Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Q39.1 What is the most important adverse effect from the most potent bisphosphonates (such as etidronate)? (Pg. 431)
Q39.2 What are the mechanisms of action by which bisphosphonates prevent and treat osteoporosis? (Pg. 431)
Q39.3 What are the reasons that oral bisphosphonates must be taken in a fasting state and in an upright position? (Pg. 435)
Q39.4 What are typical doses for (1) calcium, (2) vitamin D, and (3) various bisphosphonates in the prevention and management of corticosteroid-induced osteoporosis? (Pg. 436)
Q39.5 What is the unique subtype of hypothyroidism from bexarotene, and what is the mechanism of action regarding this complication? (Pg. 437)
Q39.6 What serum test should be monitored periodically to determine the adequacy of levothyroxine replacement in bexarotene-induced hypothyroidism? (Pg. 437)
Q39.7 Why was cerivastatin taken off the market by the US Food and Drug Administration (FDA); what is the risk of this same complication in the remaining ‘statins’? (Pgs. 438, 440)
Q39.8 Concerning drug interactions with the statins, which drugs in this class do not interact with drugs (such as cyclosporine) metabolized by the CYP3A4 pathway? (Pg. 438)
Q39.9 In which clinical settings has fenofibrate occasionally been associated with myopathy and rhabdomyolysis? (Pg. 442)
Q39.10 Concerning vitamin D therapy (1) what is the mechanistic role of vitamin D in corticosteroid osteoporosis prevention, and (2) what malignancies are possibly reduced by significant vitamin D intake? (Pg. 444)
Adverse effect(s)
Bone mineral density
Bisphosphonates
Creatine kinase (= creatine phosphokinase)
Corticosteroids
Cyclosporine (A)
Cutaneous T-cell lymphoma
Cytochrome P-450
Dual-energy x-ray absorptiometry (scan)
Glucocorticoid
Gastrointestinal
Glucocorticoid-induced osteoporosis
High-density lipoprotein
3-hydroxy-3-methylglutaryl-coenzyme A (reductase)
Intermediate density lipoprotein
Interferon
Intravenous
Low-density lipoprotein
Nonsteroidal anti-inflammatory drug(s)
Osteoclastogenesis inhibitory factor
Peroxisome proliferator activator receptors
Parathyroid hormone
Peptic ulcer disease
Retinoic acid receptor
Retinoid X receptor
Systemic lupus erythematosus
Triiodothyronine
Tetraiodothyronine (= thyroxine)
Triglycerides
Tumor necrosis factor
Thyrotropin-releasing hormone
Thyroid-stimulating hormone (= thyrotropin)
Very low-density lipoprotein
The editor would like to thank Michelle Pelle for her contribution to previous editions of this chapter.
Three of the most commonly used systemic drugs or drug classes in dermatology, corticosteroids (CS), retinoids, and cyclosporine (CsA), present the challenge of managing their adverse effects (AE). The prevention and therapy of CS-induced osteoporosis (CSIO), hypothyroidism associated with bexarotene, and retinoid- or CsA-induced hyperlipidemia are herein discussed. Featured in this chapter are the bisphosphonates (BPP), levothyroxine, the ‘statins,’ fenofibrate, and ezetimibe. A brief section on vitamin D controversies concludes the chapter.
Chronic use of systemic CS is associated with the development of osteoporosis and an increased fracture risk. Osteoporosis may be diagnosed by finding evidence of diminished bone mineral density (BMD) as measured by dual energy x-ray absorptiometry (DEXA). Much later signs of osteoporosis include the development of an osteoporotic fracture, loss of height, or kyphosis indicative of vertebral fractures.
The estimated prevalence of CSIO is 50% among treated individuals. The relative risk of a hip fracture in patients on long-term prednisone 7.5 mg daily is 2.27 (confidence interval [CI], 1.94–2.66), and the vertebral fracture risk is 5.18 (CI, 4.25–6.31). Bone loss, which occurs most rapidly during the first 6 months of prednisone therapy, results from inhibited bone formation and enhanced osteoclast-mediated bone resorption. Systemic CS promoted bone loss is caused by:
Reducing calcium absorption;
Increasing renal calcium excretion;
Reducing levels of testosterone in men and estrogen in women, by reducing pituitary secretion of gonadotropins;
Inhibiting osteoprotegerin, also known as osteoclastogenesis inhibitory factor (OCIF).
OCIF, a member of the tumor necrosis factor (TNF) receptor superfamily, is a cytokine that inhibits the differentiation of and resorption by osteoclasts, and thus increases BMD and bone volume.
BPP are antiresorptive agents with an affinity for hydroxyapatite crystals in bone. At the cellular level, they inhibit osteoclast activity. The rate of bone turnover is decreased as early as 14 days, and maximally within 6 months of treatment with BPP. Q39.1 Etidronate, the first BPP developed for clinical use, is the most potent inhibitor of mineralization, which has equal inhibition of bone resorption. All other BPP have bone resorption greater than inhibition of mineralization; drug dose is a key factor as well.
This quality is now viewed as a disadvantage, because clinical experience has revealed that such sustained inhibition leads to osteomalacia. The second- and third-generation BPP have been developed to minimize inhibition of mineralization, while maintaining the ability to prevent bone resorption. Fig. 39.1 shows the drug structure for several BPP.
Three generations of BPP are commercially available and variably indicated for the treatment of osteoporosis (including CSIO), hypercalcemia of malignancy, bone metastases, heterotopic ossification, and Paget disease. Members of this class include etidronate (Didronel), clodronate (not available in the United States), pamidronate (Aredia), alendronate (Fosamax), ibandronate (Boniva), risedronate (Actonel), zoledronate (Zometa), and zoledronic acid (Aclasta, Reclast). The BPP differ by their route and frequency of administration, as shown in Table 39.1 . Zoledronic acid is the newest oral BPP approved in the United States for the treatment of postmenopausal osteoporosis.
| Generic Name | Trade Name | Generation | Route of Administration | Dosing for Corticosteroid-Induced Osteoporosis |
|---|---|---|---|---|
| Etidronate | Didronel | First | Oral | 400 mg/day for 14 days, repeated every 15 weeks (off-label use in the US) |
| Pamidronate | Aredia | Second | IV | 90 mg initial IV dose, followed by 30 mg IV every 3 months (off-label use in the US) |
| Alendronate | Foxamax | Third | Oral | Prevention: 5 mg once daily or 35 mg once weekly (10 mg once daily if postmenopausal and off estrogen) |
| Treatment: 10 mg once daily or 70 mg once weekly. | ||||
| Ibandronate | Boniva | Third | Oral, IV | 2.5 mg once daily or 150 mg once monthly or 3 mg IV every 3 months |
| Risedronate | Actonel | Third | Oral | 5 mg once daily or 35 mg once weekly or 150 mg once monthly |
| Tiludronate | Skelild | Third | Oral | Not applicable. |
| Zoledronate | Zometa | Third | IV | Not applicable. |
| Zoledronic acid | Reclast, Aclasta | Third | IV | Prevention: 5 mg IV once every 2 years Treatment: 5 mg IV once yearly |
Absorption of oral BPP through the upper gastrointestinal (GI) tract occurs rapidly, over approximately 1 hour. Their absorption through the intestines is poor. BPP must be administered following an overnight fast and taken 30 to 60 minutes before breakfast. Coadministration with calcium, antacids, or medications containing divalent cations, interferes with their absorption. Mean bioavailabilities of standard oral doses of alendronate, risedronate, and ibandronate were 0.6% compared with intravenous (IV) dosing. Approximate plasma protein binding of BPP includes: (1) risedronate 24%, (2) alendronate 78%, (3) ibandronate 85% to 90%, and, (4) zoledronic acid 22%.
Alendronate, risedronate, ibandronate, and zoledronic acid are not systemically metabolized. Approximately half of the absorbed dose of either risedronate or alendronate is excreted unchanged in the urine within 24 and 72 hours, respectively. Steady-state conditions in the serum are observed within 57 days of daily dosing of risedronate. Plasma concentrations of alendronate, following therapeutic oral doses, are too low for analytical detection. Once absorbed, the serum concentration-time profile for risedronate is multiphasic; its initial half-life is 1.5 hours and its terminal exponential half-life, thought to represent dissociation of risedronate from bone, is 480 hours (20 days). For alendronate, the terminal half-life in humans is estimated to exceed 10 years, also reflecting its slow release from bone. Unabsorbed drug is eliminated unchanged in the feces. For ibandronate, the terminal half-life of the 150-mg tablet ranges from 37 to 157 hours. Renal excretion accounts for 50% to 60% of the total clearance of ibandronate.
Zoledronic acid concentration in the plasma decreases rapidly after infusion, owing to increased absorption of the drug by the bone. Small amounts can be detected in the plasma several days after the infusion, as the drug is gradually released during bone turnover. It is excreted intact into the urine and can be detected beginning 24 hours after the infusion until 28 days postinfusion.
Q39.2 BPP are incorporated into bone matrix. Aminobisphosphonates, such as alendronate, directly inhibit multiple steps in cholesterol synthesis, which are required for prenylation (the addition of hydrophobic molecules to a protein to facilitate protein attachment to the cell membrane) of osteoclast-associated proteins. Ultimately, BPP inhibit bone resorption and increase bone volume and strength by slowing the formation and dissolution of hydroxyapatite crystals. The mechanism by which nitrogen-containing BPP promote osteoclast apoptosis is distinct from that of the nonnitrogen-containing BPP. As elegantly illustrated in recent studies, nitrogen-containing BPP bind to and inhibit the activity of farnesyl pyrophosphate synthase, a key regulatory enzyme in the mevalonic acid pathway, critical to the production of cholesterol, other sterols, and isoprenoid lipids ( Fig. 39.2 ). As such, the posttranslational modification (isoprenylation) of proteins (including the small guanosine triphosphate–binding proteins Rab, Rac, and Rho, which play central roles in the regulation of core osteoclast cellular activities including stress fiber assembly, membrane ruffling, and survival) is inhibited, ultimately leading to osteoclast apoptosis. 9 Interestingly, whereas farnesyl pyrophosphate synthase is ubiquitously expressed in mammalian cells and has a critical role in lipid production, cellular apoptosis induced by nitrogen-containing BPP appears to occur only in osteoclasts. This is likely a direct function of the ability of BPP to selectively adhere to and be retained within bone before endocytosis within osteoclasts during osteoclast-mediated bone mineral dissolution and matrix digestion.
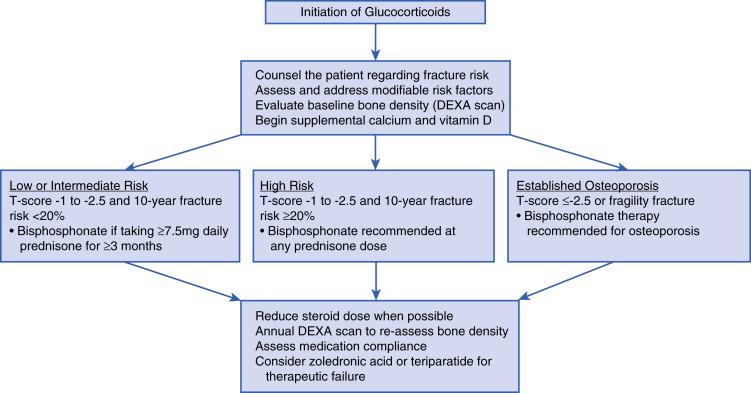
It is important to know when BPP is indicated, whether prescribing or collaborating with other providers. For patients with an anticipated 3 months or longer course of CS, screening should take place at baseline for osteopenia (T-score between –1 and -2.5) and osteoporosis (T-score < –2.5) with a DEXA scan to estimate BMD. Oftentimes, clinical findings and DEXA scan results can guide management. Additional tools are available, such as the World Health Organization fracture prevention algorithm (FRAX) to prevent 10-year fracture risk, but this tool does underestimate CS-induced fracture risk. (FRAX is available at http://www.shef.ac.uk/FRAX/ ). There is no current model to accurately predict fracture risk in premenopausal women and men under age 50 years. Fig. 39.3 shows how to approach treating CSIO using BPP.
Alendronate has been shown to increase BMD in patients receiving CS therapy in two 48-week randomized, controlled trials of 477 women aged 17 to 83 years, receiving CS therapy. Subjects were randomized to placebo, 5 mg daily alendronate, or 10 mg daily alendronate. All patients received 800 to 1000 mg elemental calcium and 250 to 500 IU vitamin D daily. There were fewer new vertebral fractures in the alendronate groups (overall incidence 2.3%) than in the placebo group (3.7%), but this number did not reach statistical significance (relative risk 0.6; 95% CI, 0.1–4.4). There were no differences in serious AE, although a small increase in mild upper GI effects was noted in the 10-mg alendronate group.
BPP have been shown by meta-analysis to be the most effective therapy to increase BMD in patients receiving CS therapy, with a 4.6% difference in percent change in the lumbar spine relative to no treatment or treatment with calcium. The efficacy of BPP was further enhanced when used in combination with vitamin D (6% difference in BMD). BPP, including alendronate, risedronate, and ibandronate, have also been shown to reduce fracture risk in patients receiving CS treatment. A meta-analysis of nine randomized clinical trials that each included more than 50 patients in each treatment arm found that the overall reduction in risk or vertebral fractures was 37% (CI, 0.49–0.80) ( Box 39.1 ).
Alendronate: 5 mg daily, 70 mg weekly, or 150 mg monthly (70 mg weekly dose for treatment is often favored)
Risedronate: 5 mg daily or 35 mg weekly
Ibandronate: 150 mg/mo (only weak recommendation for this medication for use in corticosteroid-induced osteoporosis)
Zoledronic acid: 5 mg once yearly infusion for patients who cannot tolerate oral BPP (monitor for flu-like symptoms for 2–3 days after first injection; can treat with acetaminophen or NSAID; use caution in patients with history of AF)
Consider referring for dental examination for all patients; avoid BPP therapy when dental work is needed
Correct hypocalcemia and vitamin D deficiencies
Assess for comorbidities that may preclude BPP therapy:
Check serum creatinine (avoid BPP if creatinine clearance is <30–35 mL/min, and consider referral to endocrinology or nephrology for additional management)
Ensure patient has no swallowing difficulties and can remain upright for 30 minutes after taking BPP
Avoid use in patients with active upper gastrointestinal (GI) disease
Take alone on an empty stomach first thing in the morning with 8 oz of water (enteric-coated, delayed-release risedronate is taken immediately after breakfast with 4 oz of water)
Avoid food and drink and other medications or supplements for 30 min after taking alendronate or risedronate and 1 hour after taking ibandronate
Remain upright for 30 min after taking
Discontinue if patient develops esophagitis
Do not prescribe for any patients with swallowing difficulties or active upper GI disease
AF , Atrial fibrillation; BPP , bisphosphonates; NSAID , nonsteroidal anti-inflammatory drug.
BPP are first-line for treatment of CIOP and evidence supports their efficacy in preventing and treating bone loss in these patients. They should be considered in all patients taking CS chronically who have accelerated bone loss or a history of fragility fractures. Those most likely to benefit include those at highest fracture risk, including patients over age 50 years with known osteoporosis, osteopenia taking 7.5 mg or more of prednisone for 3 months or longer, and osteopenia taking any dose of prednisone considered high risk. Evidence is less well defined in premenopausal women and younger men. One must consider potential long-term risks and teratogenicity when considering therapy in these patients.
Alendronate or risedronate are typically first-line agents, and IV zoledronic acid is an option for patients who cannot tolerate oral BPP. BPP should be avoided in patients with creatinine clearance less than 30 mL/min, and such patients should be referred to a specialist for comanagement. Dosing and counseling details can be found in Box 39.1 . Several studies provide evidence of the efficacy of these medications and their benefits.
Monthly ibandronate is considered and alternative therapy but is not first line. It has been shown to decreased vertebral fracture risk and to increase BMD in women with postmenopausal osteoporosis. However, there is not currently specific data to support its use first-line for treatment of CSIO. A study in rabbits demonstrated potential to prevent and treat CSIO in animals, but human studies are lacking.
BPP may cause upper GI disorders, such as dysphagia, esophagitis, and esophageal or gastric ulcer. Oral BPP should not be used in patients with serious esophageal disease. Other contraindications include hypocalcemia, renal insufficiency, osteomalacia, and hypersensitivity to any component of the product.
Risedronate and alendronate are classified as pregnancy category C. At least two of the parenteral BPP, pamidronate and zolendronic acid, are rated pregnancy category D. Regarding the oral formulations, there is a theoretical risk of fetal harm, predominantly skeletal, if a woman becomes pregnant during or relatively soon after completing a course of a BPP. BPP should be avoided during pregnancy and lactation, and used only when the potential benefit justifies the potential risk to both mother and fetus.
Of note, BPP are lipid soluble and may be stored in body fat for months to years. With the potential for fetal harm with abnormal bone development, as seen in animal studies, caution should be used when considering treatment of premenopausal women who may still become pregnant.
Adverse Effects
The AE profile of the BPP class is described in Table 39.2 . Abdominal pain is the most common AE, followed less commonly by nausea, heartburn, irritation or pain of the esophagus, emesis, dysphagia, bloating, constipation, diarrhea, melena, and peptic ulcers.
| All bisphosphonates | Hypocalcemia |
| Increased parathyroid hormone (PTH) | |
| Skin rash | |
| Osteonecrosis (primarily of jaw) | |
| Skin rash | |
| Oral bisphosphonates | Esophagitis |
| Esophageal and gastric ulceration | |
| Intravenous bisphosphonates | Fever |
| Transient leukopenia | |
| Acute-phase reaction | |
| Bone pain | |
| Ocular inflammation | |
| Nephrotic syndrome | |
| Etidronate | Osteomalacia |
| Hyperphosphatemia |
Q39.3 Serious AE that have occurred during BPP therapy, including upper GI complications, have been similar to those of placebo in large controlled trials. Taking the oral BPP, while upright, before eating, probably reduces the risk of this complication.
Serum calcium and phosphorus are slightly decreased at 6 months in patients receiving BPP (0.8% and 2.7%, respectively, in those receiving risedronate), but this was transient and normalized at 3 years of therapy. A compensatory increase in serum parathyroid hormone (PTH) levels is often observed.
Osteonecrosis, primarily of the jaw, has been reported in association with BPP. Most reported cases have occurred in cancer patients, patients on concomitant therapies (chemotherapy, radiotherapy, and CS), and with IV administration. Risk factors for jaw osteonecrosis include a history of periodontal and dental abscesses, diabetes, smoking, alcohol use, and poor oral hygiene. Symptoms of osteonecrosis of the jaw include toothache, jaw pain or altered sensation, loose teeth, recurrent soft tissue infections, and/or exposed bone. Diagnosis is made by visual inspection, and early diagnosis can be achieved by magnetic resonance imaging (MRI). The incidence of this potential complication is low but increases with treatment duration of BPP therapy. Nearly 80% of cases in one series were initiated by tooth removal and other dental procedures. Surgical intervention is not recommended, as it often leads to further exposure of bone. Osteonecrosis can lead to chronic pain and disfigurement; therefore early diagnosis and discontinuation of the BPP are important to reduce morbidity. Preventative measures may include: (1) a routine dental examination before BPP treatment initiation, (2) postponing BPP treatment initiation, until invasive treatment is completed, and (3) discontinuing therapy in asymptomatic patients, if systemic conditions permit, for 3 months before and 3 months, following elective invasive dental procedures.
Atypical subtrochanteric and femoral fractures may be associated with BPP use. They present with groin or thigh pain without preceding trauma. They are more common in patients who have taken BPP for more than 3 years. They can also occur in patients not taking BPP. Fortunately, this is a rare complication and the number needed to harm for atypical femoral fracture is 1282, if receiving BPP for 8 years. Any patient taking CS and presenting with new, dull, or aching pain in the groin, thigh, or hip should have a plain radiograph of the affected side, and the radiologist should be made aware of the concern for atypical femoral fracture. Unlike the more common fractures of the femoral neck or intertrochanteric region that are not associated with prodromal symptoms, BPP-treated patients with subtrochanteric fractures are described as having weeks or months of discomfort at the site before the fracture occurs. Radiologic features at the fracture site include thickening of the femoral cortex, the presence of a transverse fracture, and a cortical beak. These findings can also be found in patients never treated with BPP.
BPP drug holidays are currently not specifically recommended for patients who are at risk for CSIO. Studies guiding such recommendations did not include CSIO, and results are not generalizable. A retrospective observational study of patients on extended BPP for CSIO found that patients who stopped alendronate after 1 year, while remaining on more than 6 mg/day of prednisone, had significantly decreased BMD versus those who continued on alendronate. It has been recommended continuing BPP for CSIO, while taking glucocorticoids, with annual repeat of DEXA scans to monitor BMD.
There have been conflicting reports regarding the association of atrial fibrillation (AF) and BPP use. In a recent review, pooled data from randomized control trial (RCT) and observational studies suggested that risk of AF is increased with both oral and IV BPP, and risk is greater with IV preparations. The current available evidence does not support the need to avoid BPP in patients who are at increased risk for AF. The absolute risk of AF is considered negligible, and the benefits of fracture reduction outweigh the risk of AF. BPP may aggravate AF in patients predisposed to it, but further prospective studies are needed. Caution should be used when prescribing BPP to patients with a history of AF, especially with IV zoledronic acid.
BPP are not metabolized and do not induce or inhibit hepatic microsomal drug-metabolizing enzymes of the cytochrome P-450 (CYP) system. Careful use of aspirin and nonsteroidal anti-inflammatory drugs (NSAID), in conjunction with BPP, is necessary because of the risk of potentiation of upper GI AE.
A baseline DEXA scan should be obtained if possible in patients who are beginning long-term (>3 months) CS therapy. The DEXA scan can be repeated after 3 to 6 months of BPP total therapy to monitor changes and should be repeated yearly. Patients should be counseled on minimizing behaviors that predispose to bone loss (smoking cessation and decreased alcohol consumption) and should be encouraged to perform weight-bearing exercise. Serum 25-hydroxy vitamin D should be measured annually. Q39.4 All patients taking any dose of CS with an anticipated duration of 3 months or longer, should maintain through either diet or supplements, a daily calcium intake of 800 to 1200 mg daily and vitamin D of 800 to 2000 units daily. Supplements should be started concomitantly with the CS, as most bone loss occurs early in treatment. Special considerations should be given for sarcoid patients who may have a higher baseline vitamin D level, as well patients with history of hypervitaminosis D, hypercalcemia, hypercalcuria, and those with chronic kidney disease. Higher doses of calcium (1500 mg daily) can be considered in patients with more pronounced reduction in bone density.
Either alendronate (5 or 10 mg daily) or risedronate (5 mg daily) are indicated for adjunctive prevention and treatment of CSIO. For this indication, weekly dosing regimens with either alendronate (35 mg once weekly for prevention or 70 mg once weekly for treatment of CSIO) or risedronate (35 mg once weekly) are also now available; in both cases, the efficacy and tolerability profiles of weekly regimens have been similar to those of once-daily doses. Weekly dosing has been shown to promote better compliance and persistence with therapy than daily BPP doses.
Certain patient populations who require sustained CS therapy have inherent and/or additional risk factors for osteoporosis that should be kept in mind to maximize their prevention strategy. Reduced mobility, caused by arthritis and myositis, can predispose individuals to osteoporosis, as can renal impairment. Bone-resorbing cytokines (interleukin [IL]-1, TNF-α, and IL-6) are upregulated in systemic lupus erythematosus (SLE) and rheumatoid arthritis. Limited sun exposure predisposes to low vitamin D levels in SLE and dermatomyositis patients. Endocrine abnormalities, including amenorrhea, premature menopause, low androgen levels, hyperprolactinemia, and a history of Graves thyrotoxicosis, contribute to bone loss. The long-term use of heparin, other anticoagulants, and immunosuppressive therapies has been associated with osteoporosis and vertebral fractures. Box 39.2 summarizes the overall recommendations for the prevention and treatment of CSIO.
Patients beginning CS therapy (prednisone equivalent of ≥5 mg/day) with plans for treatment duration of ≥3 months:
Encourage weight-bearing exercise, reduce alcohol consumption, and recommend smoking cessation.
Consider hormone replacement therapy (HRT) in postmenopausal women.
Begin supplementation with both calcium (800–1200 mg per day) and vitamin D (800–2000 IU per day). Calcium alone does not prevent bone loss in patients receiving CS therapy.
Obtain BMD measurement of lumbar spine and/or hip with DEXA scan before initiating therapy and in patients already treated with CS for 3 or more months.
CS-treated premenopausal women, a
a Patients should be counseled regarding pregnancy risks.
postmenopausal women on HRT and men should be treated with either alendronate 5 mg/day or risedronate 5 mg/day. FLOAT NOT FOUND
CS-treated postmenopausal women not receiving HRT should be treated with either alendronate 10 mg/day or risedronate 5 mg/day.
Calcitonin is not recommended for prevention of bone loss in patients beginning CS treatment, but it may serve as alternative agent in patients who have contraindications to, or cannot tolerate, bisphosphonates.
Consider special patient populations who may have additional risks for osteoporosis (see text).
BMD, bone mineral density; CS , corticosteroids, DEXA , dual energy x-ray absorptiometry.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here