Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Perhaps the most frequent use of diagnostic immunohistochemistry (IHC) in the surgical pathology laboratory pertains to breast biopsies by virtue of the volume and sheer difficulty of cases. In addition to utilizing IHC for diagnostic problems with breast biopsies, breast biopsies lend themselves to the frequent use of IHC for prognostic and predictive tests. In addition, the diagnosis of breast carcinoma in the metastatic setting requires thorough knowledge of IHC along with pitfalls for each stain.
This chapter addresses diagnostic issues involving stromal invasion, ever expanding spectrum of papillary lesions, atypical proliferative lesions, discrimination of ductal and lobular neoplasia, and identification of breast tumor types, Paget disease of the breast, fibroepithelial lesions, and metastatic breast carcinoma. The diagnostic section is followed by theranostic applications in breast cancer and a discussion regarding immunogenomics.
Epithelial lesions of the breast are not only the most frequent lesions encountered by the surgical pathologist but also are the greatest source of concern in the differential diagnosis of benign versus malignant lesions. The lesion categories that typically need to be differentiated include: nonneoplastic proliferative lesions versus malignant lesions, in-situ carcinoma versus invasive malignancy, and pseudo-invasive lesions (adenosis, radial scar, sclerosing lesions etc.) versus invasive malignancies. , In addition, atypical ductal epithelial hyperplasia (ADH), papillary lesions, and microinvasive carcinoma (invasive focus less than or equal to 1 mm) lend themselves to IHC clarification in many instances.
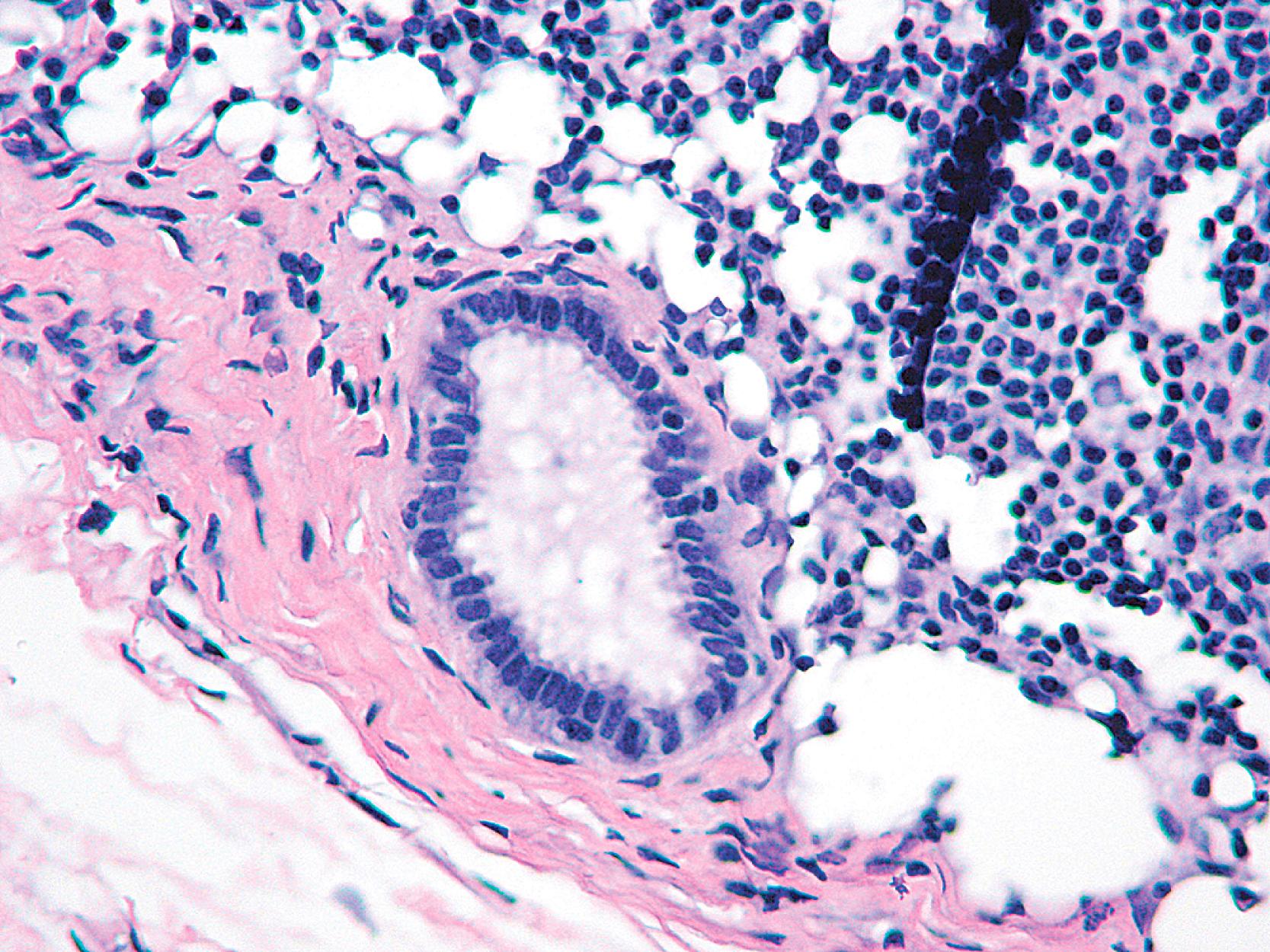
In all of these diagnostic situations, it is the presence of the myoepithelial cell (MEC) in intimate relationship with the epithelial cells of the lesion that determines the difference between in-situ and invasive disease and between benign pseudo-invasive lesions and invasive carcinoma. Microglandular adenosis (MGA), a distinct non-organoid benign form of adenosis, is a well-known exception to this statement (see below). An extremely rare lesion called “infiltrating epitheliosis” also lacks a peripheral layer of MECs. Some other sclerosing lesions (such as a radial scar) can also show patchy loss of myoepithelium. Adenomyoepitheliomas show loss of some of the myoepithelial markers in the periphery of the lesion. Barring some exceptions, the presence of MECs that envelop ductal-lobular epithelium, situated on the epithelial basal lamina, has always been considered to be an important criterion that separates invasive from non-invasive neoplasms. MECs can be visualized rather easily in normal breast ductules and acini, but when these structures dilate and fill with proliferating cells, or are compressed, it is virtually impossible to visualize them on hematoxylin and eosin (H&E) stain. Replacing the yesteryear antibodies to S-100 protein, high-molecular-weight keratin (HMWK), smooth muscle actin (SMA), calponin, and smooth muscle myosin heavy chain (SMMHC) are the more sensitive and specific antibodies to cytoplasmic components of MECs, along with the nuclear marker, p63 ( Table 19.1 ).
| Antibody | Localization | MEC | Myofibroblast | Microvasculature | Carcinoma |
|---|---|---|---|---|---|
| S100 | Cytoplasm | Weak | Variable | Negative | Variable |
| SMA | Cytoplasm | Strong | Moderate | Strong | Rare |
| Calponin | Cytoplasm | Strong | Weak-mod | Strong | Rare |
| SMMHC | Cytoplasm | Strong | Rare | Strong | Negative |
| p63 | Nucleus | Strong | Negative | Negative | Rare nuclei |
Antibodies to S-100 protein are not sensitive or specific for MEC and stain MEC in an erratic manner. The use of antibodies to maspin and CD10 have been tempered by the fact that they stain a variety of cell types, including luminal cells of the terminal duct lobular unit and tumor cells.
Cytokeratin cocktail antibodies, in addition to CK5, CK14, and CK17 identify MEC, but the staining is often inconsistent. They also immunostain acinar cells, which makes it difficult to differentiate MECs because of their proximity to the acinar cells. Anti-smooth muscle actins react with stromal myofibroblasts in addition to MECs and thus are not specific for MECs. The cross-reaction with myofibroblasts makes it difficult to identify MECs specifically, especially in ductal carcinoma in situ (DCIS), in which there may be periductal stromal desmoplasia.
Although anti-smooth muscle actin and muscle-specific actin HHF-35 stain MECs in the majority of benign breast lesions, there is substantial cross-reaction with stromal myofibroblasts, especially with SMA. Calponin and SMMHC are two antibodies that are more specific for MECs. SMMHC is a structural component (200 kD) unique to smooth muscle cells, which functions within the hexagonal array of the thick-thin filament contractile apparatus. Calponin, a 34 kD polypeptide, modulates actomyosin adenosine triphosphatase (ATPase) activity in the smooth muscle contractile apparatus and is unique to smooth muscle. , , In their analysis of 85 breast lesions, Werling and colleagues found that calponin and SMMHC always detected MEC in benign lesions and SMMHC stained myofibroblasts in 8% of cases compared to calponin which stained 76% of cases. It is also our experience that SMMHC and calponin are excellent antibodies, but calponin does stain stromal myofibroblasts to a greater extent than SMMHC.
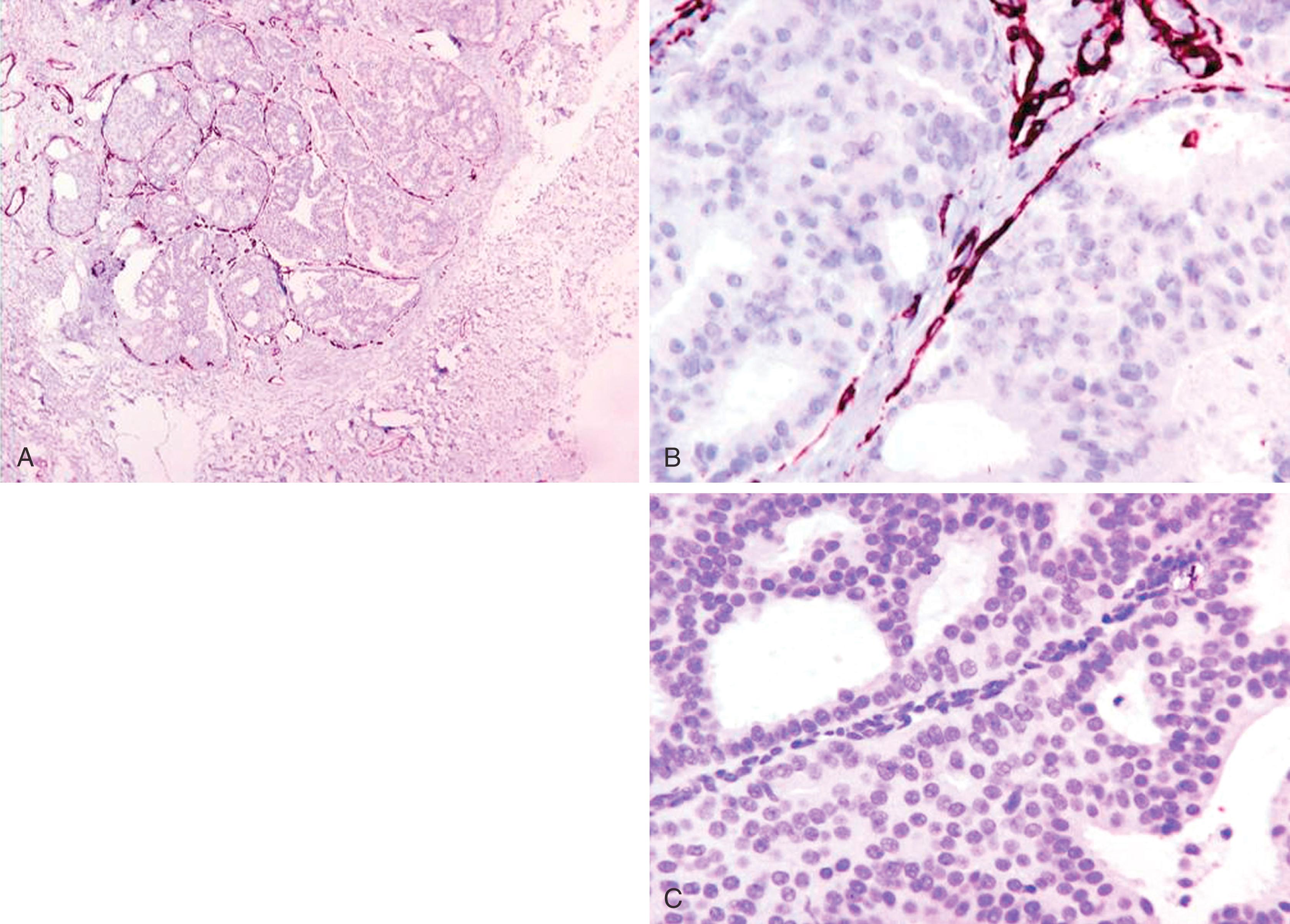
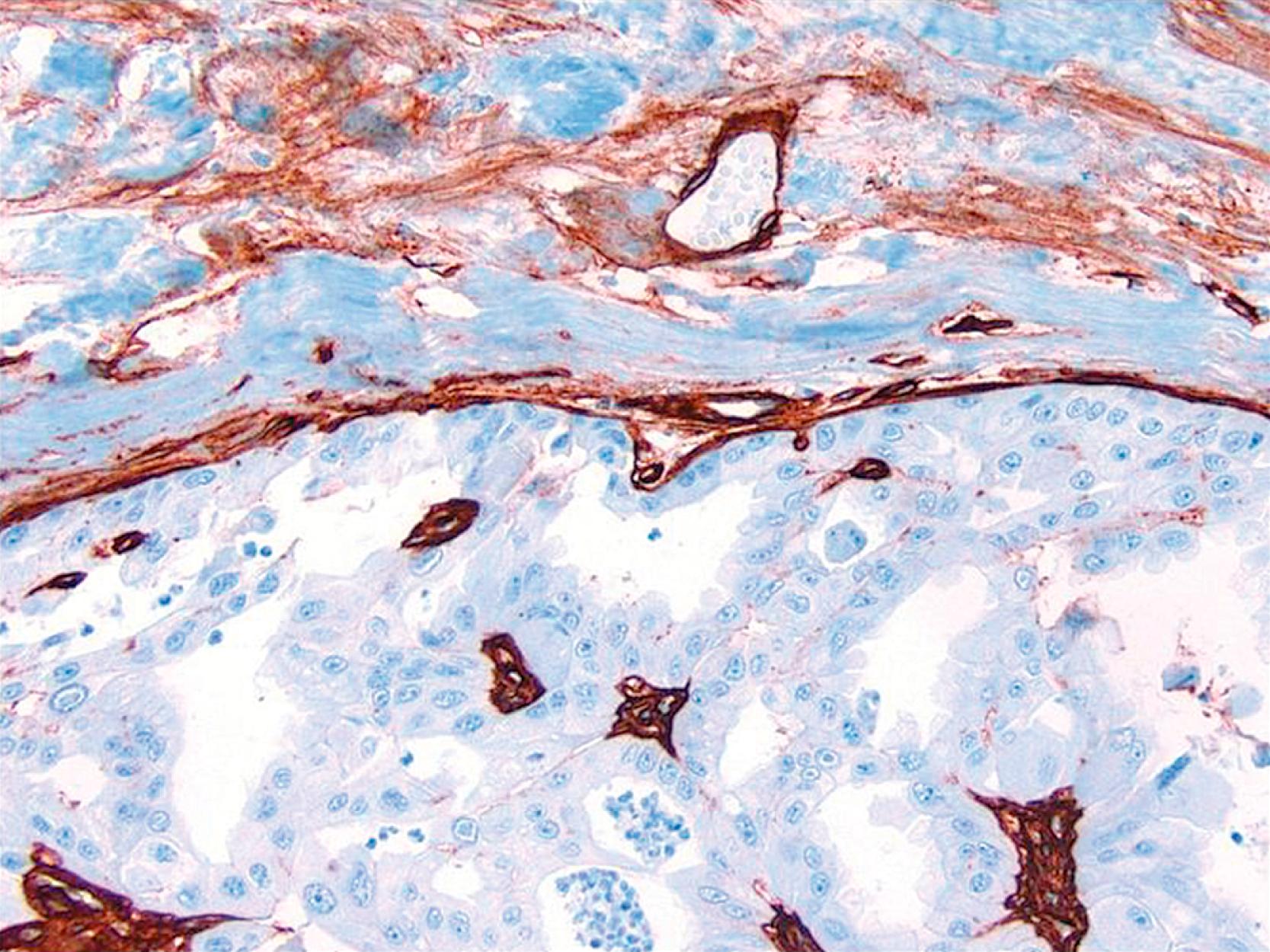
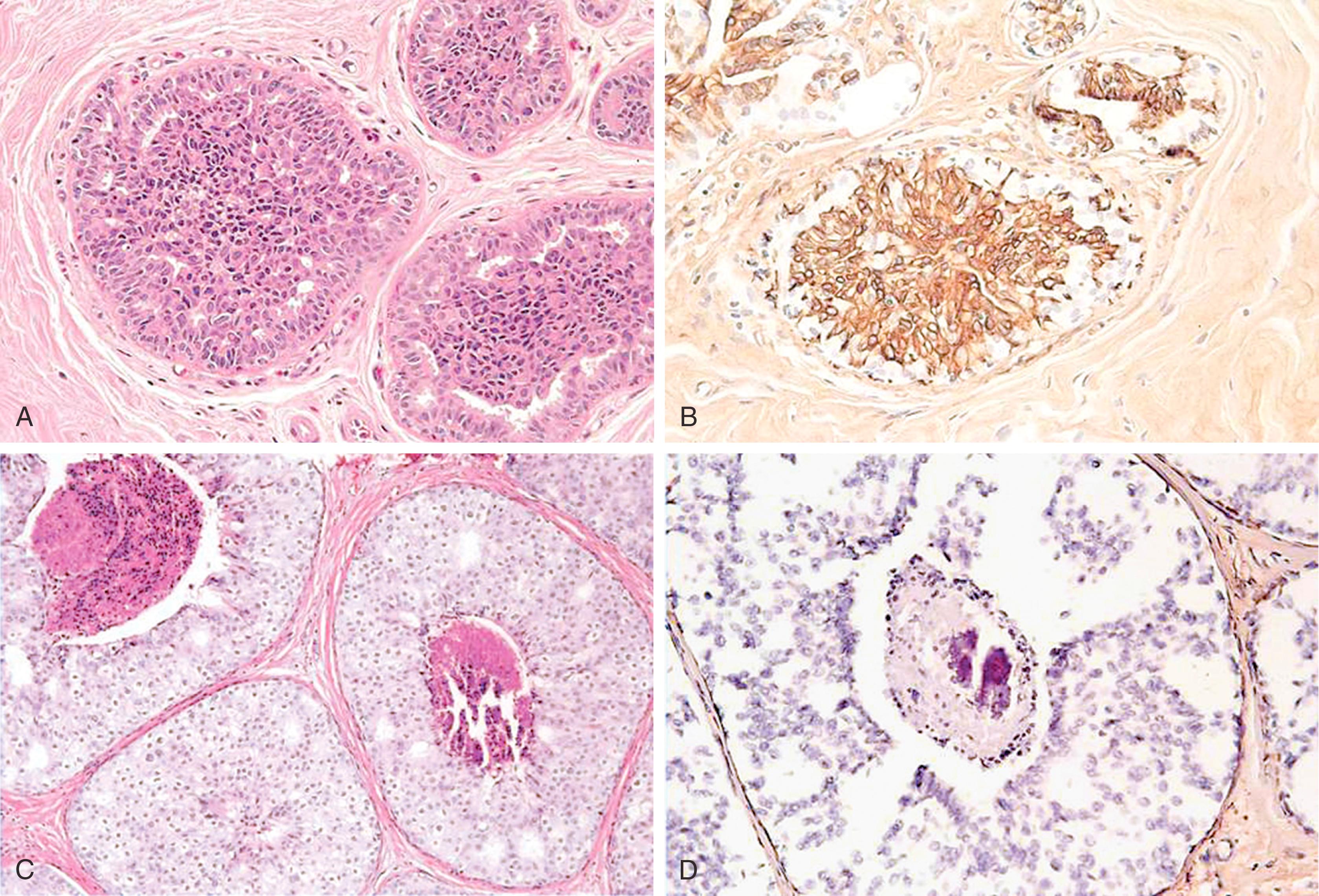
p63, a homologue of the tumor suppressor protein p53, is used as a multi-tasker in multiple organs for the detection of MEC, basal cells (prostate), myoepithelial differentiation (breast metaplastic carcinoma and salivary gland tumors), and as a marker for squamous differentiation. , , The advantage of p63 in the diagnosis of stromal invasion is that it is present only in the nucleus, which renders it most specific for MEC in the breast. Also, it does not stain myofibroblasts. Some have used a cocktail of dual-staining for SMMHC and p63 together. In our experience, using SMMHC and p63 is optimal for discerning MECs on difficult breast biopsies, especially diagnostic core biopsies ( Figs. 19.1–19.5 ). Distinguishing DCIS from invasive carcinoma on core biopsy can be crucial, as almost all patients with invasive carcinoma will have a sentinel lymph node (SLN) biopsy. An isoform of p63 (ΔNp63) is recognized by antibody p40. Studies comparing p63 and p40 reactivity in breast tissue and lesions have shown similar staining pattern for both antibodies. An antibody to thymidylate synthase has also been described to be at par with p63, but the experience is limited.
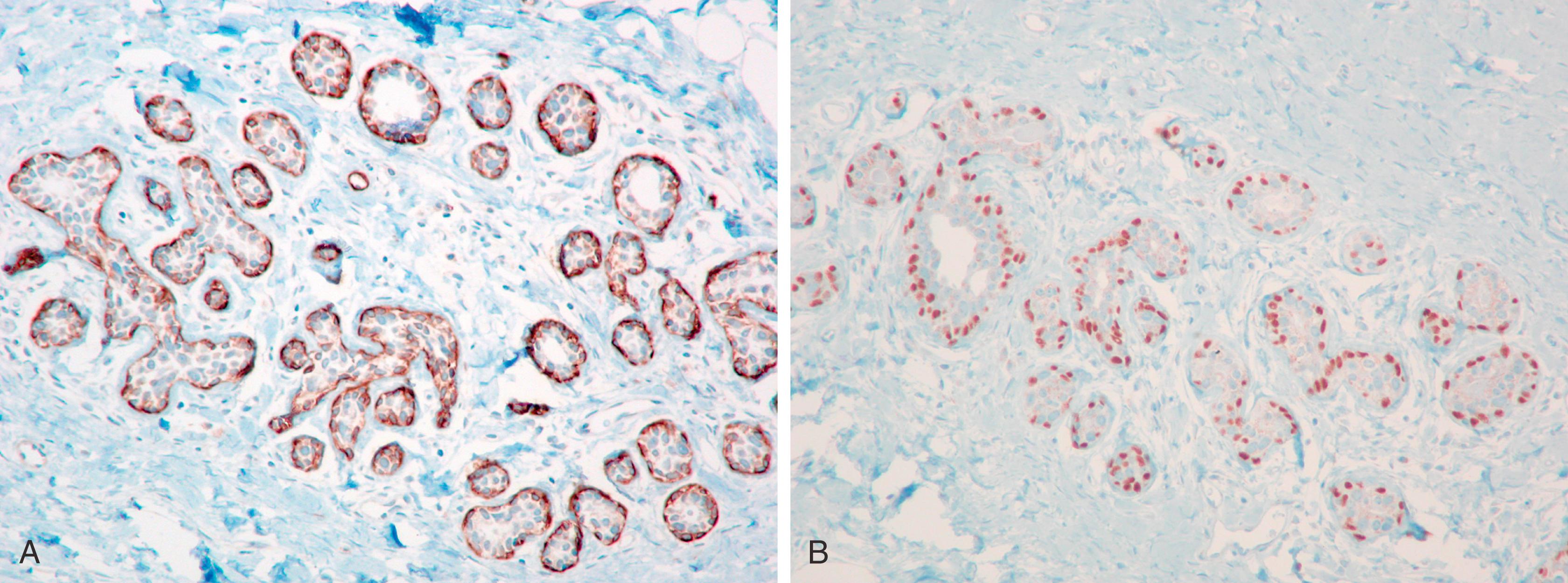
An important pitfall to note is that around 5% of DCIS cases (especially the DCIS in the background of papillary lesion) completely lack MECs using any antibody ( Fig. 19.6 ). In these situations, a critical appraisal of the histologic section is crucial to arrive at the correct diagnosis. For a lesion to be diagnosed as invasive carcinoma, the tumor cells should show “frank” infiltration in addition to a lack of MECs in the periphery. It is also important to remember that p63 nuclear immunostaining results in apparent “gaps” of immunostaining because staining of cytoplasm of the MEC does not occur ( Fig. 19.7 ). Any nuclear staining around nests of tumor cells can be construed as evidence of the presence of MECs. Special care must be taken to exclude nuclear staining of tumor cells around the periphery of neoplastic ducts, as p63 stains tumor cells in approximately 10% of cases (see Fig. 19.7 ).
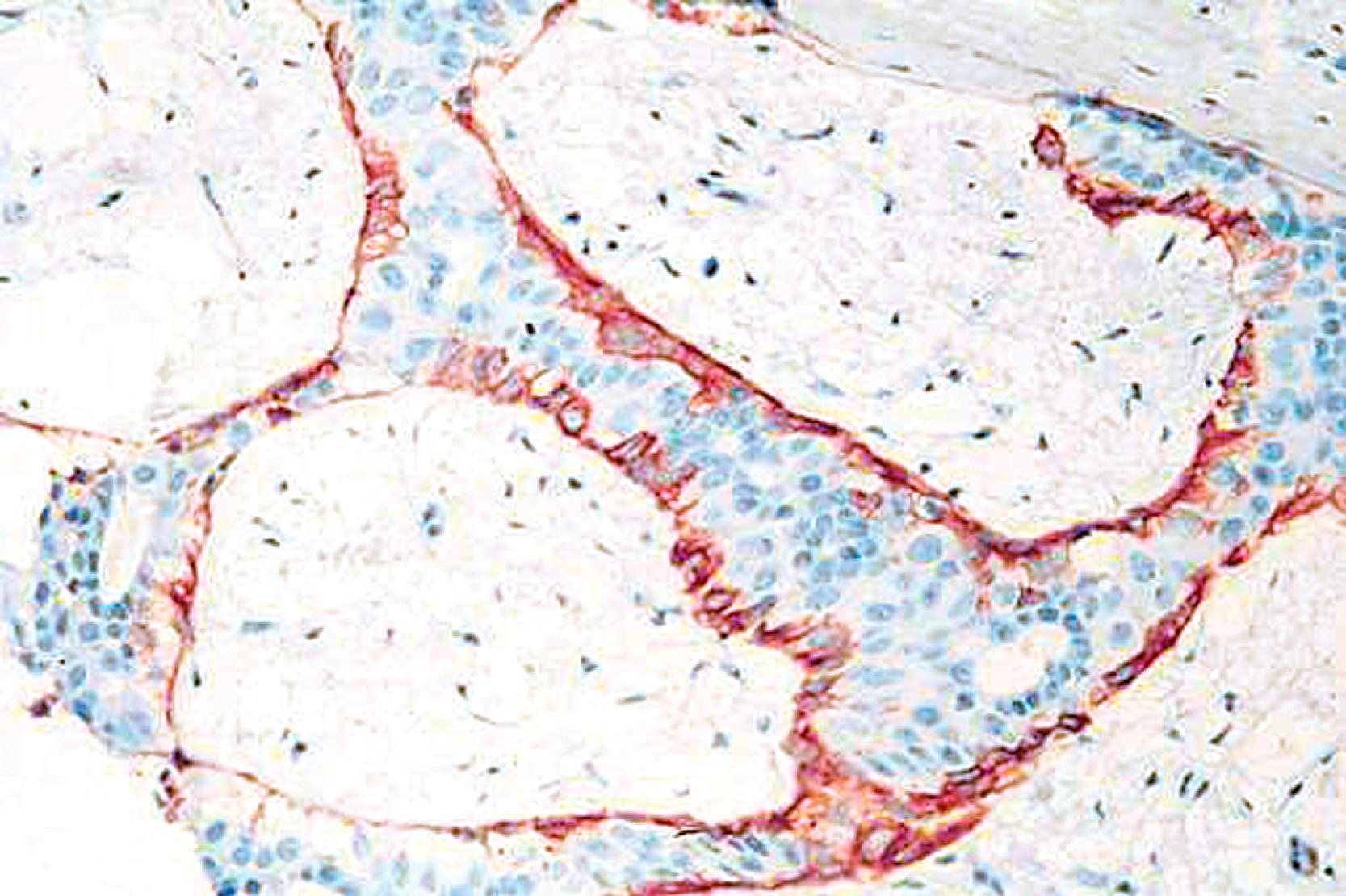
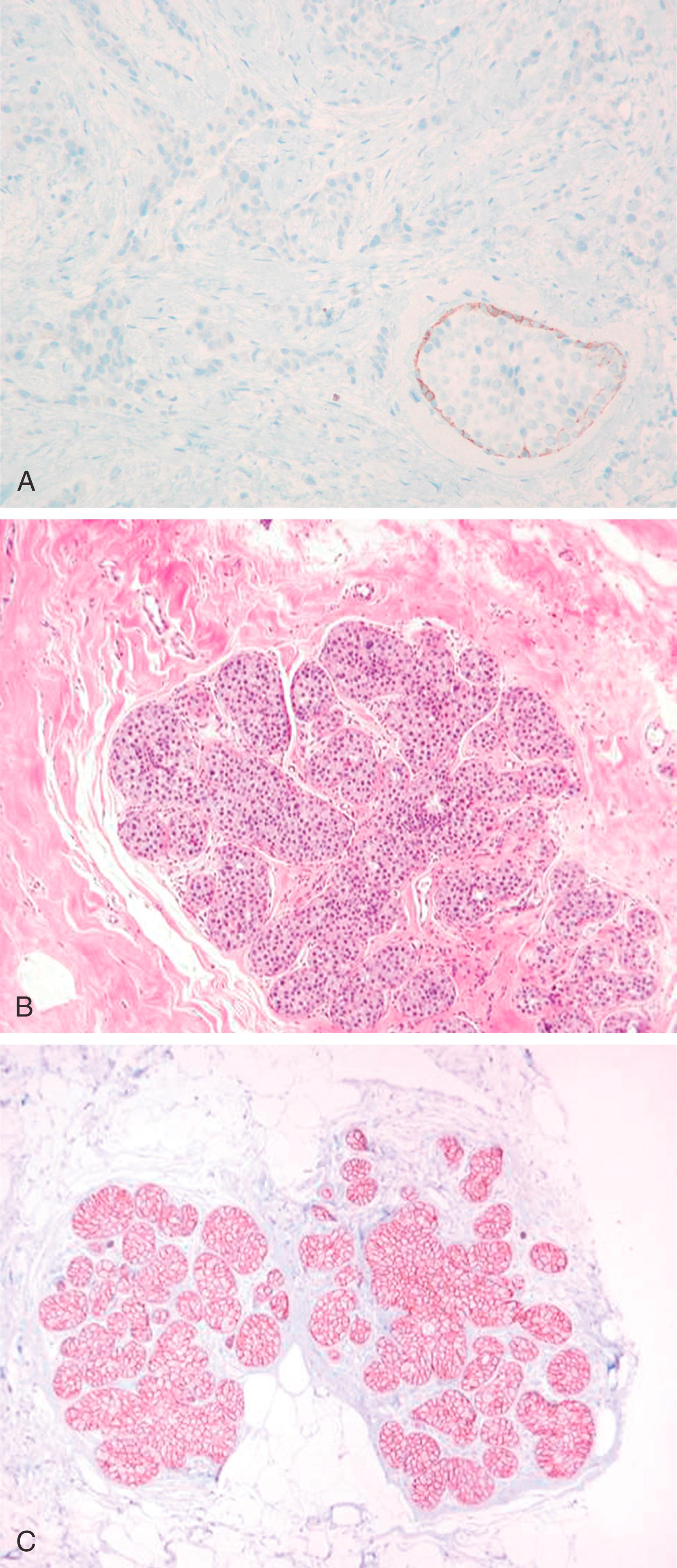
Lesions that are especially difficult on core biopsies include the distinction of carcinoma in-situ from invasive carcinoma in the presence of prominent periductal stromal desmoplasia (“regressive changes”) or heavy lymphoid infiltrates; lobular growth of rounded sheets of tumor cells; infiltrating cribriform carcinoma; sclerosing adenosis (with or without DCIS involvement); cancerization of lobules; radial scars with stromal elastosis-desmoplasia; tubular carcinoma; and sclerosing papillary lesion. The optimal MEC antibodies needed to attack these difficult cases include both SMMHC and p63 ( Figs. 19.8–19.12 ).
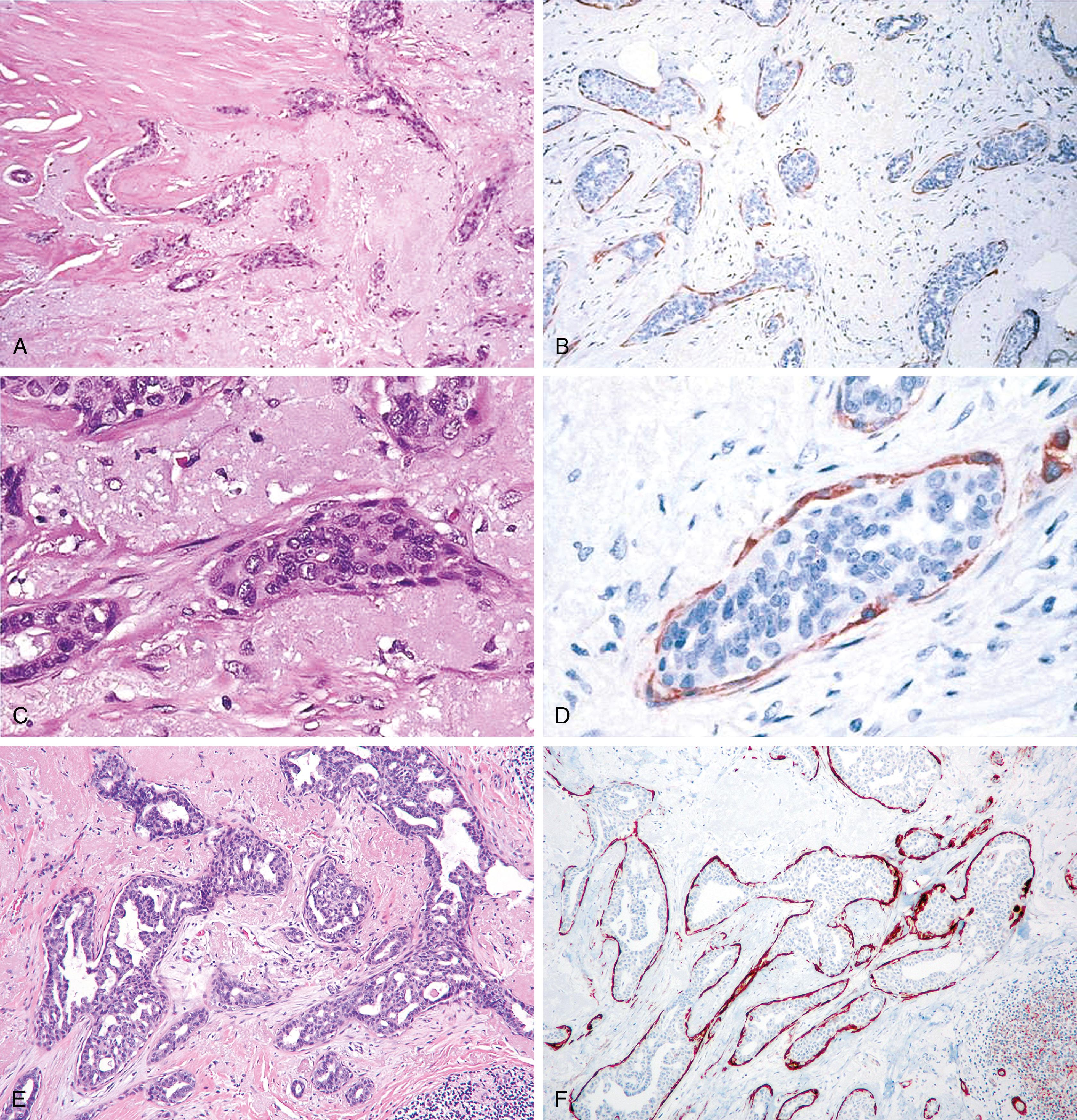
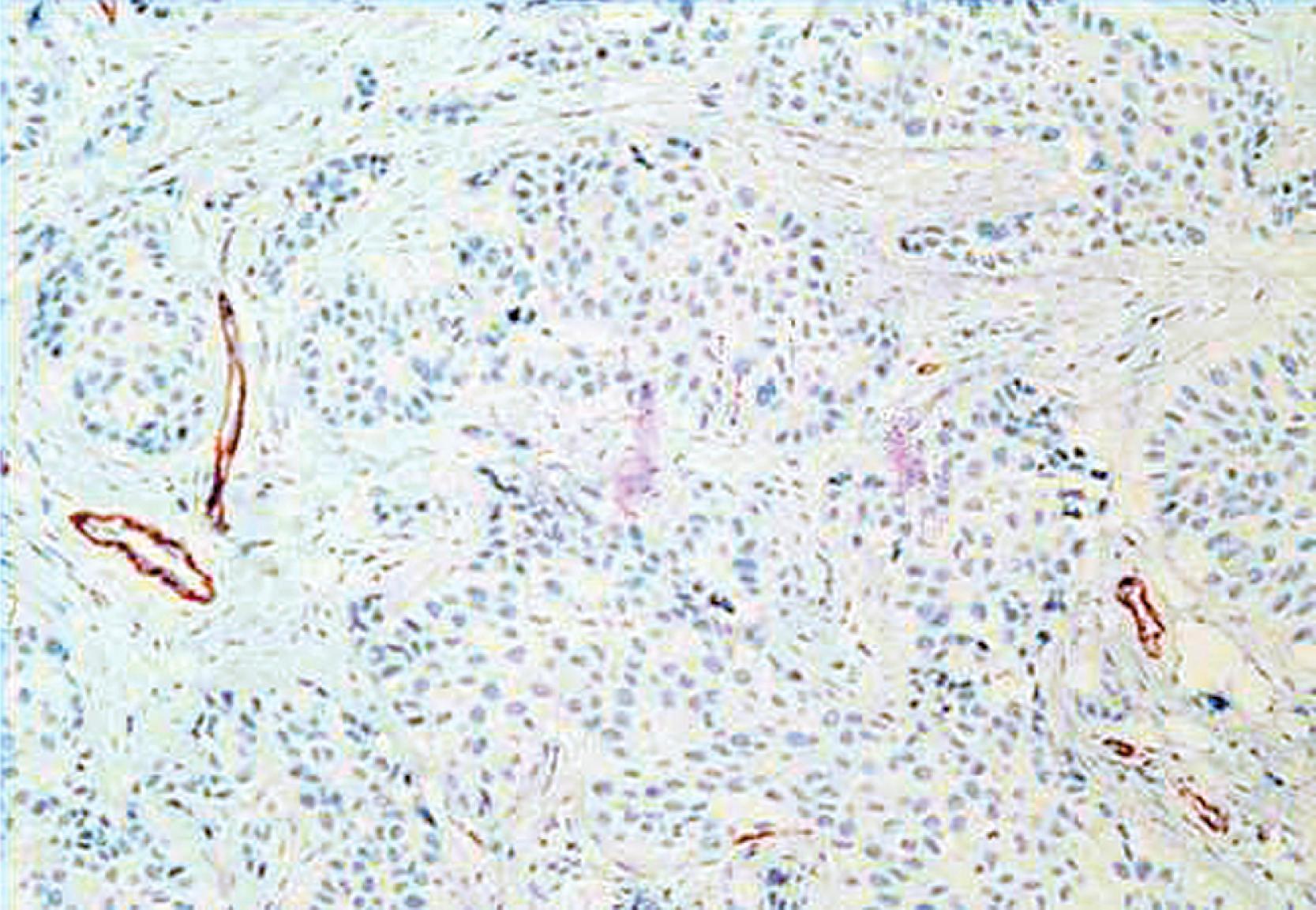
A significant pitfall for misinterpretation of MEC antibodies such as calponin and even SMMHC is that these antibodies may immunostain the microvasculature around tumor nests. Initial examination of such a case with SMMHC will reveal immunostaining hugging the tumor nests, suggesting the presence of MECs ( Fig. 19.13 ). Examination at a higher magnification will reveal the microvasculature around the tumor nests. When one then examines the p63, it is negative (see Fig. 19.13 ).
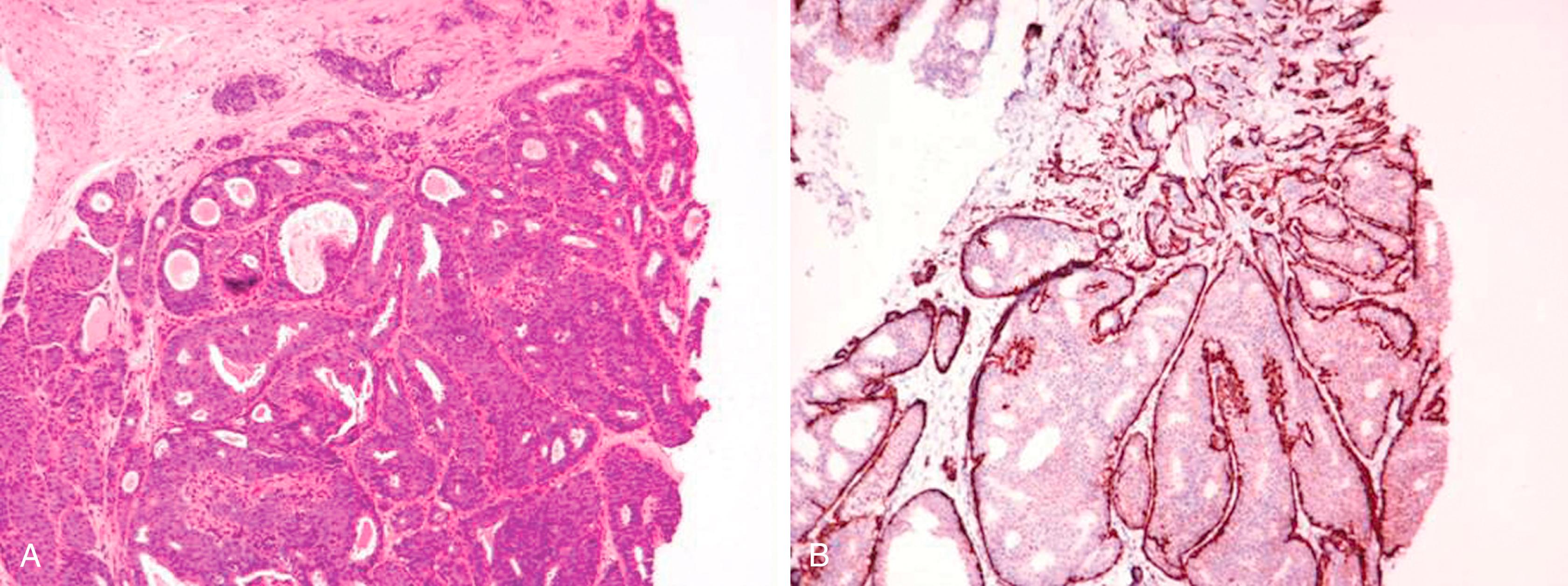
IHC for MEC is useful to help discriminate the three dominant benign lesions of the breast—sclerosing adenosis, MGA, and tubular carcinoma ( Table 19.2 ), but a detailed morphologic study of the lesion is essential. , The MECs are seen by IHC in all forms of adenosis except the microglandular form, a benign lesion that is known not to contain MECs. In addition to the distinct non-organoid morphology of MGA, tubular adenosis, described by Lee and colleagues, may mimic both MGA and carcinoma but differs from MGA by containing MECs. MGA is positive with S-100 protein, whereas sclerosing adenosis and tubular carcinomas are S-100 negative.
| Diagnosis | Histology | MECs | Collagen IV | Other IHC |
|---|---|---|---|---|
| Tubular carcinoma | Invasive tear-drop shape tubules, apical snouts, desmoplasia | Absent | Absent | EMA+, ER/PR+ |
| Microglandular adenosis | Round glands in fat lined by flat to cuboidal epithelium. Inspissated secretions within glands | Absent | Present | S100+, EMA neg, ER/PR neg, GCDFP15 neg |
| Tubular adenosis | Tubules sectioned longitudinally and lacks lobulocentric distribution | Present | Present | S100 negative |
| Sclerosing adenosis | Lobular growth pattern, epithelial cell atrophy, and lobular fibrosis | Present (relative abundance) | Present | S100 negative |
Although the presence of a peripheral layer of myoepithelium indicates a benign or non-invasive lesion and the absence of a peripheral layer of myoepithelium indicates an invasive and potentially malignant lesion, there are some exceptions to the rule. The benign lesions that show complete loss of peripheral layer of myoepithelium include MGA and infiltrating epitheliosis. Patchy loss is not uncommonly seen in several sclerosing lesions such as radial scar and sclerosing papillomas. A peculiar phenomenon is seen in benign adenomyoepitheliomas where expression of some myoepithelial markers is significantly reduced, while other markers are still expressed. The non-invasive or other indolent lesions that show absence of myoepithelium in the periphery include encapsulated and solid papillary carcinomas and tall cell carcinoma with reverse polarity (see section “Immunohistochemistry of Papillary Lesions”). In contrast, some “malignant” tumors express staining for myoepithelial markers such as expression of p63 around tubules in low grade adenosquamous carcinoma and frequent p63 staining in adenoidcystic carcinomas. Presence or absence of peripheral layer of MECs is an important characteristic to consider in diagnosis of breast lesions; however, a diagnosis of invasion or potential for malignancy is defined by overall features of a particular lesion rather than by a single stain.
The presence of MEC enveloping proliferating and sclerosing breast lesions is indicative of a benign or noninvasive process.
A combination of cytoplasmic SMMHC and nuclear p63 antibodies are the best discriminators for the presence of MECs, especially in desmoplastic-sclerotic proliferations.
MEC antibodies may be confirmatory for diagnosing microinvasive carcinoma (i.e., invasive carcinomas measuring no greater than 1 mm in largest dimension).
Microglandular adenosis: SMMHC-, p63-, S-100+, ER−.
Pitfall: Immunostaining of myofibroblasts and vascular walls with SMMHC; and p63 occasionally stains neoplastic cells.
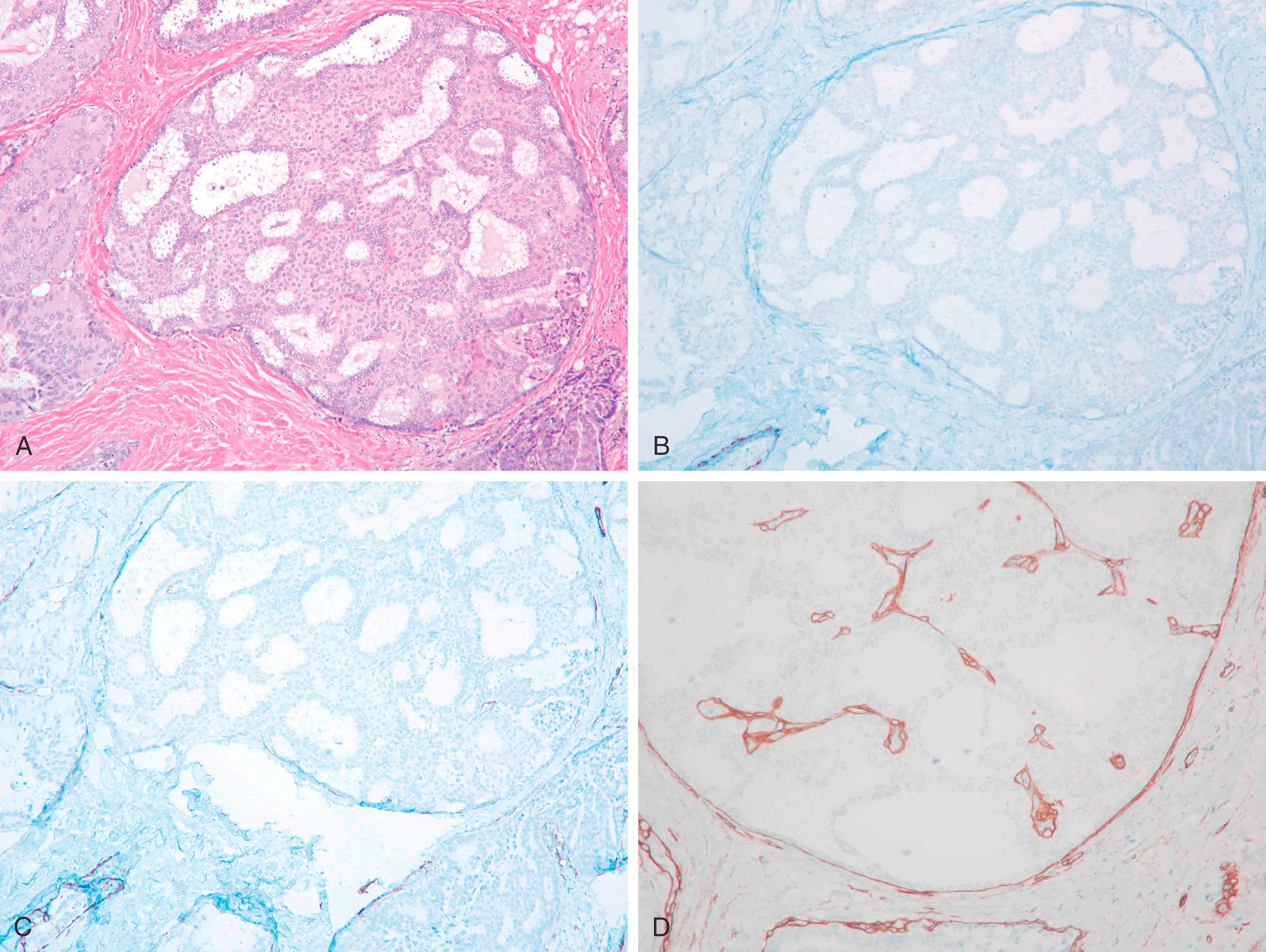
Papillary lesions range from benign papilloma to atypical papilloma to papillary carcinoma (in situ and invasive). There are several reports on the use of MEC markers to distinguish between different categories. , A papillary lesion is classified as a papilloma if there is a uniform layer of MECs in the proliferating intraluminal component of the lesion, whereas the absence of MECs would suggest an atypical papillary lesion. Atypical papilloma is a term often used when ADH overgrows the papilloma. These atypical areas generally lack MECs by immunohistologic examination. However, the absence of staining for HMWKs such as 34βΕ12, CK5 and CK5/6 within the proliferative component is more helpful in classifying a lesion as atypical ductal hyperplasia or in-situ carcinoma. The distinction between a papilloma, atypical papilloma and papillary DCIS (either de novo or DCIS involving papilloma) is not that problematic in the majority of cases and diagnosis can be made using morphology and IHC staining ( Fig. 19.14 ). The more difficult and confusing area is the distinction between a large cystic in-situ lesion and an invasive carcinoma. In-situ papillary carcinoma has been referred by different names in the literature. The term intra-cystic papillary carcinoma has been used for a single mass forming cystic lesion with malignant papillary proliferation. Papillary DCIS is a term that has been used for more diffuse lesions. The use of MEC markers to assess invasion in these lesions has yielded variable results. In an immunohistochemical study of papillary breast lesions, Hill and Yeh found consistent staining patterns in cases originally diagnosed as papilloma or invasive papillary carcinoma, but found variable staining in cases diagnosed as intra-ductal papillary carcinomas. Of the nine intra-ductal papillary carcinomas in their series, four cases showed unequivocal basal MECs by IHC, one case showed partial discontinuous staining, and four cases were predominantly negative for basal MECs. The authors found that lesions originally classified as intra-ductal papillary carcinoma but lacked basal MECs by IHC were uniformly large, expansile, papillary lesions with pushing borders and a fibrotic rim. The authors hypothesized that such lesions form a part of the spectrum of progression intermediate between in-situ and invasive disease and suggested that these lesions should be termed as “encapsulated papillary carcinoma.” Collins et al. have also favored such designation. Subsequent reviews classified papillary lesions more uniformly using morphology and IHC. The papillary lesions are now classified as papilloma, papilloma with ADH (atypical papilloma), papilloma with DCIS, papillary DCIS, intracystic papillary carcinoma (encysted or encapsulated papillary carcinoma), solid-papillary carcinoma, and invasive papillary carcinoma. ,
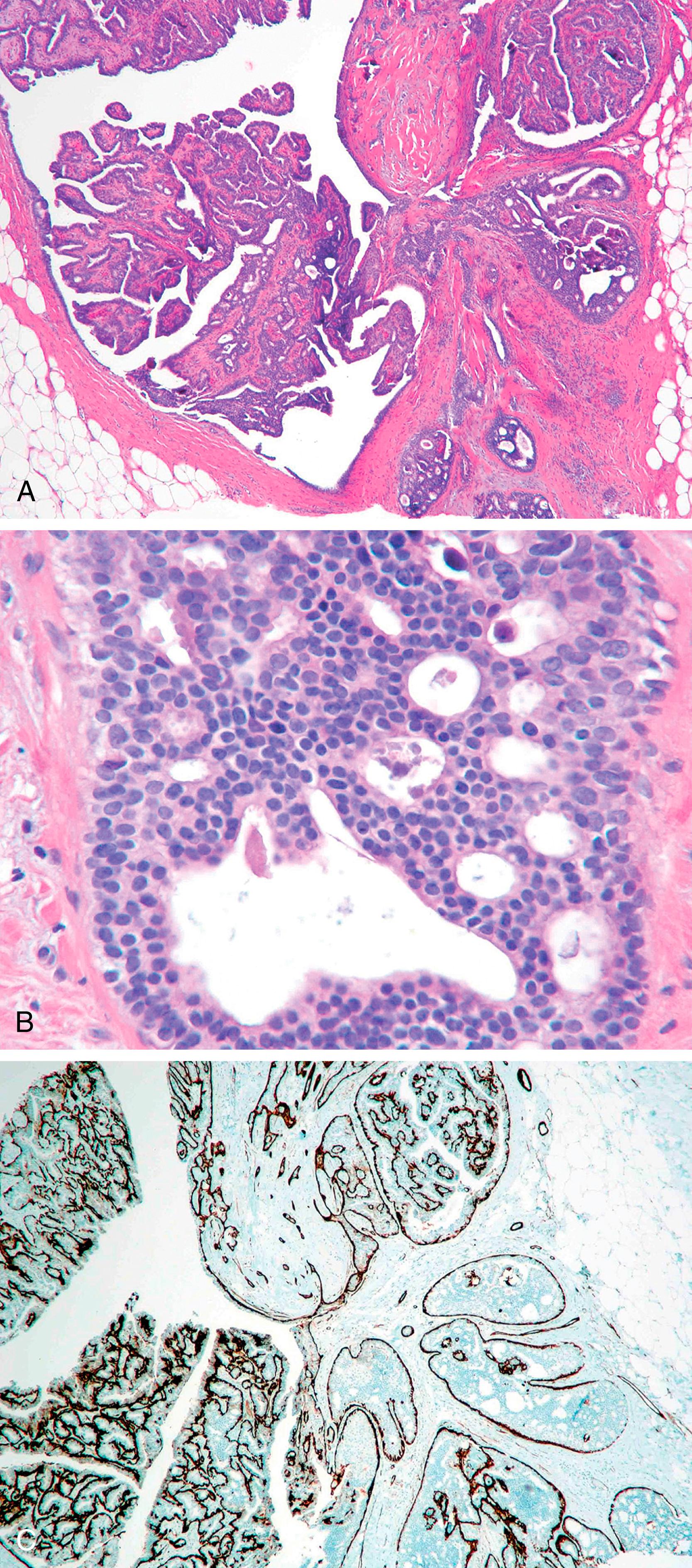
An intraductal papilloma with usual ductal hyperplasia shows the presence of MECs within and around the lesion and the proliferative component is positive for HMWK CK5. Atypical papilloma/papilloma with DCIS/papillary DCIS show a peripheral layer of MECs, but the proliferative component is negative for myoepithelial makers and CK5.
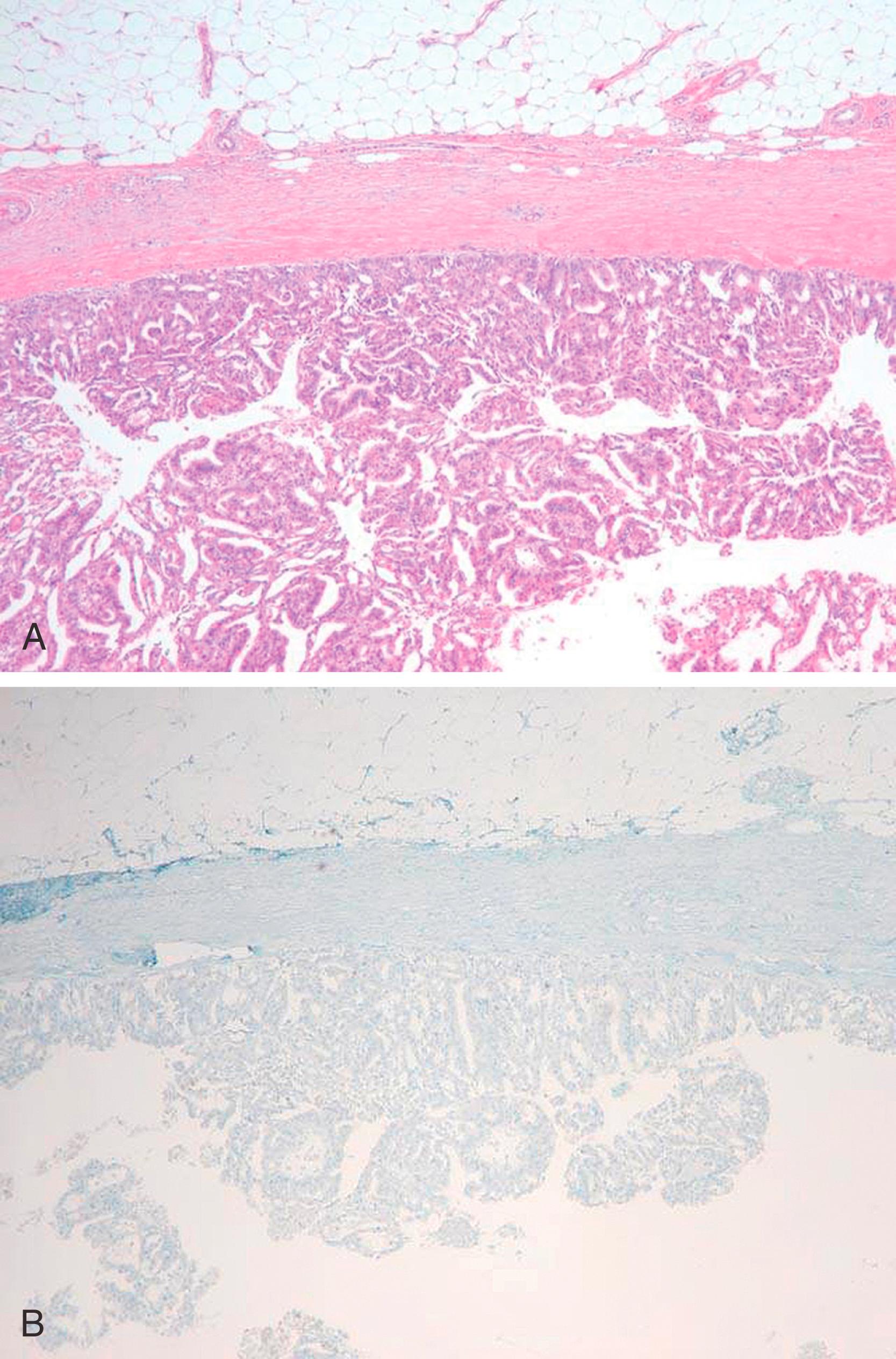
The problem in diagnosis arises from the fact that intracystic and solid-papillary carcinomas have the morphology of an in-situ lesion, but they lack the presence of MECs around the periphery ( Fig. 19.15 ). Intra-cystic papillary carcinomas generally retain strong expression for collagen IV completely around the lesion ( Fig. 19.16 ) suggesting that these are non-invasive lesions. However, it is to be noted that collagen can also be laid down around the invasive front of carcinoma, but true invasive cancers generally show a weak and discontinuous type of staining. , We don’t recommend using collagen IV in routine diagnostic testing due to inconsistent staining and interpretation issues. Conceptually, encapsulated and solid papillary carcinomas are somewhere in the spectrum between in-situ and invasive carcinomas, but the clinical behavior of these lesions is more akin to in-situ disease, , Therefore, these lesions are best managed as variants of an in-situ carcinoma for practical purposes. However, it is extremely important to analyze the resection specimen on these lesions in entirety by histologic evaluation due to the not so infrequent presence of frank invasion (invasion within or beyond the fibrotic rim) in the periphery of these lesions. We believe it is the presence of these minute foci of invasive carcinoma that are responsible for occasional metastatic disease reported with intracystic papillary carcinomas. The MEC staining pattern for each papillary lesion is summarized in Table 19.3 .
| Papillary Lesions | Myoepithelial Markers (p63 and SMMHC) | CK5 or CK5/6 | Clinical Behavior |
|---|---|---|---|
| Papilloma | Positive within and around ducts | Positive in proliferative epithelium | Benign |
| Papilloma with ADH/DCIS or papillary DCIS | Reduced/absent within, positive around ducts | Negative within proliferative epithelium | Risk for invasive malignancy |
| Encapsulated papillary carcinoma | Reduced/absent within, mostly negative around | Negative within proliferative epithelium | Similar to DCIS, unless frankly invasive |
| Solid papillary carcinoma | Reduced/absent within, mostly negative around | Negative within proliferative epithelium | Similar to DCIS, unless frankly invasive |
| Solid papillary neoplasm with reverse polarization a | Reduced/absent within, mostly negative around | Positive in lesional epithelium | Generally indolent clinical course |
a New WHO name is tall cell carcinoma with reverse polarity (TCCRP).
There are two other lesions that have papillary architecture but are usually not considered in the differential diagnosis of papillary lesions due to their uncommon occurrence. First is an adenomyoepithelioma, which as the name suggests, shows admixture of glandular and MECs. Adenomyoepitheliomas often arise in a background of adenosis or a papillary lesion. A pancytokeratin stain highlights glandular elements and the MECs (which are often in abundance) are highlighted by myoepithelial markers. However, the reactivity for myoepithelial markers varies from case to case, and it is not uncommon to see patchy loss of some myoepithelial markers in the periphery of the lesion. This latter finding should not be misconstrued as an evidence for invasion. Most adenomyoepitheliomas are clinically benign with only occasional cases reported to undergo malignant transformation. Malignancy in adenomyoepithelioma is diagnosed based on morphologic features and infiltration into the surrounding tissue rather than based on IHC stains. The second uncommon lesion is the so called “breast tumor resembling tall cell variant of papillary thyroid carcinoma” (BTRPTC). It has characteristic cyto-morphologic features, but often comes across as a solid-papillary lesion. Clinically, it is identified as an incidental mass lesion, 1 to 3 cm in largest dimension which shows solid-papillary nests of bland appearing proliferating epithelium. The epithelium can show grooves and occasional nuclear inclusions. The lesional nuclei are arranged away from the stromal aspect (known as reverse polarization), and hence it is also named as solid papillary neoplasm with reverse nuclear polarization (SPNRP). BTRPTC and SPNRP are now called tall cell carcinoma with reverse polarity (TCCRP). The proliferating epithelium of TCCRP stains strongly for CK5 (staining similar to usual ductal hyperplasia), but is only weak and patchy positive for estrogen receptor (ER). There is no staining for myoepithelial cells around the lesion (staining similar to EPC and SPC). The cells often express S100 and calretinin and occasionally SMMHC indicating MEC differentiation. , Since TCCRP often demonstrate mutation of IDH2 gene, a mutant antibody for IDH2 protein has been shown to stain TCCRP cells, while other papillary lesions have been reported to be negative. , The exact clinical course of this lesion is uncertain, but limited clinical experience suggests an indolent clinical course. The lesion may locally recur if incompletely excised. The potential for distant recurrence is questionable. TCCRP is a distinct lesion with characteristic staining pattern that needs to be distinguished from other established papillary lesions for proper patient management.
The MECs and CK5 are present in the proliferative cellular component of a papilloma, but are absent in the areas of atypical ductal hyperplasia and DCIS.
MECs are uniformly present around the periphery of the lesion in a papilloma, atypical papilloma, papilloma with DCIS, papillary DCIS, but absent at the periphery of intracystic and solid-papillary carcinomas.
Caution is advised in diagnosing invasion based on MEC antibodies in a papillary lesion on a core biopsy. Recommend complete excision for assessing invasion.
TCCRP demonstrate loss of MECs around the periphery, but the lesional cells are CK5 positive.
Differences in cytokeratin expression have been described between hyperplasia and DCIS. , The antibody 34βΕ12 recognizes CK1, CK5, CK10, and CK14, and these keratins are typically found in myoepithelium and squamous epithelium. Normal breast MECs and proliferative duct epithelium express 34βΕ12 ( Fig. 19.17 ). The expression is lost in ADH. Low- to intermediate-grade DCIS is also largely negative for 34βΕ12 (see Fig. 19.17 ). Most low-/intermediate-grade DCIS are uniformly positive for CAM5.2, reflecting a shift away from HMWKs to the more simple keratins 8 and 18. The 34βΕ12 immunostaining profile for DCIS and ADH is very similar and cannot be used to help distinguish DCIS from ADH but can be an aid to histomorphology in separating DCIS from florid usual ductal epithelial hyperplasia (UDH) in difficult cases. CK5/6 antibody (clone D5/16B4) antibody has also been shown to be largely negative in DCIS. Similar results have been obtained with CK5 antibody. This expression of HMWKs (or basal keratins) in usual hyperplasia with loss in ADH and DCIS suggests that atypical lesions try to acquire a more “luminal” phenotype. Adding to the same theme, usual hyperplasia is generally negative or patchy positive for ER, but atypical hyperplasia and low/intermediate grade DCIS are often strongly and diffusely ER positive. So, in a lesion with ambiguous morphology for ADH, a combination of CK5 and ER may be helpful in rendering a more definitive diagnosis. A CK5+ and ER low/negative immunophenotype of the proliferative component would favor usual hyperplasia, while the opposite (CK5 negative, ER strong+) profile would favor ADH/DCIS.
There are a few pitfalls for using these IHC stains for making a diagnosis of ADH/DCIS. First of all, this panel is not valid for columnar cell lesions as even benign columnar cell changes strongly express ER. Second, apocrine lesions (benign/atypical/or malignant) are generally negative for ER and are negative for CK5 or show variable reactivity for CK5. Finally, the basal-like DCIS are usually positive for CK5 and negative for ER. Another aspect to remember is that native luminal epithelium, that is, the normal epithelium that rests upon the myoepithelium is usually negative for CK5 and should not be interpreted as “atypia.” The results for CK5 should be interpreted in the intra-ductal proliferative component for atypia assessment. Therefore, CK5 and ER should be used in conjunction with defined morphologic criteria for diagnosing ADH/DCIS (see Fig. 19.17 ).
The diagnosis of atypia in papillary lesions is also very challenging. Fortunately, the same cytokeratin patterns of immunostaining hold up for the differential of ADH/DCIS in a papilloma versus florid hyperplasia in a papilloma.
Cytokeratin antibody 34βΕ12, CK5/6, and CK5 intensely stain florid ductal hyperplasia of breast, which may be useful in separating florid hyperplasia in ducts or papillomas from ADH/DCIS.
Both ADH and DCIS lack 34βΕ12, CK5/6, and CK5 antibody staining and cannot be distinguished by IHC.
Estrogen receptor expression is diffuse and strong in ADH and low-grade DCIS, but is either negative or patchy in ductal hyperplasia.
Based on cell cohesiveness, the two broad categories of breast carcinoma (invasive or in situ) are ductal and lobular types. Ductal carcinoma in-situ increases the risk of invasive malignancy at the local site whereas lobular carcinoma in-situ is considered a marker of generalized increased risk of invasive malignancy, although some recent data suggests precursor properties for lobular carcinoma in-situ. Invasive ductal carcinomas (IDCs) are often unifocal lesions compared to invasive lobular carcinoma, which are often multifocal and/or more extensive than what is estimated on clinical and mammographic examination. Distant metastases from ductal carcinoma preferentially involves lung and brain, whereas metastases from lobular carcinoma more often involve the peritoneum, bone, bone marrow, and visceral organs of gastrointestinal and gynecologic tracts. Despite the above differences, at present, with the combined multi-modality therapy, there appears to be no difference in disease free or overall survival between ductal and lobular carcinomas. , , However, there are enough significant differences in patient preoperative evaluation and subsequent treatment, that an accurate diagnosis is warranted at the time of core biopsy. At some breast cancer centers, a preoperative (before lumpectomy or mastectomy) magnetic resonance imaging (MRI) of the breast is performed to evaluate the extent of disease with a core biopsy diagnosis of invasive lobular carcinoma. , , , A core biopsy diagnosis of ductal versus lobular carcinoma is also important if the patient will be treated by neo-adjuvant chemotherapy. Although therapy response is better predicted with tumor receptor status and grade rather than morphologic tumor type, studies do show response to neoadjuvant chemotherapy (NACT) only in a subset of ductal cancers with no or minimal effect on lobular cancers. Therefore, pathologists have to strive hard to give the best diagnosis possible for current management and also for the future as specific therapies become available. One study has shown relative effectiveness of aromatase inhibitor, letrozole over tamoxifen for patients with lobular carcinoma.
Strong E-Cadherin (ECAD) membranous staining has been long used to define ductal carcinomas. Ductal carcinomas (in situ or invasive) retain membranous ECAD because they do not show homozygous mutation/silencing of the ECAD gene. Mutation of the ECAD gene either leads to a mutant protein that loses its adhesive properties or there is not enough protein to function as adhesive molecule.
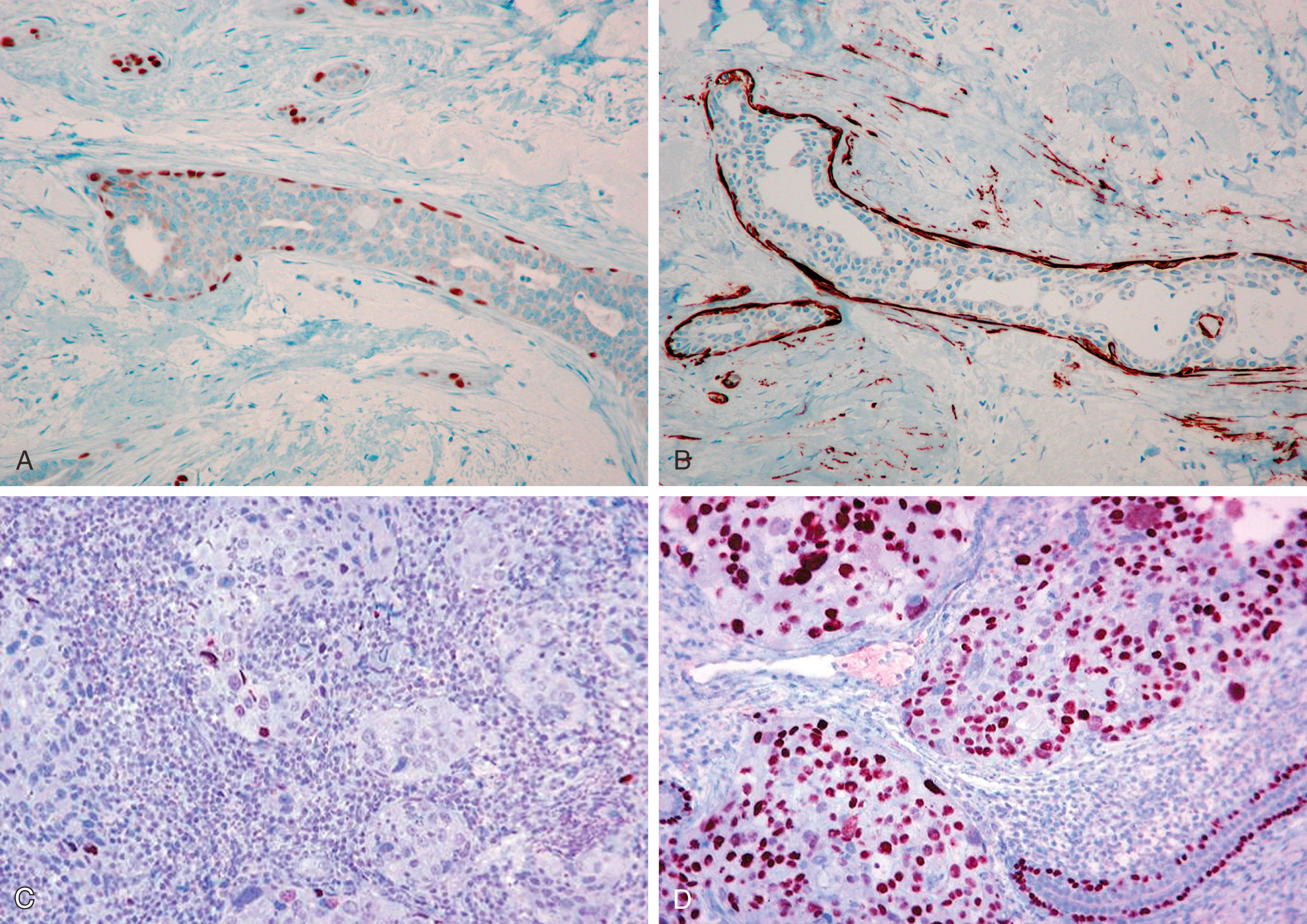
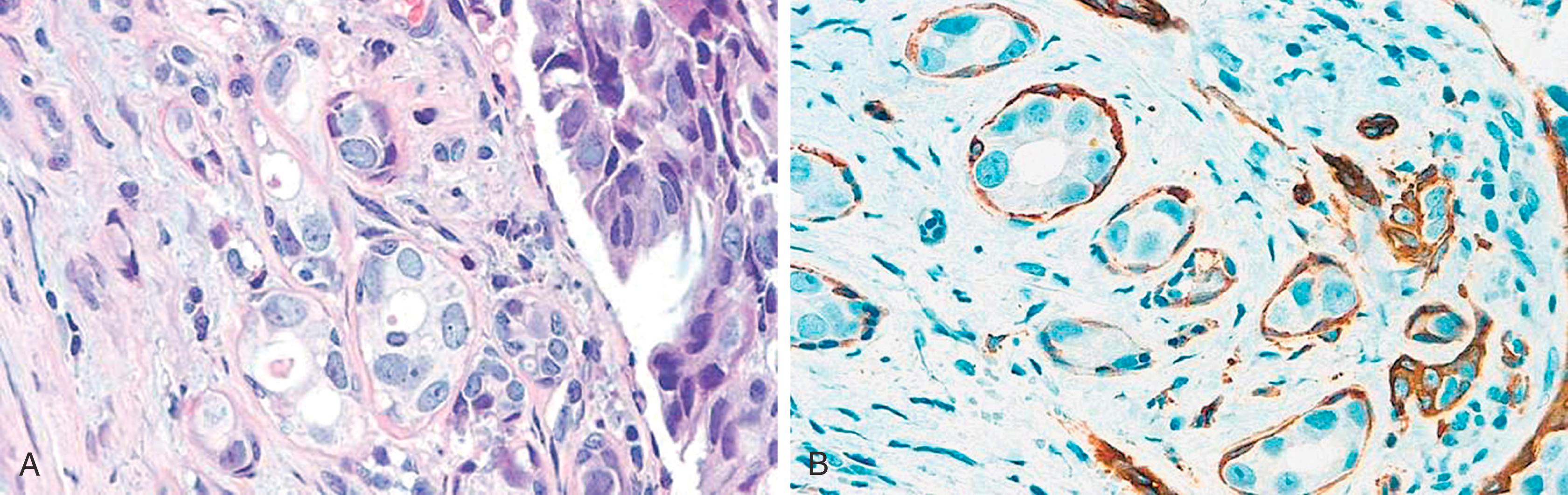
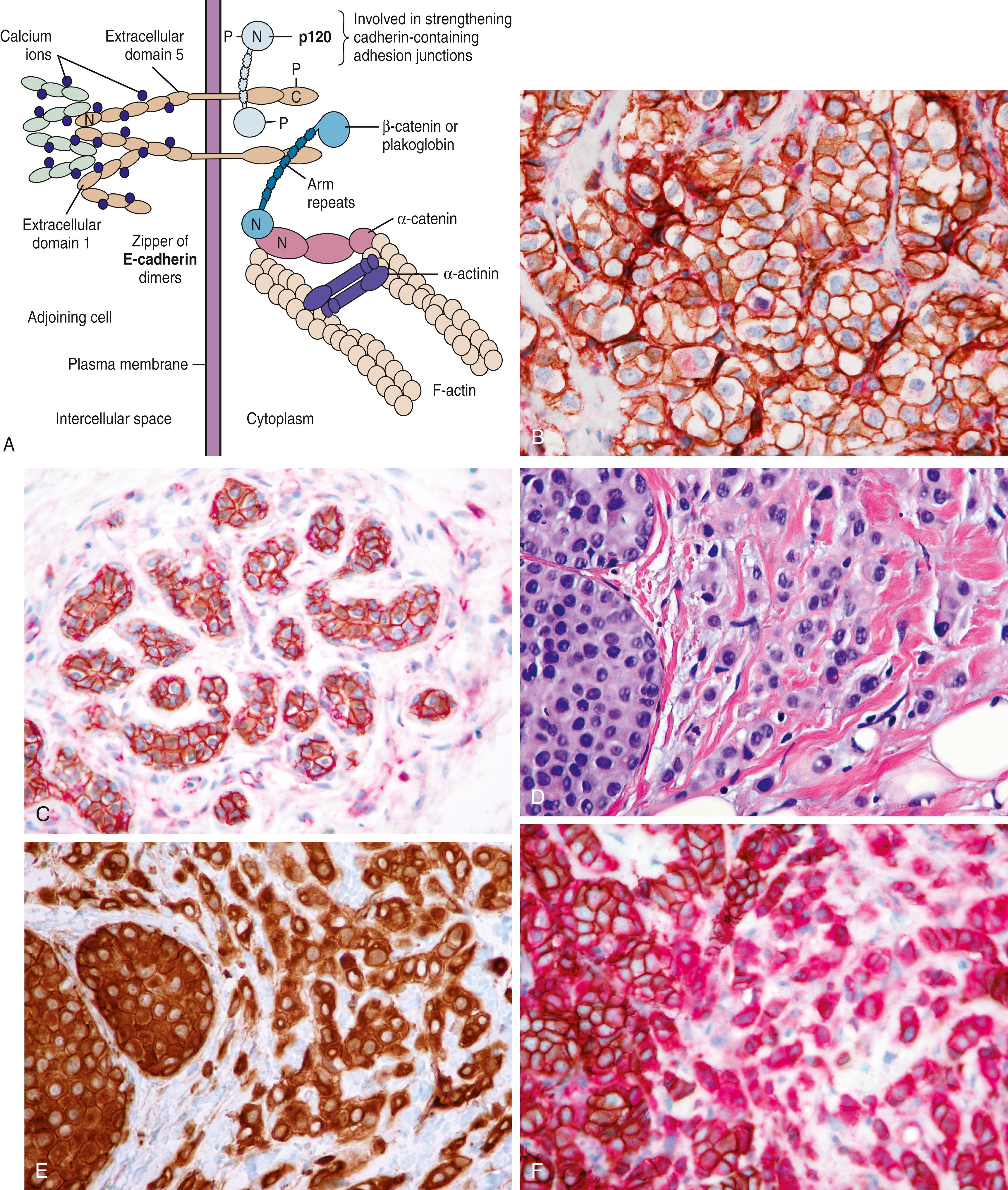
The ECAD gene, CDH1 , is a large gene located on 16q22.1. The ECAD protein has an intracytoplasmic portion, an intramembranous portion, and an extracellular domain. Cell-to-cell adhesion through ECAD is also critically dependent on the subplasmalemmal cytoplasmic catenin complexes (α, β, γ and p120 isoforms) that link ECAD to the actin cytoskeleton of the cell. Abnormalities of the catenins or ECAD gene expression can result in a variety of immunohistochemical ECAD pathologies. Lobular carcinomas studied at the genetic level have often shown ECAD mutation that accounts for the loss of cohesiveness of the tumor cells. , The majority of these mutations have been found in combination with a loss of heterozygosity (LOH) of the wild type ECAD locus (16q22.1), a hallmark of classical tumor suppressor genes. Immunohistochemically, this correlates with either a complete absence of the ECAD protein or abnormal localization (apical or perinuclear). This abnormal localization may be dependent on the type of mutation. Truncation mutations produce an ECAD product that is inept at binding to neighboring cells, resulting in a histologic pattern of widely dyshesive cells that are completely negative for ECAD by IHC (e.g., classic infiltrating lobular carcinoma [ILC]) ( Fig. 19.18 ). Loss of membrane staining may be associated with a granular cytoplasmic immunostaining ( Fig. 19.19A and B ) that represents cytoplasmic solubilization of a portion of the truncated protein. Proximal truncation mutations may result in the inability of ECAD to bind to the catenin complex, resulting in a short ECAD represented by focal or dot-like membrane immunostaining ( Fig. 19.19C ). Patients with focal staining of LCIS cells with ECAD may have an ipsilateral risk of carcinoma akin to DCIS. Mutations in the catenin complex can also lead to dysfunctional ECAD and loss of membrane staining. , While deletions of the CDH1 gene as a result of LOH are seen in ductal carcinomas, they are not early events and are not usually associated with the point mutations seen in lobular neoplasia.
In most cases, ECAD staining is unequivocal (positive or negative) and can be solely used in distinguishing ductal from lobular carcinomas. In a minority (approximately 15% cases) of cases, the stain may be difficult to interpret. This aberrant staining for ECAD is often seen as partial membranous and beaded. Another stain that could be used in such situations is p120. This stain represents p120 catenin, which binds with ECAD on the internal aspect of the cell membrane to form the cadherin-catenin complex ( Fig. 19.20A ). The complex is essential for the formation of intercellular tight junctions, and is composed of an external domain of calcium-dependent ECAD, and an internal domain of ECAD to which is bound the α, β, and p120 catenins. The α and β catenin are complexed with the carboxy-terminal cytoplasmic tail of ECAD, whereas the p120 catenin is anchored to ECAD in a juxta-membranous site. P120 is actively involved in the status of cell motility, ECAD trafficking, ECAD turnover, promotion of cell junction formation, and regulation of the actin cytoskeleton. The binding of p120 to ECAD stabilizes the complex and increases the half-life of membrane ECAD by slowing the normal turnover of ECAD that normally occurs by cellular endocytosis. P120 that is bound to ECAD exists in equilibrium with a small cytoplasmic pool of p120. When ECAD is absent, the cytoplasmic pool of p120 increases. Therefore, in normal ducts and ductal carcinomas, p120 shows a membranous pattern of staining ( Fig. 19.20B and C ). In contrast, lobular carcinomas (with absent or non-functional ECAD) show strong cytoplasmic p120 immunoreactivity ( Fig. 19.20D and E ). This positive cytoplasmic staining for lobular carcinoma is much easier to interpret than ECAD negative staining. A combination of ECAD and p120 drastically reduces the number of ambiguous diagnoses and better delineates (or diagnosed with increased confidence) the category of mixed ductal and lobular carcinoma ( Fig. 19.20F ). These mixed carcinomas comprise no more than 10% of all breast carcinomas and probably arise due to “late” ECAD inactivation within a ductal carcinoma. In contrast, loss of ECAD protein occurs very early in lobular carcinogenesis. Lack of ECAD staining and strong p120 cytoplasmic staining is observed in all morphologically characterized lobular carcinoma in situ and atypical lobular hyperplasias ( Fig. 19.21 ). Additionally, lack of ECAD within minimal epithelial proliferation in the breast terminal duct lobular unit defines atypical lobular hyperplasia and distinguishes it from mild ductal hyperplasia. This distinction is important because patients with ALH are typically referred to a risk clinic. Although some studies have advocated surgical excision with core needle biopsy diagnosis of atypical lobular hyperplasia, , most breast care providers recommend referral to high risk clinic rather than surgical excision. The IHC stains support the notion that the term “lobular hyperplasia” has no significance in breast pathology.
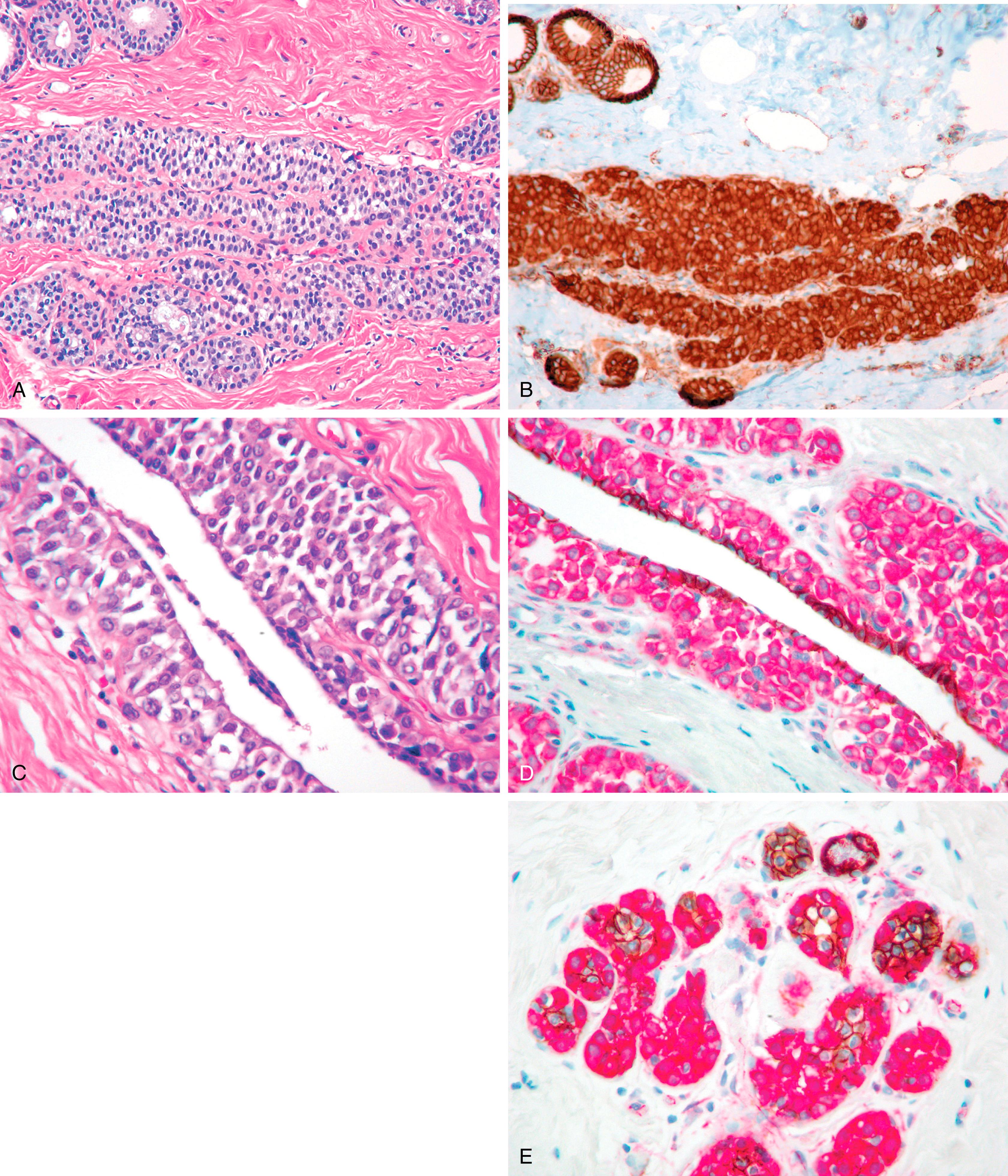
Immunohistochemical and molecular methods not only aid with the diagnostic issues, but to some extent they also demand that one looks at the morphology that has been learned in a new light. All invasive breast carcinomas that infiltrate in a “single-file” pattern with a low nuclear grade are not lobular carcinomas, as IDC also have this pattern.
The morphologic assessment of a ductal or lobular phenotype is not without controversy and has limitations. The classic ILC is composed of small cells with bland cytology and some “plasmacytoid” features. The growth pattern is completely dyshesive. Breast tissue can grossly appear normal (as well as the mammogram) yet show widespread dyshesive carcinoma of the classic type. These tumors are uniformly ECAD negative and associated with specific patterns of systemic metastases. IDC can also show patterns seen in ILC, for example, “single-filing” of tumor cells, targetoid patterns, and regional dyshesiveness. Such patterns may be confusing but are readily resolved with ECAD immunostaining. There are subgroups of morphologically “indeterminate” lobular/ductal phenotypes. ECAD separates these groups distinctly in most cases and demonstrates the existence of mixed lobular-ductal phenotypes in a minority of cases. , , ECAD stains MECs, a pitfall for misinterpretation of LCIS as DCIS (see Fig. 19.18A ).
The morphologic reproducibility of distinguishing IDC from ILC and LCIS from DCIS is less than optimal. There can be substantial variation in the interpretation of ILC versus IDC and LCIS versus DCIS. For this reason alone, ECAD and similarly p120 IHC could be justified to aid in correctly classifying these lesions.
ECAD stain is a useful diagnostic adjunct in cases with indeterminate morphology.
p120 further enhances diagnostic accuracy by being a “positive” stain for lobular carcinoma.
Lobular lesions are characteristically negative for ECAD and demonstrate intense cytoplasmic immunoreactivity for p120.
Aberrant ECAD immunostaining may be cytoplasmic or punctate/partial membranous, depending on the type of EACD mutation. p120 catenin in these instances proves the lobular phenotype.
Normal ducts and ductal lesions demonstrate membranous staining for ECAD and p120.
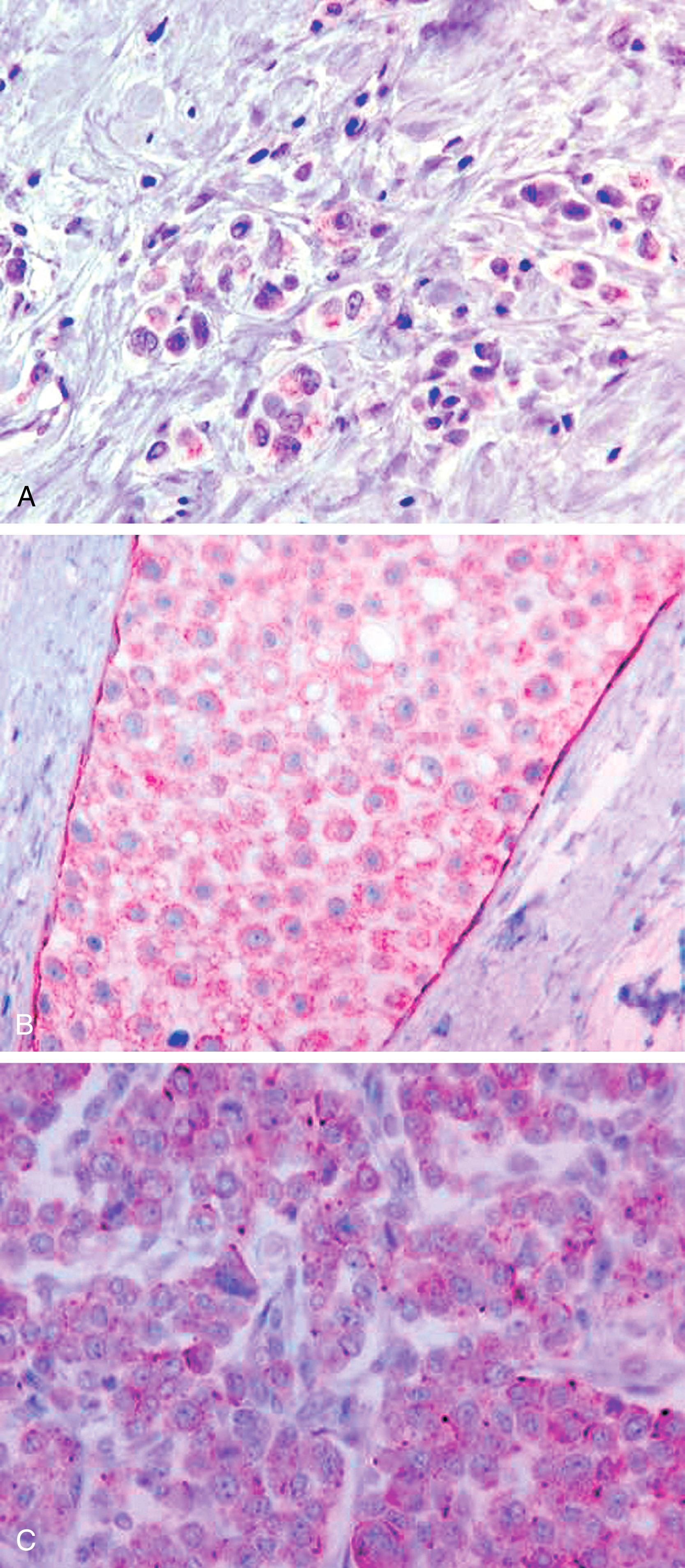
Described by Bassler in 1980 and further detailed by Weidner, Eusebi, and Reis-Filho, the genetic, immunohistologic, and clinical features have been sufficiently detailed to recognize invasive pleomorphic lobular carcinoma (PLC) and pleomorphic lobular carcinoma in situ (PLCIS) as a distinct clinicopathologic entity. Based on cell cohesiveness, PLC and PLCIS are basically a subtype of lobular carcinoma. The histologically recognizable PLC and PLCIS are almost always ECAD negative (or show aberrant staining) and demonstrates strong cytoplasmic immunoreactivity for p120. Histologically, these show grade 3 nuclei with a dyshesive pattern of growth in both in situ and infiltrating varieties ( Fig. 19.22 ). The in-situ component may be discovered on mammograms as calcifications. The core biopsies demonstrate in-situ dyshesive grade 3 nuclei with some cases showing comedonecrosis and calcification. A comprehensive analysis of 26 PLCs revealed a closer association between PLC and classic lobular carcinoma than between PLC and ductal carcinoma. The authors analyzed 26 cases of PLC, 16 cases of classic lobular carcinoma and 34 cases of IDC by IHC, array comparative genomic hybridization (aCGH), fluorescence in situ hybridization (FISH), and chromogenic in situ hybridization (CISH). Comparative analysis of aCGH data suggested the molecular features of PLC (ER/PR+, ECAD-negative, 1q(+), 11q(−), 16p(+) and 16q(−)) were more closely related to those of classic ILC than IDC. However, PLCs also showed some molecular alterations that are more typical of high-grade IDC than ILC (p53 and HER2 positivity in some cases, 8q(+), 17q24-q25(+), 13q(−) and amplification of 8q24, 12q14, 17q12 and 20q13). Some of these IDC-like alterations may be responsible for the aggressive biology of PLC. However, it is to be noted that pleomorphic histology may not always be associated with increased mitotic activity which is important for breast cancer prognosis. In fact, in a multivariable model, pleomorphic morphology was not prognostic but rather mitosis score, receptor subtype and pathologic stage were independently and significantly associated with disease free survival.
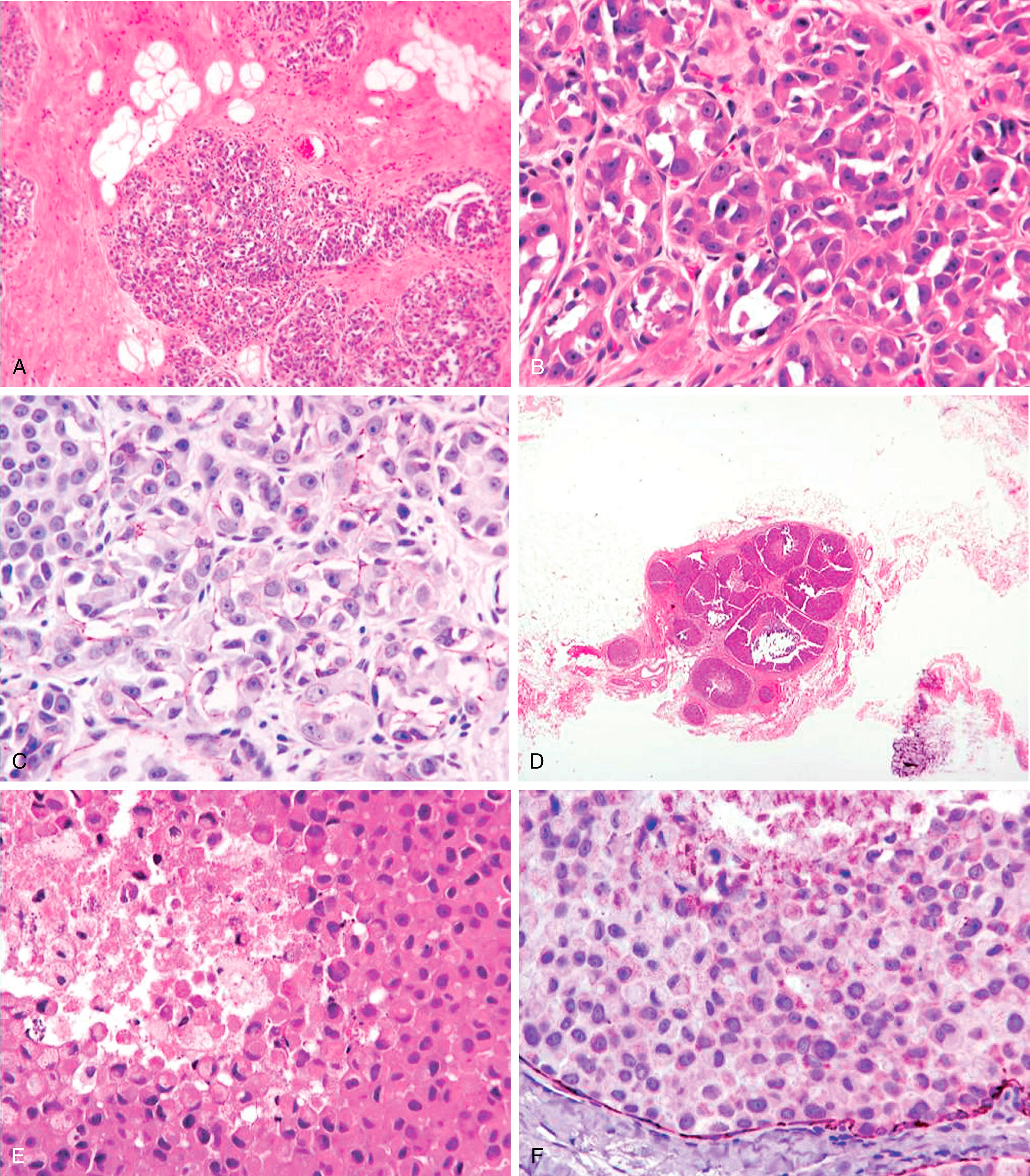
Sneige et al. studied 24 cases of PLCIS by IHC and found them to be universally positive for ER (100%). They also showed frequent p53 reactivity (25%) and moderate to high proliferative activity; and HER2 positivity was seen in 1/23 cases (4%). Of these 24 cases, 14 were associated with PLC, which showed similar IHC profile. Our experience with PLC and PLCIS is also very similar. Although HER2 over-expression/amplification may be seen in PLCs, the HER2+ rate is not very high as previously reported, and PLCs are ER+ in the majority of cases, albeit expression levels may vary from case to case. Since, there is high likelihood for developing invasive carcinoma in the vicinity of PLCIS, these lesions should be managed similar to DCIS.
Described originally by Fisher in 1977 as a lobular growth pattern with tiny tubules and single-filing characteristic of lobular carcinoma, the prognosis was described as intermediate between that of pure tubular carcinoma and ILC. , This lesion had been categorized as a variant of ILC because of the small cells and characteristic ILC pattern of single-filing, and targetoid infiltration.
Wheeler as well as Esposito documented uniform membranous ECAD immunostaining in the tubules and lobular-appearing components ( Fig. 19.23 ), , and discovered that pure LCIS and mixed LCIS/DCIS predominate in these lesions. The combination of a small, rounded tubule profiles with infiltrating lobular-like patterns that is ECAD+ is a ductal immunoprofile.
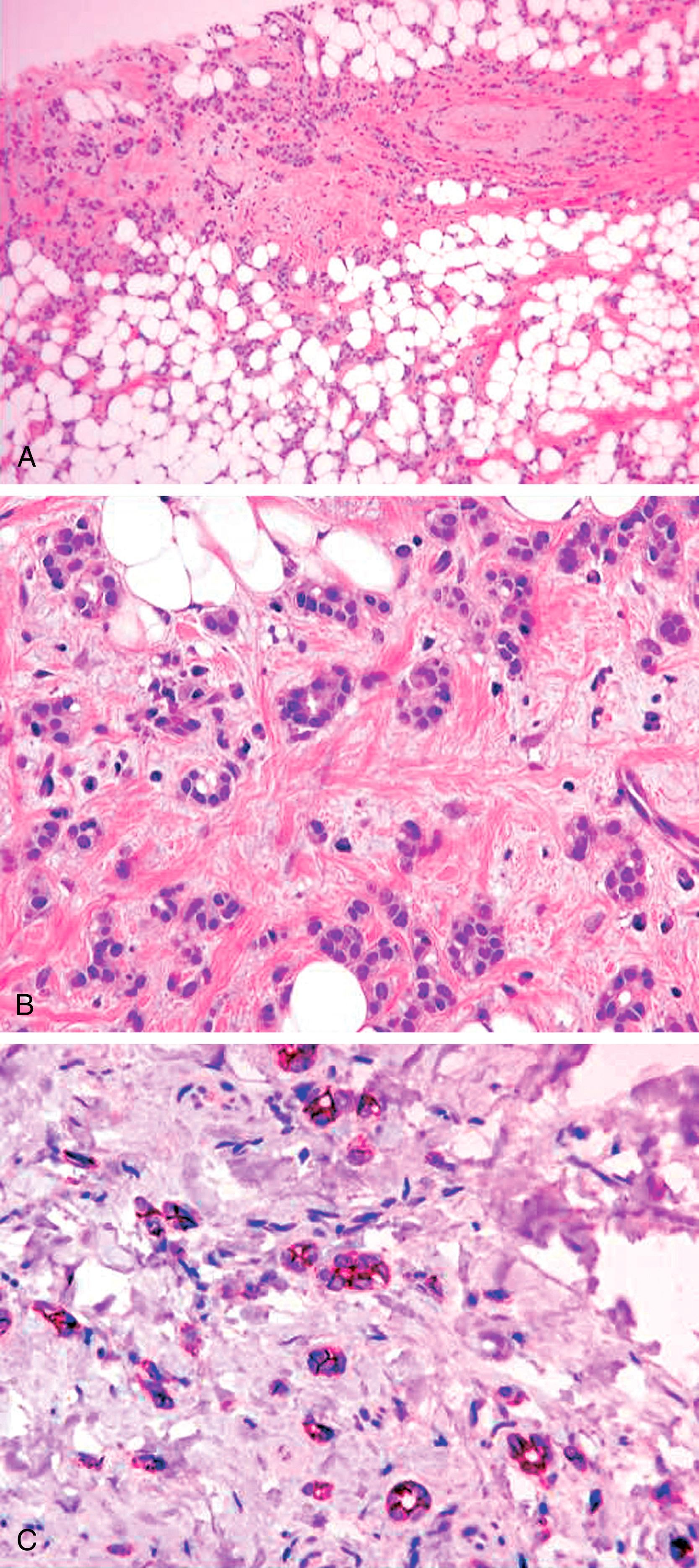
The term histiocytoid breast carcinoma (HBC) was coined by Hood et al. due to tumor cells resemblance to histiocytes. In 1983, Filotico described a case of lobular-appearing carcinoma with histiocytic features. Subsequent reports assumed that this variant was of lobular type by virtue of the characteristic infiltrating pattern. Immunohistologic studies have provided further clarification. Gupta et al., studied 11 cases of histiocytoid carcinoma and found that 8 of 11 cases lacked ECAD and 8 of 11 had LCIS. The authors concluded that histiocytoid carcinoma lacked distinct morphologic and clinical features and has an immunohistochemical profile that is consistent with both ductal and lobular differentiation. The current evidence suggests that HBC is a morphologic pattern that can be observed in ductal, lobular and apocrine tumors and is not a distinct entity by itself. It is interesting to note that classical lobular carcinoma with histiocytoid morphology have a higher likelihood for HER2 over-expression/amplification compared to lobular carcinomas without histiocytoid morphology.
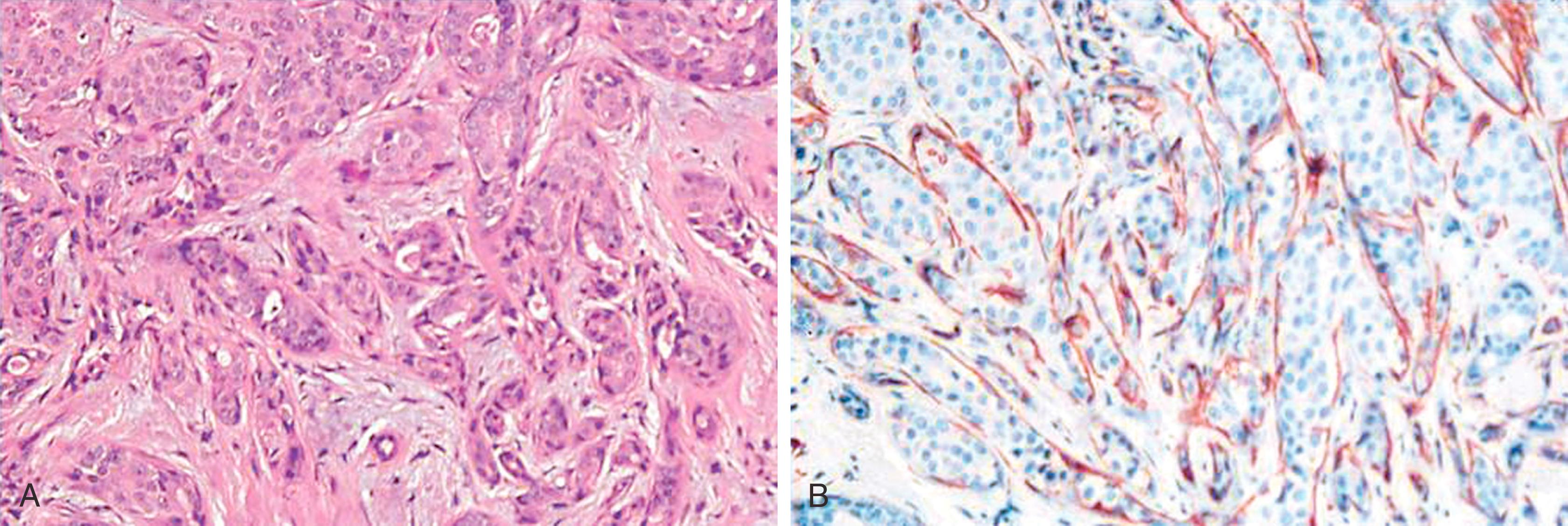
Tight clusters of neoplastic cells surrounded by clear spaces characterize the invasive micropapillary carcinoma. , The cell clusters are devoid of fibrovascular cores (unlike papillary carcinoma) and often display tubular structures in the center. The stroma is typically described as “spongy” with little or no desmoplasia of the surrounding tissue. , Some ductal carcinomas of “no special type” (NST) also show clear spaces around neoplastic cells, which are likely due to retraction of the intervening fibrotic stroma and should not be confused with micropapillary morphology. Fortunately, the distinction between true micropapillary carcinoma and NST carcinoma with retraction artifact can be easily made by epithelial membrane antigen (EMA or MUC1) stain. , , Ductal carcinoma of NST shows an apical or cytoplasmic staining with EMA. In contrast, invasive micropapillary carcinomas show accentuation of the basal surface (stroma facing) of the neoplastic cells ( Fig. 19.24 ). This “reverse polarity” of the neoplastic cells is a characteristic feature of invasive micropapillary carcinoma. The distinction between a ductal NST and micropapillary carcinoma may not be clinically very significant, because stage for stage, there is no significant difference between the two entities. However, micropapillary morphology (even when small) is highly predictive of lymph node metastases, and these tumors also more commonly tend to involve the skin and chest wall. The incidence of axillary lymph node metastasis has been reported to be as high as 95%. However, we believe the actual incidence of axillary lymph node involvement in routine clinical testing is close to 60%. Acs et al. showed that even partial reverse cell polarity, defined as prominent linear EMA reactivity on at least part of the periphery of tumor cell clusters, has the same implication as micropapillary differentiation and these tumors may represent part of a spectrum of invasive micropapillary carcinoma. A confident diagnosis of a true micropapillary carcinoma on a core biopsy helps the surgeon to plan appropriate management, viz. special attention and clinical exam of the axilla, to perform fine needle aspirate if axillary nodes are slight enlarged (but not definitely suspicious), and to request intraoperative frozen section even if the lymph node is grossly negative.
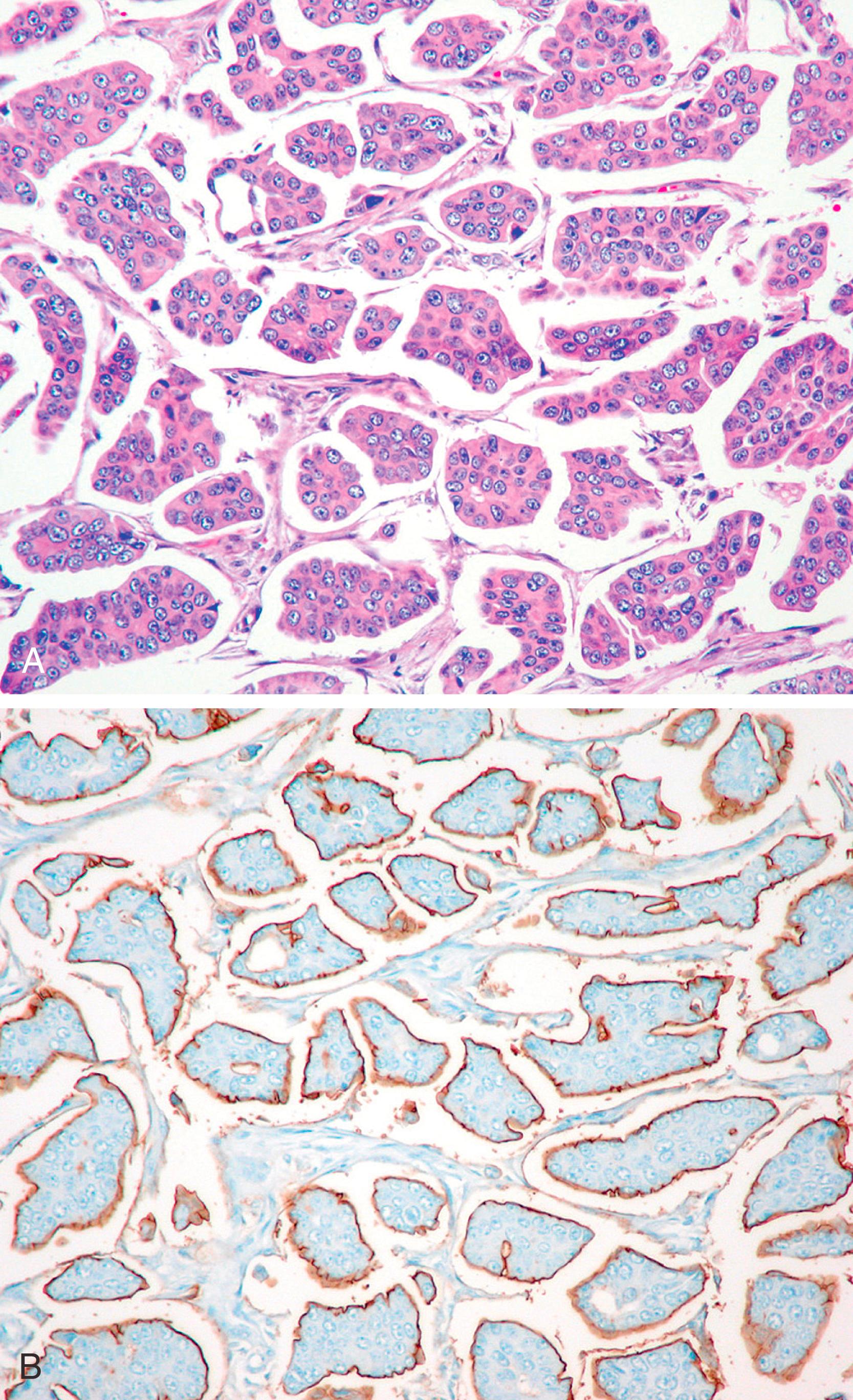
Routine hormone receptor (HR) protein and HER2 oncoprotein analysis on invasive breast carcinomas in the last decade has delineated clinically significant subgroups. One such group is that of “triple negative” tumors, that is, tumors that are negative for all three biomarkers ER, progesterone receptor (PR), and HER2. These tumors have been known to be clinically aggressive, and therapeutic options are limited since these are not amenable to HR-based therapy or HER2 targeted therapy. The so called “basal-like” breast carcinomas constitute at least 80% of the “triple negative” tumors. The “basal-like” subtype was initially recognized by gene expression profiling studies. , Basal-like carcinomas are histologically characterized by high Nottingham grade, geographic necrosis, good circumscription, and mild to moderate host lymphocytic response. These tumors generally show reduced (not absent) ECAD membranous expression, but show strong membranous p120 immunoreactivity (unpublished data); therefore, we consider them as subtypes of ductal carcinomas. Basal-like carcinomas are characteristically “triple negative” and show expression of basal-type cytokeratin (CK5/6, CK14, CK17), EGFR, vimentin, and p53. , Often, a panel of basal-type cytokeratins and EGFR in triple negative tumors is used to identify basal-like carcinomas ( Fig. 19.25 ). The antibodies used to identify the basal-like variant are an example of “genomic application” of IHC. This details fundamental immunohistologic profiles, used as surrogate markers, that reflect a genomic profile. Antibody to CK5 (clone XM26) is more sensitive (but equally specific) than CK5/6 (clone D5/16B4) for identifying basal-like breast carcinomas. The immunohistochemical studies have also confirmed the existence of in-situ carcinoma of basal phenotype. , Gene-expression studies have consistently identified basal-like carcinomas to have poor prognosis. , , These tumors occur in both pre and post-menopausal patients; however, identifying basal-like carcinoma in a young pre-menopausal patient may suggest the presence of hereditary breast and ovarian carcinoma syndrome. At present, triple negative basal-like breast carcinomas are not treated any differently compared to non-basal triple negative breast carcinomas. Immunotherapy and poly ADP-ribose polymerase (PARP) inhibitors are frequently used to treat triple negative basal-like breast cancers. At present, PARP inhibitor are only effective in patients who have germline mutations in BRCA1/2 genes or defective homologous recombination repair pathway. The basal cytokeratins in triple negative breast cancers can be used for diagnostic purposes to define true basal-like breast cancers as a surrogate to molecular profiling. Basal cytokeratins can also be used in clinically problematic weak ER+ cases to determine if these tumors represent luminal or basal-like breast cancers. Other markers such as nestin and inositol polyphosphate-4-phosphatase (INPP4b) have been utilized for this distinction. In a small study of 58 cases, positive nestin expression and loss of INPP4b in weak ER+ tumors (Allred score 3 to 5) showed a worse medial survival of 45.8 months versus 65 months for other weak ER+ tumors ( P = .012).
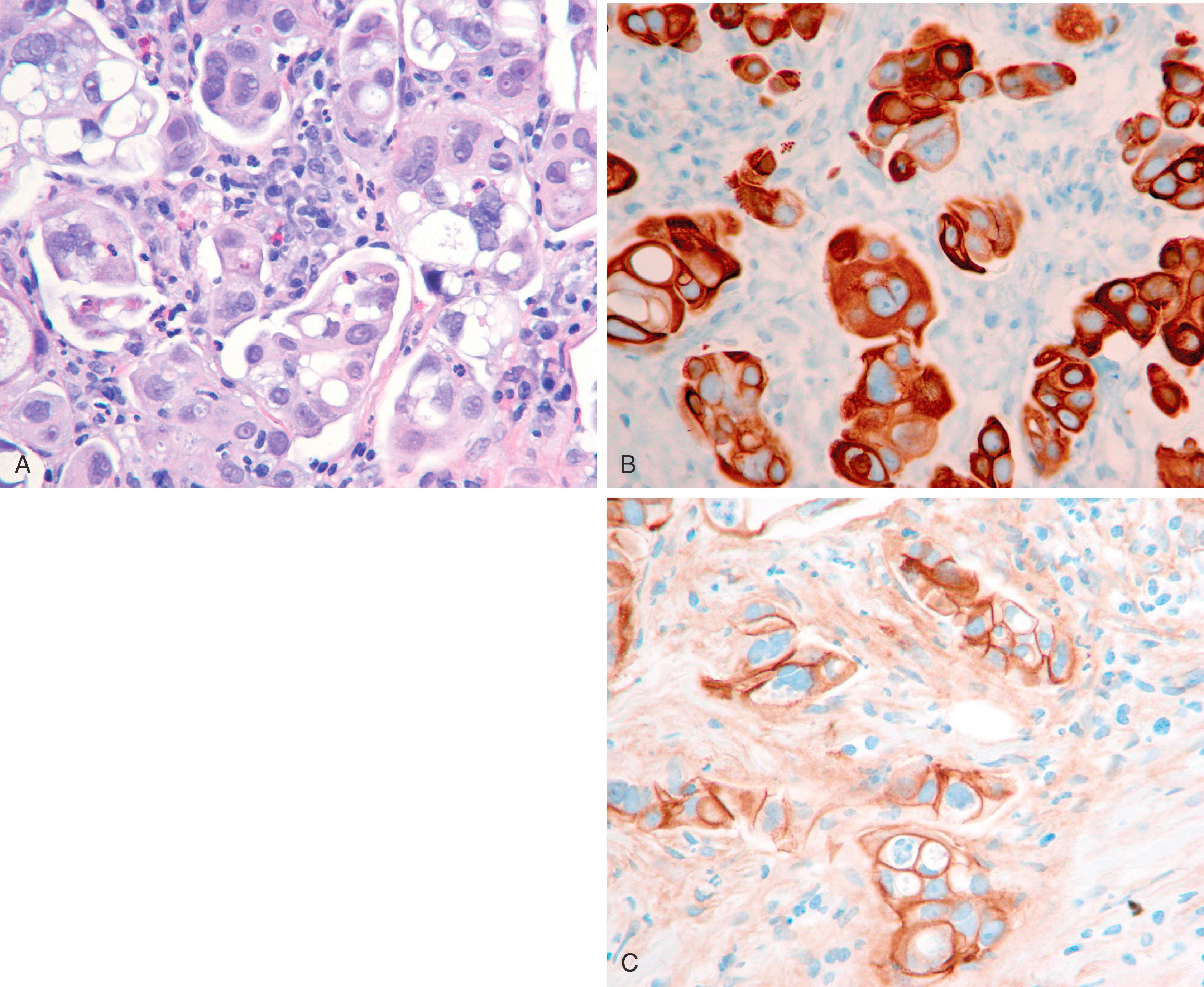
Metaplastic carcinoma comprises a group of heterogeneous neoplasms that exhibit pure epithelial or a mixed epithelial and mesenchymal phenotypes. , Diagnosis is not problematic when there is a recognizable component of metaplastic carcinoma, that is, an obvious adenocarcinoma, adenosquamous or squamous cell carcinoma, osseous or chondroid differentiation. The most problematic cases are the ones, which predominantly show spindle cell morphology without an obvious epithelial or DCIS component ( Fig. 19.26 ). This is usually the issue on a core biopsy rather than on an excision specimen. IHC stains can be helpful in this situation. A panel composed of multiple keratin stains (CAM5.2, AE1/3, 34βΕ12, CK5, and CK7) and EMA is more useful than a single keratin. Another sensitive and specific marker for spindle cell metaplastic carcinoma is p63 and should always be included in the panel. , Vimentin expression in the tumor does not exclude a spindle cell carcinoma. , , Vimentin expression has been found in 50% of hormone-independent cell lines, and since metaplastic carcinomas are usually negative for receptors, vimentin expression is actually expected. If all the keratins, EMA, and p63 fail to show any immunoreactivity on a core biopsy, complete excision of the lesion should be recommended. In many cases, an epithelial component is present only focally. Although every effort should be made to prove an atypical/malignant-appearing spindle cell lesion to be a metaplastic carcinoma, the differential diagnosis also includes melanoma, angiosarcoma, and stroma of phyllodes tumor. At least two melanoma markers should be performed. S100 is a very sensitive melanoma marker, but has been reported to stain between 20% and 50% of metaplastic breast carcinomas and therefore is not the best stain for this differential diagnosis. , Strong keratin reactivity or multiple keratin positivity would also exclude a melanoma. However, CAM5.2 positivity alone is not enough to exclude a melanoma unless it is strong and diffuse. , Another significant malignant lesion with which metaplastic carcinoma can be confused is an angiosarcoma. These tumors may occur after radiation treatment or de novo . It is obvious to think about angiosarcoma in a malignant spindle or epithelioid lesion of the breast if there has been a prior history of radiation treatment. However, in absence of such a clinical history, the lesion should be extensively examined by available IHC stains. More than one vascular marker should be used due to the heterogeneous expression of vascular markers. Of the 3 commonly used vascular markers (CD31, CD34, and Factor VIII), CD31 is generally considered as the most specific vascular endothelial marker, but occasional weak staining of carcinomas has been described. We have also seen staining of carcinoma cells and histiocytes with CD31. It is a diagnostic pitfall, especially in small samples. A diagnosis of a de novo primary angiosarcoma of the breast should be made only if there is unequivocal IHC staining for vascular markers, negative staining for p63 and HMWKs, and appropriate histology of the lesion. Other vascular markers that can be positive in angiosarcoma include D2-40, ERG, and FLI-1. In absence of staining for epithelial markers, the possibility of phyllodes stroma should also be considered. The stroma of phyllodes tumor is often (not always) positive for CD34, but is negative for other vascular markers. P63 is often negative in phyllodes tumor. However, p63 and p40 reactivity has been reported in 57% and 29% of malignant phyllodes tumor ( n = 14), respectively. In the same publication, focal cytokeratin reactivity was also reported in 21% of malignant phyllodes tumor. In our experience this is uncommon, but we suggest taking overall morphology and results of all IHC markers into account for making a definitive diagnosis. In summary, a malignant spindle cell lesion is a metaplastic carcinoma unless proven otherwise. A panel comprising of multiple keratins (especially basal cytokeratins), EMA, p63, melanoma, and vascular markers is required in the workup of a malignant spindle cell lesion.
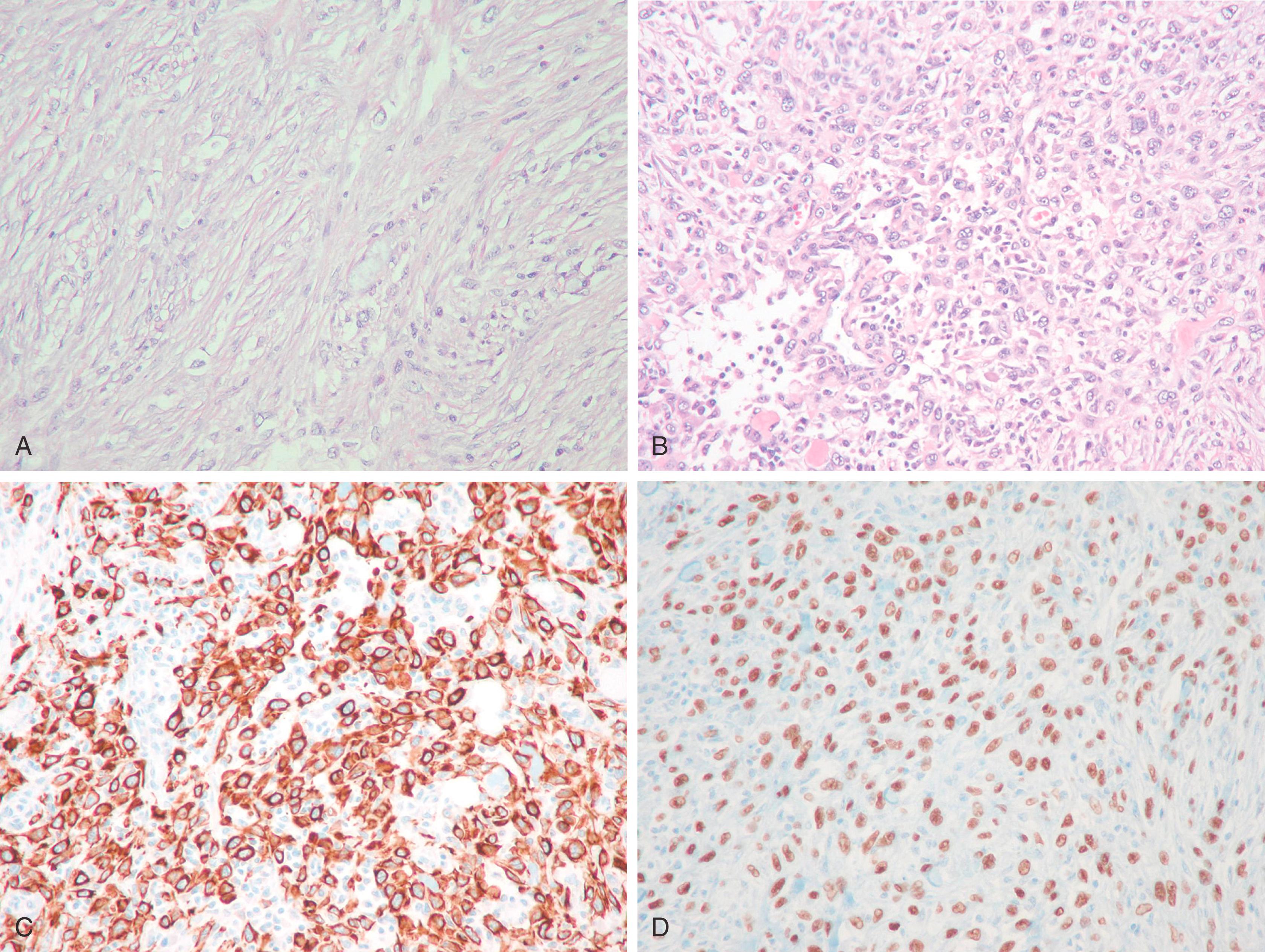
Tumors of the breast in which MEC differentiation predominantly include adenomyoepithelioma, myoepithelioma, and myoepithelial cell carcinoma (MECC). Although the majority of myoepithelial tumors are benign, occasional tumors may exhibit aggressive behavior in the form of carcinoma or myoepithelial carcinoma. , The typical immunostaining pattern of the myoepithelial components of these tumors is strong cytoplasmic staining for 34βE12, CK5, and nuclear p63. Tumor cells are typically positive with S-100 protein (90%) and may be positive with muscle markers such as calponin (86%), muscle-specific actin, desmin (14%), and α-smooth muscle actin (36%). , Occasional cells exhibit immunostaining with glial fibrillary acidic protein (GFAP). The presence of smooth muscle markers and immunostaining for GFAP is more in keeping with pure myoepithelial differentiation as opposed to metaplastic carcinomas (discussed above), which are largely negative for these markers. , Expression of SMA is very nonspecific and is not a definitive marker for muscle differentiation. Metaplastic carcinomas of the breast (carcinosarcoma, spindle cell carcinoma, sarcomatoid carcinoma) have an immunoprofile very similar to myoepithelial differentiation, as they regularly coexpress weak cytoplasmic CAM5.2 for low molecular weight keratins, strong cytoplasmic immunostaining for HMWK 34βΕ12, CK5/6 or CK5, vimentin, and nuclear immunostaining for p63 (90%). However, GFAP and SMMHC are largely negative. Immunostaining with the muscle markers is most indicative of a pure myoepithelial neoplasm as opposed to a metaplastic carcinoma. The immunoprofile of metaplastic carcinoma is shared to a great degree with myoepithelial neoplasms, with some investigators suggesting that the MEC is the progenitor cell for metaplastic carcinomas. , , , Leibl also demonstrated that the experimental myoepithelial markers CD29 and 14-3-3 sigma stain metaplastic carcinomas, supplying further evidence of the myoepithelial nature to these tumors. The literature suggests that using the terms myoepithelial carcinoma versus metaplastic carcinoma is a matter of semantics and may not have any clinical significance.
Myoepithelial tumors need to be separated from the rare primary spindle cell sarcoma of the breast, which may include fibrosarcoma (vimentin positive) leiomyosarcoma and rhabdomyosarcoma (positive with muscle markers), synovial sarcoma (positive with CK7 and CK19), malignant nerve sheath tumors (patchy S-100+ and vimentin+), and the so-called malignant fibrous histiocytomas (vimentin+). Although each of these tumors may have characteristic light microscopic features, immunostaining patterns may be useful in the diagnostic distinction ( Table 19.4 ). Primary liposarcomas (S100+) of breast are rare tumors that may arise in a preexisting phyllodes tumor (CD34+ stroma).
| Diagnosis | CK5/6 | K903 | P63 | GFAP | S100 | HHF-35 |
|---|---|---|---|---|---|---|
| Metaplastic carcinoma | + | + | + | N | N | S |
| Myoepithelial carcinoma | + | S | S | S | + | + |
| Fibrosarcoma | − | − | − | − | − | R-N |
| Myosarcoma | − | − | R/− | − | − | + |
| MPNST | − | − | − | − | S | − |
| Synovial sarcoma | − | − | − | − | R/− | − |
When the spindle cell lesion in the breast demonstrates bland cytomorphologic features, then the differential diagnosis includes myofibroblastoma, fibromatosis, fibromatosis-like metaplastic carcinoma, inflammatory myofibroblastic tumor, nodular fasciitis, scar tissue, leiomyoma, and cellular pseudoangiomatous stromal hyperplasia (PASH). Most of these lesions have characteristic morphology, but IHC stains are frequently used to confirm cellular differentiation. The myofibroblastoma of the breast ( Fig. 19.27 ) is distinguished immunohistochemically from myoepithelial tumors by lack of immunostaining for keratins, S-100 protein, and other myoepithelial markers. Myofibroblastomas demonstrate CD34+ cells in vast majority of the cases. Myofibroblastomas are often positive for HRs and show variable reactivity for actin and desmin. Myofibroblastomas have been previously referred to as solitary fibrous tumor of the breast, but immunohistochemical and genetic studies fail to find the link. Solitary fibrous tumors are often STAT6 positive, and mammary myofibroblastomas have been found to be negative. The genetic studies on myofibroblastomas have shown partial monosomy of 13q and 16q with deletion of the region 13q14 in greater than 50% cases. These findings are similar to 13q and 16q rearrangements in spindle cell lipoma. Moreover, it is not uncommon to find adipose tissue within myofibroblastomas supporting the concept that myofibroblastomas are likely related to spindle cell lipomas. PASH, which could be part of fibroadenoma or phyllodes tumor, is also positive for CD34. In contrast, fibromatosis involving the breast is negative for CD34, but demonstrate abnormal (nuclear) localization of beta-catenin. , It is to be noted that nuclear β-catenin expression has been documented in phyllodes tumor and metaplastic carcinomas, but expression is diffuse and strong in fibromatosis compared to other diagnoses. Scar tissue can be extremely difficult to distinguish from fibromatosis but is thought to be negative for nuclear β-catenin expression. Fibromatosis-like metaplastic carcinoma is distinguished from fibromatosis by expression of keratin stains and p63 reactivity in the former. In addition to the characteristic morphology, inflammatory myofibroblastic tumor shows reactivity for actin and ALK protein. Leiomyomas are extremely rare in the breast but are expected to be positive for smooth muscle markers, actin, desmin, and caldesmon. Patchy actin and desmin reactivity can be seen in many other myofibroblastic lesions, and therefore diagnosis of leiomyoma requires appropriate morphology and uniform strong expression for muscle markers.
Micropapillary carcinomas can be confidently identified by using EMA (or MUC1) that demonstrates “reverse polarity” of the tumor cells.
Basal-like carcinomas are “triple negative” and show immunoreactivity for basal cytokeratins and EGFR.
Basal-cytokeratins (especially CK5) along with p63 are very sensitive markers for identifying spindle cell metaplastic carcinomas.
Although muscle differentiation in myoepithelial carcinomas separates them from spindle cell metaplastic carcinomas, they likely represent two different spectra of one entity.
Adenomyoepitheliomas are biphasic tumors that resemble (and actually a variant of) intraductal papilloma at one extreme and show pure spindle cell myoepithelioma at the other extreme.
Non-carcinomatous spindle cell proliferation in a breast core biopsy sample includes both benign (fibromatosis, myofibroblastoma) and malignant lesions (stromal component of malignant phyllodes tumor, melanoma or primary sarcomas).
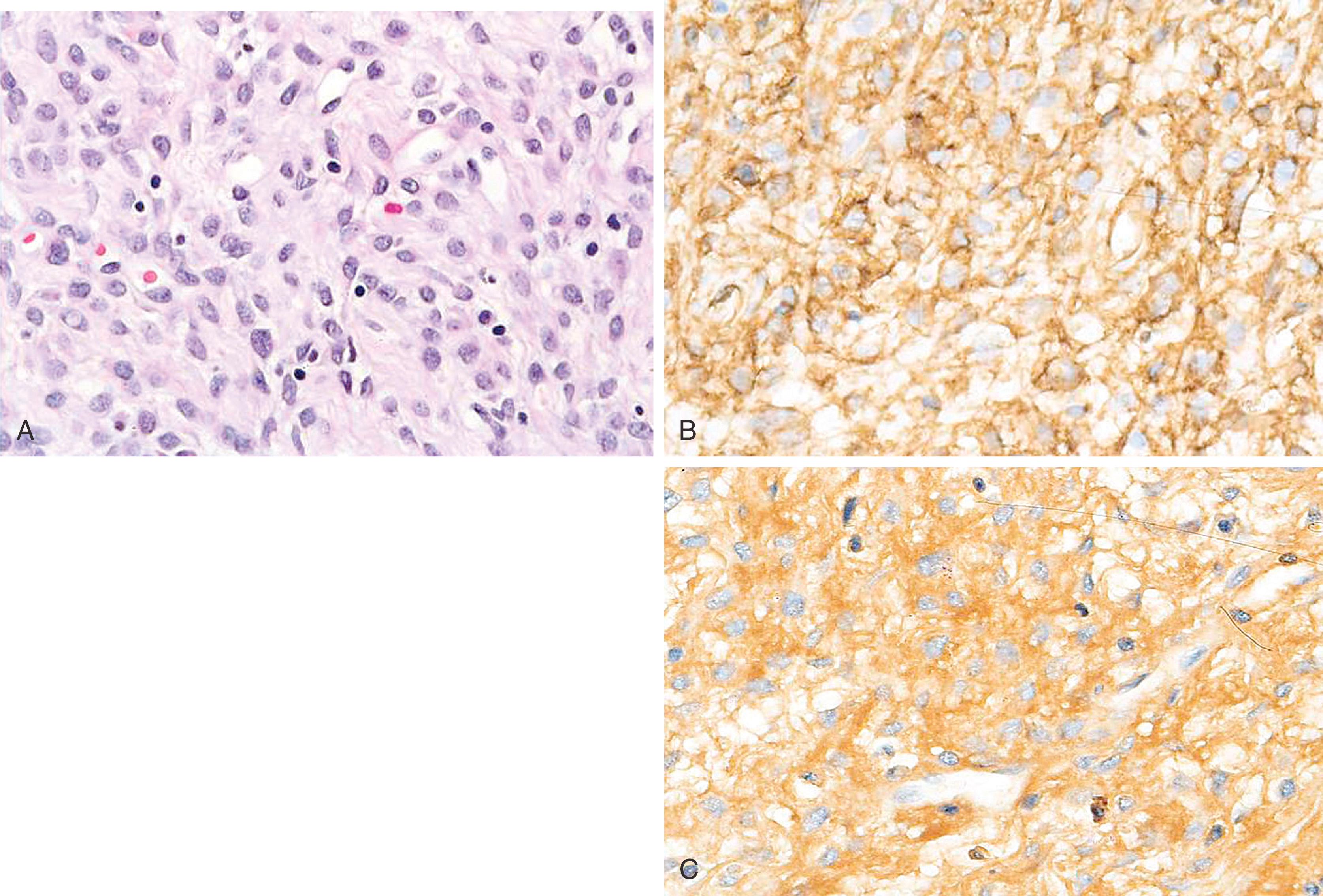
The presence of malignant epithelium (with breast cancer immunophenotype) within the nipple epidermis is termed as Paget disease of the breast. It is often the result of intra-epidermal spread of malignant cells from underlying ductal carcinoma in-situ. The presence of an associated mass lesion indicates the presence of invasive carcinoma in addition to DCIS.
Paget disease of the breast is manifested as CK7+ malignant cells infiltrating the epidermis of the nipple. Tumor cells are conspicuous by their infiltrative “shotgun” pattern, large size, abundant cytoplasm, signet-ring forms, and, sometimes, mucin positivity ( Fig. 19.28 ).
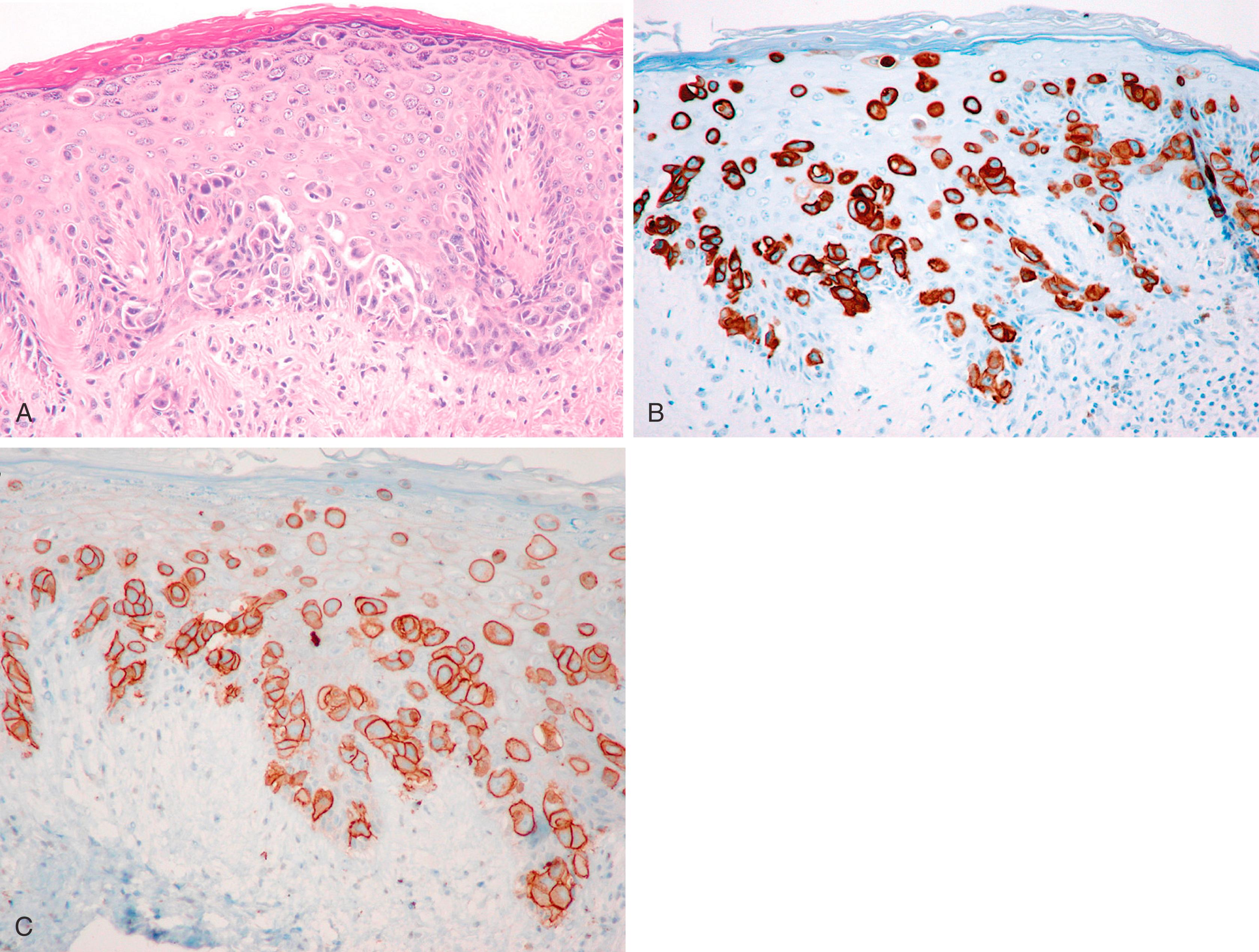
The majority (>90%) of the underlying breast carcinomas are ductal in nature. , In a nipple/areola biopsy or in cases where underlying carcinoma could not be documented, the differential for Paget disease includes a melanoma and squamous cell carcinoma in situ (Bowen disease). The single best stain for this differential diagnosis is CK7, which is positive in almost all cases of Paget disease (see Fig. 19.28 ). Cells of Paget disease are also positive for HER2 (in approximately 80% to 90% cases) ; this correlates to the IHC expression of underlying breast carcinoma, which is often a HER2 positive high-grade DCIS with apocrine differentiation and comedonecrosis. Additional stains that can be positive in Paget disease are gross cystic disease fluid protein-15 (GCDFP-15+), mammaglobin (MGB), carcinoembryonic antigen (CEA+), and HRs. However, it is important to remember that ER and PR are not good markers of Paget disease. Although Paget disease is a manifestation of underlying breast carcinoma, most often the carcinoma is a DCIS with comedonecrosis and these tumors are frequently negative for HRs. GATA3 is also positive in Paget disease, but it also stains background keratinocytes. This limits the usefulness of GATA3 in diagnosing Paget disease. If the possibility of a melanoma is entertained, then at least two melanoma markers should be used, since S100 can be positive in about 18% of Paget disease. However, it should be noted that malignant melanoma on the nipple is extraordinarily rare. Pagetoid squamous carcinoma (Bowen disease) of the breast is rare and can be distinguished from Paget disease. Cells of Bowen disease are negative for CK7 and squamous nature of the cells can be confirmed by CK5 or CK5/6 and p63 stains, while Paget shows reverse result for these antibodies.
Toker cells are CK7+ and may be present in the skin of the normal nipple, but generally they are inconspicuous compared with Paget cells, are cytologically bland, and do not cause diagnostic problems. It has been suggested that Toker cells may be the origin of intraepidermal Paget cells, based on similarity of immunophenotypes. In cases of florid papillomatosis of the nipple, some CK7+ cells may be found in the epidermis, a pitfall to be aware of in diagnosing Paget disease of the nipple. In addition, the intraepidermal portion of nipple ducts can be a pitfall for intraepidermal CK7+ cells.
Pseudopaget disease may on occasion be seen in the major ducts. Large histiocytes infiltrate the epithelium and impart a picture simulating Paget disease. These large cells are CK7 negative and strongly positive for CD68 ( Fig. 19.29 ).
Most often positive in Paget disease: CK7, HER2.
Other positive but less helpful stains: GCDFP-15, MGB, CEA, ER, PR, GATA3.
Pitfall: CK7+ cells in the epidermis in cases of florid nipple duct papillomatosis, Toker cells, or intraepidermal extension of lactiferous duct cells.
Pagetoid Bowen disease: CK7 negative, CK5 and p63+.
Melanoma: Keratin negative, melanoma markers +.
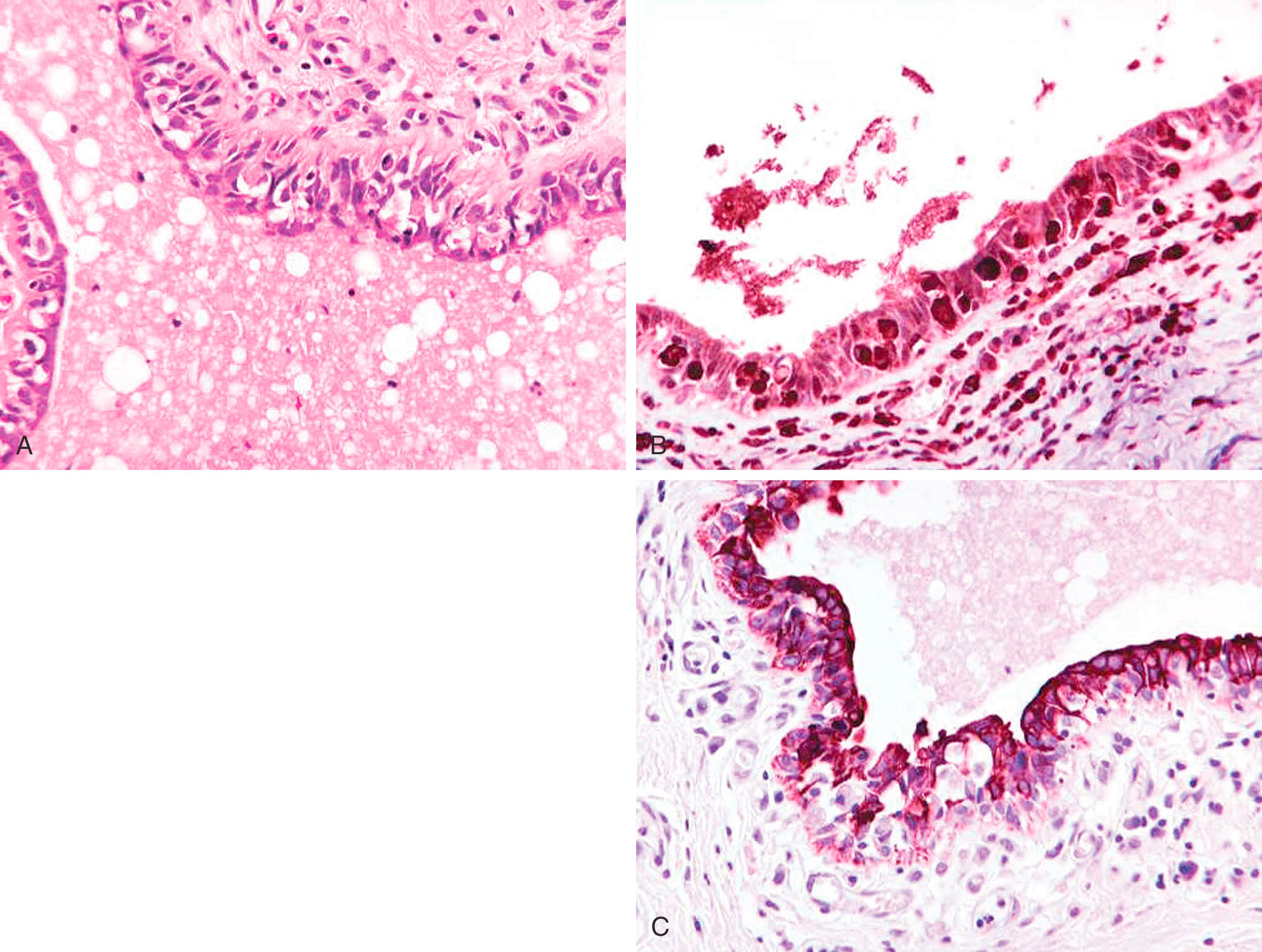
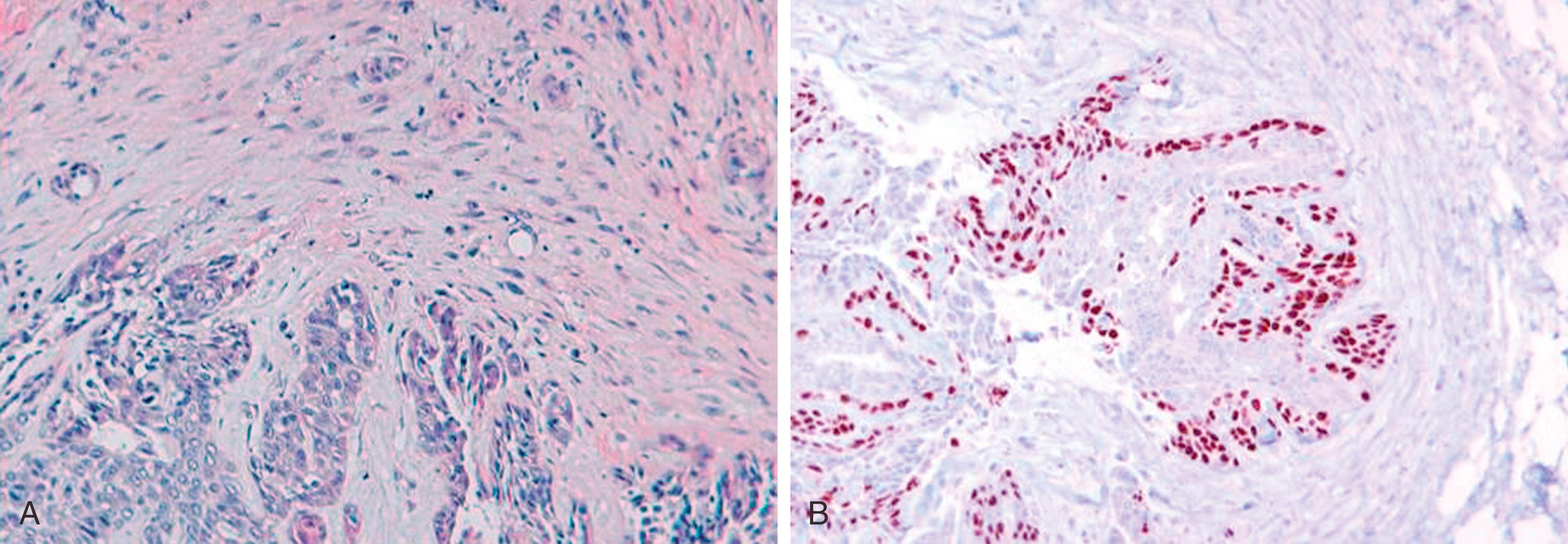
Lympho-vascular space invasion in breast carcinoma is an independent predictor of axillary lymph node metastases, which in turn is one of the most important prognostic factors in breast carcinoma. One recent study has shown that peri-tumoral lymphatic space invasion (and not blood vessel invasion) was determinant of lymph node metastasis. In addition, identification of tumor emboli within dermal lymphatics is also important for correlation purposes in cases of inflammatory carcinomas. However, the pitfalls of interpretation of lymphatic channels in paraffin-embedded breast tissue are well known. Retraction artifacts, ducts with misplaced epithelium, and artifactual displacement of cells commonly complicate the interpretation of biopsy samples. D2-40 shows high sensitivity and specificity for normal lymphatic channels in a variety of tissues. , D2-40 stains the lymphatic endothelium crisply and intensely, but does not stain the normal vascular endothelium ( Fig. 19.30 ). , It is highly sensitive and specific in identifying lymphatic space invasion. , In the breast, D2-40 stains lymphatic channels with a crisp, intense membrane staining of lymphatic endothelium. The D2-40 shows a smudgy immunostaining pattern with MECs and reactive stromal myofibroblasts. It is a pitfall, and this faint to occasionally moderate staining around the periphery of a small duct may be mistaken for lymphatic space invasion; however, it is important to remember that lymphatic vessels are stained very intensely with D2-40 ( Fig. 19.30C ).
Historically, complete axillary lymph node dissection (CALND) had been performed with lumpectomy or mastectomy specimens primarily for staging purposes, providing information that was used to determine adjuvant chemotherapy. The CALND may not change the course of the disease, although with removal of involved axillary nodes, the control of local recurrence in the axilla is easier. The morbidity associated with this procedure is substantial in terms of limitation of arm motion, arm pain, and chronic lymphedema.
The concept of an SLN was spawned by Cabanas in his study of penile carcinoma. The pioneering studies of sentinel lymph node metastasis (SLNM) originated with the study of melanoma patients; the goal was to spare these patients the morbidity of large regional lymph node dissections. Patients with melanomas who had SLN surgery were found to have a relatively orderly progression of lymph node metastases, with the SLN receiving the initial deposits of metastatic cells, followed by metastases in more distal lymph node groups. The same rationale was subsequently used for breast cancer patients. , The SLN is identified by injecting a radioisotope and blue dye before planned surgical excision. The SLN, identified by a combination of visual inspection for blue dye and intraoperative scanning for radioactivity, is harvested and submitted for pathologic study. The rationale is that for patients who are SLN-negative, a further morbid procedure of axillary cleanout is unnecessary, but for SLN-positive patients, an axillary dissection is indicated for proper staging and possibly to provide better control for local recurrence. The controversy in this approach arises from several valid questions:
What is the natural history of micrometastatic (MM) disease in the axilla?
Is MM SLN disease an obligate pathway to clinically manifested local recurrence in the axilla?
Is MM SLN disease an indication for adjuvant chemotherapy?
How should the excised SLN be examined pathologically?
Does MM SLN disease affect overall survival?
What are the biological parameters of MM disease that can predict the behavior of the disease in an individual patient?
Is it possible to recognize “benign transport” of epithelial elements in a SLN?
These are interesting and provocative questions for the care of the breast cancer patient. The American Joint Commission on Cancer defines micrometastasis as a cluster of cells that are no larger than 2 mm. One study with more than 10 years of follow-up conclude that micrometastases are associated with a small but statistically significant decrease in tumor-free survival and overall survival when compared with truly node-negative cases, but they are not an independent prognostic factor. The size of the metastatic deposit, taken together with tumor size and other factors, may additionally stratify patients at risk for further disease.
In most institutions, SLN biopsy with lumpectomy or mastectomy as indicated has become the standard of care. The vast majority of SLN metastases are found in the first three SLN that are submitted. Additionally, with completion and reporting of American College of Surgeons Oncology Group (ACOSOG) Z0011 study data, axillary lymph node staging has further changed. This study randomized patients undergoing lumpectomy for invasive cancer to either have completion axillary dissection or no axillary dissection for up to two positive sentinel nodes. Follow-up on these two randomized groups showed no statistical difference in disease free and overall survival. The lack of worse prognosis in patients who did not receive complete axillary dissection is likely related to the beneficial effect of radiation therapy after lumpectomy and also due to the fact that in approximately 60% cases of positive sentinel node(s), it is only the sentinel node(s) that is/are positive. In view of the Z0011 data, extensively sampling (i.e., multiple levels of the lymph node) and use of highly sensitive methods for identifying metastatic tumor seems unnecessary. ,
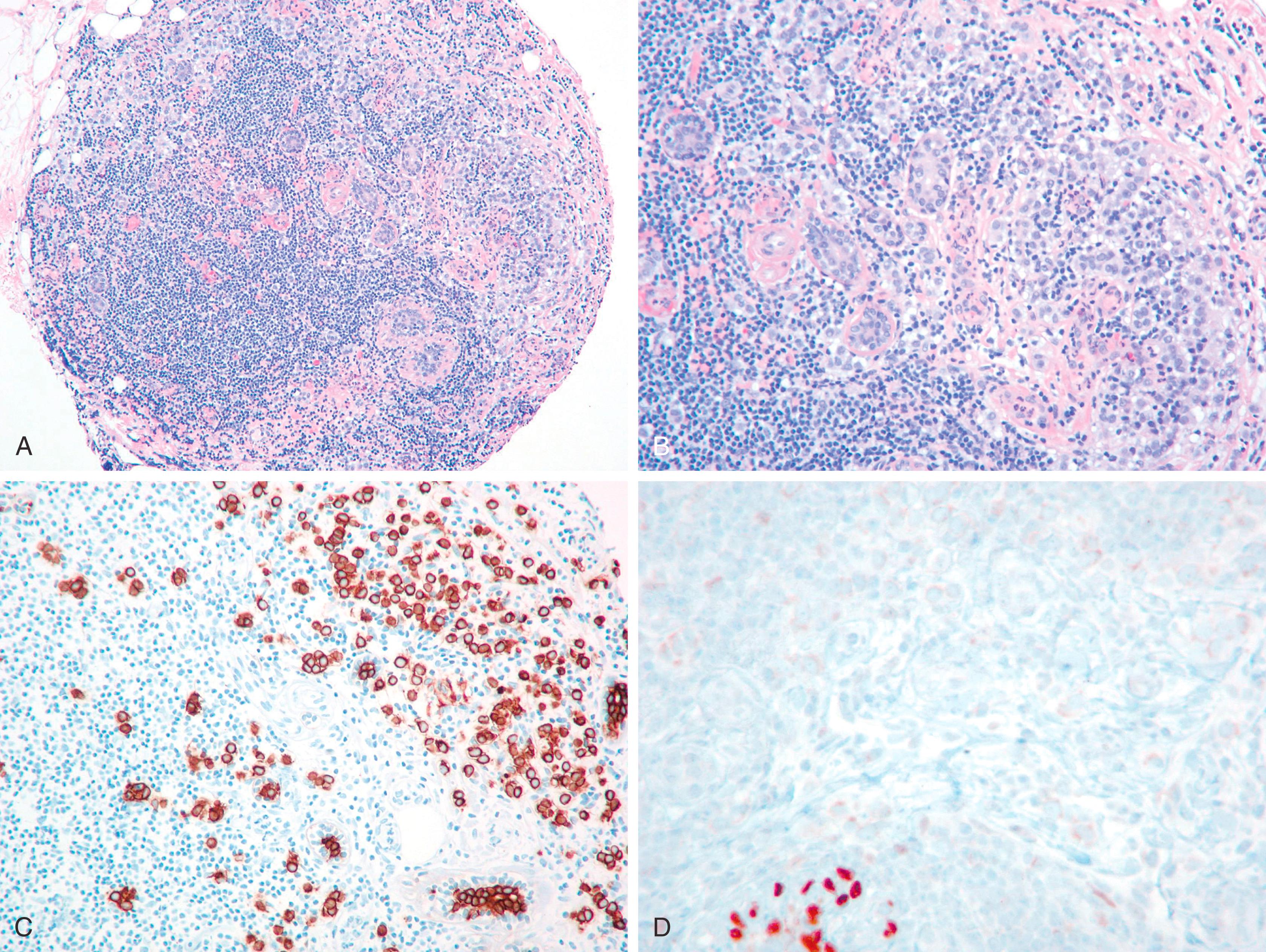
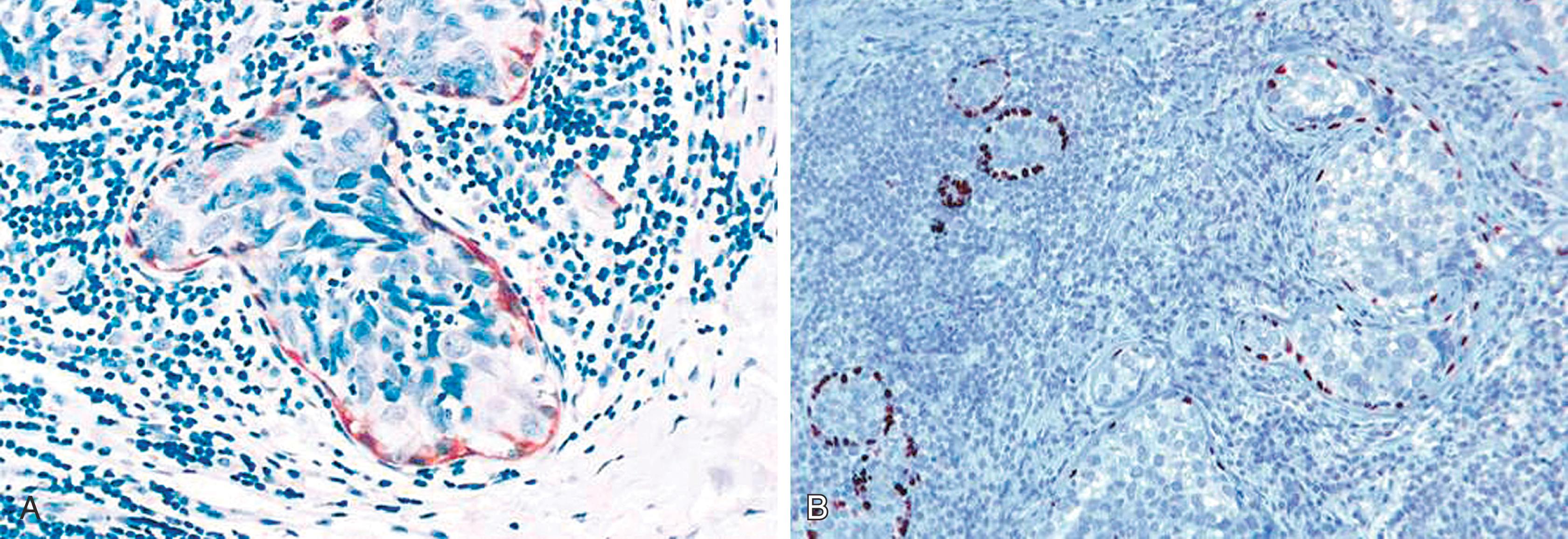
For the surgical pathologist, the appropriate triage and examination of the SLN is of utmost importance, but even here some controversy exists. When SLN mapping procedure began to be the standard of care a few years ago, the SLNs were histologically examined by multiple levels and cytokeratin stains on at least two levels. Since then, more experience has been gained with the procedure and the reporting of SLNs. It was soon realized that majority of micrometastases (metastases between 0.2 and 2 mm) can be identified by H&E alone and IHC for cytokeratin stains generally highlight isolated tumor cells (tumor cell aggregates ≤0.2 mm). Although the exact clinical significance of isolated tumor cells, and even micrometastases, remains uncertain, studies have shown that they both are associated with non- SLN positivity in approximately 10% of cases, especially when the tumor size is larger than 1 cm (pT stage 1C or more). However, completion axillary dissection with one to two positive sentinel nodes in lumpectomy patients, does not translate into improved disease free or overall survival as shown in Z0011 trial. If the primary breast carcinoma is of ductal type, it would be difficult (not impossible) to identify isolated tumor cells by H&E stain, and most pathologists would agree that they would be able to identify micrometastases ( Fig. 19.31A ). Therefore, cytokeratin stains on SLN do not add any significant information beyond H&E stain in a primary ductal cancer. However, there are significant differences, when the primary breast tumor shows a lobular morphology. Due to single cell infiltration, small (micro) metastases of lobular carcinoma (of the classical type) in a lymph node are extremely difficult to identify ( Fig. 19.31B and C ). Occasionally, cytokeratin stains would identify macro-metastases, not readily apparent on H&E stain. Cserni et al. have reported that sentinel node positivity detected by IHC in lobular carcinomas was associated with further nodal metastases in 12 of 50 (24%) cases. Therefore, it is not unreasonable (although not required) to perform cytokeratin stains on SLNs in cases of lobular carcinoma. As far as lymph node examination after neoadjuvant therapy is concerned, routine cytokeratin staining of the lymph node is not required. However, IHC staining can be performed if findings are equivocal or suspicious on H&E staining.
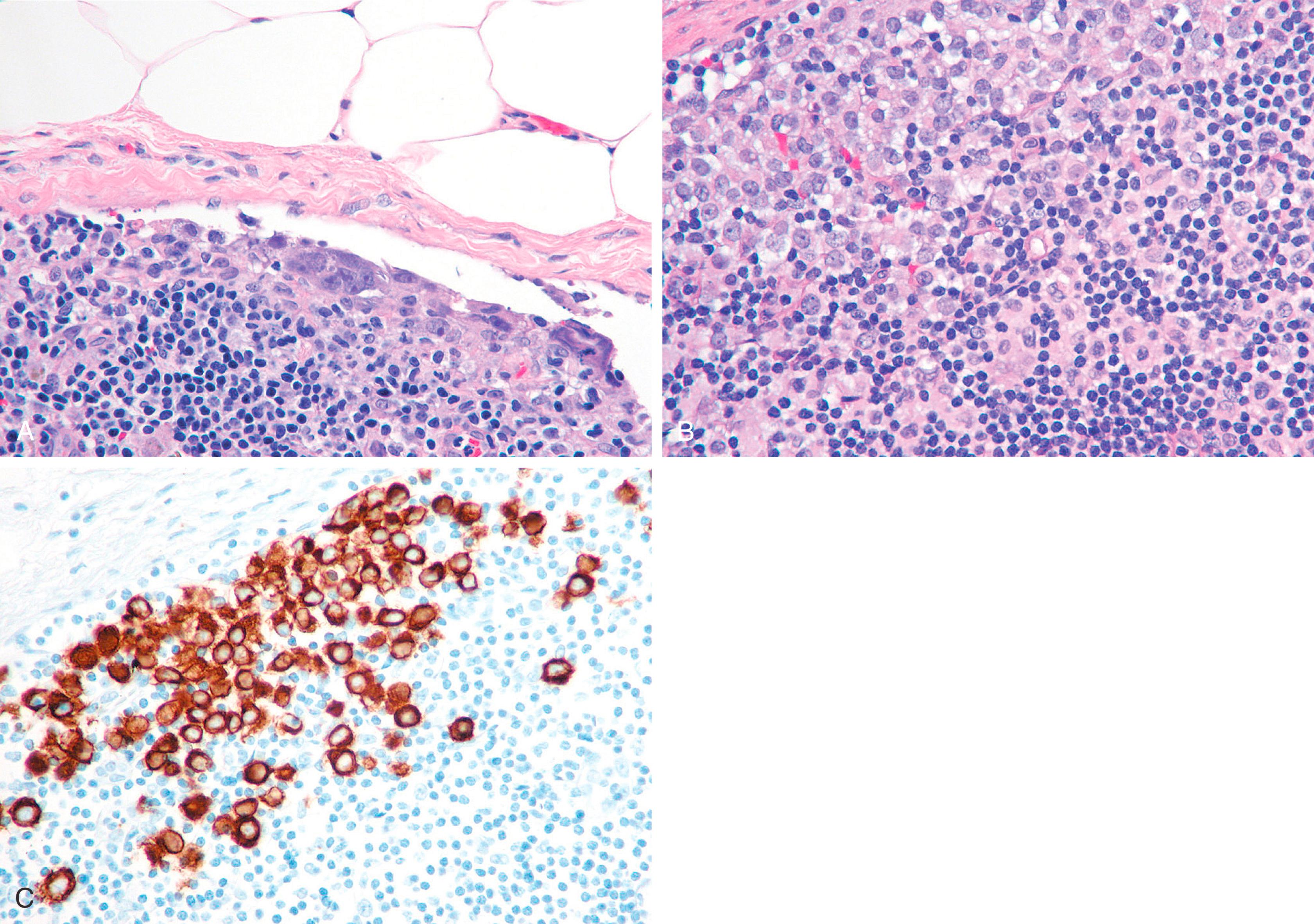
When performing cytokeratin immunostaining of SLNs, one should use a cocktail such as AE1/AE3 cocktail; CAM5.2 is less desirable because of the manner in which it stains dendritic cells in the lymph node. MM cells occur in small clusters less than 2 mm in diameter within the lymph node or subcapsular sinus, and they need to be distinguished from the dendritic appearance of the interstitial reticulum cells of the lymph node, which are also keratin positive. Studies with larger numbers of patients are needed to discern if the site of lymph node micrometastasis (peripheral sinus vs. parenchyma of lymph node) is clinically significant.
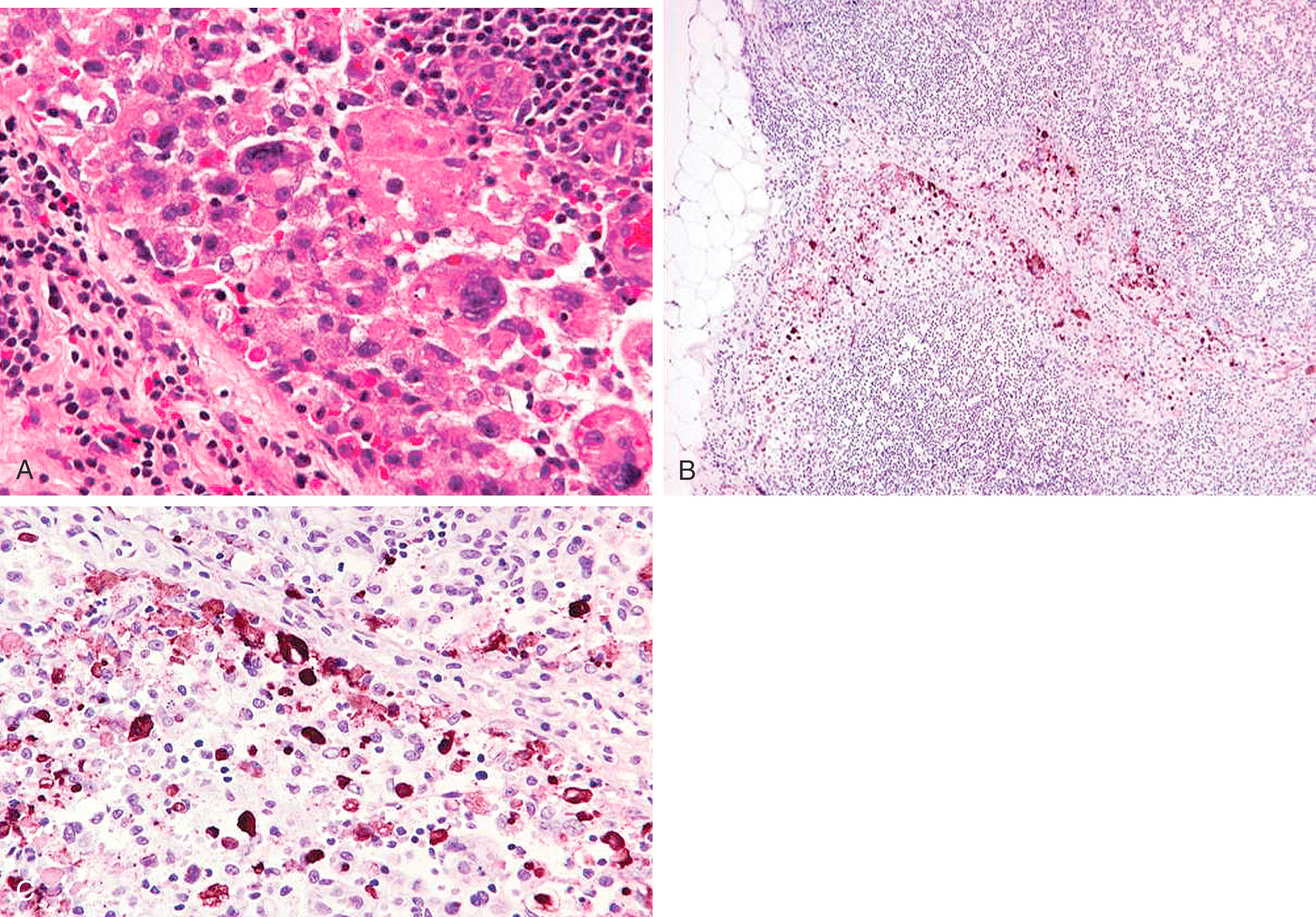
Aggregates of breast epithelial cells in the subcapsular sinus of axillary lymph nodes have been described by Carter and associates as occurring as a result of “mechanical transport” after a breast biopsy. Some impugn the core biopsy itself or the breast massage that follows isotope/dye injection as sources of mechanical displacement of cells into the SLN. Solitary keratin-positive cells may be transported to the SLN, and the histologic feature often associated with true benign transport is the association of CK-positive cells with altered red blood cells and hemosiderin and macrophages ( Fig. 19.32 ). Diaz et al. described benign epithelial tissue in skin dermal lymphatics and SLN(s) from patients with pure DCIS. This lends morphologic documentation to the concept of “benign mechanical transport.” The distinction between benign transport and “true” metastasis is easy if the cells in lymph node appear “benign,” but there is no objective way to distinguish benign transport from true metastasis when the cells appear cytologically malignant.
In the past few years, a few studies have shown the usefulness of intraoperative molecular tests in determining metastatic disease. , These are reverse transcriptase polymerase chain reaction assays (RT-PCR) which use a completely closed system and are fully automated from RNA extraction to final interpretation. One such assay was called the GeneSearch Breast Lymph Node (BLN) Assay (Veridex LLC, Warren, NJ), which was approved by United States Food and Drug Administration (FDA) for axillary lymph node testing. The GeneSearch BLN assay was composed of sample preparation kit, all reagents required for performing RT-PCR and protocol software to be used with the Cepheid SmartCycler System (Sunnyvale, CA). According to the company, the test was optimized for detecting metastatic disease larger than 0.2 mm. The test analyzed the expression of CK19 and MGB genes. The studies showed high sensitivity, specificity, positive, and negative predictive values for the test. Overall, this molecular assay was very much comparable to the frozen section examination, permanent sections, and even IHC. However, just like any other test, one should be aware of the false positive and false negative test results as well as the usefulness and pitfalls of a particular test. Since molecular tests are not morphologic assays, one has to be extremely careful with any sources of contamination. A cutting bench metastasis (“floater”) can be easily recognized on an H&E-stained slide as such, but will give a false positive result by RT-PCR, and there will be no definite way to identify this as an error. The SLNs identified in the axillary tail may contain minute amount of breast tissue in the surrounding adipose tissue which may also give a false positive result. Therefore lymph nodes should be completely trimmed of the adipose tissue before sectioned for the molecular analysis. Moreover, fat interferes with the assay itself, and this could be an issue when the SLN is diffusely replaced by adipose tissue. Occasionally a benign epithelial inclusion (>0.2 mm) within the lymph node could also be a source of a false positive result ( Fig. 19.33 ). Given the significance of the treatment decision based on a positive SLN result (CALND, which cannot be undone) and several sources of false positive result with molecular tests, we believe that currently there is insufficient data to replace the morphologic methods with molecular assay. At present, we suggest that a positive molecular result should be confirmed by morphology either by frozen or permanent sections before a final decision is made. In contrast a negative result is highly valuable given the very high negative predictive value of the molecular tests. Notably, the commercial assay mentioned earlier (GeneSearch BLN assay) was taken off the market due to a variety of reasons including workflow problems and lack of interest in identifying micrometastasis and isolated tumor cells not seen with routine testing. However, one test that is still in clinical use is One Step Nuclei Acid (OSNA) amplification assay. It is used in 12% of the UK hospitals. It is a quantitative RT-PCR for CK19. The original publication correlated the mRNA copy number to size of metastasis (negative, micro, or macrometastasis). In a meta-analysis, OSNA was found to be highly sensitive (87%) and specific (98%) for detecting a macrometastasis. However, up to 21% of cases classified as macrometastasis by OSNA would have tumor reclassified as micrometastasis on corresponding microscopic examination. To address this limitation, a new cutoff has been proposed. However, due to the reasons mentioned earlier, it is unclear if this test will be widely adopted.
Section the gross lymph node at 2-mm intervals, examine with H&E (no levels required).
For primary ductal carcinomas, AE1/AE3 keratin stain should be avoided.
AE1/AE3 can be performed for lobular carcinomas as even large tumor aggregates may be missed on H&E examination alone.
Ninety-seven percent of all SLN metastases will be found in the first three SLN when multiple SLN are submitted.
Intraoperative molecular tests are comparable to morphologic examination, but there are potential sources of false positive results.
Molecular tests to identify micrometastatic tumor in the sentinel lymph node seems unnecessary for individual patient management.
IHC not routinely required for post neoadjuvant therapy lymph nodes, but can be used if suspicious cells are identified.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here