Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Noninvasive cross-sectional imaging with computed tomography angiography (CTA) and magnetic resonance angiography (MRA) plays a crucial role in the diagnosis and preintervention assessment of patients with vascular disease. Because imaging of vascular disease imposes stringent requirements in terms of high spatial and temporal resolution, many of the developments in computed tomography (CT) and MR have been spurred by the demands that cardiovascular imaging places in terms of speed, temporal resolution and length of scan volume. Modern protocols satisfy the requirement to image vascular territories during a short acquisition time as dictated by either the patient's breath-holding capability and/or the duration between peak arterial enhancement and onset of the venous phase in the case of CTA and MRA. For some vascular pathologies, especially with MRA, intravenous contrast medium (CM) can be avoided completely albeit at the expense of lower resolution, longer scan times and increased artefacts. Although most vascular territories can currently be addressed with application of (almost) the same CT or MR protocol, there are many instances where bespoke imaging protocols incorporate either specific precontrast imaging or additional postcontrast imaging.
CTA and MRA provide an excellent roadmap for planning interventional procedures such as angioplasty and stenting of the aorta, renal and visceral arteries.
CT is a workhorse of modern vascular imaging in clinical practice. CT is widely available, fast, safe and easily performed and generates isotropic cross-sectional images that are easily comprehended and which can be easily manipulated into the multiplanar and maximum-intensity reformats that are the mainstay of image interpretation, and if required into volume-rendered and surface-shaded displays. Isotropic submillimeter three-dimensional (3D) datasets are obtained within a single short acquisition (during a single breath-hold for imaging within the thorax and abdomen) during the first pass of an intravenously delivered bolus of iodinated contrast medium (CM). Scan times are currently so fast that images can be triggered to the cardiac cycle to produce motion-free coronary and aortic root CT angiograms.
To optimise image quality, constant delivery of a substantial volume (2 to 4 mL/s) of iodinated contrast agent via an automated pump is required, with CM volumes tailored to the region of interest. To minimise contrast-induced nephropathy, the lowest dose of CM possible must be used, which mandates use of a robust bolus detection algorithm to ensure data acquisition during peak arterial enhancement. If required, multiphase acquisitions can be performed, albeit at the expense of increased radiation dose: for example, to detect sources of gastrointestinal (GI) bleeding and to demonstrate late filling of endoleaks following endovascular aneurysm repair.
Single-phase postcontrast CTA is the mainstay of most arterial imaging. As iodinated contrast is delivered intravenously, and considering that patients with suspected vascular disease may have impaired cardiac function, a significant challenge is to match data acquisition with peak arterial enhancement. This can be ensured by measuring the circulation time from the injection site in the antecubital fossa to the region-of-interest be means of a test bolus and setting the scan delay time accordingly or by using a method of ‘bolus detection’ that monitors the attenuation within a large artery in the imaging fields (e.g. the pulmonary trunk for pulmonary CTA and abdominal aorta for abdominal CTA) and initiating data acquisition once a sharp increase in Hounsfield units signals contrast arrival. Accurate bolus timing allows the lowest dose of CM possible to be used. Poor timing reduces the quality of the examination, exposes the patient to increased risk of nephrotoxicity due to multiple injections and significantly increases radiation dose. Intravascular contrast is determined by the amount of iodine within the artery at the time of imaging and is influenced by the concentration of iodine injected and the injection rate.
Because modern CTA protocols are not limited in temporal and spatial resolution, the same protocol can be used for virtually all regions of interest, which greatly increases the ease of use.
One potential problem because of the rapid speed of CTA may be encountered in peripheral CTA whereby false-positive occlusions in the lower leg and foot can result from data acquisition ‘outstripping’ the bolus. This is addressed by slowing down the speed of table movement (effectively altering the pitch) to allow for slow flow as a result of poor cardiac output and proximal stenoses. Another problem is the presence of vascular calcification which complicates interpretation of the degree of stenosis and determination of patency of some arterial segments. Although the effect of calcium can be eliminated to some degree by use of postprocessing techniques, subtraction techniques have not gained traction in clinical practice owing to disappointing results and doubling of radiation dose. Potentially, dual-energy CT (DECT) may offer some benefit in this regard.
Noncontrast CT gives limited information but can be performed to look for acute vascular rupture (abdominal aortic aneurysm [AAA] rupture) and as a baseline to ensure that foci of vascular calcification are not mistaken for endoleaks. In addition, for stroke imaging, vessels occluded by embolus frequently present a dense appearance (the hyperdense vessel sign).
DECT (also called spectral imaging) is an exciting development that offers great promise to further increase the modality's potential. Conventional CT uses single energy x-ray beam energy (70 to 140 kVp, typically 120 kVp for adults) which generates image contrast depending on differences in photon attenuation of the various body tissues such as soft tissue, fat, air and calcium. The degree that a material will attenuate the x-ray beam is proportional to tissue composition and photon energy level and how closely it matches the k-edge (the inner electron shell binding energy) of the material. Therefore tissue attenuation can be manipulated by using two energy levels (typically 80 and 140 kVp) to acquire images that are processed into additional datasets. These features can be exploited in several ways to improve vascular imaging. For example, DECT CTA utilises lower iodine dose and lower radiation dose compared with conventional CTA. DECT images can be constructed to produce iodine images (looks like standard postcontrast CTA) and water images (looks look like a noncontrast study) in a single acquisition and can differentiate between calcium and iodine in diseased arteries and in suspected endoleaks by implementing calcium subtraction techniques during postprocessing. Another area where DECT can improve CTA quality is around joint prostheses, where image quality is often compromised by beam hardening and photon starvation. Beam hardening from attenuation of low-energy photons, which contribute to radiation scatter and artefact but not image quality, can be preferentially removed by (postprocesssing) with artefact reduction software and DECT-generated iodine images can help to reduce streak artefact in the assessment of vascular patency and endoleak detection in patients who have been treated with coil embolisation.
In the mid-1990s, time-inefficient noncontrast MRA based on time-of-flight (TOF) and phase-contrast techniques was replaced by contrast-enhanced techniques which generated improved signal-to-noise ratio (SNR), increased image resolution and shortened scan times, which allowed arterial phase and breath-hold imaging. Since this time, progressive improvements in technology such as faster gradients, improved surface coils, parallel imaging, k-space sharing techniques and noncartesian methods of data sampling and the adoption of the moving table along with better understanding of CM kinetics and side effects of gadolinium contrast agents have combined to provide a robust technique that can evaluate almost any vascular anatomy during single arterial and multiple subsequent phase imaging. Because of the unique properties of k-space, selective arterial phase imaging can be performed during an acquisition that lasts substantially longer than the arterial phase provided collection of the crucial contrast-defining k-space data is synchronised with onset of arterial enhancement by use of a bolus detection (or test bolus) technique. Because MRA technology lags behind CTA in terms of speed, the last station in Mutli-station (3 station) better moving table peripheral MRA can be contaminated by venous overlap. Although this is simply a matter of image acquisition speed in relation to circulation time, onset of venous enhancement can be delayed by application of tourniquets inflated to between arterial and venous pressure. With the newest, fastest techniques and anticipating further advances in speed over the next few years, it is likely that MR speed will progress to the point now occupied by CT: namely, that the limitation will no longer be venous contamination related to slower acquisitions.
Newer noncontrast MRA methods aimed at eliminating the need for CM injection which are based on cardiac phase dependent flow, flow-encoding, spin labelling and relaxation techniques may in the future provide a completely noninvasive method for assessing the peripheral run-off vessels. In the meantime, techniques such as steady-state free precession (SSFP) have been implemented with some success for imaging of target volumes.
Although time-of-flight (TOF) techniques have been largely forgotten, in specific instances (e.g. assessing patency of pedal arteries when other methods fail) they provide an accurate fall-back method which may identify vessels onto which grafts can be placed.
Both CTA and MRA are highly efficient for evaluation of the vascular tree. Because of the widespread availability of CT, rapid scan times, ease of patient setup (no concerns regarding safety of implanted devices) and ease of monitoring, CTA has become the investigation of choice for most acute vascular conditions. Despite the advantage of the absence of ionising radiation, MR is mostly used in an outpatient setting for less acute medical indications. Although it was previously widely held that MR contrast agents had an unrivalled safety profile, concerns about a risk of nephrogenic systemic fibrosis (NSF) in patients with renal impairment and brain deposition of gadolinium chelates in all subjects have negatively impacted use of MRA. Both of these conditions are agent specific and associated with high-dose use—NSF is only encountered in patients with markedly impaired renal function (although there are many confounding factors such as coexisting inflammatory conditions, blood phosphate level, type of contrast agent used and cumulative dose) and can be completely avoided by avoiding gadolinium completely in patients with severe renal impairment and by judicious choice of CM type and dose when CM injection is deemed necessary. In the case of basal ganglia hyperintensity attributed to deposition of gadolinium, this is solely an observation made on T 1 weighted brain images and no clinical sequelae have been attributed to it.
Because of such concerns surrounding use of gadolinium contrast-enhanced MRA, there has been a resurgence in interest in noncontrast techniques. Time-of-flight MRA, the grandfather of modern noncontrast MRA techniques, suffered from long scan times related to the need to use long repetition times (TRs) to maximise inflow and the requirement to scan axially which is highly time inefficient. Images were prone to overestimation of stenosis secondary to flow-related artefacts. The demand for new, more time-efficient noncontrast MRA techniques has led to the development of novel noncontrast methods based on cardiac phase-dependent flow, flow-encoding, spin labelling and relaxation. Although excellent results have been reported, results are highly operator dependent and to some extent manufacturer dependent and techniques rivalling contrast-enhanced techniques have been slow to appear. Promising techniques include four-dimensional (4D) phase-contrast MRA, cardiac-gated 3D fast spin-echo, flow-sensitive dephasing, arterial spin labelling and balanced SSFP (bSSFP).
CTA is the preferred method for evaluating aortic aneurysms and dissection prior to intervention.
Measurements derived from CTA are used to select EVAR type and stent size.
Endoleaks are visualised on multiphase CTA as persistent contrast accumulation outside the graft. Delineation of their origin and morphology guide future management.
CTA is sometimes limited in its ability to differentiate vessel wall calcification from intravascular contrast, which may result in both false-positive and false-negative occlusions, a drawback which can be addressed by dual-energy computed tomography.
CTA , Computed tomography angiography; EVAR, endovascular aneurysm repair.
Assessment of abdominal aortic morphology prior to endovascular stent placement is easily achieved with CTA and MRA. The relationship of the neck to the renal and mesenteric arteries, the shape of the neck and the status of the iliac arteries can be accurately determined by both CTA and MRA, but the presence and extent of calcification are better evaluated by CTA and, in practice, CTA is preferred and is the basis for determination of appropriate stent size and type. Based on measurements from CTA (dimensions of neck distance from renal arteries, size of iliac arteries) stents are selected and, in the case of juxtarenal aneurysms involving or extending close to the renal and mesenteric artery origins, may be fenestrated and customised to individual patients ( Fig. 77.1 ).
Aortic stent grafts used for endovascular aneurysm repair (EVAR) exclude the aneurysm from the circulation by providing a conduit for blood to bypass the sac. Endoleaks are characterised by persistent blood flow within the aneurysm sac and are most commonly asymptomatic and detected on follow-up imaging. Some endoleaks expose the aneurysm sac to arterial pressure which may result in continued expansion with risk of rupture. As a result, lifelong post-EVAR imaging surveillance is required. Endoleaks are visualised on CTA and MRA as contrast opacification of the aneurysm sac outside the graft and may require multiphase imaging as occasionally recent thrombus and calcification can mimic contrast on single phase imaging only.
Endoleaks are classified as follows:
| Type | Features |
|---|---|
| Type I | Secondary to inadequate seal at the EVAR anchor site; occurs in up to 10% of case, almost never resolves and usually requires treatment to reseal the defect |
| Type II | The sac fills retrogradely via branch vessels arising from the lumbar, inferior mesenteric or internal iliac arteries and occurs in up to 25% of cases |
| Type III | Mechanical failure due to defect on the graft or separation of the modular components |
| Type IV | Secondary to graft porosity and almost always resolves within a few days post repair |
| Type V | Endotension—expansion of aneurysm sac without demonstrable abnormality on computed tomography angiography |
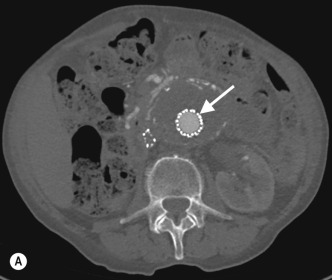
Visceral aneurysms at all other locations can also be diagnosed with CTA and MRA ( Fig. 77.2 ).
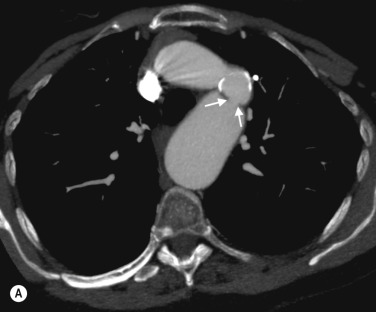
CTA is the investigation of choice for diagnosing acute aortic dissection, particularly in unstable patients, and for determining the extent of the flap. MRA offers similar information and in stable patients also gives excellent cardiac functional assessment. In patients presenting with suspected acute dissection but no flap on CT, MR can demonstrate intramural haematoma, although the penetrating ulcers that often lead to this condition are also well shown on CT ( Fig. 77.3 ).
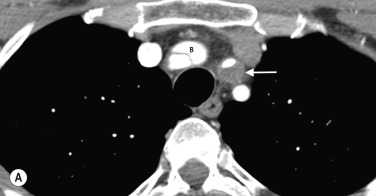
Anatomical mapping of thoracic or abdominal coarctation are well performed by both CT and MR, although by using phase-contrast techniques both the extent of collateral flow and peak velocities (V) through the area of maximum stenosis can be measured with MRI. The pressure gradient can then be estimated using a modified Bernoulli equation (Δ P = 4V 2 ) which can be useful for assessing change at follow-up ( Figs 77.4 and 77.5 ).
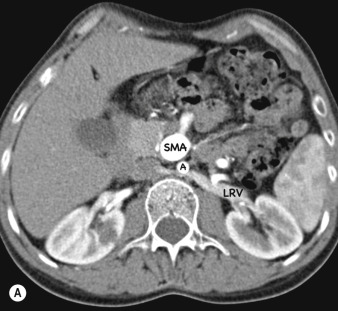
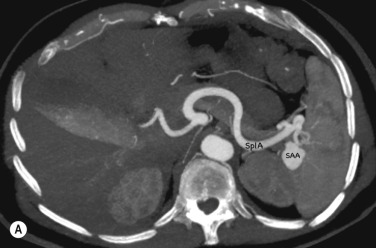
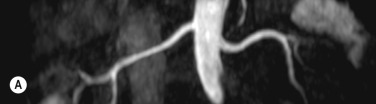
Imaging of the renal arteries for suspected renal artery stenosis can be easily performed with CTA and MRA, and the decision to use one of these modalities over the other usually depends on individual preference and experience. Renal artery stenosis is a cause of hypertension and can be caused by either atherosclerosis (which almost always affects the ostium) and fibromuscular dysplasia (FMD), which affects the vessel beyond its proximal part. The main renal arteries are medium-sized arteries that arise from the upper abdominal aorta around L1, in the vicinity of the coeliac and superior mesenteric artery (SMA) origins. The arteries can be single or multiple, and if multiple there is usually a dominant artery. Visualisation of the origin is easily performed with CTA or MRA, but challenges remain for evaluating the more distal artery (and therefore exclusion of FMD) owing to progressive decrease in artery size with distance from its origin and motion of the artery, even during perfect breath-holding owing to substantial excursion of the artery during the cardiac cycle. Secondary signs such as diminished renal perfusion and reduced kidney size are equally evaluated on CTA and MRA, although MRA offers benefit in allowing measurement of blood flow by phase-contrast techniques. Estimation of renal perfusion can also be performed directly by MR using either a contrast bolus or by arterial spin labelling ( Fig. 77.6 ).
Transplant arteries are equally well demonstrated by CTA and MRA, although additional postprocessing may be required related to variation in the position of the transplant artery.
Imaging of the renal arteries should always be accompanied by imaging of the adrenals (to include noncontrast imaging on CT or in-phase and out-of-phase T 1 weighted imaging on MRI).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here