Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The granulomatous reaction pattern is defined as a distinctive inflammatory pattern characterized by the presence of granulomas. Granulomas are relatively discrete collections of histiocytes or epithelioid histiocytes with variable numbers of admixed multinucleate giant cells of varying types and other inflammatory cells. Conditions in which there is a diffuse infiltrate of histiocytes within the dermis, such as lepromatous leprosy, are not included in this reaction pattern. The group is subdivided by way of
the arrangement of granulomas;
the presence of accessory features such as central necrosis, suppuration, or necrobiosis; and
the presence of foreign material or organisms.
It is difficult to present a completely satisfactory classification of the granulomatous reactions. As Hirsh and Johnson remark in their review of the subject, “Sometimes a perfect fit can be achieved only with the help of an enlightened shove.” Many conditions described within this group may show only nonspecific changes in the early evolution of the inflammatory process and in a late or resolving stage show fibrosis and nonspecific changes without granulomas. Occasionally, a variety of granuloma types may be seen in one area, such as in reactions to foreign bodies or around ruptured hair follicles.
It is necessary in any granulomatous dermatitis to exclude an infectious cause. In some countries, up to 90% of granulomas have an infectious etiology. Culture of fresh tissue as well as histological search increases the chances of identifying a specific infectious agent. The time-consuming examination of multiple sections may be necessary to exclude such a cause. Special stains for organisms may be indicated. Occasionally, fungi are shown only by silver stains, such as Grocott methenamine silver, and not by periodic acid–Schiff (PAS) staining. All granulomas should be examined under polarized light to detect or exclude birefringent foreign material.
Considerable advances have been made in the understanding of the formation and maintenance of granulomas in tissue reactions and the roles played by B and T lymphocytes and cytokines. It is also clear that there are several types of macrophages in granulomas, particularly at an ultrastructural level. The different forms of multinucleate giant cells seen in granulomas may simply reflect the types of cytokines being produced by the component cells. This new information has not so far been shown to be useful in routine diagnostic problems. The glioma-associated oncogene homologue gli-1 is expressed in lesional skin in patients with cutaneous sarcoidosis, granuloma annulare, and necrobiosis lipoidica. These findings provide a rationale for clinical trials of inhibitors of gli-1 signaling, including tacrolimus and sizolimus, for the treatment of these conditions. Polymerase chain reaction (PCR) techniques have proved useful in detecting infectious agents in tissue sections, particularly mycobacterial species.
The following types of granulomas form the basis of the subclassification of diseases in this reaction pattern:
Sarcoidal : Granulomas composed of epithelioid histiocytes and giant cells with a paucity of surrounding lymphocytes and plasma cells (“naked” granulomas)
Tuberculoid : Granulomas composed of epithelioid histiocytes, giant cells of Langhans and foreign body type with a more substantial rim of lymphocytes and plasma cells and sometimes showing central “caseation” necrosis. Granulomas have a tendency toward confluence.
Necrobiotic (collagenolytic) : Granulomas are usually poorly formed and there are collections, and a more diffuse array, of histiocytes, lymphocytes, and giant cells with associated “necrobiosis” (collagenolysis). The inflammatory component may be admixed with the necrobiosis or form a palisade around it.
Suppurative : Granulomas composed of epithelioid histiocytes and multinucleate giant cells with central collections of neutrophils. Chronic inflammatory cells are usually present at the periphery of the granulomas.
Foreign body : Granulomas composed of epithelioid histiocytes, multinucleate (foreign body–type) giant cells, and variable numbers of other inflammatory cells. There is identifiable foreign material, either exogenous or endogenous in origin.
Xanthogranulomas : Granulomas composed of numerous histiocytes with foamy/pale cytoplasm with a variable admixture of other inflammatory cells and some Touton giant cells.
Miscellaneous : A category in which the granulomas may be variable in appearance or do not always fit neatly into one of the previous categories.
Many of the conditions exhibiting a granulomatous tissue reaction are discussed in other chapters; they are mentioned only briefly.
The various granulomatous diseases are discussed, in order, according to the classification listed previously.
Sarcoidal granulomas are found in sarcoidosis and in certain types of reaction to foreign materials and squames.
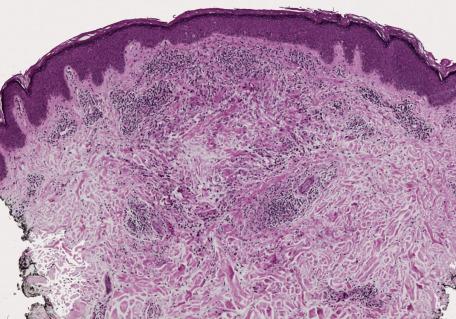
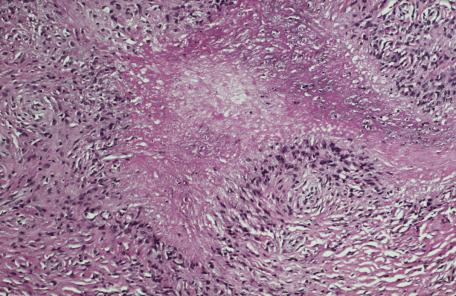
The prototypic condition in this group is sarcoidosis. Sarcoidal granulomas are discrete, round to oval, and composed of epithelioid histiocytes and multinucleate giant cells that may be of either Langhans or foreign body type. Generally, the type of multinucleate histiocyte present in a granuloma is not helpful in arriving at a specific histological diagnosis. Giant cells may contain asteroid bodies, conchoidal bodies (Schaumann bodies), or crystalline particles. Typical granulomas are surrounded by a sparse rim of lymphocytes and plasma cells, and only occasional lymphocytes are present within them. Consequently, they have been described as having a “naked” appearance. Although the granulomas may be in close proximity to one another, their confluence is not commonly found. With reticulin stains, a network of reticulin fibers is seen surrounding and permeating the histiocytic cluster.
Sarcoidal granulomas can be found in the following circumstances:
Sarcoidosis
Blau's syndrome
Foreign body reactions ( Table 8.1 )
| Silica (including windshield glass) |
| Silicone |
| Tattoo pigments |
| Zirconium |
| Beryllium |
| Zinc |
| Acrylic or nylon fibers |
| Keratin from ruptured cysts (uncommon) |
| Exogenous ochronosis |
| Sea-urchin spines |
| Cactus |
| Wheat stubble |
| Desensitizing injections |
| Ophthalmic drops (sodium bisulfite) |
| Sulfonamides |
| Interferon injection sites |
Secondary syphilis
Sézary syndrome
Metastatic Crohn's disease
Orofacial granulomatosis
Granuloma annulare (sarcoidal type)
Herpes zoster scars
Systemic lymphomas
Breast cancer
Common variable immunodeficiency (see p. 236 )
X-linked hyper–immunoglobulin M (IgM) syndrome
Tumor necrosis factor α (TNF-α) inhibitors
Nijmegen breakage syndrome
Sarcoidal granulomas are exceedingly rare in secondary syphilis (see p. 713 ), Sézary syndrome, herpes zoster scars (see p. 768 ), systemic lymphomas, and breast cancer. The granulomas in metastatic Crohn's disease and orofacial granulomatosis are often of mixed sarcoidal and tuberculoid types. These diseases are considered with the miscellaneous granulomatous diseases (see p. 232 ). The sarcoidal variant of granuloma annulare is discussed with other types of this condition (see p. 221 ). They are not considered further in this section.
Sarcoidosis is a multisystem disease that may involve any organ of the body but most commonly affects the lungs, lymph nodes (mediastinal and peripheral), skin, liver, spleen, and eyes. Central nervous system involvement is uncommon. There is an increased incidence of sarcoidosis in people of Irish and Afro-Caribbean origin. Cutaneous sarcoidosis is rare in Asia.
Between 10% and 35% of patients with systemic sarcoidosis have cutaneous lesions. Although sarcoidosis is usually a multiorgan disease, chronic cutaneous lesions may be the only manifestation. The skin lesions may be specific, showing a granulomatous histology, or nonspecific. The most common nonspecific skin lesion is erythema nodosum, which is said to occur in 3% to 25% of cases. Sarcoidosis of the knees is often associated with erythema nodosum. An erythema nodosum–like eruption with the histological changes of sarcoidosis has also been described. Sarcoidosis predominantly affects adults; skin lesions are rarely seen in children. There are reports of its occurrence in monozygotic twins. Children presenting before the age of 4 years usually have the triad of rash, uveitis, and arthritis, without apparent pulmonary involvement. This early-onset sarcoidosis (OMIM 609464 ) is caused by mutations in the NOD2/CARD15 gene on chromosome 16q12, similar to Blau's syndrome (see later). Vasculitis may also occur in this group.
A diversity of clinical forms of cutaneous sarcoidosis occurs. These forms include the following:
Papules, plaques, and nodules, which may be arranged in an annular or serpiginous pattern.
A maculopapular eruption associated with acute lymphadenopathy, uveitis, or pulmonary involvement.
Plaques with marked telangiectasia (angiolupoid sarcoidosis).
Lupus pernio, consisting of violaceous nodules, particularly on the nose, cheeks, and ears.
Nodular subcutaneous sarcoidosis. This form was diagnosed in 10 of 85 patients (11.8%) in one series. Lesions were most commonly located on the extremities, particularly the forearms, where indurated linear bands were sometimes found. It may occur on the breast, mimicking breast carcinoma. Subcutaneous sarcoidosis is the only specific subset of cutaneous sarcoidosis commonly associated with systemic disease.
A miscellaneous group that includes cicatricial alopecia ; an acral form ; ichthyosiform sarcoidosis ; ulcerative, necrotizing, morpheaform, and mutilating forms ; verrucous lesions ; atrophic lesions ; discoid lupus erythematosus–like lesions ; and erythroderma. Cutaneous sarcoidal granulomas have also been reported at the site of cosmetic filler injections in patients with or without systemic sarcoidosis.
This miscellaneous group represents rare cutaneous manifestations of sarcoidosis. Lupus pernio may resolve with fibrosis and scarring and is often associated with involvement of the upper respiratory tract and lungs. Oral, eyelid, and scrotal lesions have been reported. The majority of skin lesions resolve without scarring. Hypopigmented macules without underlying granulomas have been described, particularly in patients of African descent, although larger hypopigmented patches with underlying granulomas have also been described. Other rare presentations have included leonine facies, faint erythema, palmar erythema, vasculitis, alopecia, and lesions confined to the vulva. Sarcoidosis may follow the use of interferon therapy (IFN-α) for melanoma or hepatitis C. Sometimes the lesions are limited to injection site scars in these patients. Cutaneous sarcoidosis has also developed after treatment with pegylated IFN. It may also occur in patients with hepatitis C unrelated to treatment. It has also followed BCG vaccination. A seasonal, photo-induced variant of sarcoidosis has been rarely described. There have been several reports of cutaneous sarcoidosis, or sarcoid-like granulomas, associated with TNF-α inhibitors. This is particularly problematic because these agents have also been used for treatment of the disease. Sarcoidosis has also developed after rituximab therapy for pemphigus vulgaris. The authors suggest that one explanation may be that the immunological environment in the postrituximab period is similar to that in idiopathic sarcoidosis (e.g., via elevated levels of B-cell activating factor).
It is generally considered that the presence of cutaneous lesions in association with systemic involvement is an indicator of more severe disease. Skin lesions may occur in scars after trauma (including surgery, desensitizing injection sites, and venepuncture), cosmetic tattoos, irradiation, and chronic infection. In some cases, these lesions may be the first manifestation of sarcoidosis. Other cases do not appear to be related to systemic sarcoidosis and may be a sarcoidal reaction to a foreign body. Dermoscopy has been used to enhance the diagnosis of cutaneous sarcoidosis; findings suggesting granulomatous processes such as sarcoidosis include grouped translucent orange globules and linear vessels of various diameters.
Various systemic diseases have been reported in association with cutaneous sarcoidosis. The association in many of these conditions is probably fortuitous. They include B-cell chronic lymphocytic leukemia, cutaneous lymphoma, pyoderma gangrenosum, hypoparathyroidism, cryptococcal infection, dermatomyositis, systemic sclerosis, primary biliary cirrhosis, autoimmune thyroiditis and/or vitiligo, polycythemia vera, Wegener's granulomatosis, and HIV infection. In cases of HIV infection, sarcoidosis may develop after the commencement of highly active antiretroviral therapy (HAART) and the restoration of some immune function. The term immune restoration disease has been used for this circumstance. Subcutaneous granulomas and granulomatous tenosynovitis have been reported in an organ transplant recipient. Various malignancies have been reported in patients with sarcoidosis, perhaps somewhat more often in those with cutaneous involvement; these neoplasms usually follow the diagnosis of sarcoidosis and include a variety of solid tumors as well as leukemias/lymphomas. In fact, the coexistence of sarcoidosis and various lymphomas is known as the sarcoidosis–lymphoma syndrome . Among the associated lymphomas are Hodgkin's disease, non-Hodgkin lymphomas, and cutaneous T-cell lymphomas, including the recently reported association with folliculotropic peripheral T-cell lymphoma. The authors of the latter report found dense infiltration in lesional skin of CD30 + /CD163 + tumor-associated macrophages and suggest that these cells might play a role in recruitment of neoplastic T cells in lesional skin.
The cause of sarcoidosis remains controversial, although an infectious origin has long been suspected. The main candidate is a cell wall–deficient form of an acid-fast bacillus similar, if not identical, to Mycobacterium tuberculosis . In one study, DNA sequences coding for the mycobacterial 65-kDa antigen were found in 11 of 35 cases of sarcoidosis, whereas in a recent study, using only cutaneous specimens, mycobacterial DNA was demonstrated by PCR in 16 of 20 cases. Nontuberculous (atypical) mycobacteria constitute the largest group of the species identified in this study. Other findings supportive of a mycobacterial cause are the beneficial effects of long-term tetracyclines and the activation of a case after concurrent Mycobacterium marinum infection of the skin. In addition, CD4 + and CD8 + T-cell responses to mycobacterial epitopes are present in sites actively involved with sarcoidosis. In a 2005 study of 35 patients with sarcoidosis, M. tuberculosis rRNA was not detected in any case. Based on these results, the authors concluded that this organism cannot be considered as the causal agent of the disease. Evidence regarding a relationship between mycobacteria and sarcoidosis has been well summarized by Brownell et al. These authors conclude that molecular analysis and studies of humoral and cellular immune responses support the idea that mycobacterial antigens are involved in at least some cases of sarcoidosis. There has been recent commentary on the evidence for involvement of microbial antigens in the pathogenesis of sarcoidosis.
Another study concluded that there was no role for human herpesvirus type 8 (HHV-8) in the etiology, despite earlier reports suggesting a role.
Several studies have reported increased T helper (Th) 1 cytokines with increased levels of TNF-α in patients with sarcoidosis. Genes involved in Th1 and Th17 pathways are upregulated in skin lesions of sarcoidosis patients, at least when assessed at a single point in time.
An updated review of the etiology and immunopathogenesis of sarcoidosis has been published.
Regarding the influence of genes in the HLA region of chromosome 6 on the risk of the disease, Darlington et al. identified the allele combination of HLA-DRB1*04/*15 as a risk factor for extrapulmonary manifestations (including skin) of sarcoidosis.
The exact relationship of Blau's syndrome (OMIM 186580 ) to sarcoidosis is becoming clearer. It is characterized by the familial presentation of a sarcoid-like granulomatous disease involving the skin, uveal tract, and joints, but not the lung. Camptodactyly is another defining sign. Ichthyosis has been reported in one case. Onset is in childhood, and the mode of inheritance is autosomal dominant. It shares with early onset sarcoidosis mutations in the NOD2/CARD15 gene that maps to chromosome 16q12. Some patients with Crohn's disease also have mutations in this gene.
For limited cutaneous sarcoidosis, parenteral therapies have been the mainstay of therapy, but systemic therapy has been used for severe cutaneous disease, particularly if there is an aesthetic impact. Therapies that have been used include corticosteroids, hydroxychloroquine, chloroquine, allopurinol, methotrexate, isotretinoin, mepacrine, tacrolimus, thalidomide, various inhibitors of TNF-α such as adalimumab and infliximab, and thalidomide. The various therapies used to treat cutaneous sarcoidosis have been reviewed.
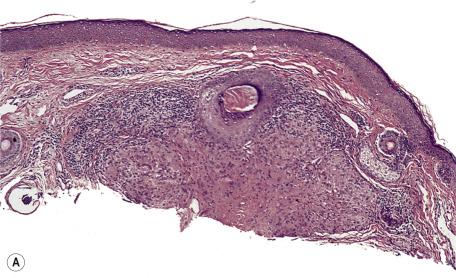
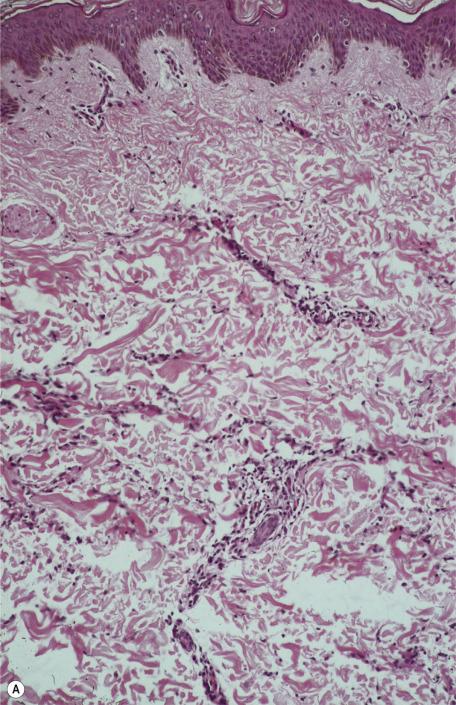
There is a dermal infiltrate of naked sarcoidal granulomas ( Fig. 8.1 ). Granulomas may be present only in the superficial dermis or they may extend through the whole thickness of the dermis or subcutis, depending on the type of cutaneous lesion. Interstitial and tuberculoid granulomas are uncommon. Both perineural and periadnexal localization of the granulomas have been reported. In one study, perineural granulomas were seen in 62% of patients and 55% of biopsies; interestingly, the predominant anatomical distribution of this finding (face, proximal extremities, trunk) was found to be similar to that of sarcoidosis small fiber neuropathy , a condition associated with sensory disturbances. A recent case of syringotropic sarcoidosis showed not only granulomas surrounding sweat glands but also decreased expression of dermicidin and aquaporin 5 in the involved sweat glands; both of these are expressed in sweat glands of normal controls. A granulomatous vasculitis is exceedingly rare. Necrosis is not usually seen in granulomas but has been reported ( Fig. 8.2 ). Small amounts of fibrinous or granular material may be seen in some granulomas. Increased mucin is present in approximately 20% of cases. Fibrinoid necrosis is said to be quite common in the cutaneous lesions of black South Africans. Slight perigranulomatous fibrosis may be present, but marked dermal scarring is unusual except in lupus pernio or necrotizing and ulcerating lesions. Fibrosis is often present in subcutaneous sarcoidosis. Touton-like giant cells may rarely be present.
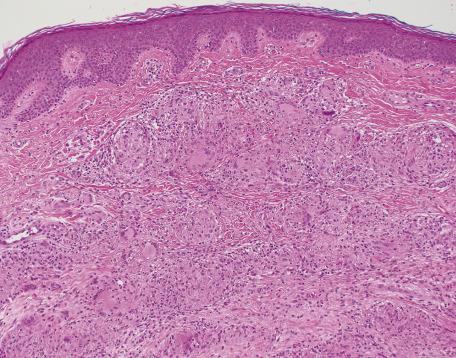

In subcutaneous sarcoidosis, the granulomas are usually limited to the subcutis without any dermal extension. The infiltrate is predominantly lobular with little or no septal involvement. Discrete foci of necrosis may be present in the center of some granulomas in a few cases. Perigranulomatous fibrosis, sometimes encroaching on the septa, is also present.
Overlying epidermal hyperplasia occurs in verrucous lesions, and hyperkeratosis occurs in the rare ichthyosiform variant. A lichenoid variant has been reported. There was a thick band-like infiltrate of sarcoidal granulomas with basal apoptotic keratinocytes. Otherwise, in most cases, the overlying epidermis is normal or atrophic.
Transepidermal elimination has been reported in sarcoidosis, and the histology shows characteristic elimination channels. In some cases, the round cell infiltrate surrounding the granulomas is more intense and the granulomas are less discrete. The diagnosis of sarcoidosis may then become one of exclusion.
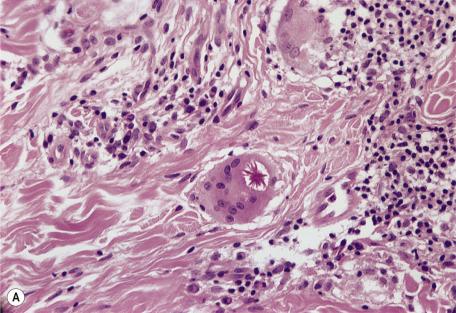
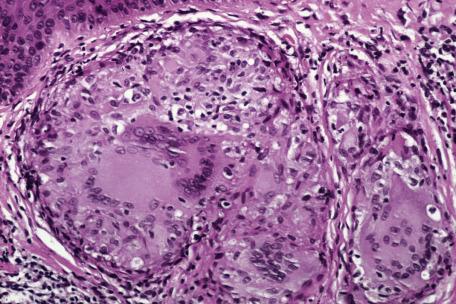
Asteroid bodies and conchoidal bodies (Schaumann bodies) may be seen in multinucleate giant cells but are not specific for sarcoidosis and may occur in other granulomatous reactions including tuberculosis ( Fig. 8.3A,B ). Schaumann bodies, which are shell-like calcium-impregnated protein complexes, are much more common in the granulomas of sarcoidosis than in those of tuberculosis. Mycobacterial antigens and lysosomal components have been found immunohistochemically within these bodies. Birefringent material has been found in the granulomas from 22% to 50% of cases. Foreign bodies are more common in sarcoidal granulomas from sites subject to minor trauma, such as the knee. Furthermore, patients with sarcoidosis can develop granulomas at the sites of implantation of foreign material. Electron probe microanalysis has identified calcium, phosphorus, silicon, and aluminum in the birefringent material referred to previously. It is thought that the calcium salts are probably the precursors of Schaumann bodies. Another explanation for the material is that it represents foreign material inoculated during a previous episode of inapparent trauma leading to granuloma formation subsequently in a patient with sarcoidosis. Asteroid bodies are said by some to be formed from trapped collagen bundles or from components of the cytoskeleton, predominantly vimentin intermediate filaments.
Biopsies taken from Kveim–Siltzbach skin test sites, sometimes used in the past for the diagnosis of sarcoidosis, show a variety of changes ranging from poorly formed granulomas with a heavy mononuclear cell infiltrate to small granulomas with few mononuclear cells. Measurement of the serum angiotensin-converting enzyme (ACE) level has now replaced this skin test.
Immunohistochemical marker studies have shown that the T lymphocytes expressing the suppressor/cytotoxic phenotype (CD8 + ) are found predominantly in the perigranulomatous mantle, whereas those expressing the helper/inducer phenotype (CD4 + ) are present throughout the granuloma. B lymphocytes are also present in the mantle zone.
Immunofluorescence studies in some cases have shown IgM at the dermoepidermal junction, IgM within blood vessel walls, and IgG within and around the granuloma. A fibrin network is present within granulomas.
Dermoscopic descriptions of sarcoidosis lesions vary somewhat, but among the common findings are translucent orange or yellow-orange areas, scar-like depigmented areas whitish or whitish-yellow scars, and linear and branching vessels.
When initially working up a case in which sarcoidosis is a potential diagnosis, polarization microscopy should be performed (while recognizing that sarcoidal lesions can sometimes show polarizable material), along with at least a fungal (PAS or methenamine silver) and acid-fast stain. Lesions proving to be infectious most often show a degree of suppuration (neutrophil accumulation) or tuberculoid granuloma formation. Other granulomatous lesions may show focal noncaseating granulomas, but in other areas there are often other types of granuloma. For example, the sarcoidal variant of granuloma annulare will often show other areas, in the same lesion or in other lesions, with interstitial infiltration or with mucinous necrobiosis. At the same time, it is important to note that lesions with the microscopic characteristics of necrobiosis lipoidica or granuloma annulare have been reported in patients with sarcoidosis on numerous occasions. So-called metastatic Crohn's disease can also show noncaseating granulomas, but a distinction can be made through an appreciation of lesional distribution and the association with inflammatory bowel disease. Another significant consideration in the differential diagnosis is tuberculoid leprosy, in which the granulomas can have a close resemblance to those of sarcoidosis. However, these granulomas tend to follow the course of nerves in the dermis, thereby often having elongated or “sausage-shaped” contours. Degenerated nerve elements can often be seen within the granulomas, demonstrable with S100 immunostaining. Although Mycobacterium leprae organisms are rarely found, at least in pure tuberculoid leprosy, their presence can be inferred through PCR studies. A reticulin stain may be helpful in difficult cases, in that there is a complex reticulin meshwork in the granulomas of sarcoidosis, but this feature is not observed in lesions of tuberculoid leprosy. In a study by Utino et al., features having a significant predictive value for tuberculoid leprosy were a predominance of tuberculoid granulomas in an adnexal and neural distribution, and granulomas replacing nerves within sweat gland glomeruli; those predictive of sarcoidosis were dermal fibrosis, back-to-back distribution of granulomas, atypical giant cells and plasma cells, greater numbers of conventional giant cells, and spared nerves beside granulomas. In their study, an analysis of reticulin fiber density using the Gomori silver impregnation stain did not discriminate between the two diseases using logistic regression analysis; however, there was support for the impression of fiber fragmentation within the granulomas of tuberculoid leprosy.
A number of foreign substances and materials when introduced into the skin may induce a granulomatous dermatitis that histologically resembles sarcoidosis. They are listed in Table 8.1 .
After some kind of trauma, silica may contaminate a wound in the form of dirt, sand, rock, or glass (including windshield glass from motor vehicles). Papules and nodules arise in the area of trauma. The granulomas seen in the dermal reaction contain varying numbers of Langhans or foreign body giant cells, some of which may contain clear colorless particles. These may be difficult to see with routine microscopy but are birefringent in polarized light. The differentiation from true sarcoidosis may be difficult because granulomas sometimes develop in scars in sarcoidosis and the granulomas can contain birefringent calcite crystals. A sarcoidal reaction to identifiable foreign material does not exclude sarcoidosis. It has been suggested that particulate foreign material may serve as a nidus for granuloma formation in sarcoidosis. Silica-rich birefringent particles have also been described in lesions without a history of injury or silica exposure. Energy-dispersive X-ray analysis techniques using scanning electron microscopy can be used to identify elements present in the crystalline material. The granulomas are thought to develop as a response to colloidal silica particles and not as a result of a hypersensitivity reaction.
A granulomatous dermatitis may occur in response to pigments used in tattooing (see p. 480 ). The skin lesions are sometimes limited to certain areas of a tattoo where a particular pigment has been used. Two patterns are seen—a foreign body type and a sarcoid type. In the latter form, there are aggregates of epithelioid histiocytes and giant cells with a sparse perigranulomatous round cell infiltrate. The histiocytes and giant cells contain pigment particles. True sarcoidosis has also been reported in tattoos, in some cases associated with pulmonary hilar lymphadenopathy. Some of these cases may represent a generalized sarcoid-like reaction to tattoo pigments rather than true sarcoidosis. Other granulomatous complications of tattoos include tuberculosis cutis and leprosy.
Sarcoidal granulomas may occur as a rare complication of ear piercing. It is not always a consequence of the trauma/scarring but may represent a contact allergy to nickel and other metals.
Zirconium compounds used in underarm deodorants and other skin preparations have been associated with a granulomatous skin reaction in sensitized individuals. Histologically, the lesions are identical to sarcoidosis. Usually no birefringent material is seen in polarized light. In one case of a reaction to an aluminum–zirconium complex, foreign body granulomas and birefringent particles were seen as well as tuberculoid-type granulomas. Ophthalmic drops containing sodium bisulfite have been implicated in the formation of pigmented papules on the face. The papules were caused by sarcoidal granulomas with brown-black pigment in foreign body giant cells.
Sarcoidal granulomas have been reported at the injection sites of interferon—both IFN-β-1b, used in the treatment of multiple sclerosis, and IFN-α-2a, used in the treatment of hepatitis C.
In the past, cutaneous granulomatous lesions with histology similar to sarcoidosis have been reported in persons exposed to beryllium compounds in industry. Other foreign bodies capable of inducing sarcoidal granulomas include acrylic and nylon fibers, wheat stubble, and sea urchin spines. Sea urchin spines can produce all types of granuloma, not just sarcoidal ones. The nature of the granulomatous reaction that followed the use of hydroxyurea is uncertain. It may have been sarcoidosis.
Occasionally, keratin from ruptured cysts or hair follicles can induce sarcoidal granulomas rather than the more usual foreign body type of reaction.
Although the granulomas in the tuberculoid group consist of collections of epithelioid histiocytes, including multinucleate forms, they tend to be less circumscribed than those in the sarcoidal group, have a greater tendency to confluence, and are surrounded by a substantial rim of lymphocytes and plasma cells. Langhans giant cells tend to be more characteristic of this group, but foreign body-type giant cells are also seen. There may be areas of caseation in the lesions of tuberculosis.
Tuberculoid granulomas are seen in the following conditions:
Tuberculosis
Tuberculids
Leprosy
Fatal bacterial granuloma
Late syphilis
Leishmaniasis
Rosacea
Idiopathic facial aseptic granuloma
Perioral dermatitis
Lupus miliaris disseminatus faciei
Crohn's disease
In addition to these diseases, tuberculoid granulomas, some with central necrosis, have been described at injection sites of protamine–insulin, as a hypersensitivity reaction to protamine. One case, consisting of both tuberculoid and sarcoidal granulomas, followed Q fever vaccination. The granulomas occurring in the homolateral limb after previous mastectomy, although described as sarcoidal, were more of a tuberculoid nature.
Typical tuberculoid granulomas can be seen in the dermal inflammatory reaction of late primary inoculation tuberculosis, late miliary tuberculosis, tuberculosis cutis orificialis, tuberculosis verrucosa cutis (“prosector's wart”), scrofuloderma, and lupus vulgaris. Cutaneous tuberculosis is discussed in detail with the bacterial infections (see p. 687 ). A similar pattern of inflammation can be seen after BCG vaccination and immunotherapy.
Santa Cruz and Strayer have stressed the variety of histological changes seen in cutaneous tuberculosis. In many forms, particularly in early lesions, there is a mixture of inflammatory cells within the dermis that includes histiocytes and multinucleate cells without well-formed epithelioid granulomas. The changes seen in the overlying epidermis are variable. In some forms, inflammatory changes extend into the subcutis. Areas of caseation may or may not be present within granulomas. In some cases, this may be difficult to distinguish from the necrobiosis seen in rheumatoid nodules (see p. 228 ). The number of acid-fast organisms varies in different lesions. In lesions with caseation, organisms are most frequently found in the centers of necrotic foci. Generally, where there are well-formed granulomas without caseation necrosis, organisms are absent or difficult to find. Neutrophils may be a component of the inflammatory infiltrate, and abscesses form in some clinical subtypes. Both Schaumann bodies and asteroid bodies can occasionally be present in multinucleate giant cells.
In lesions with caseation and demonstrable acid-fast organisms, the histological diagnosis may be straightforward. In lupus vulgaris, however, caseation necrosis, if present, is minimal and organisms are rarely found. Evidence of mycobacterial infection may be determined by PCR techniques.
There is usually a heavier round cell infiltrate about tuberculous granulomas than is seen in sarcoidosis. Remember that the small foci of fibrinous material that sometimes are seen in the granulomas of sarcoidosis may mimic and be mistaken for caseation. Epidermal changes and dermal fibrosis are not commonly part of the histopathology of sarcoidosis.
Lesions of other nontuberculous mycobacterial infections of the skin, such as those caused by M. marinum , may be histologically indistinguishable from cutaneous tuberculosis. Organisms are usually difficult to find in M. marinum infections; they are described as being longer and broader than typical M. tuberculosis . Culture or PCR is required for species identification.
The combination of marked irregular epidermal hyperplasia, epidermal and dermal abscesses, and dermal tuberculoid granulomas may be seen in tuberculosis verrucosa cutis, caused by M. tuberculosis , and in swimming pool granuloma caused by M. marinum infection. This reaction pattern is also seen in cutaneous fungal infections such as sporotrichosis, chromomycosis, and blastomycosis. Diagnosis depends on identification of the appropriate organism.
The lesions of tuberculosis cutis orificialis must be distinguished from those of oral or anal Crohn's disease and from the Melkersson–Rosenthal syndrome (see p. 232 ). This is not always possible on histological grounds and may depend on clinical history and associated lesions. Foci of caseation and acid-fast organisms may be seen in tuberculous lesions. Marked edema and granulomas related to or in the lumen of dilated lymphatic channels are present in the Melkersson–Rosenthal syndrome.
The tuberculids are a heterogeneous group of cutaneous disorders associated with tuberculous infections elsewhere in the body or in other parts of the skin (see p. 690 ). They include lichen scrofulosorum, papulonecrotic tuberculid, and erythema induratum–nodular vasculitis. Although it has previously been thought that organisms are not found in tuberculids, recent PCR studies have demonstrated mycobacterial DNA in some cases (see p. 690 ).
In lichen scrofulosorum , there is a superficial inflammatory reaction about hair follicles and sweat ducts that may include tuberculoid granulomas. Acid-fast organisms are not usually seen or cultured from the lesions. Caseation is rare.
Histopathological studies of papulonecrotic tuberculid have shown a subacute or granulomatous vasculitis and dermal coagulative necrosis with, in some cases, a surrounding palisading histiocytic reaction resembling granuloma annulare. Acid-fast bacilli are not found in the lesions. Tuberculoid granulomas were not described in one study but have been recorded in others.
In erythema induratum–nodular vasculitis , there is a lobular panniculitis, although tuberculoid granulomas usually extend into the deep dermis (see p. 570 ).
Tuberculoid granulomas are seen in the tuberculoid (TT), borderline tuberculoid (BT), and borderline (BB) groups of the classification of leprosy introduced by Ridley and Jopling. Leprosy is considered further on p. 694 .
In tuberculoid leprosy (TT), single or grouped epithelioid granulomas with a peripheral rim of lymphocytes are distributed throughout the dermis and subcutis. Unlike lepromatous leprosy, this infiltrate does not spare the upper papillary dermis (grenz zone), and it may extend into and destroy the basal layer and part of the stratum malpighii. The granulomas are characteristically arranged in and around neurovascular bundles and arrectores pilorum muscles. Granulomas, particularly in the deeper parts of the infiltrate, tend to be oval and elongated along the course of the nerves and vessels ( Fig. 8.4 ). Small cutaneous nerve bundles are infiltrated and enlarged by the inflammatory cells. There may be destruction of nerves, sometimes with caseation necrosis that may mimic cutaneous tuberculosis. In contrast, the infiltrate in tuberculosis is not particularly related to nerves. The granulomas in tuberculoid leprosy may contain well-formed Langhans-type giant cells and less well-formed multinucleate foreign body giant cells. The causative organism, M. leprae, which is best demonstrated by modifications of the Ziehl–Neelsen stain such as the Wade–Fite method, is usually not found in the lesions of tuberculoid leprosy. Rare organisms may be present in nerve fibers. PCR has been used to demonstrate DNA of M. leprae in lesions.
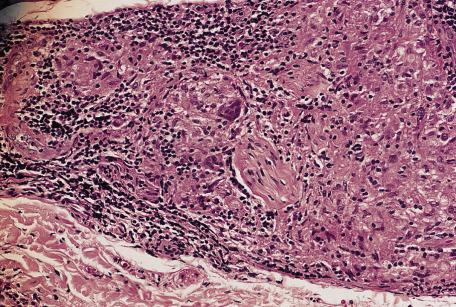
Granulomas in the borderline tuberculoid form (BT) are surrounded by fewer lymphocytes, contain more foreign body giant cells than Langhans cells, and may or may not extend up to the epidermis. Organisms may be found in small numbers or not at all. Nerve bundle enlargement is not so prominent, and there is no caseation necrosis or destruction of the epidermis.
In the borderline form (BB), the granulomas are poorly formed and the epithelioid cells separated by edema. Scant lymphocytes are present about the granulomas, and there are no giant cells. Nerve involvement is slight. Organisms are found, usually only in small numbers.
Fatal bacterial granuloma was first reported in the English literature in 2002 under the title “fatal bacteria granuloma after trauma: a new entity.” The cases were reported from rural China. They were characterized by spreading, dark red plaques that followed slight trauma to the face. The patients had severe headache and clouding of consciousness during the later stages of the disease. All patients died within 1.5 to 4 years.
Electron microscopy demonstrated two types of bacteria: one was an anaerobic actinomycete, which was sensitive to lincomycin (a forerunner of clindamycin), and the other organism was a Staphylococcus . The unknown actinomycete was regarded as the probable causative agent. One paper reported finding Propionibacterium acnes , which seems unusual. A recent publication described the culture and sensitivity results from 21 cases of fatal bacterial granuloma after facial and eyelid trauma. Twenty-two strains of anaerobic P. acnes were isolated from these patients. Sensitivity studies showed that these strains were resistant to metronidazole but sensitive to ciprofloxacin, penicillins, and a variety of other agents; combinations of antimicrobials were recommended for treatment.
The epidermis was normal and there was a heavy, diffuse dermal infiltrate of cells, mainly histiocytes. In addition, there were lymphocytes, plasma cells, neutrophils, and many multinucleated giant cells in some areas. There was vascular occlusion with focal hemorrhage and necrosis in the deep dermis.
Some lesions of late secondary syphilis and nodular lesions of tertiary syphilis show a superficial and deep dermal inflammatory reaction in which there are tuberculoid granulomas (see pp. 713, 715 ). Plasma cells are generally but not always prominent in the inflammatory infiltrate, and there may be swelling of endothelial cells. One study found that a plasma cell infiltrate and endothelial swelling, traditionally associated with syphilis of the skin, are in fact infrequently seen in biopsies. Organisms are rarely demonstrable in these lesions.
In chronic cutaneous leishmaniasis (see p. 791 ) and leishmaniasis recidivans, tuberculoid granulomas are present in the upper and lower dermis. The overlying epidermal changes are variable. Occasionally, the granulomas extend to the basal layer of the epidermis as in tuberculoid leprosy. Necrosis is not usually seen in the granulomas. Leishmaniae are usually scarce but may be found in histiocytes or, rarely, free in the dermis. The organisms have sometimes been mistaken for Histoplasma capsulatum but differ from the latter in having a kinetoplast.
Rosacea is characterized by persistent erythema and telangiectasia, predominantly of the cheeks but also affecting the chin, nose, and forehead (see p. 535 ). The lacrimal and salivary glands were affected in one case. In the papular form, papules and papulopustules are superimposed on this background. Tuberculoid granulomas are seen in the granulomatous form, which may present as a solitary plaque mimicking Morbihan's disease or solid facial edema of rosacea. Granulomatous rosacea has been reported in children as well as adults and also in association with infection with HIV. Granulomatous rosacea can be found in most clinical variants of rosacea. A rosacea-like granulomatous eruption developed in an adult patient using tacrolimus ointment in the treatment of atopic dermatitis. Granulomas may be a response to Demodex organisms or lipids. Granulomas have also been described in the lesions of pyoderma faciale, thought to be an extreme form of rosacea.
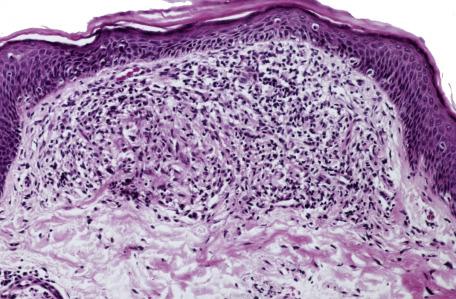
The changes seen in biopsies of the papules are variable and relate to the age of the lesion. Early lesions may show only a mild perivascular lymphocytic infiltrate in the dermis. In older lesions, there is a mixed inflammatory infiltrate related to the vessels or to vessels and pilosebaceous units. The infiltrate consists of lymphocytes and histiocytes with variable numbers of plasma cells and multinucleate giant cells of Langhans or foreign body type. In some lesions, epithelioid histiocytes and giant cells are organized into tuberculoid granulomas (granulomatous rosacea) ( Fig. 8.5 ). An acute folliculitis with follicular and perifollicular pustules and destruction of the hair follicle is sometimes seen. This corresponds to the perifollicular variant of granulomatous rosacea described by Sánchez et al. Granulomatous inflammation may be centered on identifiable ruptured hair follicles. At other times, the granulomas are distributed diffusely through the dermis. There is dermal edema and vascular dilatation. Epidermal changes, if present, are mild and nonspecific.
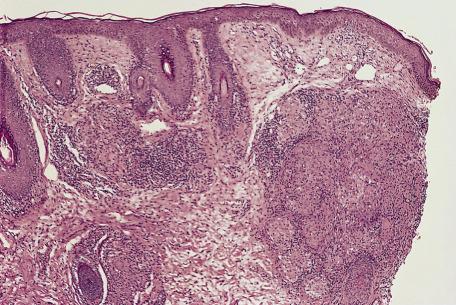
In granulomatous rosacea, the granulomas are usually of tuberculoid type and not the “naked” granulomas of sarcoidosis (see p. 536 ). Changes resembling caseous necrosis may be present, associated with a histiocytic reaction. In one series, necrosis was present in 11% of cases. Differentiation from lupus vulgaris may be difficult. In some cases of rosacea, the inflammatory changes may be related to damaged hair follicles. The presence of marked vascular dilatation is suggestive of rosacea.
Thirty cases of idiopathic facial aseptic granuloma, an unusual condition also termed pyodermite froide du visage , were originally reported from a single center in France. A number of additional cases have since been published, including cases from Italy, Spain, and the United States, and now the number of published cases totals more than 50. The disorder occurs in young children. The children present with one or several acquired painless nodules on the face, lasting for at least 1 month. There is no response to antibiotics, and no infectious agent has been identified. It has been suggested that the disease might belong to the spectrum of childhood rosacea. A granulomatous response to an embryological residue has also been considered. The putative association with rosacea has been based on both the microscopic features and the frequent association with conjunctivitis and chalazia. Ultrasound has been diagnostically useful in a number of cases. Spontaneous resolution is the rule.
The lesions are composed of perifollicular granulomas consisting of lymphocytes, plasma cells, histiocytes, epithelioid cells, some neutrophils, and numerous foreign body giant cells. In one case, the granulomas developed around a nonruptured epidermoid cyst. Foreign body giant cells would be unusual as the predominant feature in rosacea; accordingly, this etiological theory (discussed previously) seems unlikely.
Dermoscopy has shown an erythematous background, nonbranching linear vessels, a whitish perifollicular halo, and follicular plugs.
Perioral dermatitis is regarded by some as a distinct entity and by others as a variant of rosacea. The histological changes seen in perioral dermatitis and acne rosacea overlap, and clinical features are often more important in separating these two conditions.
Red papules, papulovesicles, or papulopustules on a background of erythema are arranged symmetrically on the chin and nasolabial folds with a characteristic clear zone around the lips. Lesions may occur less commonly on the lower aspect of the cheeks and on the forehead. A periocular variant has also been described. Perioral dermatitis mainly affects young women, but it has also been reported in children. In a review of patients with lip and perioral area dermatitis, Nedorost presented a useful table with the distinguishing features between a rosacea-type of perioral dermatitis, a steroid-induced type, and disease caused by irritants, allergic/photoallergic contact reactions, and atopic cheilitis. She suggests that an extended patch test series may be useful in making a diagnosis. Inhaled corticosteroids may also induce the disease.
Granulomatous perioral dermatitis of childhood is particularly seen in children of Afro-Caribbean descent. This form has been given the acronym FACE— f acial A fro-Caribbean c hildhood e ruption. This term is no longer used because of its rare occurrence in nonblack children. The term childhood granulomatous periorificial dermatitis is now preferred. Subtle clinical differences exist between this condition and perioral dermatitis and granulomatous rosacea, although it may still be a variant of one of these conditions. It has also been proposed as a childhood variant of lupus miliaris disseminatus faciei (see later). It is benign, self-limited, and typically resolves within a year of onset without scarring. Extrafacial lesions may occur. Its resolution seems to be hastened with the use of systemic antibiotics or with tacrolimus.
In many cases, perioral dermatitis appears to be related to the application of one or more cosmetic preparations that may act by occlusion. The use of strong topical corticosteroids, particularly fluorinated ones, may also have an etiological role. Recent cases have been related to the use of inhaled corticosteroids or an intranasal corticosteroid spray. Various types of toothpaste have been implicated. Perioral dermatitis has been reported in renal transplant recipients maintained on oral corticosteroids and azathioprine. It has also been associated with the wearing of the veil by Arab women.
Its relative rarity in patients with seborrheic dermatitis treated with corticosteroids has led to the postulate that perioral dermatitis may develop under fusiform bacteria-rich conditions rather than Malassezia -rich conditions as in the case of seborrheic dermatitis.
Topical metronidazole, erythromycin, and oral tetracyclines have been used as conventional treatments. Liquid nitrogen, benzoyl peroxide, and oral isotretinoin are other therapies. Three cases reported from Japan were successfully treated with a β-lactam antibiotic, cefcapene; fusobacteria were detected before treatment using a tape-stripping method and were negative after completion of treatment. Similar results have been obtained in a subsequent study. Topical pimecrolimus has been used successfully.
The histological changes in perioral dermatitis have been described as identical to those seen in rosacea. Others have found the epidermal changes to be more prominent than in rosacea, consisting of parakeratosis, often related to hair follicle ostia, spongiosis, which sometimes involves the hair follicle, and slight acanthosis. The changes in the dermis are similar to those in papular rosacea and consist of perivascular or perifollicular infiltrates of lymphocytes and histiocytes and vascular ectasia. Uncommonly, an acute folliculitis is present. Tuberculoid granulomas have been described in the dermis in some series but not in others.
In childhood granulomatous periorificial dermatitis, epithelioid granulomas are often perifollicular in distribution. This is not a feature of sarcoidosis. Furthermore, the granulomas are more tuberculoid than sarcoidal in type.
Although the cause of lupus miliaris disseminatus faciei is unknown, it may be related to rosacea. It has also been called acne agminata and acnitis. It is characterized by yellowish brown papules distributed over the central part of the face, including the eyebrows and eyelids. When widespread facial lesions are present, the appearances may mimic sarcoidosis. Occasionally, lesions occur elsewhere, including the posterior neck, palms and fingerwebs, extensor forearms and dorsa of hands and axillae. Many patients with extrafacial involvement also have facial lesions, but lesions have been limited to the axillae or posterior neck. The lesions last for months and heal with scarring. This condition occurs in both sexes, predominantly in adolescents and young adults and rarely in the elderly. It has been suggested that because the currently used name is confusing, a new title should be substituted—FIGURE ( f acial i diopathic g ran u lomas with r egressive e volution).
Studies using PCR techniques have failed to demonstrate the DNA of M. tuberculosis in lesional skin. One study using laser capture microdissection and PCR methodology found Propionibacterium acnes DNA material in all tested samples; the gene was also found in samples from normal skin, but in the latter cases the bands were regularly faint. This suggests that P. acnes may play a pathogenetic role in lupus miliaris, but it could also simply reflect the fact that lesions of lupus miliaris are follicular-based.
Successful treatment with the 1450-nm diode laser has been reported.
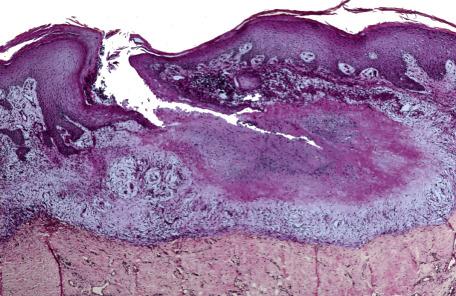
There are few histopathological studies of this condition. Biopsy appearances overlap those of both rosacea and perioral dermatitis. The characteristic lesion is an area of dermal necrosis, sometimes described as caseation necrosis, surrounded by epithelioid histiocytes, multinucleate giant cells, and lymphocytes ( Fig. 8.6A,B ). In many cases, granulomas appear related to ruptured pilosebaceous units. In one example from our files, caseation necrosis partly involved a ruptured follicle and was, in turn, surrounded by a palisade of epithelioid histiocytes. Nuclear fragments may be seen in the necrotic foci. Early lesions show superficial perivascular infiltrates of lymphocytes, histiocytes, and occasional neutrophils. Late lesions have these changes together with dermal fibrosis, particularly about follicles. Established lesions may show tuberculoid or suppurative granulomas. Demodex folliculorum were not seen in one study. Small vessel changes with necrosis of blood vessel walls, thrombi, and extravasated red blood cells have also been described.
One study of lysozyme in these lesions suggests that there is an immunological mechanism involved in the pathogenesis of this condition rather than a foreign body reaction to an unidentified dermal agent. Conversely, it has been suggested that the lesions represent a granulomatous reaction to damaged pilosebaceous units.
Those examples showing “caseous” necrosis are relatively specific for lupus miliaris disseminatus faciei, given the clinical context of the lesions and their relationship to hair follicles. In this author's view, lesions that show only perifollicular granulomas without caseation cannot be reliably distinguished from granulomatous rosacea or perioral/periorbital dermatitis and probably should not be considered diagnostic of lupus miliaris, unless other sampled lesions from the same individual show typical caseating granulomas. There is a resemblance to the palisading necrobiotic form of granuloma annulare, but the necrobiotic material in the latter is distinctly mucinous, and a relationship to a hair follicle is generally not observed in granuloma annulare. Despite the similarities to granulomatous tuberculosis with caseous necrosis, special stains are negative for acid-fast organisms in lupus miliaris. The tuberculoid granulomas of this condition differ from the naked tubercles of sarcoidosis, which do not display this type of necrobiosis but instead only occasionally display small foci of fibrinous material within granulomas; in addition, sarcoidosis can be excluded on the basis of other clinical and laboratory data.
Noncaseating granulomas of tuberculoid type may be found, rarely, in the dermis and subcutis in Crohn's disease (see p. 606 ). The term metastatic Crohn's disease is often used for the presence of multiple cutaneous lesions. Granulomas are not uncommon in the wall of perianal sinuses and fistulas. A granulomatous cheilitis has also been reported (see p. 606 ). It is important to exclude Crohn's disease in cases of apparent Melkersson–Rosenthal syndrome.
There is a report of cutaneous granulomas developing in a patient with histologically proven ulcerative colitis.
The term necrobiosis has been retained here because of common usage and refers to areas of altered dermal connective tissue in which, by light microscopy, there is blurring and loss of definition of collagen bundles, sometimes separation of fibers, a decrease in connective tissue nuclei, and an alteration in staining by routine histological stains, often with increased basophilia or eosinophilia. The term collagenolytic granuloma is favored by others. Granular stringy mucin is sometimes seen in such areas in granuloma annulare, and fibrin may be seen in rheumatoid nodules. Necrobiotic areas are partially or completely surrounded by a histiocytic rim that may include multinucleate giant cells. In some cases, histiocytes become more spindle-shaped and form a “palisade.”
Necrobiotic (collagenolytic) granulomas are found in the following conditions:
Granuloma annulare and its variants
Necrobiosis lipoidica
Necrobiotic xanthogranuloma
Rheumatoid nodules
Rheumatic fever nodules
Reactions to foreign materials and vaccines
Miscellaneous diseases
Granuloma annulare is a dermatosis, usually self-limited, of unknown cause and characterized by necrobiotic (collagenolytic) dermal papules that often assume an annular configuration. The skin or the subcutis or both may be involved. The clinical variants of granuloma annulare include localized, generalized, perforating, and subcutaneous or deep forms. Rare types include a follicular pustule variant, an acute-onset, painful acral form, and a patch form, although the latter cases are probably examples of the interstitial granulomatous form of drug reaction (see p. 237 ). The linear variant reported some years ago would now be regarded as a variant of interstitial granulomatous dermatitis (see p. 237 ). Classic granuloma annulare was preceded in one case by the development of a severe linear form along Blaschko's lines.
In the localized form, one or more erythematous or skin-colored papules are found. Grouped papules tend to form annular or arciform plaques. The hands, feet, arms, and legs are the sites of predilection in approximately 80% of cases. A papular umbilicated form has been described in children in which grouped umbilicated flesh-colored papules are limited to the dorsum of the hands and fingers. The generalized form accounts for approximately 15% of cases. Multiple macules, papules, or nodules are distributed over the trunk and limbs. Rarely, there may be confluent erythematous patches or plaques. The appearances may even simulate mycosis fungoides. It has been reported as a side effect of allopurinol, amlodipine, and TNF-α inhibitors ; the latter finding is somewhat contradictory in that TNF-α inhibitors have been used successfully as treatment of this form of the disease. Lesions in perforating granuloma annulare are grouped papules, some of which have a central umbilication with scale. The extremities are the most common site. The generalized form may also have perforating lesions. A high incidence of perforating granuloma annulare has been reported in Hawaii. It is rare in infants and young children. In subcutaneous (or deep) granuloma annulare , deep dermal or subcutaneous nodules are found on the lower legs, hands, head, and buttocks. These lesions are associated with superficial papules in 25% of cases. This group also includes those lesions described as pseudorheumatoid nodules, palisading subcutaneous granuloma, and benign rheumatoid nodules. Although arthritis does not usually occur in children with these nodular lesions, IgM rheumatoid factor has been found in serum in some cases. There is a report of one case occurring in association with juvenile rheumatoid arthritis. Computed tomography (CT) scan changes of this variant have been described. Rarely, the changes may involve deeper soft tissues and produce a destructive arthritis and limb deformity. Pseudorheumatoid nodules have also been reported in adults. A series of 14 cases all involved female patients, and most involved the small joints of the hand. Granuloma annulare was present at the periphery of the nodules in eight cases. The authors suggested the term juxta-articular nodular granuloma annulare for these cases.
Granuloma annulare has been reported as a seasonal eruption on the elbows or hands and from unusual sites such as the penis, the palms, about the eyes, and the ear. There has been one case of cutaneous granuloma annulare associated with histologically similar intraabdominal visceral lesions in a male patient with insulin-dependent diabetes.
Females are affected more than twice as commonly as males. The localized and deep forms are more common in children and young adults. The deep (subcutaneous) form has been reported as a congenital lesion. Generalized granuloma annulare occurs most often in middle-aged to elderly adults. Most cases of granuloma annulare are sporadic, but familial cases have occasionally been reported. Patients with the generalized form of the disease show a significantly higher frequency of HLA-BW35 compared with controls and with those who have the localized form of the disease.
Lesions of granuloma annulare have a tendency to regress spontaneously; however, approximately 40% of cases recur. Resolution of lesions subject to biopsy, but not other lesions, has been reported. In one series, spontaneous regression of localized lesions in children occurred from 6 months to 7 years, with a mean of 2.5 years. There has been an example of evolution of granuloma annulare to mid-dermal elastolysis. In the generalized form, the clinical course is chronic with infrequent spontaneous resolution and poor response to therapy. Nonperforating lesions are usually asymptomatic.
Although the cause and pathogenesis of the skin lesions in granuloma annulare remain uncertain, possible triggering events include insect bites, trauma, the presence of viral warts, drugs, erythema multiforme, and exposure to sunlight. Lesions have occurred in the scars of herpes zoster, localized or generalized, in a saphenectomy scar, in a Becker's nevus, and at the sites of tuberculin skin tests. The possible link between both the localized and generalized forms and diabetes mellitus remains controversial. There is more convincing evidence of this association with the generalized form than with the localized form, although there may be a weak association with nodular as opposed to the annular type of localized lesion. Significantly lower serum insulin levels have been found in children with multiple lesions of granuloma annulare. One study of two children with diabetes mellitus documented early, transient development of granuloma annulare, followed by the later development of persistent necrobiosis lipoidica. A case-control study failed to find any association between granuloma annulare and type 2 diabetes mellitus. Reported cases of the concurrence of these two diseases may represent the chance association of two not uncommon conditions. It has been reported in association with necrobiosis lipoidica ; sarcoidosis ; scabies ; Alagille syndrome ; toxic adenoma of the thyroid ; autoimmune thyroiditis ; uveitis ; hepatitis B and C infection ; photoinduction by paroxetine during treatment of hepatitis C infection with pegylated IFN-α ; Epstein–Barr virus infection ; parvovirus B19 infection ; BCG (including generalized granuloma annulare), hepatitis B, and antitetanus vaccination ; waxing-induced pseudofolliculitis ; tuberculosis ; tattoos ; granulomatous and patch-stage mycosis fungoides; a monoclonal gammopathy ; hypercalcemia ; myelodysplastic syndrome ; chronic myelomonocytic leukemia ; Hodgkin's lymphoma and non-Hodgkin's lymphoma, including cutaneous marginal zone lymphoma ; gastrointestinal stromal tumor ; and metastatic adenocarcinoma. In these conditions, the clinical presentation may be atypical. Localized, generalized, and perforating forms of granuloma annulare have been reported in patients with AIDS; the generalized form is the most common clinical pattern. It may, rarely, be the presenting complaint in AIDS. Bartonella infection has not been detected in lesions of granuloma annulare. Interstitial granuloma annulare has been reported in borreliosis, but it may represent a pattern related to the Borrelia infection.
It has been suggested that the underlying cause of the necrobiotic granulomas is an immunoglobulin-mediated vasculitis. In a recent study, neutrophils and neutrophil fragments were commonly present in early lesions, but a true vasculitis was rare. Direct immunofluorescence studies did not demonstrate immune deposits in vessel walls. Other studies have stressed the importance of cell-mediated immune mechanisms with a delayed hypersensitivity reaction of Th1 type against as yet undefined antigens. This is supported by the finding of increased levels of interleukin (IL)-18, IFN-γ, and IL-2 in lesional skin. Collagen synthesis is increased in the lesions of granuloma annulare, probably representing a reparative phenomenon.
The most widely used drugs are topical and systemic corticosteroids, but they are not always effective, and relapses may occur when they are discontinued. Other therapies have included cryosurgery; laser ; retinoids ; vitamin E and a 5-lipoxygenase inhibitor ; dapsone; chloroquine; UV-A1 phototherapy ; narrowband UV-B therapy ; combination therapy including calcineurin inhibitors ; imiquimod cream ; T-cell–directed therapies such as infliximab, efalizumab, etanercept, and adalimumab ; hydroxyurea ; methotrexate ; and various forms of laser therapy.
Three histological patterns may be seen in granuloma annulare—necrobiotic (collagenolytic) granulomas, an interstitial or “incomplete” form, and granulomas of sarcoidal or tuberculoid type. The third of these patterns is uncommon. In most histopathological studies, the interstitial form is most common.
In the form with necrobiotic (collagenolytic) granulomas , one or more areas of necrobiosis, surrounded by histiocytes and lymphocytes, are present in the superficial and mid-dermis ( Fig. 8.7 ). The peripheral rim of histiocytes may form a palisaded pattern ( Fig. 8.8 ). Variable numbers of multinucleate giant cells are found in this zone. Some histiocytes have an epithelioid appearance. An increased mitotic rate is found in the histiocytes in some cases. The histiocytes are CD68 + . Surprisingly, in one series, the histiocytic component of the infiltrate stained only for vimentin and lysozyme and not the other common histiocyte markers (HAM56, CD68 [KP1], Mac-387, and factor XIIIa). PGM1, the most specific histiocytic marker, is strongly expressed in all cases. A perivascular infiltrate of lymphocytes and histiocytes is also present; eosinophils are found in 40% to 66% of cases, but plasma cells are rare. Occasionally, there may be an accompanying superficial and deep, dense nodular lymphocytic infiltrate composed predominantly of T cells—features described in “pseudolymphoma.” Granulomatous perineural inflammation is an uncommon finding. The central necrobiotic areas contain increased amounts of connective tissue mucins that may appear as basophilic stringy material between collagen bundles. Neutrophils and nuclear dust contribute to the basophilic appearance. Special stains such as colloidal iron and Alcian blue aid in the demonstration of mucin. Heparan sulfate is present in addition to hyaluronic acid. Elastic fibers may be reduced, absent, or unchanged in the involved skin. The colocalization of granuloma annulare and mid-dermal elastolysis and granuloma annulare and elastolytic giant cell granuloma have also been reported.

Occasionally, neutrophils or nuclear fragments are present in necrobiotic areas. In the rare follicular–pustulous form, there are neutrophils in the upper portion of the follicles leading to pustule formation. Palisading necrobiotic granulomas surround hair follicles. An acute or subacute vasculitis has been described in or near foci of necrobiosis, associated with varying degrees of endothelial swelling, necrosis of vessel walls, fibrin exudation, and nuclear dust.
The lesions of subcutaneous or deep granuloma annulare have areas of necrobiosis that are often larger than in the superficial type ( Fig. 8.9 ). These foci are distributed in the deep dermis, subcutis, and, rarely, deep soft tissues. There may be overlying superficial dermal lesions. Eosinophils are said to be more common in this variant than in the superficial lesions.
In the juxta-articular nodular form of granuloma annulare (pseudorheumatoid nodules), there are deep dermal nodules with subcutaneous extension composed of epithelioid granulomas separated by thickened collagen bundles. Eosinophilic material, composed predominantly of collagen, is surrounded by histiocytes in a palisaded array. This differs from the usual form of granuloma annulare, in which this material is mucin. Scanty mucin is often present in this juxta-articular form. The interstitial form of granuloma annulare is present next to the nodules in approximately half of the cases, further supporting the notion that the nodular lesions are a form of granuloma annulare.
In the disseminated form of granuloma annulare, the granulomatous foci are often situated in the papillary dermis ( Fig. 8.10 ). Necrobiosis may be inconspicuous.
In the interstitial or “incomplete” form of granuloma annulare, the histological changes are subtle and best assessed at lower power. The dermis has a “busy” look as a result of increased numbers of inflammatory cells, mainly histiocytes and lymphocytes ( Fig. 8.11 ). They are arranged about vessels and between collagen bundles that are separated by increased connective tissue mucin. There are no formed areas of necrobiosis. In some cases, the interstitial component is minimal.
Cases of the nonnecrobiotic sarcoidal or tuberculoid type of granuloma annulare are uncommon and pose a diagnostic problem. The presence of increased dermal mucin or eosinophils may be helpful distinguishing features ( Fig. 8.12 ).
In most cases of granuloma annulare, the epidermal changes are minimal. Perforating lesions have a central epidermal perforation that communicates with an underlying necrobiotic granuloma ( Fig. 8.13 ). At the edges of the perforation, there are varying degrees of downward epidermal hyperplasia to form a channel. The channel contains necrobiotic material and cellular debris. There is surface hyperkeratosis. The lesions sometimes perforate by way of a hair follicle. Pustules are a very rare finding in the perforating variant.
Immunofluorescence studies have shown fibrin in areas of necrobiosis. IgM and C3 were present in blood vessel walls in one series. Immunoperoxidase techniques have demonstrated activated T lymphocytes with an excess of helper/inducer phenotype (CD4 + ) and CD1 + dendritic cells related to Langerhans cells in the perivascular and granulomatous infiltrates. In contrast, lymphocytes were predominantly of CD8 type in a patient with HIV infection. A study of the staining pattern of lysozyme in the inflammatory cell infiltrate suggests that this may be useful in distinguishing granuloma annulare from other necrobiotic granulomas. The distribution of the inhibitor of metalloproteinase-1 is different in granuloma annulare and necrobiosis lipoidica.
Ultrastructural studies have confirmed the presence of histiocytes in the dermal infiltrate together with cellular debris and fibroblasts. Degenerative changes in collagen in areas of necrobiosis include swelling, loss of periodic banding, and fragmentation of fibers. Elastic fibers also show degenerative changes. Fibrin and other amorphous material is present in interstitial areas.
Granuloma annulare must be distinguished from other necrobiotic granulomas. Necrobiosis lipoidica typically shows horizontally oriented foci of amorphous, eosinophilic necrobiosis, with intervening zones of granulomatous inflammation often horizontally oriented and layered between areas of necrobiosis (the “sandwich sign”). In granuloma annulare, the intervening areas of dermis between the necrobiotic granulomas are relatively normal compared with necrobiosis lipoidica, and there is no fibrosis. Plasma cells, which are inconspicuous in granuloma annulare, are readily identified and sometimes numerous in necrobiosis lipoidica. Granulomas tend to be better developed in necrobiosis lipoidica and often include multinucleated giant cells, whereas in contrast to granuloma annulare, mucin is not generally demonstrable in the necrobiotic areas. Based on these changes, Lynch and Barrett classified granuloma annulare as a “blue” granuloma, in contrast to “red” necrobiotic (collagenolytic) granulomas (of which necrobiosis is an example), in which fibrin, eosinophils, or flame figures contribute to an eosinophilic appearance. Although this is an oversimplification of a sometimes-difficult assessment, the various blue and red granulomas are listed in Table 8.2 . There is often a close resemblance between subcutaneous granuloma annulare and rheumatoid nodule . The mean age of patients with subcutaneous granuloma annulare (second decade) is significantly less than that for rheumatoid nodule (sixth decade), and patients with subcutaneous granuloma annulare usually do not have symptoms or signs of arthritis. Microscopically, rheumatoid nodules tend to have fibrinous eosinophilic necrobiosis, giant cells within palisaded foci, and significant stromal fibrosis, whereas subcutaneous granuloma annulare demonstrates edematous-appearing to mucinous necrobiosis, a lack of giant cells, and lesser degrees of fibrosis. Typically, mucin stains are positive in the necrobiotic foci of granuloma annulare and not in those of rheumatoid nodule, but there can be overlap of these findings. Therefore, one should not depend on the results of mucin stains alone in distinguishing between these two disorders. The eosinophilic material in juxta-articular, nodular granuloma annulare also contrasts with rheumatoid nodules in which the central material is fibrin. Actinic granuloma (annular elastolytic giant cell granuloma) shares with granuloma annulare the annular clinical appearance, the presence of granulomas in the dermis, and the phenomenon of elastolysis so that some authorities regard it as simply a variant of granuloma annulare. However, the granulomas of actinic granuloma tend to be less organized than those in the palisaded type of granuloma annulare, and they feature more multinucleated giant cells, a lack of significant necrobiosis, and little or no mucin deposition. An elliptical biopsy taken across the active lesional border in actinic granuloma will show granulomas with ingestion of elastic fibers in the middle zone, fibrosis with an absence of elastic fibers in the dermis inside the expanding border, and normal-appearing dermis, with preserved solar elastosis, outside the expanding border. Granuloma multiforme (Mkar disease) , a condition seen predominantly in central African countries, has clinical and histopathological resemblances to both granuloma annulare and actinic granuloma, and it may in fact represent a variant of those disorders.
| “Blue” granulomas | “Red” granulomas |
|---|---|
| Granuloma annulare | Necrobiosis lipoidica |
| Wegener's granulomatosis | Necrobiotic xanthogranuloma |
| Rheumatoid vasculitis | Rheumatoid nodule |
| Pseudorheumatoid nodules of adults | |
| Churg–Strauss syndrome | |
| Eosinophilic cellulitis (Wells’ syndrome) |
There is some superficial resemblance of lesions of disseminated granuloma annulare to lichen nitidus (see p. 62 ), although in disseminated granuloma annulare there are no acanthotic downgrowths of the epidermis at the periphery of the lesions.
With regard to interstitial granuloma annulare, a similar appearance can be seen in the interstitial granulomatous drug reaction (see p. 237 ), although in the latter condition true necrobiosis is uncommon and, if present, localized. Furthermore, eosinophils are often present, and there may be lichenoid changes at the dermoepidermal interface. In interstitial granulomatous dermatitis, there are both neutrophils and eosinophils in the infiltrate, although both cell types may be sparse. Another characteristic feature is the presence of a palisade of histiocytes around one or many collagen fibers, which often have a basophilic hue. Rarely, M. marinum infection of the skin may mimic interstitial granuloma annulare. It is uncertain whether the cases of this type of granuloma annulare associated with borreliosis are mimics or true examples of granuloma annulare. Other mimics include granulomatous mycosis fungoides and B-cell lymphoma. In fact, interstitial granuloma annulare and patch stage mycosis fungoides can also coexist. One study showed that T-cell receptor gene rearrangement analysis is helpful in differentiating granulomatous T-cell lymphomas from the benign granulomatous mimics, sarcoidosis and granuloma annulare; interestingly, however, a monoclonal T-cell population was found in 2 of 15 cases of granuloma annulare in this study. Negative staining for both hemosiderin and human herpesvirus 8 can be used to distinguish interstitial granuloma annulare from early Kaposi's sarcoma in which both are usually positive. A recent example of secondary syphilis microscopically resembled interstitial granuloma annulare; differences from granuloma annulare included a plasma cell component to the infiltrate and the finding of spirochetes with immunohistochemical staining. In another report, a case initially believed to be interstitial granuloma annulare proved to be papular mucinosis; the true diagnosis was supported by dense, diffuse dermal mucin deposition and the association with a monoclonal gammopathy.
The sarcoidal variant of granuloma annulare would by its very nature be difficult to distinguish from true sarcoidosis , although mucin deposits, eosinophils, or subtle areas suggesting interstitial granuloma annulare may be identified. Eosinophils and obvious mucin are not seen in sarcoidosis. However, granuloma annulare and sarcoidosis have been reported in the same patient.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here