Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Widespread use of medical imaging has led to a significant increase in the use of radiologic contrast media (CM). Half of the estimated 78 million computed tomography (CT) examinations and 37 million magnetic resonance imaging (MRI) examinations performed annually in the United States include the use of CM. Radiographic CM are used in imaging examinations to aid in the characterization, detection, and staging of disease. Although the CM currently in use have strong documented safety profiles, their use is not completely without risk. According to the World Health Organization, an adverse drug reaction is a harmful, unintentional, and often unavoidable response to normal therapeutic doses of a medicine. In general, most patients who receive a contrast agent have no adverse reactions (ARs), and when a reaction does occur it is usually mild and self-limiting. Therefore, establishing preventive measures against ARs from CM is essential for patient safety. Awareness of the various reactions that can occur and prompt management are critical in reducing the likelihood of an adverse outcome. Rapid evaluation and treatment of ARs requires adequate training, readily available equipment, and appropriate medications. This chapter discusses the important aspects of screening, recognizing, and managing the risks central to intravenously administered CM.
Iodinated contrast media (ICM) can be classified according to their ionicity, relative osmolality, and chemical structure ( Table 20.1 ). During the last 2 decades, the use of CM has transitioned from traditional ionic agents to safer nonionic agents, which are equally effective for imaging but cost much more. Traditional ionic agents, or high-osmolar CM, dissociate into charged particles when dissolved in water and, as a result, have an osmolality 5 to 8 times that of blood (normal blood osmolality is 285–295 mOsm/kg in adults). Charged particles are toxic irritants and can cause allergic reactions. Nonionic agents, or low-osmolar/isoosmolar CM, do not dissociate into charged particles when dissolved in water and have one-half the osmolality of ionic agents. As a result, nonionic agents are associated with a considerably lower incidence of ARs, particularly cardiovascular and allergic-like reactions. Due to the significant cost of nonionic CM, using new technologies, increasing the concentration, and decreasing the volume of CM are among the efforts to minimize the economic burden. Additionally, the cost of nonionic CM continues to decrease as manufacturers’ patents expire and competing agents enter the market.
| Name | Type | Iodine Content (mgI/mL) | Osmolality (osmol/kg) |
|---|---|---|---|
| Nonionic | |||
| Iodixanol (Visipaque 320) | Nonionic dimer | 320 | 290, isoosmolar |
| Iohexol (Omnipaque 350) | Nonionic monomer | 350 | 884, low osmolar |
| Iopamidol (Isovue 370) | Nonionic monomer | 370 | 796, low osmolar |
| Ionic | |||
| Ioxaglate (Hexabrix) | Ionic dimer | 320 | 580, low osmolar |
| Metrizoate (Isopaque Coronar 370) | Ionic monomer | 370 | 2100, high osmolar |
| Diatrizoate (Hypaque 50) | Ionic monomer | 300 | 1550, high osmolar |
The majority of CM used for MRI are based on gadolinium (Gd) chelates and are extremely well tolerated by the vast majority of patients. Currently, there are nine gadolinium-based CM (GBCM) approved by the US Food and Drug Administration (FDA) that differ in a number of properties ( Table 20.2 ). Generally, most GBCM present a distribution in the body similar to that of ICM. However, the mean dose of GBCM delivered is typically 5 to 15 times lower (ranges between 10 and 20 mL) than ICM. This is certainly one of the reasons why GBCM are considered safer to use, and their molecular structure and osmolality are less significant as far as safety is concerned in comparison with ICM. In terms of physical properties, stability and relaxivity are the most important criteria for patient safety optimization and diagnostic efficacy. Stability refers to the resistance of GBCM to break down into toxic components (Gd ions) in the blood circulation. GBCM with higher relaxivity provide greater contrast enhancement and increased signal intensity at lower doses, thereby achieving improved diagnostic efficacy while reducing the risk of an AR.
| Brand Name | Chemical Name | Structure/Charge | Stability (log K eq )/T1 Relaxivity (L/mmol × s) | NSF Incidence (Based on EMA Classification) | Comments |
|---|---|---|---|---|---|
| Magnevist | Gadopentetate dimeglumine (Gd-DTPA) | Linear/ionic | 22.5/4.1 | High | Highest relaxivity of all extracellular GBCM |
| Omniscan | Gadodiamide (Gd-DTPA-BMA) | Linear/nonionic | 16.9/4.3 | High | Low thermodynamic stability |
| Optimark | Gadoversetamide (Gd-DTPA-BMEA) | Linear/nonionic | 16.6/4.7 | High | Low thermodynamic stability |
| MultiHance | Gadobenate dimeglumine (Gd-BOPTA) | Linear/ionic | 22.6/6.3 | Intermediate | Highest relaxivity of all extracellular GBCM |
| Eovist (US) Primovist | Gadoxetate disodium (Gd-EOB-DTPA) | Linear/ionic | 23.5/6.9 | Intermediate | Designed for liver imaging |
| Ablavar (US) Vasovist | Gadofosveset trisodium (Gd-DTPA-DCHP-MS-325) | Linear/ionic | 22.1/16 | Intermediate | Highest relaxivity of any agent due to reversible albumin binding |
| Gadavist (US) Gadovist | Gadobutrol (Gd-BT-DO3A) | Macrocyclic nonionic | 21.8/5.2 | Low | Highest viscosity; above-average relaxivity |
| Prohance | Gadoteridol (Gd-HP-DO3A) | Macrocyclic nonionic | 23.8/4.1 | Low | Lowest viscosity and osmolality of all agents; below-average relaxivity |
| Dotarem | Gadoterate meglumine (Gd-DOTA) | Macrocyclic ionic | 25.8/3.6 | Low | Designed for CNS-related MRI exams |
CM administration depends on the clinical indication, vascular access, and type of examination. The method of CM delivery (hand injection vs. power injection) also varies depending on the requested procedure. Stable intravenous (IV) access is always necessary, and recognizing the importance of proper technique is critical to avoid potentially serious complications. Careful preparation of the power injection apparatus is crucial to minimize the risk of complications ( Box 20.1 ). Power injection of IV CM should be through a flexible plastic cannula; metal needles must always be avoided. Vascular access guidelines for CM administration at our institution can be seen in Box 20.2 . Power injection of CM through some central venous catheters can be performed safely, provided that certain precautions are followed ( Box 20.3 ). Furthermore, obtaining the patient’s full cooperation throughout the injection process is important to avoid or promptly identify a complication should one occur. The injection should always be discontinued if the patient reports pain or sensation of swelling at the injection site. Although not an adverse reaction of CM, a clinically significant venous air embolism can occur when IV CM is administered by hand injection. Venous air embolisms are potentially fatal but extremely rare complications commonly seen in the intrathoracic veins, main pulmonary artery, and right ventricle, and they appear as air-fluid levels or air bubbles on contrast-enhanced CT. Unintentional injection of large amounts of air into the venous system may result in dyspnea, expiratory wheezing, chest pain, cough, tachycardia, pulmonary edema, and hypotension. Stroke leading to neurologic deficits may also occur due to decreased cardiac output or paradoxical air embolism. Administering 100% oxygen and placing the patient in the left lateral decubitus position is the mainstay of treatment. To further reduce the size of air bubbles and help restore circulation and oxygenation, hyperbaric oxygen can be given. Closed-chest cardiopulmonary resuscitation should be started immediately if cardiopulmonary arrest occurs.
Standard procedures should be used to clear the syringe and pressure tubing of air, after which the syringe should be reoriented with the tubing directed downward.
Before initiating the injection, the position of the catheter tip should be checked for venous backflow.
If backflow is not obtained, the catheter may need adjustment, and a saline test flush or special monitoring of the site during injection may be appropriate.
If the venipuncture site is tender or infiltrated, an alternative site should be sought.
If venous backflow is obtained, the power injector and tubing should be positioned to allow adequate table movement without tension on the intravenous line.
Mechanical injection of intravenous contrast media may be performed through the following power/pressure injectable devices:
Injection rates for 24 g catheters up to 2 mL/s
Injection rates for 22 g catheters up to 4 mL/s
Injection rates for 20 g catheters up to 5 mL/s
Injection rates for 18 g catheters up to 7 mL/s
Saf-T-Intima catheters, not to exceed injection rates of 2 mL/s regardless of the gauge size
Power/pressure injectable PICC lines:
PowerPICC: Manufacturer guidelines indicate injection rates up to 5 mL/s 300 psi. Product identification tags indicate maximum injection rates.
Power/pressure injectable chest port (must be accessed by a physician or registered nurse):
PowerPort implantable port, Xcela Power PICC: manufacturer guidelines state that the port must be identified prior to use and accessed with an identified power injectable PowerLoc safety infusion set. The product withstands 5 mL/s power injection at 300 psi pressure limit setting.
Central lines:
Pressure injectable ARROWg+ard Blue PLUS CVC Injection: manufacturer guidelines indicate rates up to 10 mL/s distal lumen 16 g 300 psi. Medial and proximal lumens 5 mL/s 18 g 300 psi. The injection rate is indicated on the identification tag of the lumen port access.
Central lines not indicated for power/pressure injections may be used for mechanical injections with the approval of the radiologist or requesting physician and must not exceed a rate of 2 mL/s.
The following devices are not approved for mechanical injections of iodinated contrast media. They are approved for manual injections via hand or gravity drip and must be approved by a radiologist for the examination value:
PICC lines not indicated for power/pressure injections
Chest ports not indicated for power/pressure injections
Midline catheters
Accessed femoral or lower extremity lines
Peripheral internal jugular accessed or external jugular accessed jugular lines
The following devices are not approved for any injection of iodinated contrast media:
Any catheter put in for the purpose of hemodialysis
Tunneled central venous catheters (e.g., Hickman, Broviac, NeoStar)
Any catheter placed intraarterially
CVC, Central venous catheter; PICC, peripherally inserted central catheter.
Check the computed tomography scout scan or a recent chest radiograph to confirm the proper location of the catheter tip.
Before connecting the catheter to the injector system tubing, test the catheter tip position for venous backflow.
Backflow will occasionally not be obtained because the catheter tip is positioned against the wall of the vein in which it is located.
If saline can be injected through the catheter without abnormal resistance, administer contrast media through the catheter.
If abnormal resistance or discomfort is encountered, seek an alternative venous access site. Injection with large-bore (9.5-F to 10-F) central venous catheters using flow rates of up to 2.5 mL/s has been shown to generate pressures below manufacturers’ specified limits.
Contrast media should not be administered by power injector through small-bore, peripheral (e.g., arm) access central venous catheters (unless permitted by the manufacturer’s specifications) because of the risk of catheter breakage. It cannot be assumed that all vascular catheters including a peripherally inserted central catheter can tolerate a mechanical injection. A number of manufacturers have produced power injector–compatible vascular catheters. The manufacturer’s specifications should be followed.
ARs can be prevented or minimized by applying certain principles for all patients. These include reassessing if CM is necessary, determining if a different IV CM can be used, and determining if 12–24 hours of premedication with corticosteroids and antihistamines can be started prior to CM administration. In some cases alternative imaging modalities can be diagnostic, thus avoiding the use of CM. If CM is necessary, the lowest possible dose to acquire the necessary clinical information should be administered. Because different types of CM have varying safety profiles, choosing the most suitable one for the patient may help avoid complications. In patients with a history of allergies or prior reaction to CM, premedication with corticosteroids and antihistamines can be attempted, although the efficacy of premedication still remains controversial. If premedications will be administered, oral administration is preferable and should be given at least 6 hours prior to injection of CM whenever possible. Additionally, patients with prior reactions should be followed up more closely during CM administration and kept in observation for longer periods. If readministration of CM is required, it should be avoided for several days to a week after the first injection to allow the kidneys to recover. The American College of Radiology (ACR) recommends that the delay in CM readministration be balanced with the patients severity of renal disease and the medical urgency, whereas the European Society for Urological Radiology (ESUR) recommends the duration of delay be at least 7 days between two injections. If the patient did not reach the maximum daily CM dose, low contrast dose injection protocols on specific imaging scanners can be done to repeat studies without waiting.
Treating ARs requires adequate training with a proactive approach. Studies have shown that radiologists feel insufficiently prepared to manage acute incidents, particularly rare severe reactions. Didactic teaching, simulation courses, and virtual modules are great methods to help the radiologist maintain familiarity with methods for evaluation and treatment of reactions. A study by Tubbs et al. showed that radiology residents who underwent medical simulations showed considerable improvement in confidence and knowledge in management of ARs. Niell et al. demonstrated that didactic learning alone is inadequate because individuals in the study still felt that treating a reaction was difficult and distressing. Therefore, a combination of teaching methods with training repeated yearly or more often appears to be the most effective approach. All personnel who oversee CM injections are also encouraged to maintain current certification in basic life support.
For any imaging procedure, the referring physician and radiologist must weigh the risk-to-benefit profile of the requested CM-enhanced examination. Acquiring consent for the injection of CM is not customary because it is generally considered to be safe; however, most institutions collect information from patients to identify risk factors that may increase the likelihood of a reaction. Patients with a history of allergies, asthma, and those with features of atopy (e.g., urticaria) are 3 to 6 times more likely to develop a severe reaction to CM. Although there is no cross-reactivity, patients who have had allergic-like reactions to ICM are also at risk of AR to GBCM. It is important to note that seafood allergies have not been shown to predispose a patient to ARs, and patients with these allergies should be counseled appropriately. Patients with a previous AR to CM are 5 to 6 times more likely to develop a subsequent reaction and are known as high-risk patients. For individuals with kidney diseases, the risk is proportional to the preexisting renal impairment. Other miscellaneous risk factors include patients on certain medications (e.g., metformin), history of multiple myeloma or sickle cell trait, poor cardiac status, anxiety, and history of thyroid cancer or pheochromocytoma.
Corticosteroid premedication for high-risk patients is the standard of care in the United States. Corticosteroids produce antiinflammatory effects and cause decreased effectiveness of the innate immune system by impairing function of immune cells (e.g., neutrophils, mast cells). Prophylaxis with corticosteroids has been found to reduce the overall risk of a contrast reaction and is largely reserved for high-risk patients with previous anaphylactoid reactions to CM. However, its efficacy in the prevention of moderate and severe reactions is more controversial due to lack of evidence. Because steroids weaken the immune system, impaired immunity or active infection is seen as a relative contraindication. Additionally, steroids have their own side effects; therefore, the risks and benefits should be considered before administration. Typically, corticosteroids are well tolerated and cause no adverse effects when only a few doses are administered. When emergent contrast injection is required, higher doses of intravascular steroids may be considered. Elective and emergent premedication regimens with corticosteroids may also involve the use of antihistamines ( Tables 20.3 and 20.4 ). All forms of antihistamines can cause drowsiness; however, second-generation antihistamines cause less drowsiness and may be beneficial for patients who need to drive themselves home. Patients who are unable to take oral medications can have an IV dose of 200 mg hydrocortisone.
| Dose | |
|---|---|
| Steroids | Methylprednisolone, one 32-mg tablet, orally administered at 12 and 2 h before CM administration or Prednisone, one 50-mg tablet, orally administered 13 h, 7 h, and 1 h before CM administration |
| Antihistamine | Diphenhydramine 50 mg IV, IM, or orally administered 1 h before CM administration |
| Most Desirable in Emergency (First Choice) | Less Desirable Than First Choice | Least Desirable (Chosen When There Is Inadequate Time to Achieve Steroid Effect) | |
|---|---|---|---|
| Steroid | IV methylprednisolone sodium succinate 40 mg or IV hydrocortisone sodium succinate 200 mg every 4 h until CM administration |
IV dexamethasone sodium sulfate 7.5 mg or IV betamethasone 6 mg every 4 h until CM administration |
Omit IV steroids; no effect when given less than 4–6 h before CM administration |
| Antihistamine | IV diphenhydramine 50 mg, 1 h before CM administration | IV diphenhydramine 50 mg, 1 h before CM administration | IV diphenhydramine 50 mg, 1 h before CM administration |
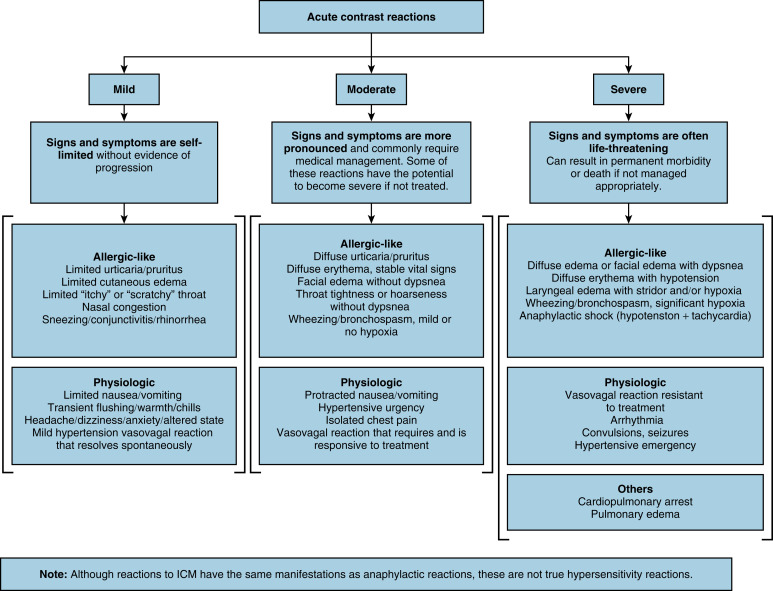
Despite recognizing predisposing risk factors, ARs remain unpredictable and sporadic due to their multifactorial nature. ARs occur more commonly in ICM, and the incidence varies between studies ( Table 20.5 ). Incidence of ARs ranges from 0.2% to 3% for ICM with severe reactions ranging from 0.005% to 0.01%. Incidence of ARs ranges from 0.03% to 0.5% for GBCM with severe reactions ranging from 0.002% to 0.01%. Most ARs occur in the first 5 minutes within the first hour of CM administration. ARs are typically classified into three categories of severity: mild (flushing, arm pain, nausea/vomiting, headache), moderate (bronchospasm, hypotension), and severe (cardiovascular collapse, laryngeal edema, convulsions, arrhythmias) ( Fig. 20.1 ). The vast majority of ARs are mild, with the most common being transient injection site discomfort, headache, dizziness, nausea with or without vomiting, paresthesia, and itching. Mild reactions typically require no treatment because they are usually self-limiting and do not progress. However, if mild reactions become more severe, patients should be treated and monitored to ensure recovery. Moderate reactions with no or only mild hypoxia are usually not life threatening (e.g., bronchospasm); however, patients should be observed closely and managed until symptoms have resolved completely. Severe reactions should be identified and treated immediately to prevent permanent morbidity or mortality. For all ARs, practitioners should maintain IV access, measure vital signs, and assess the patient’s well-being (e.g., appearance, voice quality, and symptoms). Facilities where injection of CM occurs must be equipped with the proper tools/supplies required to manage any form of reaction ( Box 20.4 ). The management for different ARs as outlined by the ACR is shown in Table 20.6 .
| Reference | Incidence |
|---|---|
| Iodinated Contrast Media | |
| Cochran et al. | Overall rate 0.2% (LOCM) |
| Hunt et al. | Overall rate 0.15 (LOCM) |
| Mortelé et al. | Overall rate 0.7% (iopromide) |
| Wang et al. | Overall rate 0.6% (iohexol, iopromide, or iodixanole) |
| Dillman et al. | Overall rate 0.18% (pediatric) |
| Callahan et al. | Overall rate 0.46% |
| Caro et al. (meta-analysis) | Fatality rate of 0.9 per 100,000 injections of LOCM |
| Gadolinium-Based Contrast Media | |
| Hunt et al. | Overall rate 0.04% |
| Dillman et al. | Overall rate 0.07% (gadopentetate dimeglumine, gadobenate dimeglumine, gadodiamide) |
| Li et al. | Overall rate 0.48% |
| Murphy et al. | 0.06% (gadopentetate dimeglumine) 0.03% (gadodiamide) 0.4% (gadoteriodol) |
The following equipment must be readily available and within or nearby any room in which contrast media is to be injected (adult or pediatric sizes are optional for facilities that do not inject adult or pediatric patients, respectively):
Oxygen cylinders or wall-mounted oxygen source, flow valve, tubing, oxygen masks a
a Although oxygen can be administered in a variety of ways, use of nonrebreather masks is preferred because of their ability to deliver more oxygen to the patient.
(adult and pediatric sizes)
Suction: wall-mounted or portable; tubing and catheters
Oral and/or nasal airways: rubber/plastic Ambu-type bag-valve-mask device; masks in adult and pediatric sizes; protective barriers for mouth-to-mouth respiration are optional if the bag-valve-mask device is stocked
Stethoscope; sphygmomanometer
Intravenous solutions (0.9% [normal] saline and/or Ringer lactate solution) and tubing
Syringes and IV cannulas: various sizes; tourniquets
Needles: various sizes
Necessary medications:
Epinephrine 1:10,000, 10-mL preloaded syringe (for IV injection) and/or
Epinephrine 1:1000, 1 mL (for IM injection) and/or
Epinephrine IM auto-injector b
b Examples: EpiPen Jr. or Auvi-Q 0.15 mg; injects 0.15 mg epinephrine (as 0.3 mL of 1:2000 or 0.15 mL of 1:1000 epinephrine, respectively) or EpiPen or Auvi-Q 0.3 mg; injects 0.3 mg epinephrine (as 0.3 mL of 1:1000 epinephrine).
(sizes for patients 15–30 kg and patients ≥30 kg)
Atropine, 1 mg in 10-mL preloaded syringe
Beta-agonist inhaler with or without spacer
Diphenhydramine for PO, IM, or IV administration
Nitroglycerin: 0.4-mg tabs, sublingual
Aspirin, 325 mg (for chest pain where myocardial ischemia is a consideration)
Optional medications:
Lasix, 20–40 mg IV
Labetalol, 20 mg IV
Dextrose 50% 25 mg/50 mL syringe
The following items should be on the emergency/code cart c
c If in a hospital or clinic, the emergency/code cart should conform to hospital or departmental policies and procedures; it often includes these items.
or within or near any room in which contrast media is injected:
Defibrillator or automated external defibrillator
Blood pressure/pulse monitor
Pulse oximeter
The contact phone number of the cardiopulmonary arrest emergency response team should be clearly posted within or near any room in which contrast media is injected. For magnetic resonance imaging, to avoid any resuscitative equipment becoming a magnetic hazard, patients requiring treatment should be taken away from the imaging room immediately.
| Type of Reaction | Adults | Children |
|---|---|---|
| Urticaria (Hives) | ||
| Mild (scattered and/or transient) |
|
|
| Moderate (more numerous/bothersome) | ||
| Severe (widespread and/or progressive) | ||
| Diffuse Erythema | ||
| All forms |
|
|
| Normotensive |
|
|
| Hypotensive | ||
| Bronchospasm | ||
| All forms |
|
|
| Mild |
|
|
| Moderate | ||
| Severe |
|
|
| Laryngeal edema (all forms) | ||
| Hypotension (definition varies for children of different ages) | ||
| All forms |
|
|
| With bradycardia (vasovagal reaction), pulse <60 beats/min | If mild:
If severe (patient remains symptomatic despite above measures):
|
If mild:
If severe (patient remains symptomatic despite above measures):
|
| With tachycardia (anaphylactoid reaction), pulse >100 beats/min | If hypotension persists: |
If severe (symptoms persist): |
| Unresponsive and pulseless |
|
Same as for adults |
| Hypertensive crisis ¶ |
|
|
| Pulmonary edema |
|
|
| Seizures/convulsions |
|
|
| Hypoglycemia | ||
| All patients |
|
|
| If patient is able to swallow safely |
|
|
| If patient is unable to swallow safely | IV access is available:
If no IV access is available:
|
IV access is available:
IV access is not available:
|
| Anxiety (panic attack) |
|
|
| Reaction rebound prevention § | ||
a 1 mg/kg (max 50 mg) PO, IM, or IV; administer IV dose slowly over 1–2 min.
b 25–50 mg IM or IV (administer IV dose slowly over 1–2 min).
c 1 mL of 1:10,000 dilution (0.1 mg); administer slowly into a running IV infusion of fluids; can repeat every few minutes as needed up to 10 mL (1 mg) total.
d 0.3 mL of 1:1000 dilution (0.3 mg); can repeat every 5–15 min up to 1 mL (1 mg) total or epinephrine autoinjector (EpiPen or equivalent), (0.3 mL of 1:1000 dilution, fixed [0.3 mg]); can repeat every 5–15 min up to 3 times.
e 0.1 mL/kg of 1:10,000 dilution (0.01 mg/kg); administer slowly into a running IV infusion of fluids; can repeat every 5–15 min as needed; maximum single dose: 1.0 mL (0.1 mg); can repeat up to 1 mg total dose.
f 0.01 mL/kg of 1:1000 dilution (0.01 mg/kg); max 0.30 mL (0.30 mg); can repeat every 5–15 min up to 1 mL (1 mg) total or epinephrine autoinjector (1:1000 dilution equivalent). If <30 kg, pediatric epinephrine autoinjector (EpiPen Jr or equivalent) 0.15 mL equivalent (0.15 mg); if ≥30 kg, adult epinephrine autoinjector (EpiPen or equivalent) 0.30 mL (0.30 mg).
g Two puffs (90 μg/puff) for a total of 180 μg; can repeat up to 3 times.
h IV 0.2 mL/kg of 0.1 mg/mL solution (0.02 mg/kg); minimum single dose of 0.1 mg and maximum single dose of 0.6–1.0 mg; maximum total dose of 1 mg for infants and children, 2 mg for adolescents; administer into a running IV infusion of fluids.
i Two sugar packets or 15 g of glucose tablet or gel or ½ cup (4 oz) fruit juice.
j IV 5 mg/kg; administer over 1–2 min.
k IV 1 mg/kg; administer over 1–2 min.
∗ All forms can cause drowsiness; IM/IV form may cause or worsen hypotension.
† Second-generation antihistamines cause less drowsiness and may be beneficial for patients who need to drive themselves home.
‡ In hypotensive patients, the preferred route of epinephrine delivery is IV because the extremities may not be perfused sufficiently to allow for adequate absorption of IM- administered epinephrine. With respect to IM delivery of epinephrine, the EpiPen Jr package insert does not provide dosing recommendations for children <15 kg. Also, it can be difficult to dose medications accurately in neonates and infants.
§ Although IV corticosteroids may help prevent a short-term recurrence of an allergic-like reaction, they are not useful in the acute treatment of any reaction. However, these may be considered for patients having severe allergic-like manifestations prior to transportation to an emergency department of an inpatient unit.
¶¶ Also see BLS and ACLS booklets published by the American Heart Association.
¶ Diastolic BP >120 mm Hg; systolic BP >200 mm Hg; symptoms of end organ compromise.
The most clinically important ARs are anaphylactoid (allergic-like) reactions, which typically begin within several minutes of CM exposure. The incidence of allergic-like reactions to low-osmolar CM (LOCM) is relatively rare, with estimates in the range of 0.2% to 3.1%. Most of these reactions are mild to moderate in severity. The incidence of allergic-like reactions is higher in certain age and gender combinations (e.g., young females) compared to the general population. The increased incidence of ARs seen among younger patients may be due to psychological effects. Anaphylactoid reactions are mediated by type 1 hypersensitivity mechanisms, which involve the release of histamine and other mediators. Many patients have allergic-like reactions at initial exposure, unlike the classic allergic reaction that requires an initial sensitizing exposure. This is because mediators directly stimulate an allergic response through mast cell degranulation and other pathways, without involvement of immunoglobulin E (mediator of the classic allergic reaction). Because no previous exposure is required, patients do not usually have a more serious reaction with subsequent administration of IV CM. The small number of severe reactions that are mediated by immunoglobulin E can be identified by positive results at skin testing.
Physiologic reactions that are chemotoxic in nature are believed to be caused by the dose and molecular toxicity of ICM as well as physical and chemical aspects such as viscosity or osmolality, among others. For example, high osmolality of ICM can lead to extracellular fluid shifts resulting in cellular dehydration and dysfunction. Physiologic effects of CM can also affect several organ systems leading to various symptoms such as nausea, vasovagal reactions, bradycardia, and hypotension to name a few. It is important to distinguish allergic-like reactions from dose-dependent reactions due to the difference in management. For example, physiologic reactions do not require premedication in the future. On the other hand, patients who suffered an allergy-like reaction may likely need premedication with steroids for future CM-enhanced examinations.
Delayed reactions (DRs) occur from 30 to 60 minutes up to 1 week after CM administration, with the majority occurring between 3 hours and 2 days. The incidence of delayed ARs is 5% to 6% for low-osmolar monomeric CM and 10.9% for isoosmolar dimeric CM. DRs are mostly seen in woman, young adults, and patients with a history of allergy. They are typically mild to moderate in severity and tend to be self-limiting cutaneous reactions (e.g., urticaria, maculopapular rash) that often require only symptomatic or no treatment. Cutaneous events in DRs have been seen significantly more often with dimeric nonionic CM than with monomeric nonionic CM (16.4% vs. 9.7%, respectively). Noncutaneous symptoms and signs include nausea, vomiting, fever, drowsiness, and headache. Symptoms such as salivary gland swelling (iodide mumps ) and acute polyarthropathy are rare and more common in patients with renal disease. Premedication for future administration of CM is only warranted if the patient experienced a previous severe DR.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here