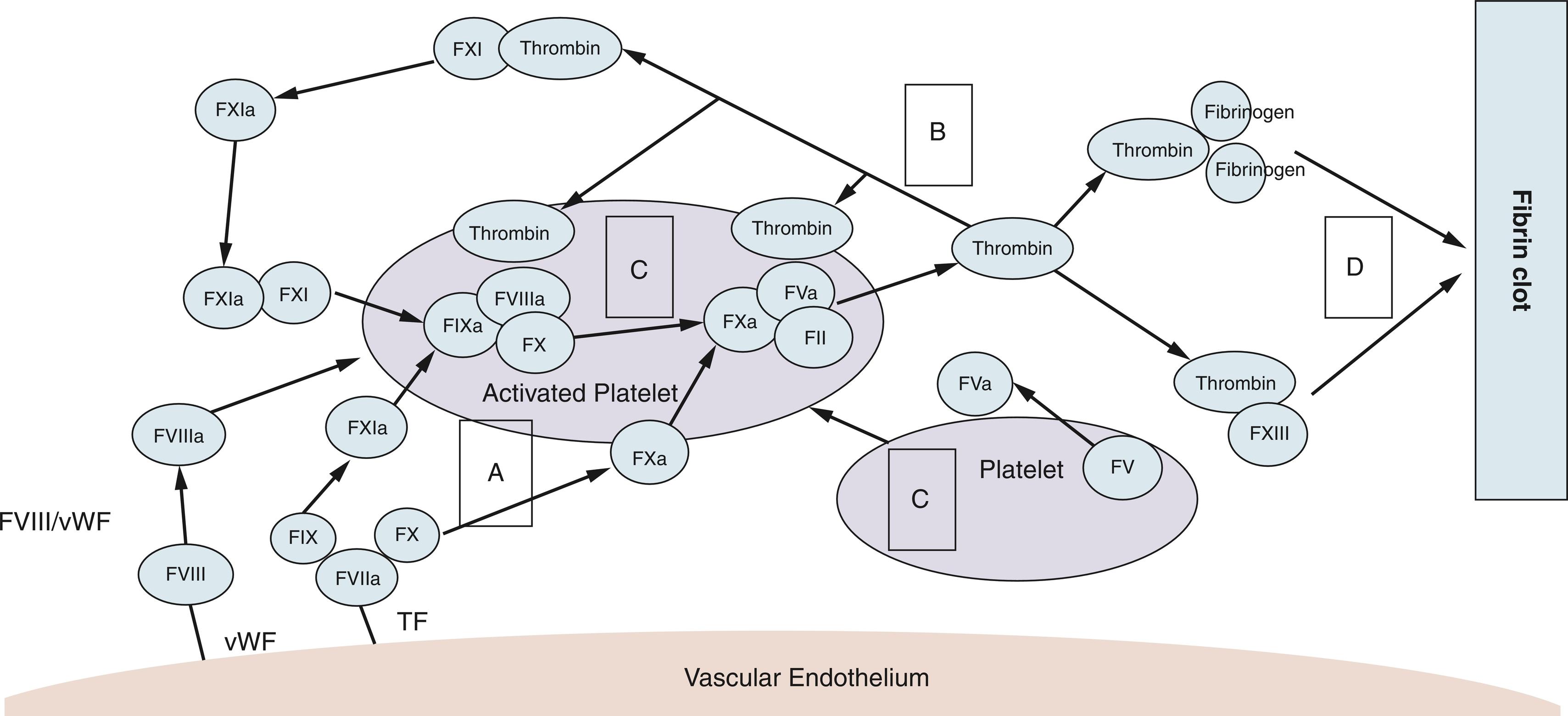
The endothelium plays a pivotal role in the activation of the extrinsic coagulation pathway because injury to the endothelial glycocalyx exposes TF. Under normal conditions, natural anticoagulant systems protect the endothelium to limit fibrin generation and deposition to the specific injury site. The negatively charged glycocalyx is rich in heparinoids and interacts with antithrombin. Other natural anticoagulants include TFPI, proteins C and S, and antithrombin III (ATIII).
Fibrinolytic proteins function to regulate fibrin accumulation. Tissue and urokinase plasminogen activators initiate the plasminogen conversion to plasmin, which directly degrades fibrin. The fibrinolytic system is controlled by plasminogen activator inhibitors (PAIs). Increased PAI expression has been reported in several pathological situations, impairing fibrinolysis and therefore creating a hypercoagulable environment. To control the vascular injury in a normal host, surgical trauma generally promotes a more procoagulant state.
In addition to endothelial TF exposure secondary to surgical trauma, injured cells release endogenous molecules to communicate the cell injury to other pathways. TF activates immunologically active cells (monocytes and neutrophils). The TF activation leads to excess fibrin deposition. Natural anticoagulation mechanisms (including TFPI, antithrombin, protein C) are reduced or dysfunctional secondary to inflammation or tissue hypoperfusion. Protease-jactivated receptors (PARs) establish the link between coagulation and inflammation on the molecular level. Of the four PAR subtypes identified, PAR1 is particularly relevant in inflammatory pathophysiology. The cytoprotective effect of PAR1 is stimulated by activated protein C (APC) or a low amount of thrombin. When activated by high-dose thrombin, PAR1 has a more disruptive effect on the endothelial barrier.
The observation that a patient in septic shock had low APC levels and that microvascular thrombosis was partially responsible for impaired tissue oxygenation became the rationale for exploring APC in septic shock to improve patient outcome. Clinical trials failed to demonstrate a treatment benefit of APC or protein C zymogen but was associated with increased bleeding.
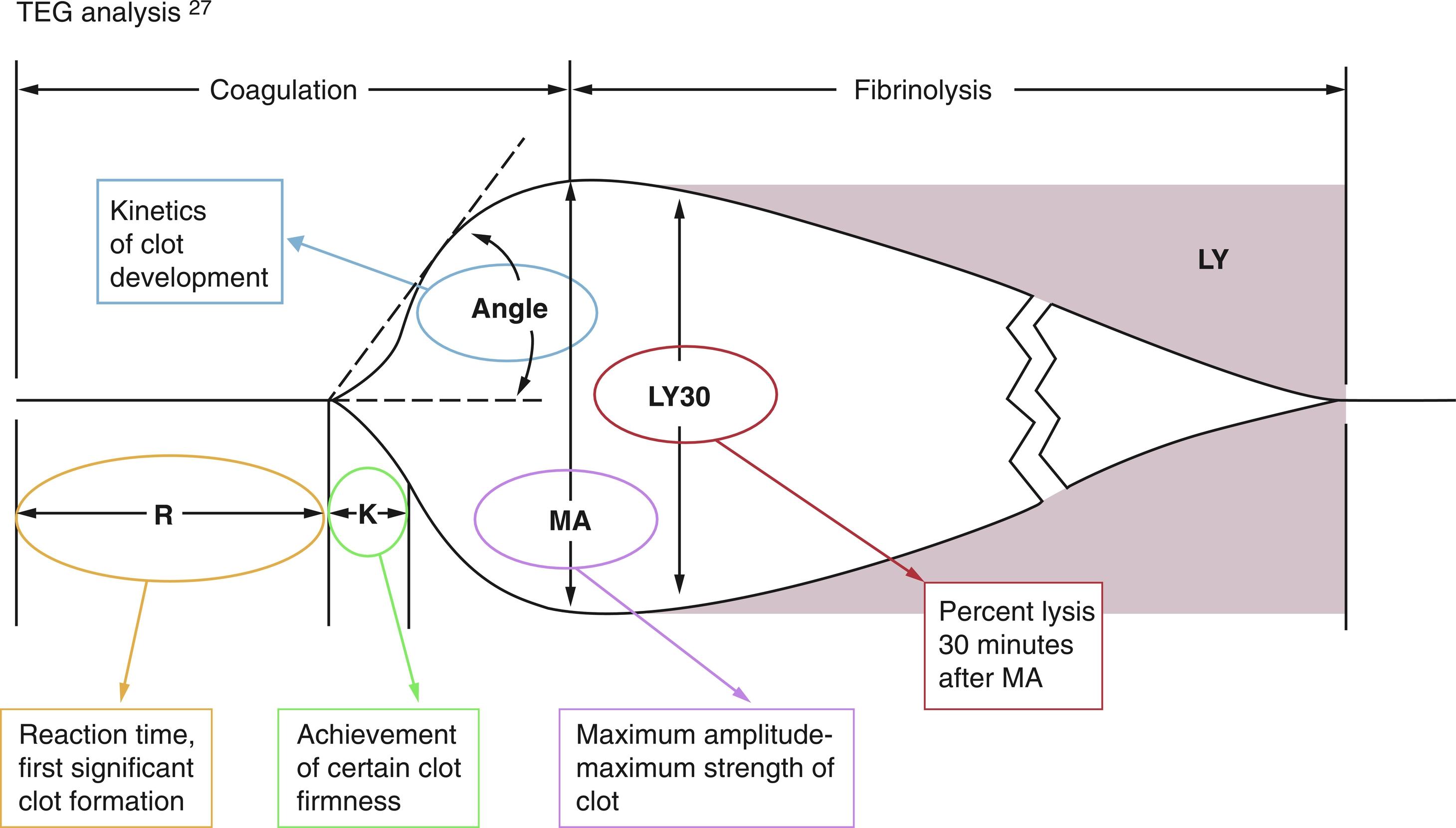
Due to the complexity of the coagulation abnormalities in the perioperative period, conventional coagulation assessment with prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen levels may not be sufficient or timely enough. The long turnaround times may render laboratory test results irrelevant before the information is available. In the operating room or in the intensive care unit, activated clotting time is a perioperatively available point-of-care test. The most concerning limitation of coagulation monitoring is its artificial nature, assessing isolated components of the coagulation system under artificial laboratory conditions. Recent publications are favoring perioperative coagulation monitoring using viscoelastic point-of-care testing such as thromboelastography (TEG) or rotational thromboelastometry (ROTEM). Although the technology is still FF and artificial, it more closely reflects the in vivo coagulation status. There are different variants of this technique currently available: TEG (Haemonetics Corp., Braintree, MA), ROTEM (Tem International GmbH, Munich, Germany), and Sonoclot (Sienco, Inc, Arvada, CO). The information obtained using this technology has been shown to be helpful for clinical decision-making during high-blood loss surgical procedures (trauma, cardiac surgery, and liver surgery) and for coagulation assessment during pregnancy.
Information obtained from TEG and ROTEM indicates (1) time to initiation of clot formation (R), (2) speed or rate of clot propagation (a), and (3) clot strength (amplitude A, or shear elastic modulus G) ( Fig. 3.2 ). The test takes into account both platelet function and fibrin–platelet interaction. Modifications of the method use inhibitors of platelet–fibrin interaction and inhibitors of platelet aggregation, and they can distinguish coagulation factors from platelet contributions to the clot formation. Viscoelasticity-based coagulation assessment and treatment have been shown to be helpful in multiple clinical situations with complex coagulopathies (trauma, liver transplantation, sepsis, and cardiopulmonary bypass). A test to assess not only platelet quantity but also quality is the platelet function analyzer (PFA-100; Dade International Inc., Miami, FL), which measures platelet aggregation or plug time. D-dimer assays are widely available to detect fibrin degradation products, and the presence of these products indicates activation of fibrinolysis, but the D-dimer is not necessarily a marker of increased fibrin formation.
Surgical procedures and surgical stress commonly unbalance this elaborate arrangement, therefore causing hypo- or hypercoagulability. In addition to direct surgical injury to vascular structures and blood loss, fluid therapy-induced dilution of coagulation components, hypothermia, or tissue acidosis may promote hypocoagulability and bleeding. Surgical stress and tissue trauma release of TF may activate the coagulation process. The stress response also results in a downregulation of the anticoagulant response. The result of decreased anticoagulant activity, in combination with immobility and other patient factors (malignancy, drugs, and comorbidities), may be hypercoagulability.
During the perioperative period, the function of the coagulation system is altered by activation of the procoagulant mechanisms and depression of the anticoagulant and fibrinolytic mechanisms. The primary outcomes and events measured for perioperative activation of coagulation are vascular thrombotic complications, including stroke, myocardial infarction, and venous thromboembolism. Specific pathways and mechanisms have been implicated in surgery-associated hypercoagulability. Extrinsic coagulation is activated by vascular injuries that expose or upregulate TF on the endothelial surfaces and by platelet thrombi at sites of injury that also generate fibrin clot formation. Other surgical responses include increased levels of coagulation factors, decreased levels and activity of endogenous anticoagulants such as APC and ATIII, and inhibition of fibrinolytic function.
Several variables related to the surgical procedures and concurrent interventions affect the coagulation function in the perioperative period. Although several specific variables have been studied with respect to activation of the coagulation response, identifying the role of individual factors in the complexity of the whole surgical setting has been difficult. Variables that have been implicated include the pain or stress response, the mode of anesthesia, the type and quantity of intravenous fluid administration, body temperature, and the site of surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here