Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
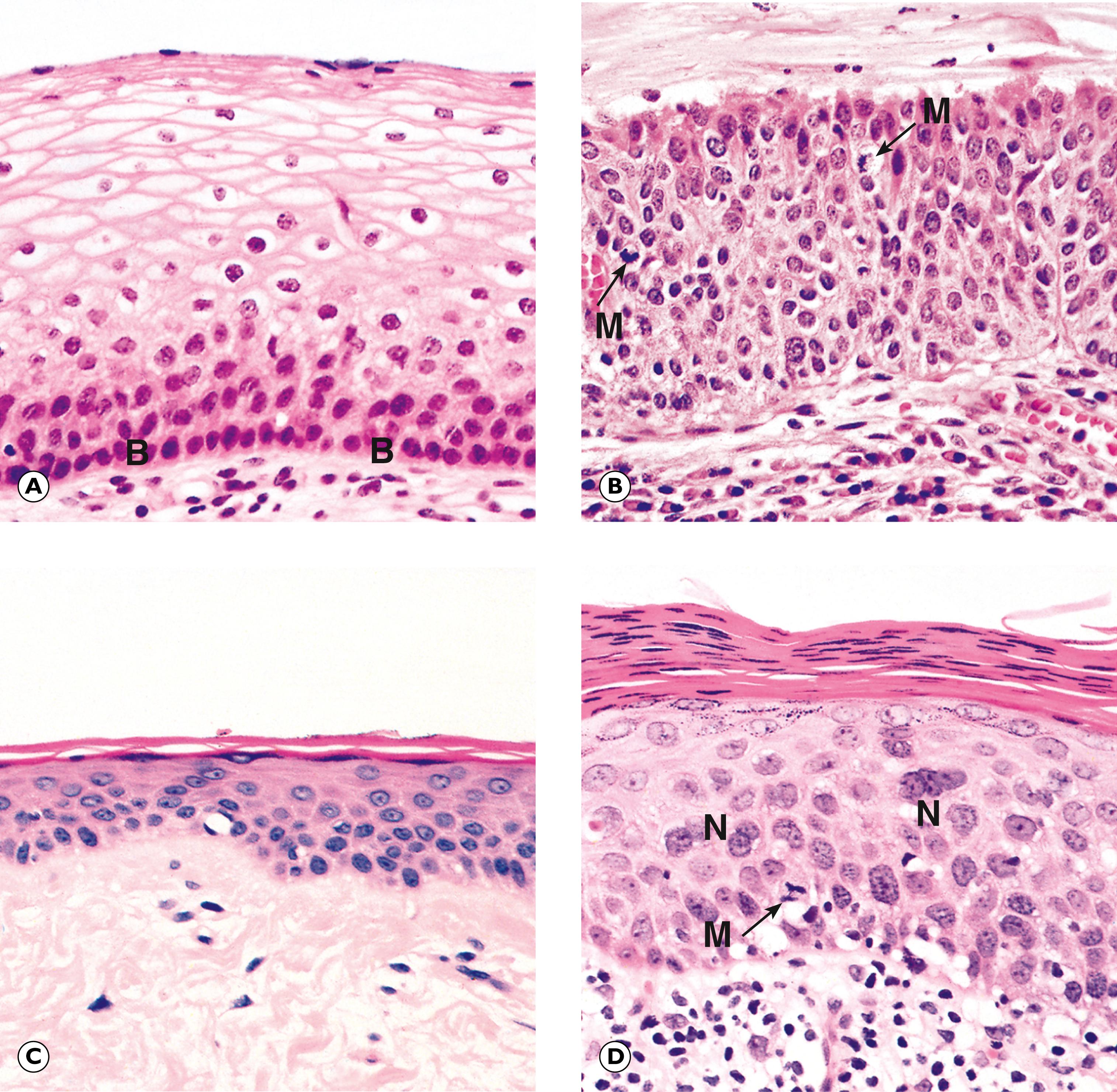
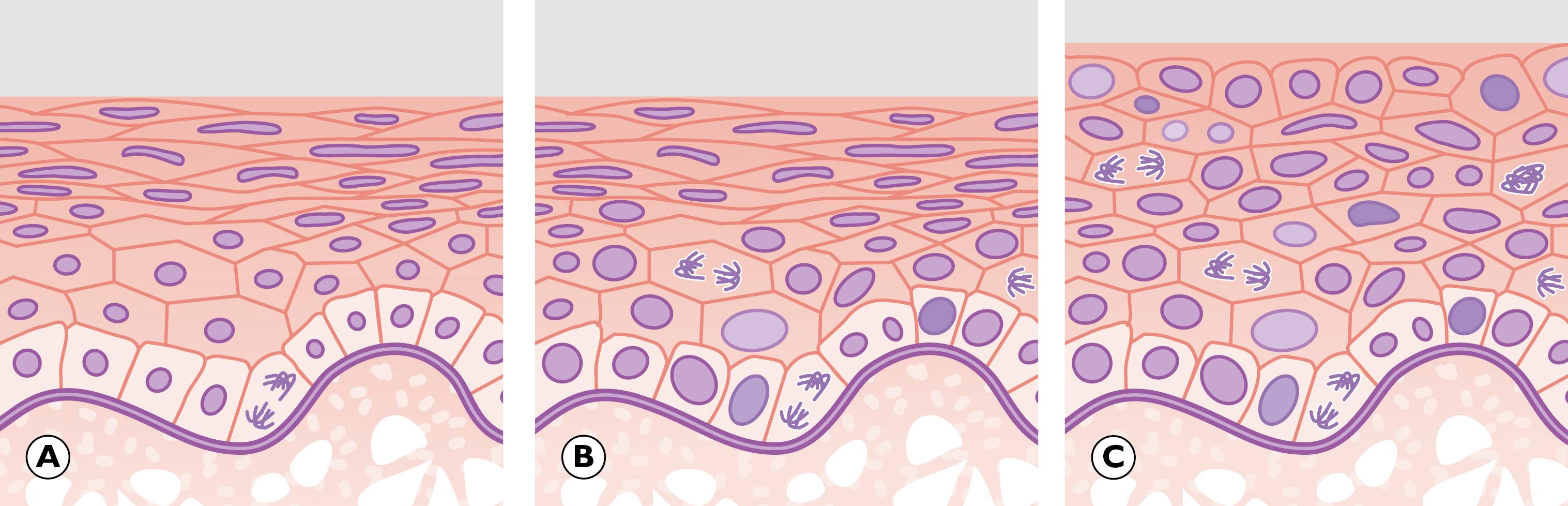
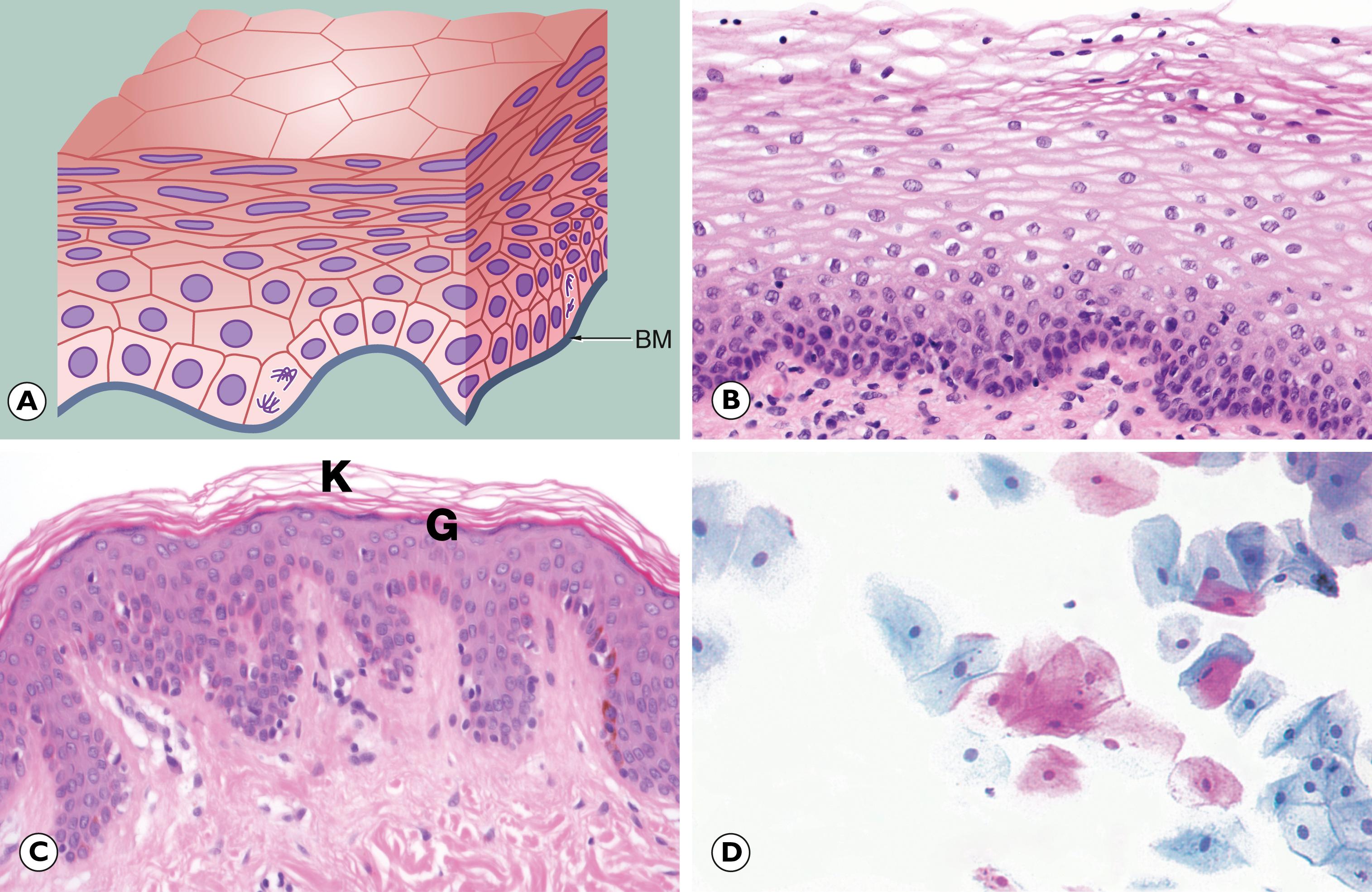
In the previous chapter, we considered cellular adaptations to a variety of stimuli resulting in alterations in their pattern of growth, whilst still achieving full differentiation/maturation. Occasionally, cells have an increased rate of division such that they do not have time to reach complete maturation (with full development of cytoplasmic specialisation) before another cycle of cell division supervenes. This leads to a population of cells that are structurally abnormal, having a high nuclear to cytoplasmic ratio with large nuclei containing abundant, dark-staining chromatin and prominent, occasionally multiple, nucleoli. These nuclear changes are associated with evidence of incomplete maturation of the cytoplasm, which may be lacking in the specialised structures normally seen in that cell type, such as mucin vacuoles or surface cilia. This is called failure of differentiation and the cells are said to show atypia .
Cellular atypia may be a consequence of rapid multiplication of cells as a response to cell destruction due to a persistent damaging stimulus. In this case, atypia is a result of the high turnover of cells attempting to regenerate damaged epithelium. When the damaging stimulus is removed, these abnormalities should revert to normal with full differentiation of the cells returning. However, sometimes the atypia is persistent and is not simply the result of regeneration. In these cases, the population of atypical cells may eventually be the focus from which invasive cancer develops. This type of persistent cellular atypia is called dysplasia ( Fig. 7.1 ). The problem for the pathologist is in distinguishing between cellular atypia, representing a reactive, regenerative response to tissue damage, and dysplasia, which is indicative of early pre-cancer. There is no simple answer.
Dysplasia often arises in metaplastic tissues and it is likely that the stimuli that cause dysplasia are also responsible for metaplastic change. Thus metaplastic epithelium in the cervix and Barrett’s oesophagus is at risk of dysplasia and subsequent neoplasia. In dysplastic cells, molecular biological techniques have demonstrated some of the genetic abnormalities found in invasive neoplasms. Because of the sinister association of dysplasia with the subsequent development of neoplasia, treatment of dysplastic conditions is undertaken to minimise the risk of subsequent development of a malignancy. Recognition of dysplastic changes by cytological examination of cervical smears forms the basis of screening for cancer of the cervix (see clinical box ‘Screening and pathology’ and Fig. 17.6 ).
Screening is an important part of disease prevention and involves assessing a subset of the population for a disease or condition using a relatively non-invasive test. This allows early detection and treatment and therefore improves survival rates. It is important to remember that screening tests only identify those at high risk of having the disease/condition and a further diagnostic test, usually in the form of a biopsy, is required for confirmation and/or treatment. Most developed countries now offer several different screening programmes, examples of which are given below. Pathologists can be involved in assessing the screening test sample itself or the diagnostic test sample (e.g. a core biopsy), which follows a positive screening result.
| Disease/condition | Screening test | Diagnostic test |
|---|---|---|
| Cervical cancer | Cervical smear: cytology | Biopsy or large loop excision of transformation zone (LLETZ) |
| Breast cancer | Mammography | Fine needle aspirate (FNA) or core biopsy |
| Colorectal cancer | Faecal occult blood (FOB) test +/- colonoscopy | Removal of polyp/ biopsy of lesion |
| Prostate cancer | Prostate specific antigen (PSA) blood test and digital rectal examination | Trans-rectal ultrasound (TRUS) and core biopsy |
B basal layer M mitotic figure N multinucleated cells
In a neoplasm, cellular proliferation and growth occur in the absence of any continuing external stimulus. Therefore, the term neoplasia (from the Greek for new growth) describes a state of autonomous cell division and the abnormal mass of cells that results is termed a neoplasm . The state of neoplastic growth contrasts with hyperplasia , discussed in Ch. 6 , where, although there is abnormal proliferation of cells, this ceases with removal of the causative stimulus.
As well as abnormal cell proliferation, neoplasia is characterised by abnormal maturation of cells. A feature of normal tissue growth is the maturation of constituent cells into a form adapted to a specific function. Such adaptation may involve the acquisition of specialised structures such as mucin vacuoles, neurosecretory granules, microvilli or cilia. This process of structural and functional maturation is called differentiation . A fully mature cell of any particular cell line is said to be highly differentiated , whereas its primitive precursor cells are described as being undifferentiated . In any given tissue, the normal cells have a characteristic state of differentiation. In contrast, neoplastic cells exhibit variable states of differentiation and commonly fail to achieve a highly differentiated state. Neoplasms are divided clinically into two main groups ( Table 7.1 ):
benign neoplasms grow slowly and remain localised to the site of origin
malignant neoplasms grow rapidly and may spread widely.
| Benign | Malignant | |
|---|---|---|
| Behaviour | Expansile growth only; grows locally | Expansile and invasive growth; may metastasise |
| Often encapsulated | Not encapsulated | |
| Histology | Resembles cell of origin (well differentiated) | May show failure of cellular differentiation |
| Few mitoses | Many mitoses, some of which are abnormal forms | |
| Normal or slight increase in ratio of nucleus to cytoplasm | High nuclear to cytoplasmic ratio | |
| Cells are uniform throughout the tumour | Cells vary in shape and size (cellular pleomorphism) and/or nuclei vary in shape and size (nuclear pleomorphism) |
There is a broad correlation between histological appearances and biological behaviour, allowing prediction of the likely prognosis. In general, the cells of benign neoplasms are well differentiated. In the case of malignant neoplasms, there is a variable degree of differentiation. At one end of the spectrum, the constituent cells may closely resemble the tissue of origin, in which case the tumour is described as being a well differentiated malignancy . Alternatively, the constituent cells may bear little resemblance to the tissue of origin, in which case the neoplasm is described as being poorly differentiated . At the extreme end of the spectrum, neoplasms that exhibit no evidence of differentiation are termed anaplastic and, in many cases, it may not be possible to identify the cell of origin on morphological grounds alone. Generally, the degree of differentiation of a neoplasm is related to its behaviour. A poorly differentiated neoplasm tends to be more invasive and more aggressive than a well differentiated neoplasm.
Transformation from normally functioning and growing cells to cancer, called carcinogenesis , is regarded as a complex multistep process involving acquisition of genetic mutations, failure of normal DNA repair mechanisms and reduced apoptosis, along with developing the capacity for invasion and metastasis.
As discussed in Chapter 1 , the consequences of a mutation in a single oncogene invariably would be overcome by otherwise normally functioning tumour suppressor genes. This concept is summarised by the Knudson hypothesis , which states that multiple mutations, or ‘hits’, are required for cancer to develop. In the typical example of familial cancer syndromes, the first hit is in inheriting the abnormal DNA which is balanced by normally functioning genes until the second hit, where these too are damaged and cancer develops.
In addition to a growing list of inherited cancer syndromes, a considerable number of environmental agents associated with the development of cancer are now recognised, including chemical agents, ionising radiation and infections such as viruses. These are known as carcinogens and examples of these are given below.
| Inherited cancer syndromes | Chemical agents | Infections | |||
|---|---|---|---|---|---|
| Gene | Association | Agent | Association | Agent | Association |
| NF1, NF2 | Neurofibromatosis types 1 and 2 | Asbestos | Mesothelioma, lung cancer, GI tract cancers | HPV | Cervical carcinoma |
| APC | Familial adenomatous polyposis, colon cancer | Nitrosamines | Gastric carcinoma | EBV | Burkitt, Hodgkin lymphomas |
| BRCA1/ BRCA2 | Breast and ovarian cancers | Benzene | Leukaemia, Hodgkin lymphoma | Hep B/C | Hepatocellular carcinoma |
| RB | Retinoblastoma | Nickel | Lung cancer | HHV8 | Kaposi’s sarcoma |
| P53 | Li-Fraumeni syndrome | Azo dyes | Bladder cancer | H. pylori | Gastric carcinomaMALT lymphoma |
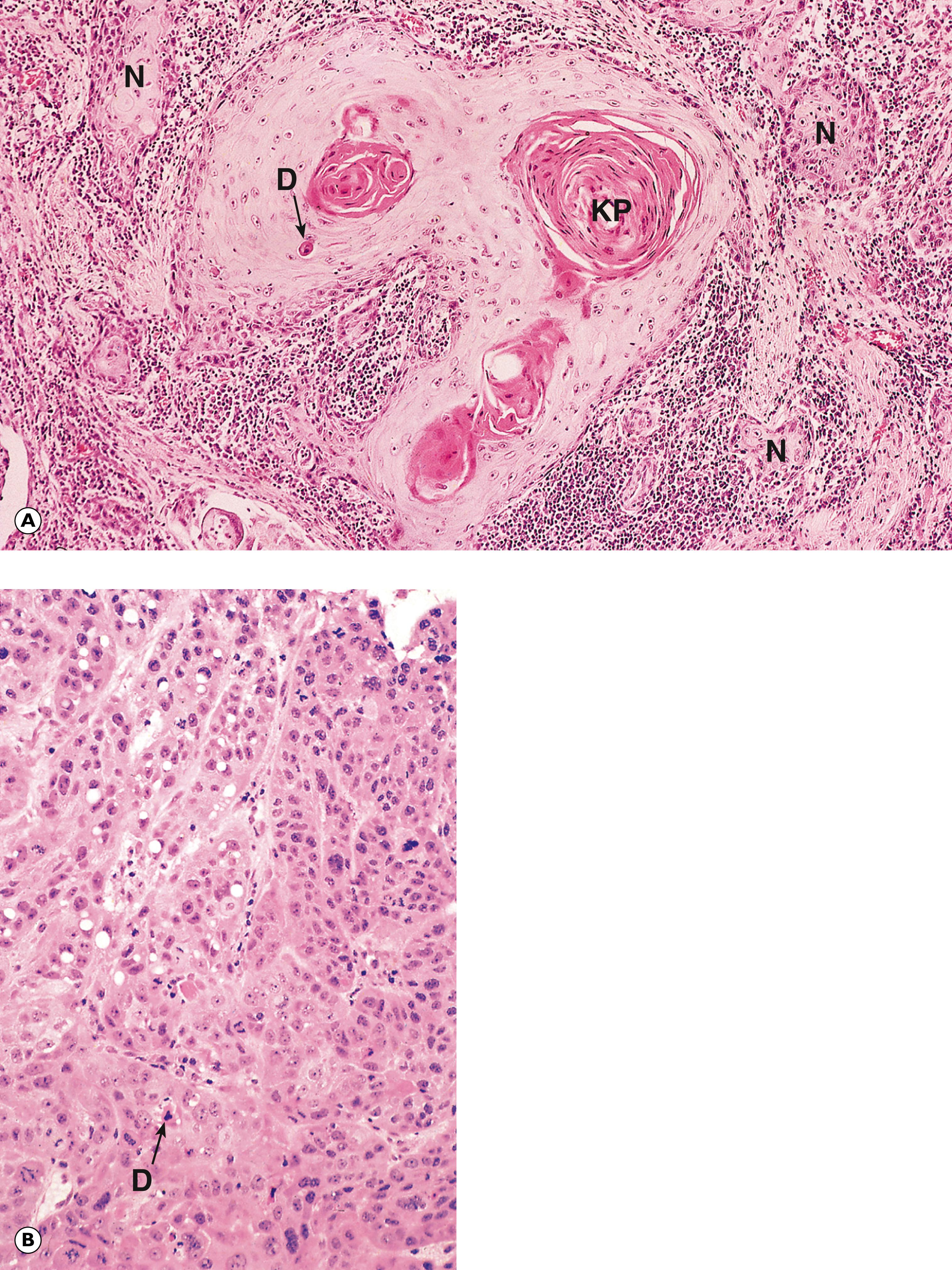
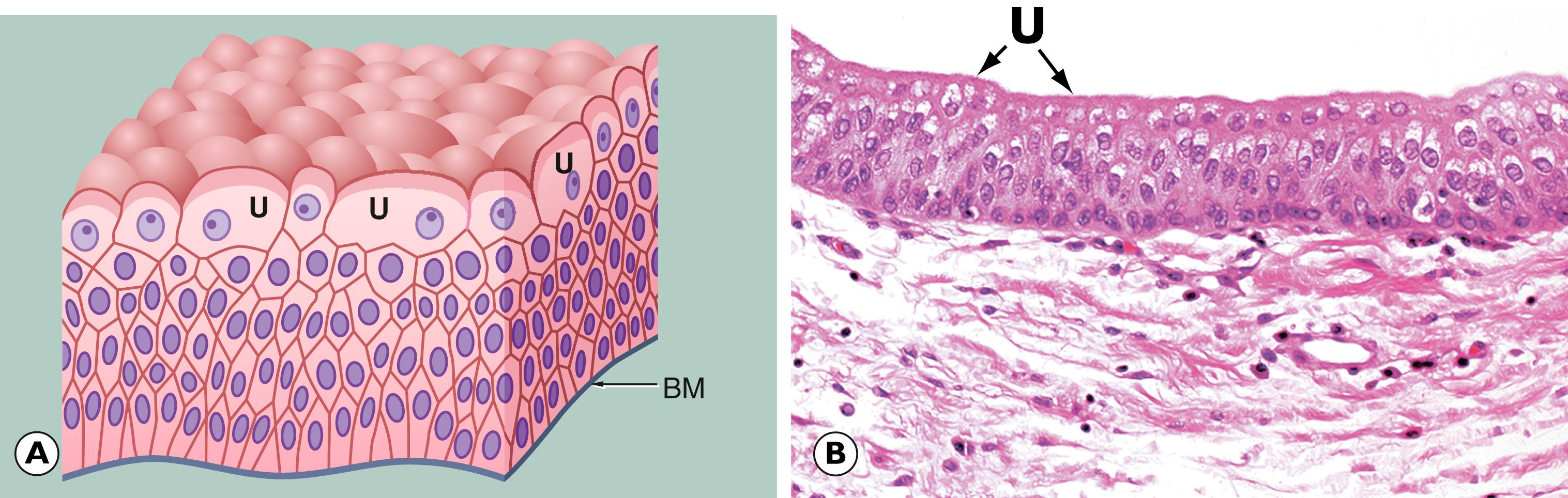
The term carcinoma in situ is used when an epithelial tissue shows the cytological and histological features of carcinoma (architectural and cytological abnormalities such as cell crowding, pleomorphism, increased and abnormal mitotic activity, which may involve the full thickness of the epithelium), but there is no evidence that the basement membrane bounding the abnormal epithelial tissue has been breached and there is no encroachment of atypical cells into the underlying stroma. The process whereby tumour cells breach the basement membrane and extend into the surrounding tissues is described as invasion . Thus, with carcinoma in situ the epithelial cells show the cytological, but not the behavioural, characteristics of malignancy. However, many forms of carcinoma in situ will become invasive if left untreated.
Certain benign epithelial tumours may progress to form an invasive malignant tumour by development and selective persistence of mutations in key oncogenes. A sequence from benign neoplasm through increasing severity of dysplasia to carcinoma in situ and on to invasive carcinoma is well recognised in certain sites.
Carcinoma in situ is synonymous with severe/high grade dysplasia. However, terminology varies depending on the anatomical location. For example, the term carcinoma in situ is used in the skin (see Fig. 21.14 ) and bladder (see Fig. 15.21 ). At other sites such as the cervix, carcinoma in situ is called CIN3 (cervical intraepithelial neoplasia; see Fig. 17.7 ), in the vulva, VIN3 (vulval intraepithelial neoplasia) and in colonic adenomatous polyps, ‘high grade dysplasia’ (see Fig. 13.21 ). It also occurs in solid glandular organs, most notably the breast (ductal and lobular carcinoma in situ; see Figs. 18.9 and 18.11 ).
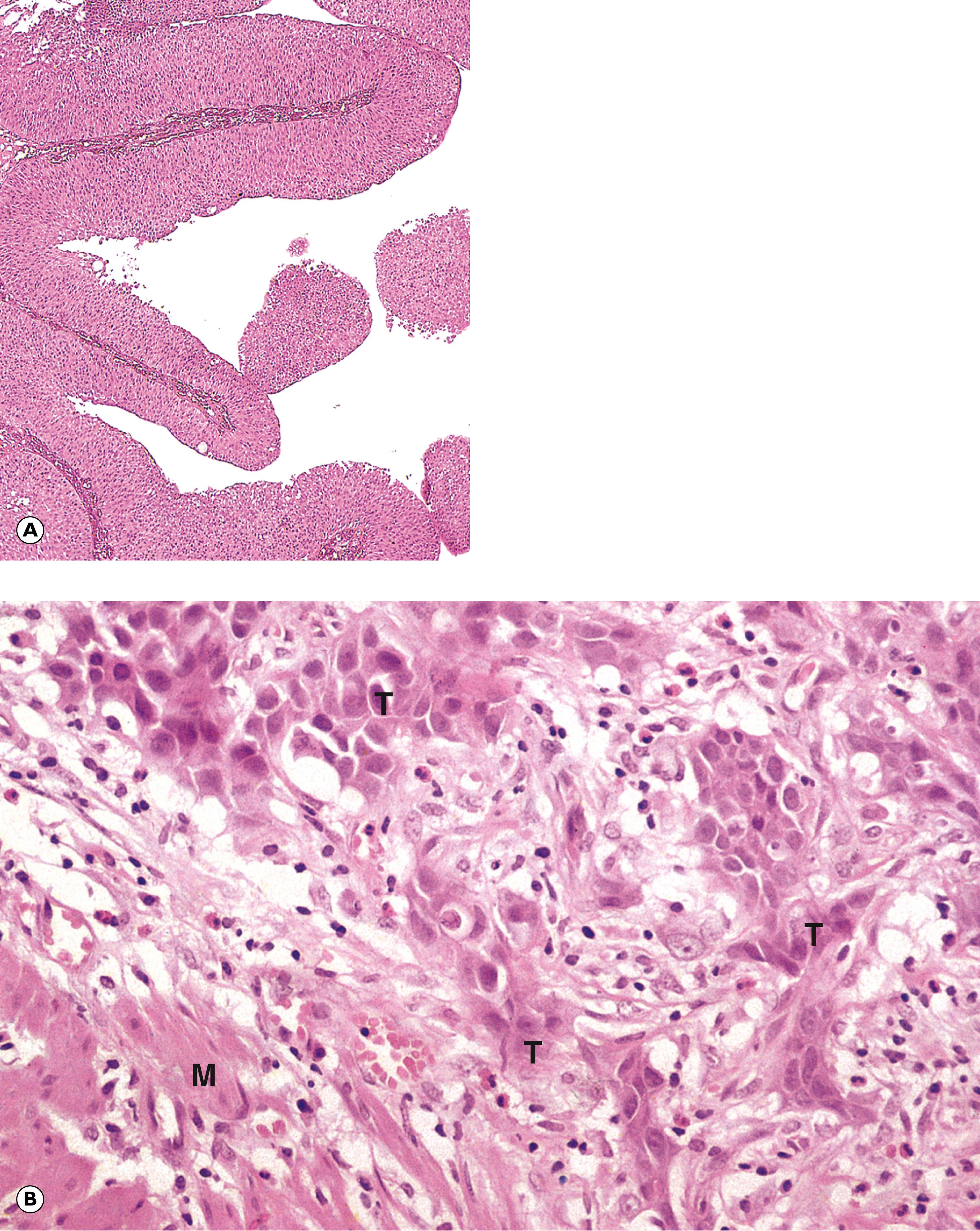
As mentioned before, the property whereby a malignant tumour can grow into the surrounding tissue is termed invasion. A further property of malignant neoplasms is distant spread of neoplastic cells away from the main neoplasm (the primary tumour ) to form subpopulations of neoplastic cells that are not in continuity with the primary tumour. Distant spread of tumour from a primary site is termed metastasis . The secondary tumours that result from this distant spread are termed metastases .
In general terms, a benign tumour will behave in a relatively innocuous manner and a malignant tumour will have deleterious effects, often leading to death. There are, however, exceptions to these generalisations and factors other than the biological growth pattern of a tumour may be important in influencing the outcome. Of these, the most notable is the location of the tumour. For example, a benign tumour of the brain stem may lead to rapid death, whereas a malignant tumour of the skin may progress slowly over many years.
In addition to local effects, malignant tumours can cause a variety of systemic symptoms such as weight loss (cachexia) , loss of appetite, fever and general malaise. In most cases, the pathophysiology is poorly understood, but includes effects of secreted cytokines such as tumour necrosis factor . Some tumours, benign and malignant, retain the function of their organ of origin. If this happens to be an endocrine function, then the tumour may exert harmful effects by secretion of excess hormone. Other tumours secrete hormones not usually secreted by that tissue, for example the secretion of parathyroid hormone by lung carcinomas. This is known as ectopic hormone secretion and the clinical manifestations are described as paraneoplastic syndromes (see clinical box ‘Paraneoplastic syndromes’).
In addition to direct, local invasion and metastatic spread, cancers may give rise to distant symptoms and/or signs through two main mechanisms:
An immune response to the malignancy producing antibodies to tumour cells, which cross-react with normal cells. In many cases, the antibodies may target normal neurones producing well characterised, complex neurological presentations.
Release of cytokines/hormones by malignant cells, such as ectopic adrenocorticotrophic hormone (ACTH; see Ch. 20 ), which may be released by a variety of cancers, including pulmonary carcinomas.
The associated clinical syndromes may precede, follow or present at the same time as the primary malignancy.
Histological assessment of a tumour provides a useful guide to its behaviour, i.e. whether the tumour is benign or malignant, and provides a rational basis for treatment. Histological assessment should establish the following features:
The type of tumour. This is based on the presumed tissue of origin and/or differentiation of the tumour.
The degree of differentiation ( Fig. 7.10 ). This is known as grading and takes into account some or all of the following features:
the similarity of the tumour to the supposed tissue of origin both architecturally and cytologically (differentiation)
the degree of variability of cellular shape and size (pleomorphism)
the proportion of mitotic figures (dividing cells).
The extent of spread of tumour (staging) is partly assessed histologically, particularly:
size of the primary tumour
histological assessment of local, vascular, lymphatic and perineural invasion
the presence of metastatic tumour deposits, for example in lymph nodes and bones.
The presence or absence of other prognostic factors. For instance, the presence of oestrogen receptors in breast carcinoma cells confers an improved prognosis. The expression of certain oncogenes is increasingly being related to prognosis in some tumours.
The classification and nomenclature of neoplasms have developed from gross morphological, histological and behavioural observation. Ideally, the name given to a tumour should convey information about the cell of origin and the likely behaviour (either benign or malignant). While this is the case for the majority of tumours of epithelial and connective tissues, there are many tumours that are given eponymous or semi-descriptive names out of either poor understanding of pathogenesis or long-established tradition. Some tumours have several different names that are synonymous, but derive from different classifications.
Benign neoplasms of surface epithelia, for example skin, are termed papillomas. This term is preceded by the cell of origin, for example squamous papilloma of skin or larynx.
Benign neoplasms of both solid and surface glandular epithelia are termed adenomas . This is prefixed by the tissue of origin, for example thyroid adenoma, salivary gland adenoma. Frequently, a benign tumour of surface glandular epithelium (almost always in the large bowel) assumes a papillary growth pattern, termed a villous adenoma .
A malignant tumour of any epithelial origin is termed a carcinoma. Tumours of glandular epithelium (including that lining the gut) are termed adenocarcinomas . Tumours of other epithelia are preceded by the cell type of origin, for example squamous cell carcinoma and transitional cell carcinoma . To classify a carcinoma further, the tissue of origin is added, for example adenocarcinoma of prostate, adenocarcinoma of breast and squamous cell carcinoma of larynx.
The nomenclature of epithelial tumours is summarised in Table 7.2 .
| Tissue of origin | Benign | Malignant |
|---|---|---|
| Surface epithelium | Papilloma/adenoma | Carcinoma |
| Examples: | ||
| Squamous | Squamous cell papilloma | Squamous cell carcinoma |
| Glandular (columnar) | Adenoma (villous or tubular) | Adenocarcinoma |
| Transitional | Transitional cell papilloma | Transitional cell carcinoma |
| Solid glandular epithelium | Adenoma | Adenocarcinoma |
| Examples: | ||
| Thyroid | Thyroid adenoma | Thyroid adenocarcinoma |
| Kidney | Renal adenoma | Renal cell carcinoma |
| Liver | Hepatic adenoma | Hepatocellular carcinoma |
In connective tissues, there is a simpler and more descriptive classification of neoplasms. First, the tissue of origin is designated, with the addition of the suffix - oma for a benign tumour or - sarcoma for a malignant tumour. As an example, a benign tumour of adipose tissue is termed a lipoma, whilst a malignant tumour of the same origin is termed a liposarcoma. A summary of other connective tissue tumours is shown in Table 7.3 .
| Tissue of origin | Benign | Malignant |
|---|---|---|
| Fibrous tissue | Fibroma | Fibrosarcoma |
| Bone | Osteoma | Osteosarcoma |
| Cartilage | Chondroma | Chondrosarcoma |
| Adipose tissue | Lipoma | Liposarcoma |
| Smooth muscle | Leiomyoma | Leiomyosarcoma |
| Skeletal muscle | Rhabdomyoma | Rhabdomyosarcoma |
| Blood vessel | Haemangioma | Haemangiosarcoma |
There are other neoplasms that do not fit into either the epithelial or the connective tissue category described above and these are grouped according to their tissue of origin. The main categories are as follows:
Melanoma: tumours of melanocytes ( Fig. 7.8 and Ch. 21)
Lymphomas: tumours of solid lymphoid tissue ( Fig. 7.7 and Ch. 16)
Leukaemias: tumours derived from haematopoietic elements that circulate in the blood and only rarely form tumour masses (see Ch. 16)
Gliomas: tumours derived from the non-neural support tissues of the brain (see Ch. 23)
Germ cell tumours: tumours derived from germ cells in the gonads ( Fig. 7.9 , Ch. 17 and Ch. 19)
Neuroendocrine tumours : tumours derived from cells of the neuroendocrine system. Examples include phaeochromocytoma (see Fig. 20.11), carcinoid tumour (see Fig. 12.22) and medullary carcinoma (see Fig. 20.7).
Finally, there is a group of tumour-like lesions known as hamartomas that represent non-neoplastic overgrowths of tissues indigenous to the site of their occurrence. These are thought to be developmental abnormalities. A common example is the ‘port-wine stain’ of the skin composed of blood vessels and known as a haemangioma . An example of a haemangioma in the liver is shown in Fig. 11.8.
D dyskeratotic cell KP keratin pearl M smooth muscle N cell nest T tumour cells
Tumour markers are substances produced by cancer cells, usually in large quantities and can be detected by biochemical assays of the blood or urine. These markers are also produced by normal cells usually at much lower levels and also by benign conditions, therefore the markers are not particularly sensitive or specific. Tumour markers can be used in screening for cancers, monitoring response to therapy and detecting recurrent disease.
There are a variety of types of tumour markers in clinical use, including hormones and specific proteins; examples of which are shown below. More recently, there has been a marked increase in molecular tests for specific genetic signatures in certain cancers to help with guiding targeted therapy and in predicting response to treatments such as chemotherapy and immunotherapy (see Ch. 1). Furthermore, there is currently a large field of research involving the detection of circulating tumour DNA in the blood as a possible method of early cancer detection.
| Hormones | Proteins | Molecular | |||
|---|---|---|---|---|---|
| Marker | Tumour(s) | Marker | Tumour(s) | Marker | Tumour(s) |
| hCG | Choriocarcinoma, germ cell tumours | PSA | Prostate cancer | EGFR mutation and ALK gene rearrangement | Non–small cell lung cancer |
| Calcitonin | Medullary thyroid carcinoma | Ig | Multiple myeloma | HER2 amplification | Breast cancer, gastric cancer |
| αFP | Hepatocellular carcinoma, germ cell tumours | CA 125 | Ovarian carcinoma | BRAF V600E mutation | Melanoma |
| Gastrin | Gastrinoma | CA 19.9 | Pancreatic cancer, cholangiocarcinoma | KRAS mutation | Colorectal cancer |
| ER/PR | Breast cancer | Chromogranin A | Neuroendocrine tumours | PD-L1 expression | Non–small cell lung cancer |
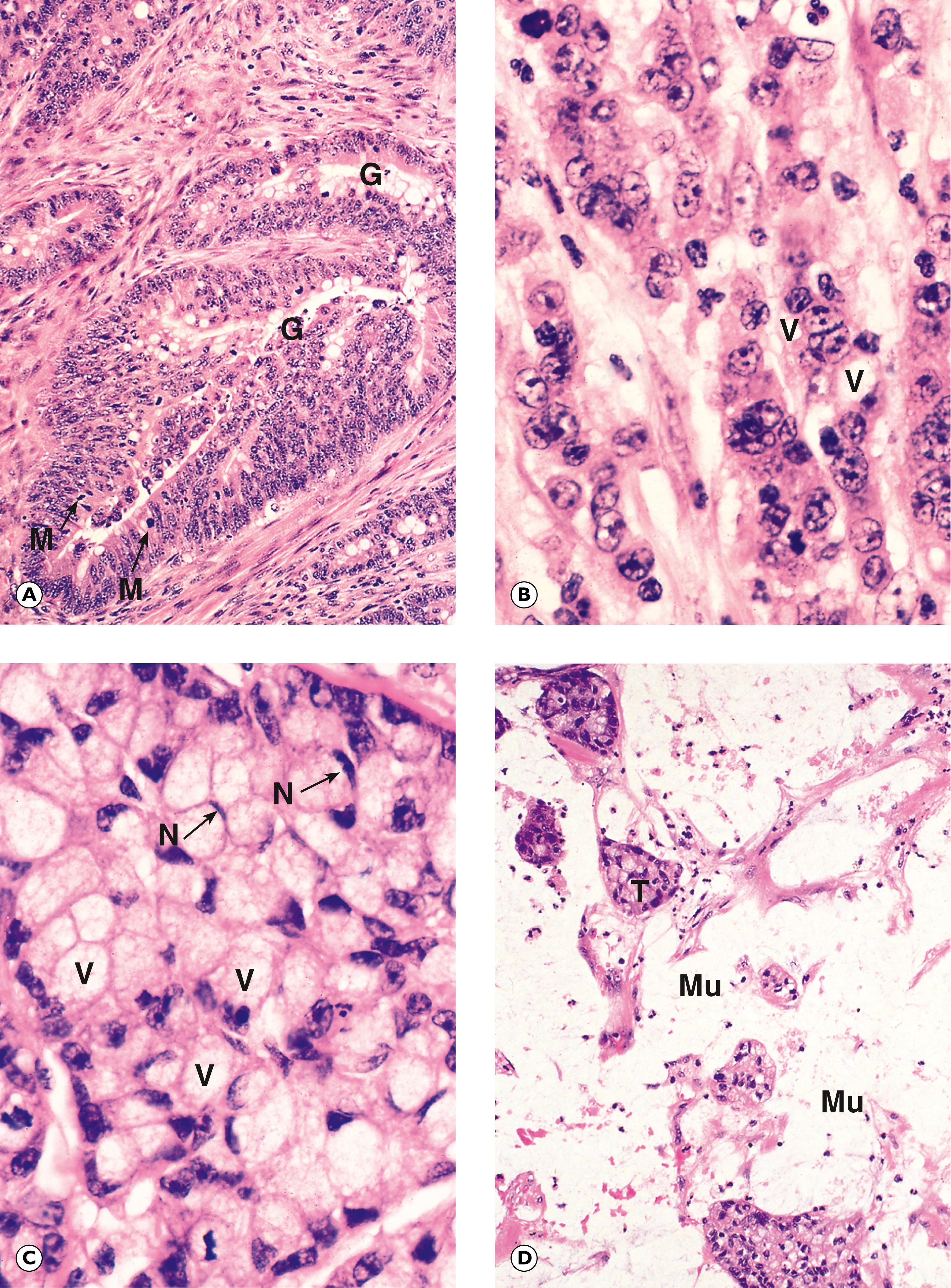
G glandular pattern M mitotic figure Mu mucin N nucleus T nest of tumour cells V mucin vacuole
C cystic space F fat G sebaceous glands H hair follicles MF abnormal mitotic figure N neural tissue
There are four main modes of tumour spread:
Local invasion. Invasive tumours tend to spread into surrounding tissues by the most direct route. Examples include carcinoma of the breast invading overlying skin or deeply into underlying muscle and carcinoma of the cervix invading rectum or bladder.
Lymphatic spread. Tumours may spread via lymphatic vessels draining the site of the primary tumour. Neoplastic cells are conducted to local lymph nodes where they may form secondary tumours, e.g. breast cancer spreading to lymph nodes in the axilla or carcinoma of the tongue spreading to lymph nodes in the neck.
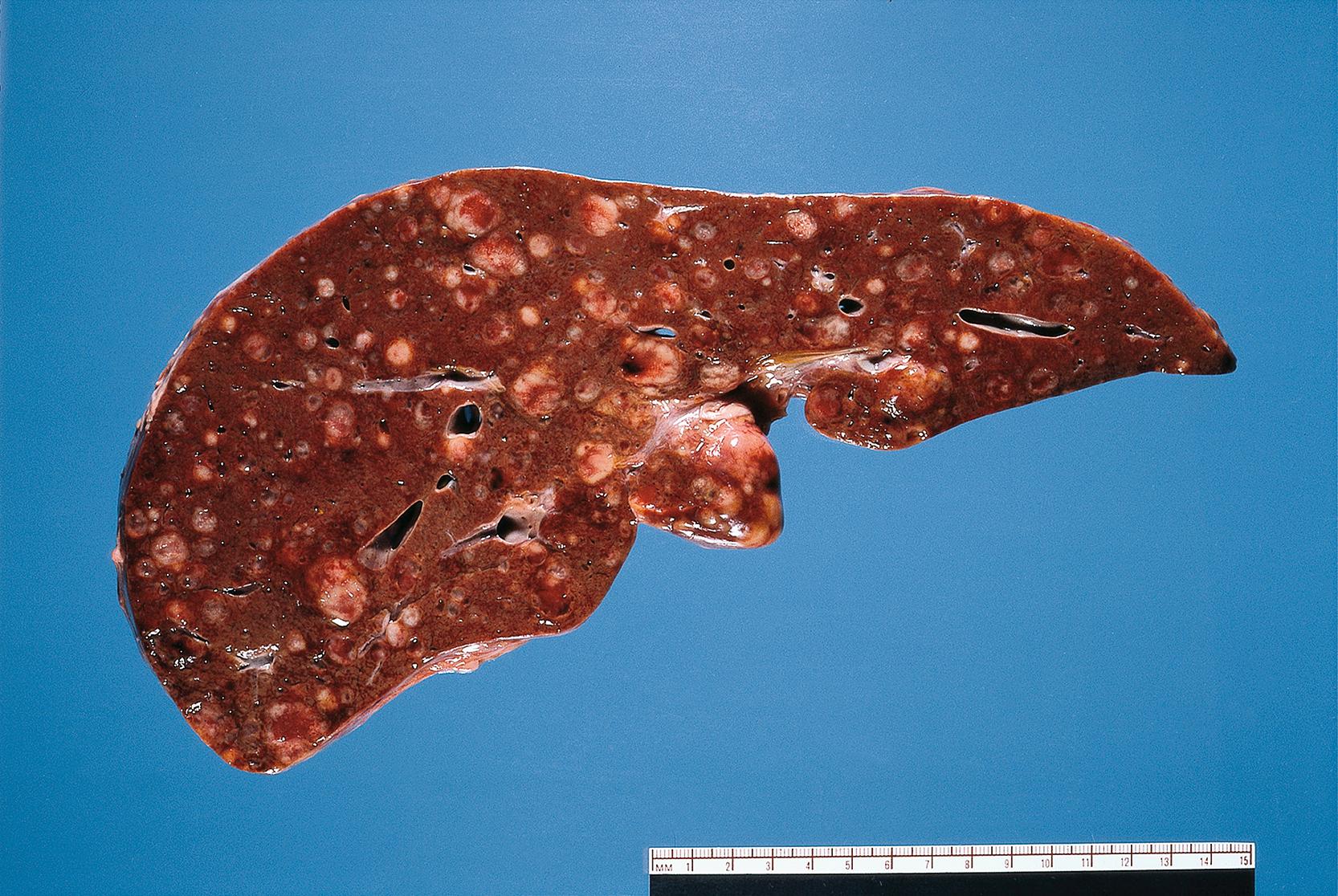
Vascular spread. Tumours can spread via the veins draining the primary site. Gut tumours tend to spread via the portal vein to the liver where secondary tumours are very common. In the systemic circulation, neoplastic cells may be trapped in the lung to form pulmonary metastases ( Fig. 7.16 ).
Transcoelomic spread. Certain tumours can spread directly across coelomic spaces, for example across the peritoneal or pleural cavities. Carcinoma of the ovary may spread transcoelomically to produce large numbers of metastatic deposits on the peritoneal surfaces.
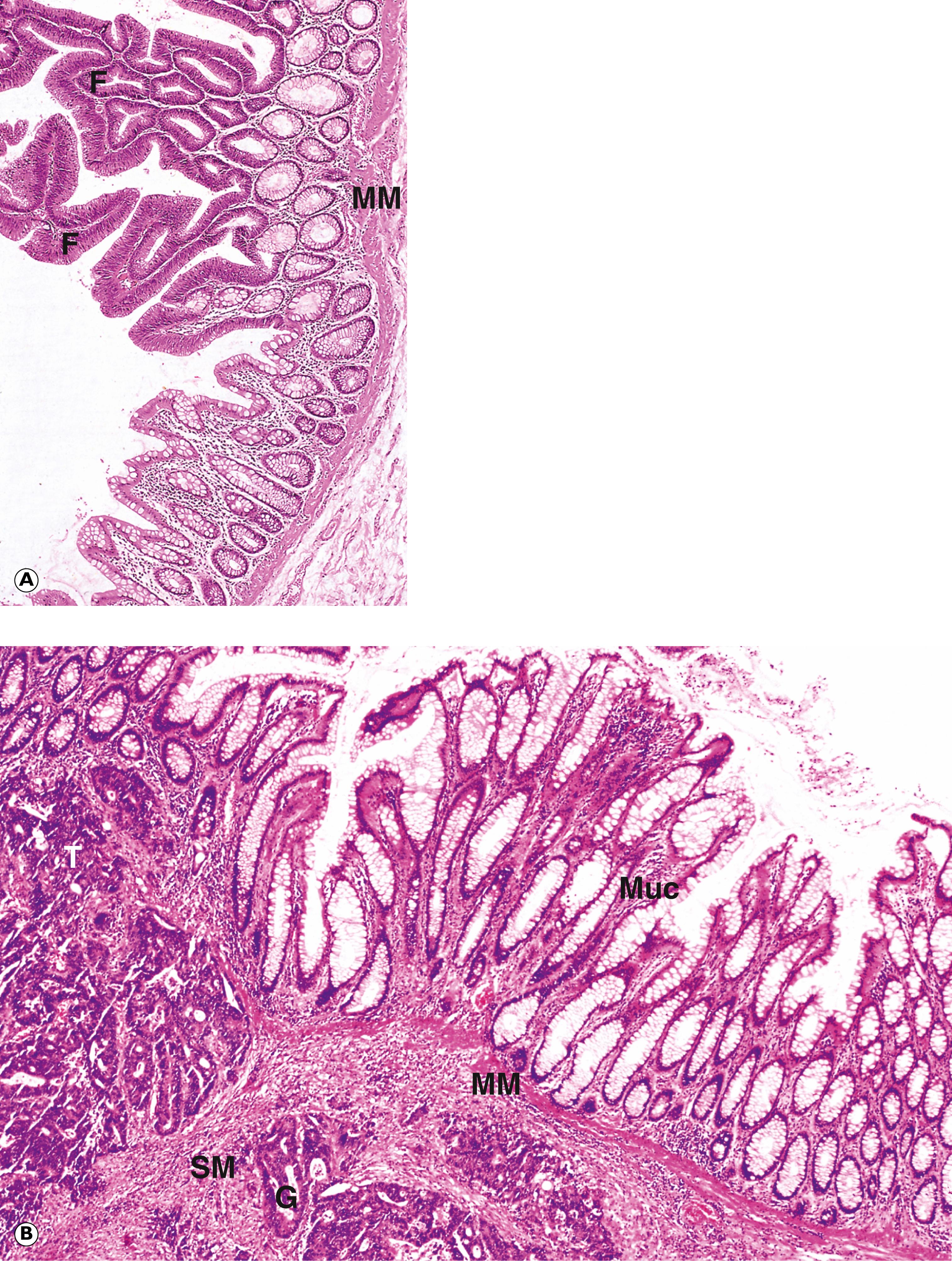
E epithelial cells F papillary fronds M mitotic figure MM muscularis mucosae Mu mucin Muc normal mucosa SM submucosa T tumour
C pseudocapsule Fa fatty tissue L leiomyoma Ly lymphatic M myometrium MS medullary sinuses S sinus St stroma T tumour cells
A arterioles L lymphatics M metastasis Me mesothelial cells N nerve fibres T tumour cells V venule
The extent of local, regional and distant tumour spread is an important determinant of tumour management and prognosis. Several systems have been devised for defining these characteristics in a standardised fashion; this is known as staging of a tumour. This is different from grading , described earlier in the chapter, which involves pathological assessment of the degree of differentiation of the tumour.
The TNM system is the most widely used method and involves scoring the extent of local T umour spread, regional lymph N ode involvement and the presence of distant M etastases. Despite advances in diagnostic techniques, the stage of a tumour is still a very good indicator of likely prognosis. Tumour stage assessment is also important in planning therapy. Tumours at an advanced stage (extensive spread) may require aggressive treatment, while early-stage tumours (localised) can be treatable by more conservative measures such as surgical excision alone. Staging is usually performed by a combination of histopathology, radiology and clinical assessments.
By way of example, the TNM (8 th Edition) for colonic cancer staging is illustrated in Table 7.4 .
One common route of the spread of cancer cells is via the lymphatic system to local (regional) lymph nodes ( Fig. 7.13 ). Cancer typically spreads in a predictable fashion to local nodes before involving more distant nodes.
Lymph node clearance (lymphadenectomy) of all the regional nodes in the vicinity of a tumour can result in significant morbidity for the patient due to lymphoedema (accumulation of excess tissue fluid due to disruption of normal draining after surgery). Sentinel node procedures aim to reduce this morbidity by identifying those patients with pathological evidence of lymph node spread and offering only these patients completion lymphadenectomy. Sentinel node biopsy can also be used to stage patients with advanced disease.
The sentinel node is the first node (or nodes) draining a tumour. In a sentinel node biopsy, radioactive tracer material is injected near to the tumour along with blue dye. A lymphoscintigram can be carried out pre-operatively to identify the exact location of a sentinel node on imaging. Intra-operatively, the sentinel node is identified as it should stain blue and is radioactive using a gamma probe. The node is often described as ‘hot and blue’ and is removed and sent to pathology for assessment. The node is often examined at multiple histological levels with additional tests such as immunohistochemistry performed to look for small groups or single tumour cells. Post-operative assessment using paraffin sections is more accurate than intra-operative frozen section in the identification of metastatic tumour cells. If metastatic tumour is present in the node, it is regarded as ‘positive’ and the patient will undergo a completion lymphadenectomy. Current research in this field involves rapid assessment of nodes intra-operatively by molecular techniques so that the patient only requires one operation.
Sentinel lymph node surgery is now commonplace for the surgical management of breast cancer and malignant melanoma.
| T0 | No evidence of primary tumour | N0 | No nodes involved | M0 | No distant metastases |
|---|---|---|---|---|---|
| T1 | Tumour invades submucosa | N1 | Spread to 1 to 3 regional nodes: N1a: 1 node N1b: 2–3 nodes N1c: no nodes positive but discontinuous tumour deposits in mesentery, sub-serosa or non-peritonealised tissues |
M1 | Distant metastases M1a: 1 organ without peritoneal metastasis M1b: 2+ organs without peritoneal metastasis M1c: metastasis to peritoneum |
| T2 | Tumour invades muscularis propria | N2 | Spread to 4 or more regional nodes N2a: 4–6 nodes N2b: 7+ nodes |
||
| T3 | Tumour invades subserosa | ||||
| T4 | a: Tumour invades visceral peritoneum OR b: Tumour invades other organs/structures |
| Definition/main features | Examples | Figure | |
|---|---|---|---|
| Dysplasia | Increased cell proliferation with failure of maturation | Uterine cervix | 7.1 |
| Skin | 7.1 | ||
| Carcinoma in situ | Features of carcinoma in an epithelium, but no invasion | 7.2 | |
| Uterine cervix | 7.7 | ||
| Skin | 21.14 | ||
| Breast | 18.9 | ||
| Epithelial tumours | Benign tumours either papillomas or adenomas | 7.11 | |
| Malignant tumours termed carcinomas | |||
| Squamous cell carcinoma | 7.3 | ||
| Transitional cell carcinoma | 7.4 | ||
| Colonic adenocarcinoma | 7.5 | ||
| Connective tissue tumours | Benign tumours named after tissue of origin with suffix - oma | 7.12A | |
| Malignant tumours named after tissue of origin with suffix - sarcoma | 7.6 | ||
| Leiomyosarcoma | 7.6 | ||
| Lymphoma | Solid tumours derived from cells of lymphoreticular system | Diffuse large B cell lymphoma | 7.7 |
| Melanoma | Tumour derived from melanocytes | 7.8 | |
| Teratoma | Tumour derived from germ cells | 7.9 | |
| Invasion | Spread of tumour to surrounding tissues | Breast carcinoma | 7.12C |
| Colonic adenocarcinoma | 7.11B | ||
| Perineural invasion | 7.17 | ||
| Metastasis | Distant spread of tumour by one of several mechanisms | Lymphatic spread | 7.13 |
| Haematogenous | 7.14 | ||
| Transcoelomic | 7.15 |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here