Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) guidelines defined supraventricular tachycardia (SVT) as:
An umbrella term used to describe tachycardias (atrial and/or ventricular rates in excess of 100 bpm at rest), the mechanism of which involves tissue from the His bundle or above. These SVTs include inappropriate sinus tachycardia, atrial tachycardia (including focal and multifocal AT), macroreentrant AT (including typical atrial flutter), junctional tachycardia, atrioventricular nodal reentrant tachycardia (AVNRT), and various forms of accessory pathway-mediated reentrant tachycardias (AVRT).
They further define paroxysmal SVT (PSVT) as: “A clinical syndrome characterized by the presence of a regular and rapid tachycardia of abrupt onset and termination. These features are characteristic of AVNRT or AVRT, and, less frequently, AT. PSVT represents a subset of SVT.”
When a patient complains of palpitations, this can reflect a broad range of differing symptoms that will frequently point to the correct diagnosis. For example, some patients may simply develop a subjective awareness of their heartbeat, which is often described as a slow forceful beating. Frequently these symptoms will be most obvious at night and will often be reported while lying on the left-hand side presumably as the cardiac apex is felt more clearly against the chest wall.
Symptoms of ectopic beats are most usually reported as a skipped beat associated with a strange sensation in the throat or an impulse to cough. This may be repetitive, and patients can often describe the frequency of these (every third beat, etc.). The symptoms may occasionally be associated with transient light-headedness and shortness of breath, although these are usually mild and momentary. If repetitive, the symptoms may be described as lasting for hours and it is important to ascertain whether this is a continuous rapid arrhythmia lasting for hours or alternately intermittent symptoms coming and going over hours. Many patients with ectopic beats, including high-burden ectopy, are completely asymptomatic, and the reason for this symptom variance remains unclear.
Symptomatic sinus tachycardia produces regular palpitations with heart rate generally in the range of 120 to 130 bpm, although it can be much faster depending on the underlying cause. Onset of symptoms in inappropriate sinus tachycardia (IAST) may be gradual or sudden as patients may become aware of sinus tachycardia only when a particular heart rate is reached. Termination is gradual usually over many minutes, and episodes can last for hours. Patients may feel breathless on minor exertion, mildly light-headed, and fatigued. Symptoms can overlap with those of SVT.
SVT is usually described as sudden in onset with rapid racing (often too fast to count) with a sensation that the heart is trying to beat out of the chest. The episodes may be triggered by sudden movements such as sudden running for the bus or by bending and standing up. Episodes may last continuously from minutes to hours and terminate suddenly. They may respond to vagal maneuvers. SVT may be associated with transient light-headedness at onset, which is occasionally severe and resulting in presyncope, although syncope is unusual. Accompanying symptoms include anxiety as a secondary phenomenon, although some patients (particularly women) have been given an erroneous diagnosis of panic attacks or primary anxiety disorder. Patients may also become breathless and develop chest discomfort during prolonged episodes. Polyuria may be reported during and early after SVT episodes due to release of atrial natriuretic peptide at these elevated rates. Symptoms are generally more severe in older age-groups, although rate is also an important determinant of symptom severity. Recurrent short bursts (seconds to minutes) of rapid palpitations with normal rhythm interspersed suggests an automatic focal AT. Prolonged irregularly irregular racing points to atrial fibrillation (AF). Sudden syncope is rarely associated with SVT and when arrhythmic in origin generally suggests ventricular tachycardia (VT) or a significant pause as a result of sinus arrest or atrioventricular (AV) block.
The physical examination may be helpful in demonstrating findings associated with valvular pathology, cardiac failure, or thyrotoxicosis or when an incessant or persistent arrhythmia is present. Investigations include a 12-lead electrocardiogram (ECG) in sinus rhythm. This will determine whether preexcitation is present and identify P wave abnormalities and bundle branch block patterns.
Routine blood tests include biochemistry and thyroid function tests. When patients present to an emergency department with tachyarrhythmias, troponin will often be elevated. In this setting, this is a nonspecific response frequently secondary to the tachycardia and not necessarily indicative of obstructive coronary artery disease. An echocardiogram is usually performed to evaluate ventricular function and atrial size and to rule out significant valvular pathology.
Most important is documentation of the tachycardia. This may involve finding ambulance traces or emergency department ECGs. For patients without documented arrhythmias, a range of monitoring strategies are available and may be chosen according to symptom frequency and patient preference. When symptoms occur daily, simple 24-hour Holter monitoring will obtain the diagnosis. Documentation of onset and termination of the arrhythmia may add important diagnostic information. For example, an IAST may have gradual increase in rate over 30 seconds to several minutes, whereas a focal AT usually has sudden onset with a “warm-up” over several beats. For less frequent episodic events, a range of wearable technologies or devices used with a smart phone are now available and this has greatly facilitated documentation of the arrhythmia (see Chapter 61 ). Alternative approaches include more prolonged monitoring of up to 30 days with a variety of different devices of variable patient tolerability. An implanted loop recorder may be considered in the unusual event that documentation cannot be obtained with simpler monitoring approaches. Finally, in patients with classic symptoms of sudden onset and offset tachycardia highly suggestive of SVT, documentation is not essential and an initial approach of a diagnostic electrophysiologic study (EPS) with a view to catheter ablation may be considered.
Atrial premature complexes are very common in the general population. In an unselected population over the age of 50, the average frequency was approximately 1 or 2 per hour and increased with each decade of life. Increase in atrial ectopy occurred not only in relation to advancing age but also in association with other cardiovascular disease. Regular physical exercise was protective. Transient increase in atrial ectopics may occur in response to intercurrent illness, in stress and anxiety, and in response to alcohol and caffeine.
Usually ectopic beats are asymptomatic but may be associated with a range of sensations, including a heightened awareness of the heart beating, the sensation that the heart has given an extra beat or missed a beat, a fluttering sensation in the chest or throat, and occasionally a momentary feeling of faintness or dizziness.
A diagnosis is generally made with ECG monitoring with documentation that symptoms occur corresponding with atrial ectopic events.
Although in the vast majority of patients atrial premature complexes are benign, the seminal paper by Haissaguerre et al. described the triggering of AF by focal atrial ectopics originating from sleeves of myocardium within the pulmonary veins. Subsequent studies showed that patients with both paroxysmal and persistent forms of AF demonstrate an increased atrial ectopic burden when compared with a control population. Furthermore, increased premature atrial complex (PAC) burden is associated with incident AF risk both in the general population and in patients with cryptogenic stroke. Longitudinal studies have described an association between excess PACs (>30/hour or runs of nonsustained AT >20 beats) and the outcomes of incident AF, stroke, and death. In 15-year follow-up, patients with excess PACS and a CHADs-VASc score of 2 or greater demonstrated an annual stroke risk comparable to that of patients with AF. A number of opinions have suggested that atrial ectopy and AF may be markers of an underlying atrial myopathy that is the primary determinant of stroke risk and adverse outcomes. , Isolated case reports have indicated that frequent PACs (20% to 40% daily burden) also may be associated with development of a reversible cardiomyopathy.
Despite the association of frequent atrial ectopy with potential for adverse events, to date there is no evidence that treatment of isolated atrial ectopy reduces risk or improves long-term outcomes. Therefore, the only indication for treatment of PACs is when they are sufficiently symptomatic. The vast majority of patients with atrial ectopics will not require any treatment other than reassurance. In those with severe symptoms, treatment would initially involve a beta blocker or calcium channel antagonist. Occasionally it might be appropriate to prescribe an antiarrhythmic medication such as flecainide. In highly symptomatic patients unresponsive to or intolerant of medication, catheter ablation may be considered when the ectopic burden is high and the atrial ectopic is unifocal in origin.
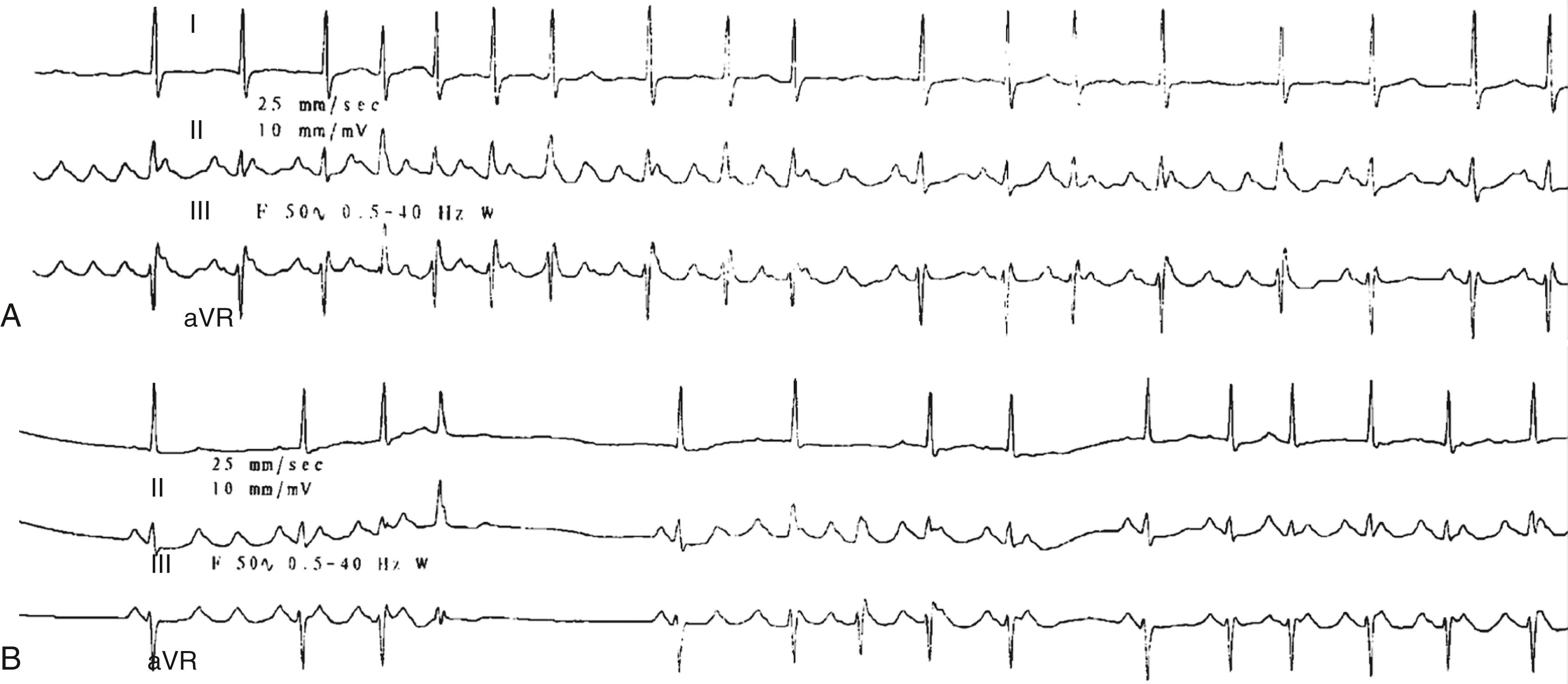
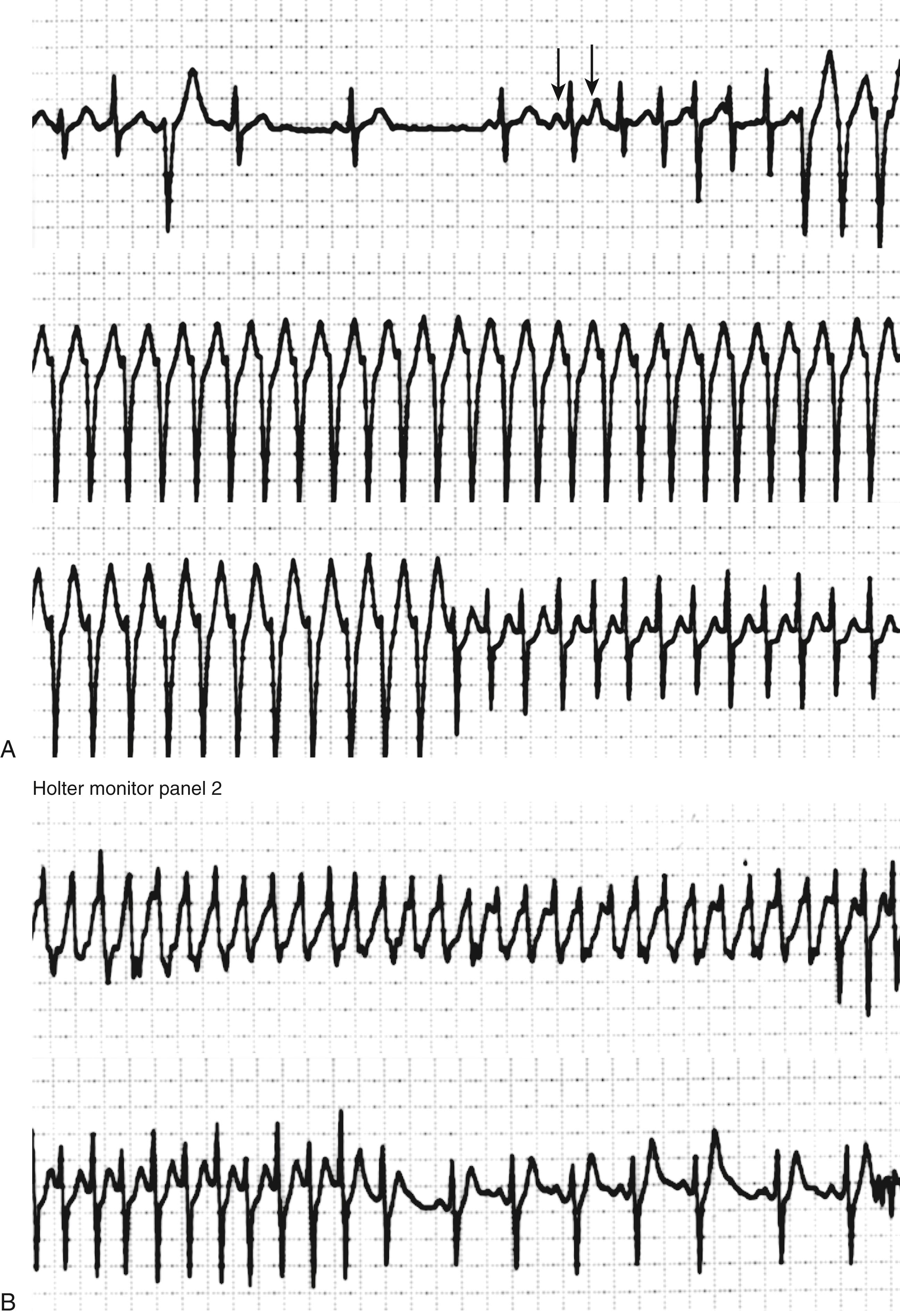
The appearance of an atrial ectopic on an ECG is characterized by an early atrial beat with a P wave morphology different from that of the sinus beat. However, the P wave is frequently inscribed within the preceding T wave and the morphology therefore unclear ( Fig. 65.1 ). An atrial ectopic may be conducted normally, with prolongation of the PR interval and possibly aberrancy or widening of the QRS but also may be nonconducted. A nonconducted or blocked atrial ectopic is one of the most common causes of an unexpected pause on an ECG ( Fig. 65.1A, B ). Nonconducted ectopics in a bigeminal pattern may create the appearance of marked sinus bradycardia (see Fig. 65.1B ). Frequent atrial ectopics accompanied by recurrent bursts of nonsustained AT indicates a very active focal trigger or triggers and is a pattern commonly seen in patients who also demonstrate paroxysms of AF ( Fig. 65.1C ). This is usually because of activity originating in the pulmonary vein sleeves.
The term atrial tachycardia encompasses a range of different tachycardias that originate in the atria and do not require the participation of the AV node for maintenance of the arrhythmia. These tachycardias have differing arrhythmia mechanisms and are often related to anatomic structures. , Broadly AT can be considered to be in one of two major categories: focal or macroreentry. Focal AT has been defined as atrial activation starting rhythmically at a small area (focus) from which it spreads out centrifugally. Impulses occur with a given periodicity separated by a quiescent interval recorded on the surface ECG as an isoelectric period. The main tenet of this definition is that, in contrast to activation seen in macroreentrant AT (also termed atrial flutter [AFL]), atrial activity originates from a focal location. In macroreentry, activation occurs around a large central obstacle, such as an anatomic structure or region of scarring; electrical activity can be recorded throughout the entire atrial cycle length. These include typical AFL and other well-characterized macroreentrant circuits in the right and left atrium, which are also frequently referred to as types of “atypical AFL.” More recently a third category of AT has been described although not routinely included in all classifications. These have been termed “small circuit” or “localized” reentry (see later).
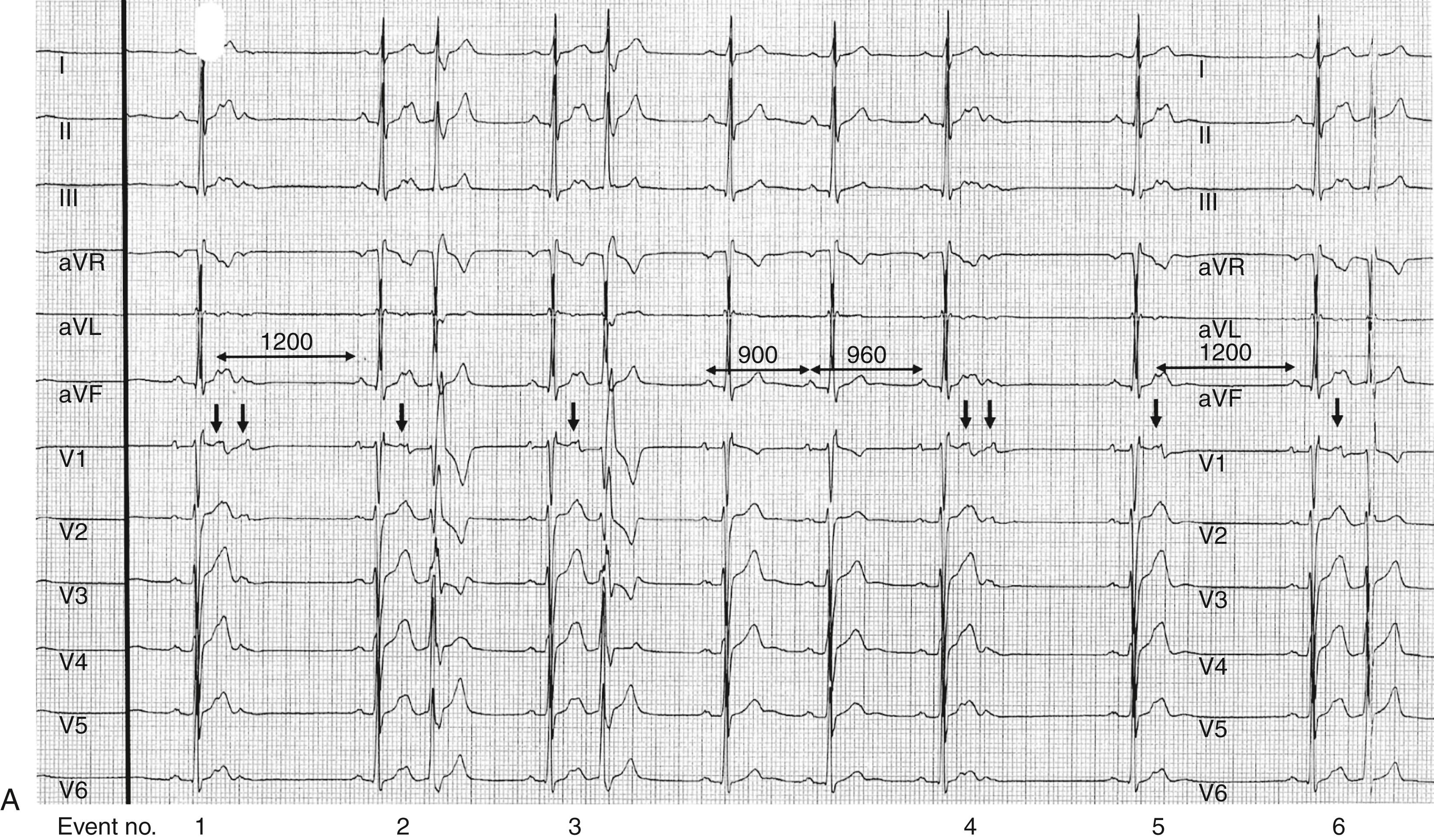
Focal AT is a form of SVT characterized by regular, organized atrial activity with discrete P waves and typically an isoelectric segment between P waves ( Fig. 65.2 , left ). However, when focal AT rate is particularly rapid, an isoelectric interval may not be apparent ( Fig. 65.2 , right ). Focal AT may display some irregularity particularly at onset (“warm-up”) and termination (“cool-down”), usually occurring over several beats. Atrial mapping reveals a focal point of origin. Mechanisms of focal AT include abnormal or enhanced automaticity (abnormal impulse initiation in an individual or cluster of myocytes), triggered activity (abnormal impulse initiation due to oscillations of membrane potential, termed early or delayed after-depolarizations) and reentry (when myocardial regions activated later in propagation reexcite regions that have already recovered excitability). In practice, there is considerable overlap in electrophysiologic properties, and it is not always possible to be certain of the underlying mechanism during a clinical EPS. For example, tachycardias due either to reentry or to triggered activity may be initiated and terminated with programmed electrical stimulation during an EPS. Tachycardias due to enhanced automaticity and triggered activity may initiate in response to isoprenaline infusion or adrenergic stimulation and may demonstrate multiple spontaneous onsets and terminations ( Fig. 65.3 ). The arrhythmia may also demonstrate cycle length variability (see Fig. 65.3 ). Finally, focal AT due to either microreentry or triggered activity may be terminated with adenosine.
Focal AT is the least common mechanism of PSVT, accounting for approximately 10% to 20% of patients with PSVT. Focal AT has a slight preponderance in women, although this has not been a consistent finding in all studies. AT can occur across the age spectrum but gradually increases in prevalence with age and peaking between the age of 40 and 60 years. Automatic AT tends to be more common in younger populations, whereas focal AT due to microreentry is more common in older populations, although many exceptions to this generalization occur. Older patients are more likely to have right-sided AT and multiple ATs. The majority of patients with focal AT do not have underlying structural heart disease or atrial abnormalities, but focal AT may also occur in this context.
Focal AT is usually manifested by atrial rates between 130 and 250 bpm but may be as low as 100 bpm or as high as 300 bpm (see Fig. 65.2 ). In general, younger patients tend to have faster AT, with rates up to 340 bpm observed in infants. The P wave morphology is usually different from that of sinus rhythm (see Fig. 65.2 ), but foci arising from the region of the crista terminalis (particularly the superior crista) may have morphology consistent with a sinus origin ( Fig. 65.4 ). The properties of the atrial focus may be similar to that of the sinus node in that they are responsive to changes in activity and autonomic tone, with the rate varying according to activity. In incessant tachycardias, rates during sleep may be up to 40 bpm less than those during waking hours.
![FIGURE 65.4, Continuous 12-lead ECG indicating short bursts of atrial tachycardia from the region of the superior crista terminalis. Note that the P wave morphology of the tachycardia beats (atrial tachycardia [AT]) is very similar to the morphology of the sinus beats (SR). FIGURE 65.4, Continuous 12-lead ECG indicating short bursts of atrial tachycardia from the region of the superior crista terminalis. Note that the P wave morphology of the tachycardia beats (atrial tachycardia [AT]) is very similar to the morphology of the sinus beats (SR).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/SupraventricularTachycardias/3_3s20B9780323722193000657.jpg)
Patients experience a variety of symptoms, including palpitations, dizziness, chest pain, dyspnea, fatigue, and presyncope. Syncope is unusual unless tachycardia rates are extremely rapid or there is associated underlying structural heart disease.
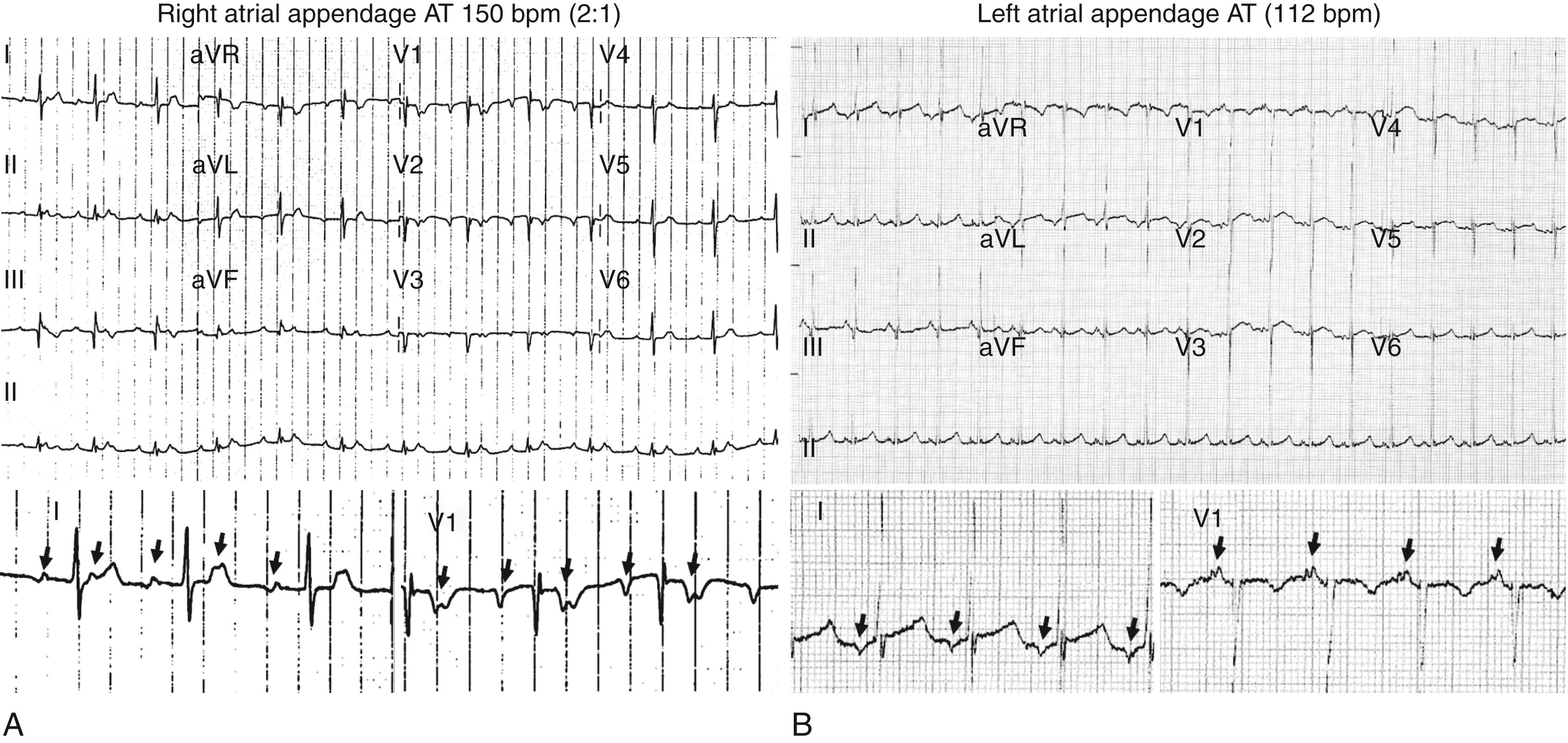
Tachycardia-mediated cardiomyopathy (TMC) has been reported to occur in a small percentage of patients with incessant ATs. In a large series of patients with focal AT, the incidence of TMC was 10%. TMC occurred exclusively in the context of incessant or very frequent (almost incessant) paroxysmal tachycardia. Patients with TMC were younger, were more frequently male, and had slower tachycardias (mean rate 117 bpm). Incessant tachycardias arise from specific anatomic locations, including the right atrial (RA) and left atrial (LA) appendages ( Fig. 65.5 ) and from the pulmonary venous ostia. Importantly, after successful ablation, left ventricular (LV) function normalizes in virtually all patients.
Embolic events and stroke have rarely been reported in patients with AT, and treatment with anticoagulation is generally not indicated. , Spontaneous remission of focal AT has been reported in both adults and children after cessation of medical therapy. In fact, the AT disappeared in 55% of patients under the age of 25, compared with just 14% of patients aged 26 or older.
Detection of AT is usually straightforward. Most patients can be diagnosed by a routine ECG during sustained tachycardia (see Fig. 65.2 ). However, those with self-reverting paroxysmal AT may require monitoring for diagnosis. If symptoms are frequent, this may require a 24-hour Holter monitor and, if infrequent, various wearable monitoring technologies are available to facilitate ECG documentation if only on a single lead. It should be noted that brief (3 to 10 beats) nonsustained AT is a common finding on routine Holter recordings and is seldom associated with symptoms. Occasionally the ECG differentiation of focal AT from other forms of SVT or from macroreentrant AT may be more challenging.
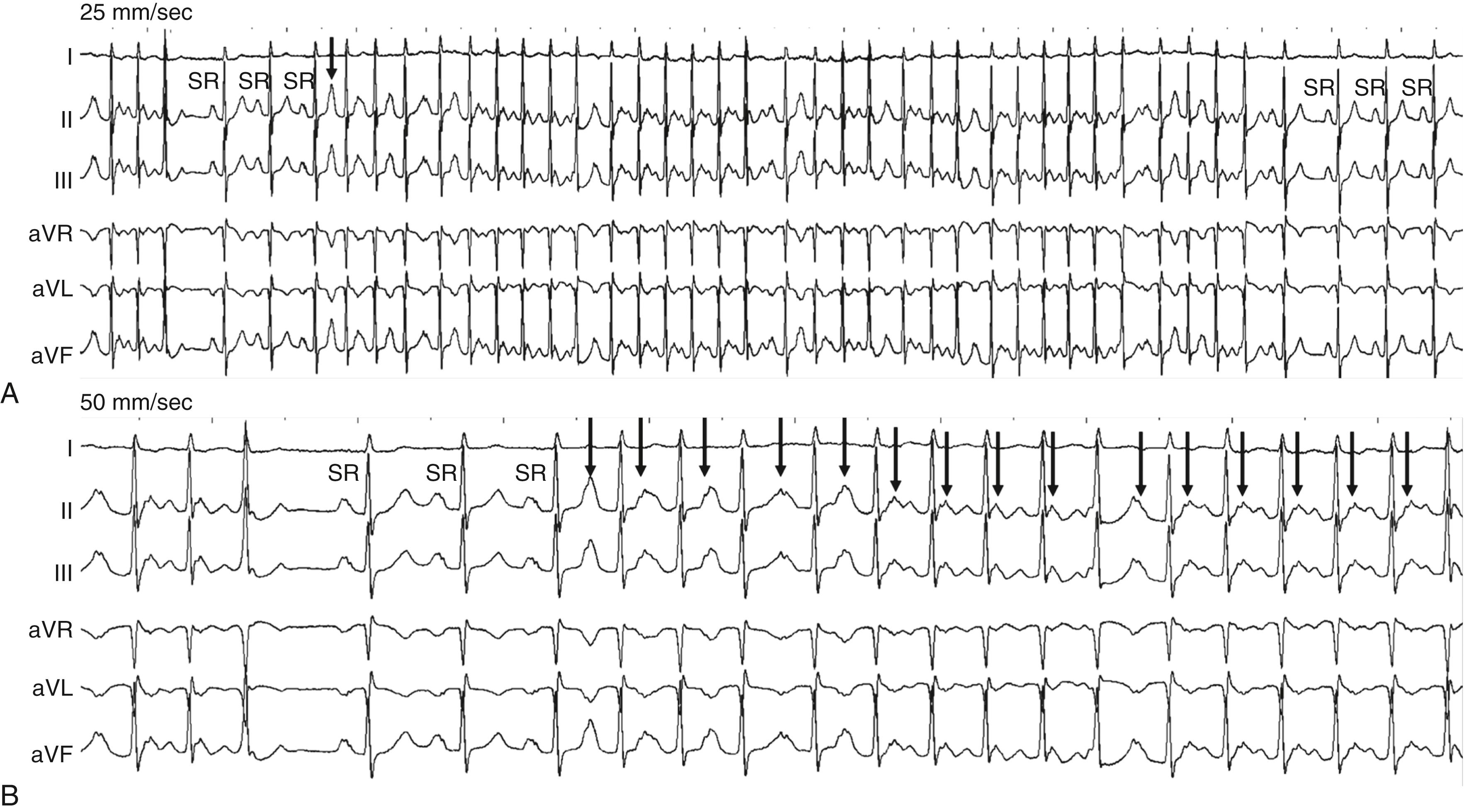
Differentiating AT from IAST on the ECG alone can at times be difficult, particularly for tachycardias originating at the superior crista terminalis. Although the P wave in AT usually has a morphology different from that of the sinus P wave (see Fig. 65.2 ), in which case the diagnosis will be clear, when AT arises from the superior crista terminalis these differences may be subtle (see Fig. 65.4 ). AT usually demonstrates an abrupt onset and termination or may warm up and cool down over 3 or 4 beats. In contrast, IAST gradually increases in rate over approximately 30 seconds to several minutes. In addition, demonstration that onset occurs with a tightly coupled P wave, particularly located in the preceding T wave, is virtually diagnostic of AT (see Fig. 65.3 and eFig. 65.1 ).
The most important differentiating factor on ECG between AT and AVNRT and AVRT is the R-P relationship. Both typical AVNRT and AVRT have a short R-P interval that does not vary (the former superimposed on the QRS and the latter in the ST segment), and the P wave morphology usually cannot be clearly discerned. Although most commonly associated with a long R-P interval, AT can occur with either a short R-P interval or a long R-P interval depending on the tachycardia rate and the speed of AV nodal conduction. It can therefore mimic either AVNRT or AVRT. The ability to demonstrate “unlinking” or variability of the R to P relationship invariably indicates AT. In AT the R-P relationship is incidental and hence possibly variable. In AVRT and AVNRT this relationship will be constant because it is integral to the tachycardia mechanism. Another clue to the diagnosis is the presence of an inferior P wave axis. This excludes AVRT or AVNRT because it suggests an origin high in the atrium. A superiorly directed P wave vector may indicate AVRT or AVNRT or an AT focus originating from the coronary sinus ostium or annular structures. Of note, atypical AVNRT and a concealed accessory pathway with slow retrograde conduction may have long R-P intervals but have constant R-P intervals and a superior P wave axis. Automatic AT may also manifest with recurrent self-limiting bursts of tachycardia that can exhibit warm-up and cool-down phases.
In most cases of focal AT it is possible to observe a discrete P wave with an intervening isoelectric interval (see Fig. 65.2A ). However, when the atrial rate is very rapid and, if atrial conduction slowing is present, there may be no isoelectric baseline and the appearance may mimic that of macroreentrant AT (see Fig. 65.2 , right ). Conversely, although macroreentrant AT (including typical AFL) frequently demonstrates a continuous undulation without an isoelectric period on the ECG, patterns resembling focal AT (with an isoelectric period) also have been described. Variation in the tachycardia rate with spontaneous termination and then reinitiation is diagnostic of a focal AT, usually the result of enhanced automaticity or triggered activity (see Fig. 65.3 ). Spontaneous bursts of tachycardia do not occur with macroreentrant tachycardias.
The rate of the tachycardia does not help discriminate between focal and macroreentrant AT as the rate ranges of both focal AT and macroreentrant AT are too wide to be reliably used for determination of arrhythmia mechanism. The rate range of focal AT is usually between 130 and 250 bpm but may be as low as 100 bpm or as high as 300 bpm. Similarly, although macroreentrant atrial arrhythmias usually have a rate between 240 and 310 bpm, conduction delays within the circuit either due to atrial pathology or use of conduction slowing antiarrhythmics can slow the rate to less than 150 bpm.
True multifocal AT is a relatively uncommon arrhythmia that occurs in the context of underlying conditions, including pulmonary disease, pulmonary hypertension, coronary disease, and valvular heart disease. However, bursts of focal AT may masquerade as multifocal AT because of the variable P wave appearance when there are varying degrees of fusion with the preceding T wave or QRS complex. Indeed, during rapid focal AT it may be quite difficult to discern a clear P wave morphology because the majority of beats are at least partially obscured by the T waves and QRS complexes unless higher grades of AV conduction block are present ( Fig. 65.6 ). Similarly, when focal AT is fast, it has some P wave interval irregularity in cycle length, and there is varying AV conduction, the trace may superficially resemble AF, particularly if there is only a single monitor lead. A careful appraisal of the trace will generally indicate a P wave fused with the T wave visible before most conducted beats (see Fig. 65.6 ).
Focal ATs do not occur randomly throughout the atria but rather have a characteristic anatomic distribution ( Fig. 65.7 ). In the right atrium they tend to cluster around the crista terminalis, coronary sinus ostium (CS os), para-Hisian or perinodal region, tricuspid annulus (TA), and RA appendage. In the left atrium, the majority originate from the pulmonary veins, with the mitral annulus, LA appendage, and left septum being less common. More recently, focal AT has been described originating from the noncoronary cusp of the aortic valve. The specific reasons for this anatomic distribution remain speculative. For example, the crista terminalis is an area of marked anisotropy with poor transverse but rapid linear conduction, creating a potential substrate for reentry. In addition, the sinus node complex is located along the crista terminalis, and the presence of automatic tissue with anisotropy may favor abnormal automaticity.
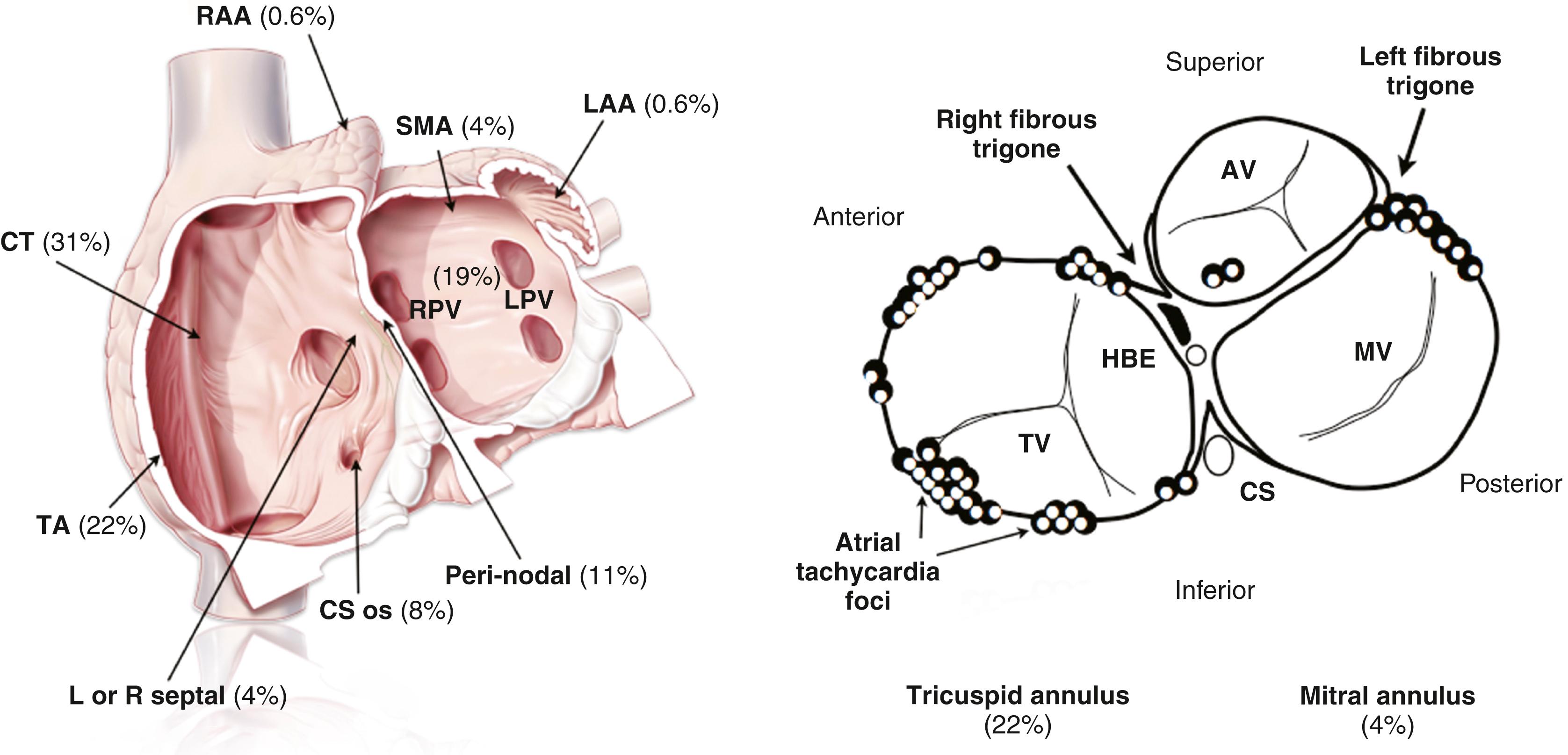
In the context of this classic anatomic distribution, the P wave morphology can provide a reliable and relatively specific guide as to the likely anatomic site of focal AT origin and a number of different P wave algorithms have been proposed. The important caveat is that no significant structural atrial disease is present and the patient has not had prior catheter ablation, because either of these factors may fundamentally change the shape of the P wave. In general, an upright P wave in V 1 suggests LA origin whereas a negative P wave in V1 suggests RA origin.
Although the sinus node reentry arrhythmia remains as a separate entity in recent guidelines documents, , there has been some debate as to whether tachycardias due to reentry within the sinus node truly exist. Sinus node reentry is clinically defined as a tachycardia that can be induced and terminated with programmed stimulation and has a P wave morphology identical or similar to that of the sinus P wave. It is now well recognized that the sinus node is not a discrete structure but rather a diffuse pacemaker complex located along the long axis of the crista terminalis. AT has been described arising from sites along the length of this structure, and it may be best to define these simply as crista terminalis AT.
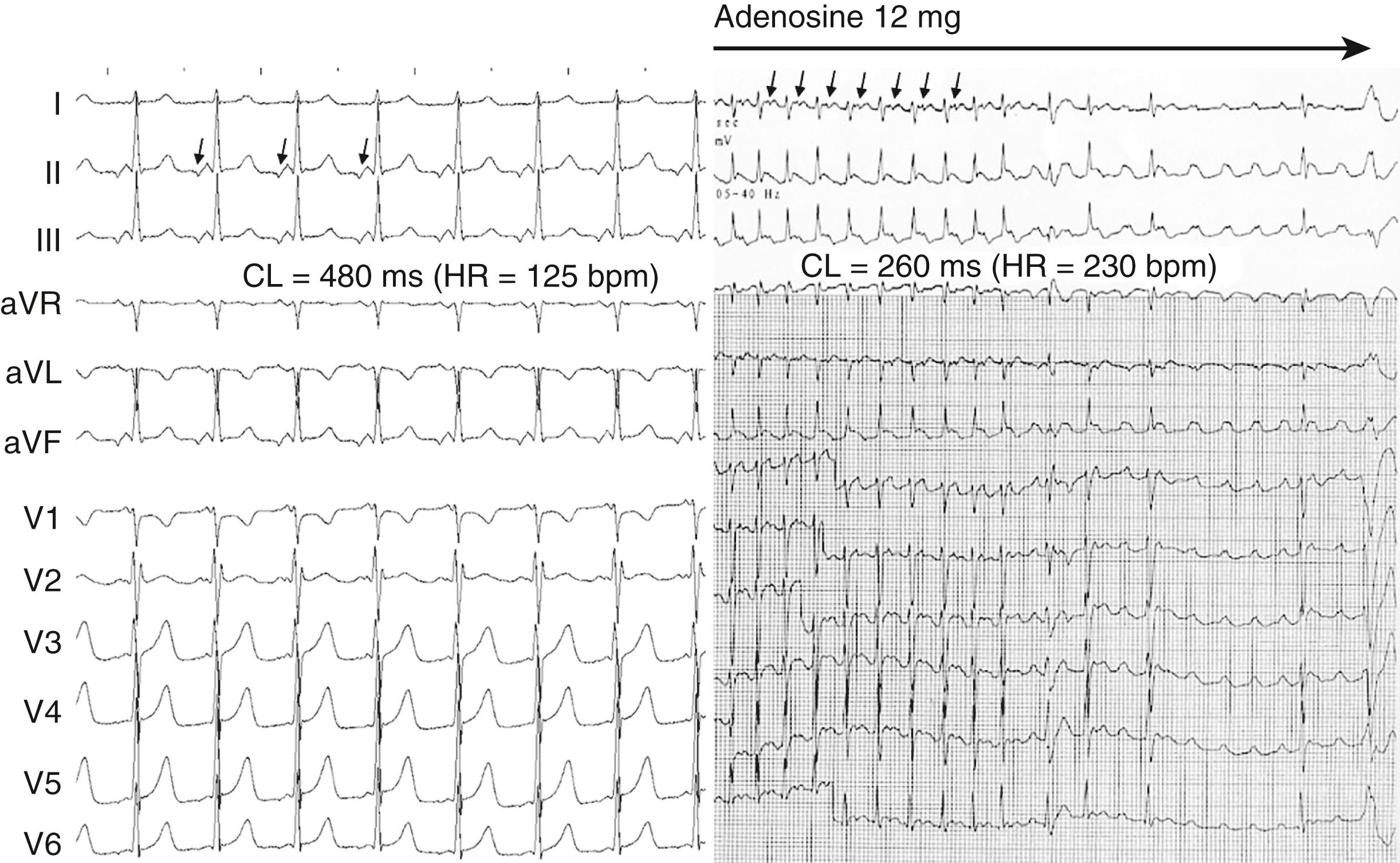
Initial management of a focal AT in the emergency department might involve administration of adenosine as for other mechanisms of SVT (6 to 12 mg intravenously). For focal AT, the arrhythmia may either terminate, transiently slow, and then return to the pre-adenosine rate or continue with AV block and unmasked P waves (see Fig. 65.2 ). If unsuccessful, intravenous beta blockers or calcium channel blockers (verapamil or diltiazem) may be effective in hemodynamically stable patients. If ineffective, antiarrhythmic agents such as flecainide, ibutilide, or amiodarone may be considered. Alternatively, if drug treatment is unsuccessful or the patient is hemodynamically unstable, synchronized DC cardioversion may be used. This would be inappropriate for automatic forms of tachycardia with recurrent bursts of tachycardia separated by 1 or more sinus beats. Similarly, for incessant automatic tachycardias, there is a high probability of recurrence of the arrhythmia after cardioversion.
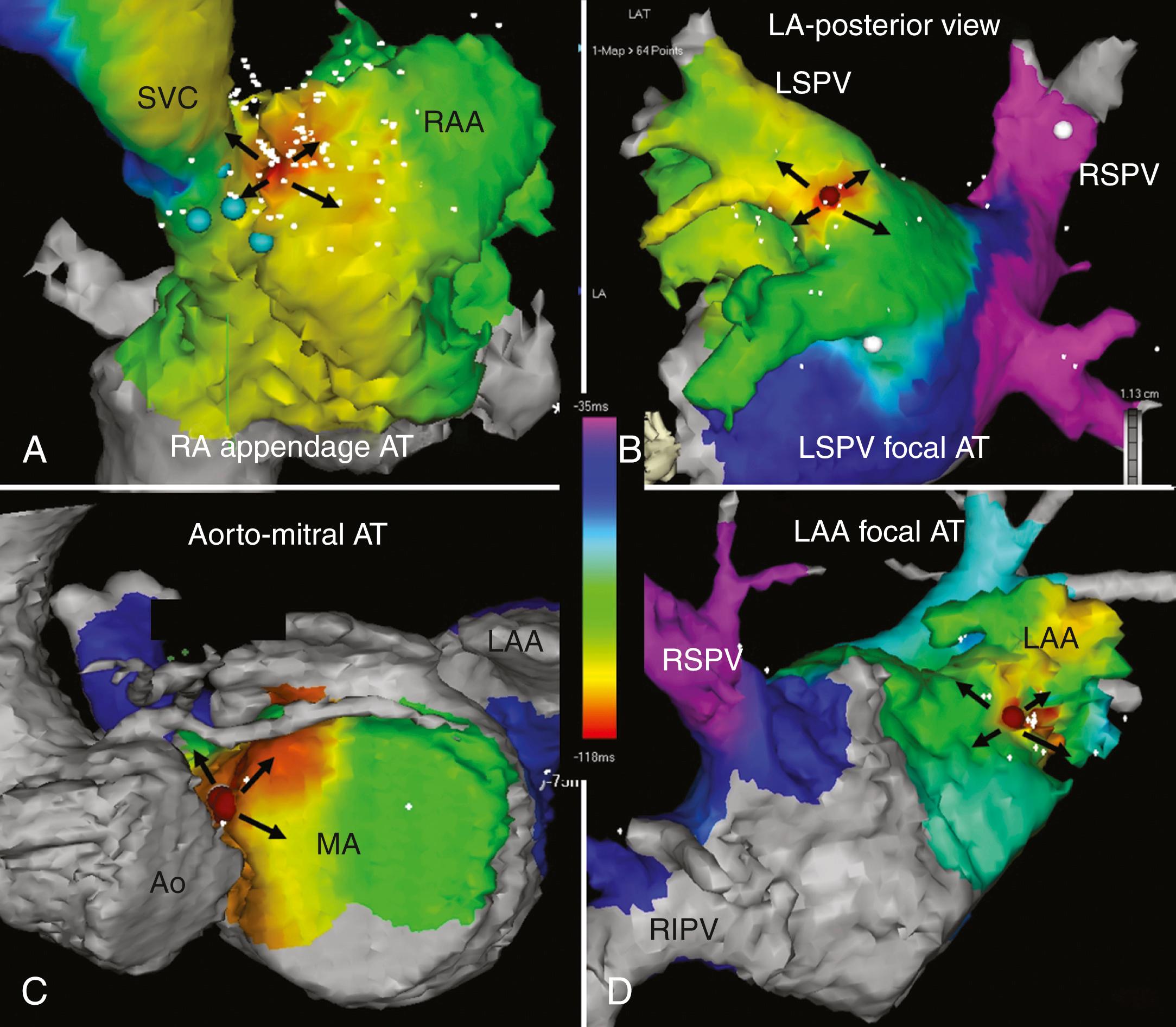
Catheter ablation is recommended as a first-line therapy in patients with symptomatic focal AT as an alternative to pharmacologic therapy (class 1). , Catheter ablation for the most part involves the use of a three-dimensional mapping system to identify the earliest site of atrial activation ( Fig. 65.8 ). Mapping will generally indicate a site from which there is centrifugal spread of activation away from that site. Contemporary ablation series indicate a high procedural success rate in excess of 85% with a very low risk of major complications. The caveat is that for some automatic or triggered forms of focal AT, on occasion all attempts at inducing the arrhythmia may be unsuccessful rendering mapping and ablation impossible on that day.
When catheter ablation is not preferred or not appropriate, pharmacologic management may be considered. However, there are no long-term, randomized, placebo-controlled studies on the use of antiarrhythmic therapy in focal AT. The available studies are observational, with small numbers and mostly conducted over a decade ago. There is widespread agreement that antiarrhythmic agents have low efficacy in the treatment of focal AT.
When drug therapy is preferred, beta blockers or non-dihydropyridine calcium channel blockers (verapamil or diltiazem) may be considered. In patients without structural or ischemic heart disease, propafenone or flecainide also may be considered. Less commonly, agents such as sotalol, amiodarone, or ivabradine have been used.
IAST is defined as an elevated heart rate of greater than 100 bpm at rest or on minimal exertion out of keeping with the level of activity or stress. , The mean 24-hour heart rate is above 90 bpm. The diagnosis can be made when there are accompanying symptoms attributable to the elevated heart rate such as palpitations, breathlessness on minor activity, light-headedness, chest pain, and fatigue. The impact on quality of life can be substantial and is frequently associated with significant psychosocial distress. , Overwhelmingly the condition manifests in young women. Other than the symptoms that may be debilitating, IAST does not have adverse prognostic significance and reports of tachycardia-induced cardiomyopathy are extremely rare. It is important to rule out secondary causes as a critical part of the initial evaluation before the diagnosis may be considered. A broad range of secondary causes of sinus tachycardia, including anxiety, anemia, volume depletion, thyrotoxicosis, fever, pulmonary embolus, cardiac failure, sepsis, stimulants, and drug withdrawal must first be excluded. The heart rate in IAST is not necessarily persistently elevated, and considerable fluctuation is often present. Resting heart rates are frequently in the normal range, and diurnal variation is generally preserved. However, significant rate increases occur with minor activity and positional changes and at other times may be unexplained. Heart rate increases generally occur gradually over 30 seconds to several minutes in contrast to onset of focal AT, which demonstrates sudden onset with a tightly coupled initiating beat and perhaps “warm-up” phenomenon over 2 to 3 beats. The P wave morphology of IAST reflects an origin in the region of the superior crista terminalis in the right atrium with a biphasic positive-negative appearance in V 1 and upright P waves in inferior leads and lead I. This resembles the morphology of a focal AT originating in the same anatomic region. The differential diagnosis also includes both postural orthostatic tachycardia syndrome (POTS) and physical deconditioning, which may have many overlapping features.
The mechanism of IAST is not well understood, and both intrinsic (sinus node hyperactivity) and extrinsic (related to dysautonomia or neurohormonal dysregulation) mechanisms have been suggested. A number of recent reports have described the development of inappropriate sinus tachycardia with elevated mean heart rates and reduced heart rate variability as a manifestation of post COVID-19 syndrome.
Treatment requires effective communication, support, and reassurance, which may improve outcomes. Lifestyle interventions such as exercise training and volume expansion may be helpful. Ivabradine, a selective blocker of the pacemaker current (I f ) has been found to be safe and effective in several small trials. , Addition of beta blockade to ivabradine therapy or the use of beta blockers alone may also be useful. However, beta blockers may increase postural dizziness and fatigue. Sinus node modification (catheter ablation) and surgical ablation are not recommended as a part of routine care for patients with IAST. Although these may have early effect and most patients have recurrent symptoms and even with complete surgical removal of the sinus pacemaker complex, fast rates are often generated from the junctional region and left atrium.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here