Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Q43.1 What are the main steps for the mechanism of action for (1) acyclovir, and (2) penciclovir? (Pgs. 494, 496)
Q43.2 What is the relative sensitivity of (1) acyclovir, and (2) penciclovir for various viruses in the human herpesvirus family? (Pgs. 494, 496)
Q43.3 What are the clinical and mechanistic advantages of penciclovir compared with acyclovir? (Pg. 496x2)
Q43.4 Describe a commonly used treatment protocol for topical cidofovir that has been shown to be effective for contagiosum. (Pg. 497)
Q43.5 Concerning the mechanisms of action for imiquimod, (1) which Toll-like receptor is induced, and (2) what are several categories of cytokines induced by the drug? (Pg. 498)
Q43.6 What are several methods used in clinical practice to enhance the penetration of imiquimod when treating common (nongenital) warts? (Pg. 498)
Q43.7 What is the controversy surrounding the treatment of molluscum contagiosum with imiquimod 5%? (Pg. 498)
Q43.8 What is the role of imiquimod, recently emerging, in the treatment of antiviral-resistant herpes simplex virus (HSV) and hypertrophic HSV? (Pg. 498)
Q43.9 What are some of the published data for and against of the efficacy of intralesional bleomycin in recalcitrant verruca? (Pg. 499)
Q43.10 What are some of the methods that help extend the bioavailability of bleomycin for intralesional use? (Pg. 500)
Q43.11 What is the mechanism of action for podofilox as an antimitotic agent in treating warts? (Pg. 501)
Q43.12 Why has podophyllin fallen out of favor in recent years for treatment of cutaneous human papilloma virus (HPV) lesions? (Pg. 501)
Q43.13 Which topical antiviral treatment is often considered first line for anogenital warts during pregnancy? (Pg. 501)
Q43.14 How does one significant mechanism of action of cantharidin relate to the source of the drug from the insect world? (Pg. 501)
5-Fluorouracil
Anogenital warts
Acquired immunodeficiency syndrome
Actinic keratosis
Cytomegalovirus
Epstein–Barr virus
US Food and Drug Administration
Granulocyte colony-stimulating factor
Granulocyte–macrophage colony-stimulating factor
Highly active antiretroviral therapy
Hydrogen peroxide
Human papilloma virus
Herpes simplex virus
Interferon-α
Interleukin
Potassium hydroxide
Molluscum contagiosum
Randomized controlled trial
Trichloroacetic acid
Thymidine kinase
Toll-like receptor
Tumor necrosis factor
Verruca vulgaris
Varicella zoster virus
The subject of topical and intralesional (IL) antiviral agents encompasses a wide variety of pharmacologic agents. The three broad categories forming the basis and sequence of discussion in this chapter are the viricidal drugs, the immune-enhancing drugs, and the cytodestructive drugs ( Box 43.1 ). Those drugs that have a proprietary formulation available in the United States are covered in Table 43.1 .
| Viricidal | Immune enhancers | Cytodestructive |
|---|---|---|
| Acyclovir | Imiquimod | Bleomycin |
| Penciclovir | Interferon | Podophyllin/podofilox |
| Cidofovir Foscarnet |
Human papilloma virus (HPV) vaccine | Trichloroacetic acid (TCA) Cantharidin |
| Idoxuridine | Intralesional Candida antigen | Salicylic acid 5-Fluorouracil Potassium hydroxide (KOH) Hydrogen peroxide (HP) |
| Generic Name | Trade Name | Manufacturer | Generic | Cream | Ointment | Additional Formulations | PPS |
|---|---|---|---|---|---|---|---|
| Viricidal Drugs | |||||||
| Acyclovir | Zovirax | GlaxoSmithKline | Yes | Yes | Yes | Oral, injectable | B—Considered safe |
| Penciclovir | Denavir | Prestium Pharma, Inc | Yes | Yes | No | B—Use as second line | |
| Cidofovir | Vistide | Various | Yes | No | No | Only in injectable forms | C a |
| Foscarnet | Foscavir | Pfizer | No | No | No | Only in injectable forms | C a |
| Immune-Enhancing Drugs | |||||||
| Imiquimod | Aldara 5% Zyclara 3.75% |
Medicis Medicis |
Yes Yes |
Yes Yes |
No No |
Pump (2.5%) | Both C—Use only after first-line therapies (cryotherapy, TCA, laser) |
| Cytodestructive Drugs | |||||||
| Bleomycin | Blenoxane | Bristol-Myers Squibb | Yes | No | No | Injectable—powder | D a |
| Podofilox | Condylox | Allergan | Yes | No | No | Solution, gel | C—Avoid use |
| Podophyllin | X | ||||||
| Salicylic acid | Multiple | Multiple | Yes | Yes | Yes | Solution, gel, film, plaster, patch, spray | C—Use over small areas for short periods; avoid use in third trimester and under occlusive dressings |
| 5-Fluorouracil | Efudex Carac Tolak |
Orthoderm Orthoderm Hill Dermaceuticals |
Yes | Yes | No | Solution, injectable | X |
| Hydrogen peroxide (HP) | Several | Several | Yes | Yes 1% | No | Solution (3% and 6%) | N/A |
| Potassium hydroxide (KOH) | Several | Several | Yes | No | No | Solution | N/A |
| Quadrivalent HPV vaccine HPV nine-valent vaccine |
Gardasil Gardasil 9 |
Merck & Co, Inc. | No | No | No | Solution | N—Not recommended |
| Trichloroacetic acid (TCA) | Tri-Chlor | Gordon Laboratories | 80% solution | N –Not recommended | |||
| Sinecatechins | Veregen | PharmaDerm | No | No | 15% | N/A | N—Not recommended |
| Candida antigen | Candin | Nielson BioSciences Inc. | Solution only | C | |||
a Pregnancy prescribing status listed is for the injectable formulation of this drug. See chapter 65 for newer ‘Pregnancy Summary Rating’ for most topical antifungals.
It should be noted that there is a large variability in treatment protocols for many of the topical and IL antivirals—several are compounded at various strengths and sometimes used once or twice daily without large randomized controlled trials (RCT) to establish a consensus on best protocol. Moreover, it is difficult to study many viral lesions, including common warts and molluscum contagiosum (MC), for example, as they typically self-resolve. Many viral lesions (especially MC and warts) are often treated with multiple modalities to increase efficacy and to decrease potential adverse effects (AE) of excessive amounts of one treatment. This makes it very difficult to determine the efficacy of each treatment individually. Multimodal treatments do appear more effective for some of the viral lesions, especially warts. Despite these limitations, with the publication of larger case series and hopefully more RCT in the future, the most effective treatment protocols continue to be established.
Acyclovir was discovered in 1974 and the first formulation, the topical form, became available in 1982. The 5% ointment is US Food and Drug Administration (FDA)-approved in the United States for the treatment of herpes simplex virus (HSV).
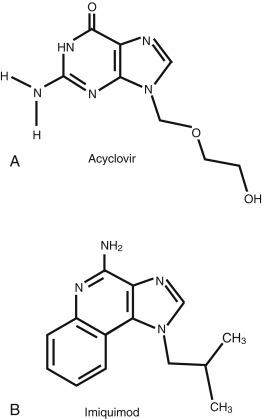
Acyclovir (9-[2-hydroxyethoxymethyl] guanine) is an acyclic analog of the nucleoside guanosine ( Fig. 43.1 ). Even when applied topically to damaged skin, including localized varicella zoster virus (VZV) infection, systemic absorption is limited. In patients treated for genital HSV with 5% acyclovir in polyethylene glycol 4 to 6 times daily for 5 to 7 days, plasma acyclovir levels are undetectable.
Q43.1 Acyclovir is specific for HSV-infected cells because the drug requires phosphorylation by the viral enzyme thymidine kinase (TK). Phosphorylation of acyclovir leads to acyclovir monophosphate, which is then further metabolized to acyclovir triphosphate by human cellular guanylate kinase. Acyclovir triphosphate then inhibits viral deoxyribonucleic acid (DNA) polymerase. In addition, acyclovir triphosphate is mistaken for deoxyguanosine triphosphate and becomes irreversibly incorporated into newly synthesized viral DNA. Because acyclovir lacks a 39-hydroxyl group for DNA elongation to continue, its incorporation leads to chain termination.
Q43.2 Acyclovir is most effective against HSV-1 and HSV-2. It is less effective against VZV owing to less efficient phosphorylation by the VZV viral TK. It is not effective against the human herpes virus cytomegalovirus (CMV) because CMV does not encode for TK.
Acyclovir 5% ointment is approved for the management of initial genital HSV and limited to nonlife-threatening mucocutaneous HSV in immunocompetent patients. Acyclovir 5% ointment applied four times daily during the first episode of genital herpes reduced the duration of viral shedding from 7 days (placebo) to 4.1 days, but did not affect duration of pain, time to healing, new lesion formation, or recurrence. In the treatment of recurrent genital HSV, there was no significant clinical improvement in symptoms or duration. Smaller European studies with the 5% ointment (polyethylene glycol base) and 5% cream base reveal more statistically significant effects for initial genital HSV and recurrent genital HSV. Early application (within 24 hours of onset of prodrome) and patient training are important.
Acyclovir 5% ointment is FDA approved for use in limited nonlife-threatening mucocutaneous HSV in immunocompetent patients, with studies showing a decrease in the duration of viral shedding and duration of pain.
Acyclovir 5% cream reduces the mean duration of an episode of recurrent HSV labialis by approximately half a day and does not prevent the development of classic lesions (progression to vesicles, ulcers, and/or crusts). A single, topical, iontophoretic application of 5% acyclovir cream for recurrent HSV labialis has been shown to reduce healing time by 1.5 days.
Application site reactions reported include mild pain and burning; however, placebo patients experienced similar AE.
The pregnancy prescribing categories for medications in this chapter are listed in Table 43.1 . Topical acyclovir is category B and generally thought of as safe to use.
An observational study of pregnant women who used topical acyclovir during the 30 days before conception, or during their pregnancy, did not show any adverse pregnancy outcome, although data on stillbirth were inconclusive.
Application of acyclovir 5% ointment for initial genital HSV is recommended every 3 hours, 6 times daily for 7 days. A finger cot or rubber glove should be used when applying the medication to prevent autoinoculation or transmission to other persons. Therapy should be initiated as early as possible after the onset of signs or symptoms.
Given the results of controlled clinical trials, dosing parameters, convenience, and cost, topical acyclovir has limited use as monotherapy for genital HSV and HSV labialis. In the author’s opinion, when medically tolerable, oral acyclovir is preferred over topical acyclovir for recurrent genital herpes, given the greater efficacy of oral acyclovir in reducing the duration of viral shedding and the time to crusting and healing of lesions.
The limited bioavailability of oral acyclovir, and the limited efficacy of topical acyclovir, led to the discovery of penciclovir. Penciclovir was approved by the FDA in 1996.
Penciclovir 2-amino-9-[4-hydroxy-3-(hydroxymethyl)butyl]-6,9-dihydro-3H-purin-6-one is an acyclic purine nucleoside analog of guanine and is structurally related to ganciclovir. Penciclovir is available only in a topical preparation because of poor oral bioavailability. Famciclovir, a prodrug of penciclovir, is available in oral form.
Q43.1 Penciclovir, like acyclovir, is selectively phosphorylated to the monophosphate form by viral TK. Penciclovir monophosphate is further phosphorylated by cellular enzymes to the active penciclovir triphosphate form, which subsequently inhibits viral replication by competing with deoxyguanosine triphosphate for viral DNA polymerase.
Q43.3 Even though acyclovir and penciclovir are qualitatively similar, penciclovir does have certain advantages over acyclovir. Penciclovir exhibits more efficient phosphorylation, a higher affinity of viral DNA polymerases for the triphosphate form, and increased stability of the triphosphate form, leading to a longer duration of activity.
Q43.2 Penciclovir exhibits inhibitory activity against several of the herpes viruses, including HSV-1, HSV-2, VZV, and Epstein–Barr virus (EBV). It has limited in vitro activity against CMV.
Penciclovir 1% cream is indicated for the treatment of recurrent HSV labialis in immunocompetent individuals 12 years of age or older. Penciclovir 1% cream reduced pain and viral shedding from 5.5 days (placebo) to 4.8 days, independent of the timing of medication initiation.
When 1% penciclovir cream was compared with 3% acyclovir cream for HSV labialis, there was no difference in efficacy. There are no comparative data between 5% acyclovir cream and penciclovir cream.
Contraindica-tions include hypersensitivity to the drug or any of the components of the formulation. The incidence of local irritation is similar for placebo. The most frequently reported AE was headache.
Penciclovir 1% cream should be applied at the earliest sign or symptom to all lesions every 2 hours (or at least 6 times daily) for 4 days.
Given the inconvenience of frequent application, the expense of penciclovir, and the reduction of symptoms and viral shedding by only half a day, the clinical benefit of penciclovir over oral antiviral therapy is limited. Q43.3 If oral therapy is contraindicated or not available, topical penciclovir is an alternative option to topical acyclovir, although no head-to-head studies of 1% penciclovir with 5% acyclovir have been performed.
Cidofovir is an acyclic nucleotide that exhibits antiviral activity against a broad range of DNA viruses, including HPV, human herpes viruses, and some pox viruses. Cidofovir is used intravenously to treat CMV retinitis in acquired immunodeficiency syndrome (AIDS) patients. No oral preparations are currently available; however, cidofovir has been compounded in topical formulations (3% or 1% cream for patients to apply at home) and IL formulations for physician administration. It has been anecdotally or in small studies shown to be effective for cutaneous viral infections such as verruca, condyloma, herpes simplex, orf, and MC.
Cidofovir ({[[S]-1-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxypropan-2-yl]oxy}methyl) phosphonic acid is a nucleoside analog of deoxycytidine monophosphate.
After incorporation into virally infected cells, cidofovir requires two stages of phosphorylation to form the active metabolite cidofovir diphosphate. Unlike acyclovir and penciclovir, however, cidofovir does not depend on viral thymidine kinase for its phosphorylation. Cidofovir diphosphate then acts as a competitive inhibitor of deoxycytosine-5-triphosphate for incorporation into viral DNA by viral DNA polymerases. After incorporation, cidofovir blocks further viral DNA synthesis.
Small studies and case reports of the use of topical cidofovir in children and adults with human immunodeficiency virus (HIV) with recalcitrant MC have revealed moderate efficacy.
In a double-blind, placebo-controlled trial of 30 immunocompetent patients, 47% of the cidofovir group (versus zero in placebo group) had complete clearance. Additional cases have been reported.
In a large series of 280 patients (both immunocompetent and immunocompromised) with recalcitrant warts, mostly palmoplantar, most patients (276 of 280 patients with at least 6 to 12 months follow-up) resolved with IL cidofovir. Treatment consisted of monthly IL injections of cidofovir 15 mg/mL until resolution of lesions, averaging two to three treatment sessions (some cases required more than four treatment sessions). Authors established a max dose of 140 mg in each infiltration. Pain and burning during injections were noted by all patients, and some had erythema and itching the days following treatment. The four patients who did not clear had small palmar warts that improved, but subsequently resolved with cryotherapy. It was thought that the small size of lesions and difficulty with medication infiltration into them attributed to their recalcitrance.
A case report with a recalcitrant plantar wart reported success with IL cidofovir. Cidofovir 75 mg/mL was mixed 1:3 with normal saline (0.75 mL of cidofovir in 2.25 mL saline) and then later diluted 1:4 in subsequent injections. A volume of 3 mL was injected into the large wart. A total of four injections over a 2-month period resulted in resolution of the wart.
No large controlled double-blind trials have been reported, thus there is not yet a consensus regarding topical cidofovir concentration (1% or 3%) and application technique (once or twice daily, with or without occlusion). However, a retrospective observational study of 35 immunocompetent patients aged from 6 to 55 years with recalcitrant plantar warts found topical cidofovir to be effective. Both 1% and 3% cidofovir cream were used and with and without occlusion. They concluded that cidofovir 3% cream applied once daily under occlusion for up to 12 weeks is likely most effective. Of their patients treated with 3% concentration, 54.3% had complete resolution of all warts. If no response is seen by 12 weeks, it is unlikely to be effective. The only AE, reported in two patients (treated with 3% cream), was local irritation. Another retrospective analysis of 126 patients, all immunocompetent, ranging in age from 3 to 67 years, found complete resolution in 53.2% of patients in an average of 12 weeks. The authors reported factors associated with resolution, including using 3% cream twice daily under occlusion for up to 12 weeks.
The initial report of topical cidofovir’s efficacy described two children treated with 3% cidofovir cream applied twice daily for 10 days to 2 weeks. A series of seven children treated with 1% cidofovir ointment applied under occlusion for 4 to 12 weeks reported clearance in four of the seven children (57%). A larger case series found complete clearance in 13 of 17 children (76%) with topical 3% cidofovir cream applied twice daily for 1 to 24 weeks without occlusion. A retrospective review of 12 children treated with 1% to 3% cidofovir cream applied once daily to every other day found complete clearance in only 25% of patients. The authors attributed their finding of lower efficacy as most likely because of the lower concentration and less frequent dosing they used.
In a series of 41 patients, both children and adults, with recalcitrant periungual warts, 23 patients (56%) had complete resolution with 3% cidofovir cream. There was some variance in application techniques, but most patients applied twice daily without occlusion with median treatment duration of 11 weeks. Only one patient reported an AE of local irritation that quickly resolved after stopping treatment, and all warts had resolved at that time.
Q43.4 Large RCT are needed to define optimal dosing of topical cidofovir. However, based upon moderately sized case series reported thus far, the medication appears safe and effective. Topical 3% cidofovir applied once daily under occlusion appears to be a good starting point for dosing. If not response seen at 12 weeks, treatment should be stopped as it is unlikely to be effective.
A randomized double-blind, placebo-controlled study of topical cidofovir gel in AIDS patients with acyclovir-resistant HSV compared 0.3% gel versus 1.0% gel versus placebo. In the treatment group, 50% had greater than 50% improvement, and 30% of treatment patients (versus zero placebo) had complete healing. Intralesional cidofovir has been reported for treatment of acyclovir-resistant HSV in an HIV patient.
In healthy patients with recurrent HSV, cidofovir gel (1%, 3%, or 5%) used within 12 hours of an outbreak reduced healing time and viral shedding by 1 to 2 days.
Topical cidofovir has been reported as a successful treatment for basal cell carcinoma (BCC), high-grade intraepithelial neoplasia in HIV-infected patients, and human polyomavirus-7 associated rash and pruritus in a lung transplant patient.
The most common AE is local irritation reported in a few patients, which resolves with treatment cessation. However, there are also a few case reports of renal failure in patients—all of whom were applying to mucosal surfaces in different compounding bases. It is strongly recommended to use caution when applying to areas at risk for high systemic absorption and when using noncream bases for compounding.
There are two reported cases of acute kidney injury (AKI) in patients treated with topical cidofovir in severely immunocompromised patients with multidrug resistant HSV. One patient was also on systemic foscarnet and had other contributing factors, and the AKI may have been exacerbated by cidofovir; however, cidofovir was not likely the causal drug. In the second patient, however, cidofovir was the probable cause; the AKI resolved with discontinuation and recurred with cidofovir resumption. Important to note, these patients were both using 5% cidofovir compounded in Ora-Plus gel and applying to mucosal (oral and perirectal) surfaces (versus cidofovir applied to the skin which is compounded in Dermovan). In the second report, they were also applying the medication three to four times daily. An additional case report of nephrotoxicity was reported in a 28-year-old renal transplant patient after topical application of 4% cidofovir to recalcitrant HPV lesions on the penis once daily. The AKI was thought to be because of propylene glycol as the excipient.
Foscarnet, a pyrophosphate analog, exhibits activity against all herpes viruses and is used in the treatment of CMV infection in immunocompromised patients. It is the oral drug of choice for acyclovir-resistant HSV. Foscarnet does not require activation by either cellular or viral enzymes. Because the drug is not a nucleoside analog, nucleoside-resistant viral polymerases are susceptible to foscarnet. Foscarnet interferes with the cleavage of pyrophosphate from deoxyadenosine triphosphate.
Topical 3% foscarnet cream did not reduce lesion numbers or the duration of ultraviolet (UV)-induced HSV labialis, compared with placebo. Topical 1% foscarnet cream was reported as a treatment for genital HSV in a healthy immunocompetent woman with acyclovir-resistant vulvar HSV. In general, however, studies in immunocompetent patients evaluating topical foscarnet for genital HSV have not shown a significant benefit over placebo. AIDS patients with acyclovir-resistant HSV improved with foscarnet 1% cream applied five times weekly.
Idoxuridine (5-iodo-2-deoxyuridine), a thymidine analog synthesized in 1959, was the first FDA-approved antiviral medication. It is no longer commercially available and not typically used in a dermatology setting with the advent of newer antivirals.
Imiquimod is an immunomodulating agent that was approved by the FDA in 1997 for treatment of external genital and perianal HPV infections for patients age 12 years and over ( Fig. 43.1 ). It has been subsequently approved for use in the topical treatment of actinic keratosis (AK; all concentrations) and superficial BCC (5% concentration) in immunocompetent adults.
Imiquimod 1-(2-2methylpropyl)-1H-imidazo[4, 5-c] quinolin-4-amine is a nonnucleoside heterocyclic amine.
Q43.5 Imiquimod, an activator of Toll-like receptor-7 (TLR-7), induces a potent antiviral and antitumor effect in vivo. The exact mechanism of action against HPV infections is not known. Imiquimod induces secretion of tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IFN-α, interleukin (IL)-6, IL-1α, IL-1β, IL-8, IL-12, granulocyte–macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF). IFN-α has an integral role in genital wart clearance and exhibits indirect antiviral activity. When used as directed, systemic absorption is minimal.
Several RCT have shown imiquimod 5% cream to be effective and safe. Complete clearance has been shown in up to 50% of treated patients (vs. 11% placebo) with once-daily, three times per week application for up to 16 weeks. Recurrence rates range from up to 19% at 3 months to 23% at 6 months. A study in HIV patients on highly active antiretroviral therapy (HAART) showed total clearance in 32% of patients at week 16.
Evaluations of imiquimod 2.5% and 3.75% in women, 12 years or older, for a shorter treatment duration of 8 weeks revealed safe and effective treatment of external anogenital warts (AGW) compared with placebo. Two studies comparing placebo with imiquimod 2.5% and 3.75%, respectively, with once daily application for a maximum of 8 weeks, revealed complete clearance rates of 14.2%, 28.3%, and 36.6%. Imiquimod 3.75% is FDA approved for the treatment of AGW as well as AK.
Total clearance has been reported in 30% of immunocompetent patients with overnight application five times weekly. A study of immunosuppressed patients found 5 of 14 patients (36%) had some benefit in the treatment of their recalcitrant hand and foot warts with an escalation dosing schedule. Because of the suspected lack of penetrance and absorption of imiquimod through common wart tissue, imiquimod monotherapy has limited efficacy for cutaneous warts. Q43.6 Imiquimod is often used in combination with cytodestructive methods such as cryotherapy and salicylic acid, and occlusion may enhance imiquimod efficacy in common warts.
Imiquimod is not FDA-approved for use in children under 12 years of age but has been reported as an effective treatment with complete clearance of warts in 16 of 18 children with twice-daily application for a mean duration of 5.8 months.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here